Abstract
Purpose
The purpose of this study was to quantify the presence of growth factors (GFs) and fibronectin in autologous platelet-rich plasma for topical ocular use (E-PRP) comparing their concentration when different preparation and preservation procedures were applied.
Methods
E-PRP was prepared with blood from healthy volunteers. The count of platelets, leukocytes, and red blood cells in the whole blood and E-PRP were performed. The concentration of the GFs platelet-derived growth factor BB (PDGF-BB), transforming growth factor β1 (TGF-β1), epidermal growth factor (EGF), vascular endothelial growth factor A (VEGF-A), and fibronectin was determined in each of the four procedures applied including fresh, frozen at −20°C for 3 months, fresh-spin, and frozen-spin at −20°C E-PRP samples. Posterior statistical analysis was performed to establish significant differences between groups and between GFs in relation to the amounts of platelets.
Results
Platelets in the E-PRP doubled in the number of basal values of whole blood (P ≤ 0.01). The blood cells in the E-PRP decreased drastically in red cells (99%) and also in leukocytes (82%). The concentration of PDGF-BB and EGF was significantly higher (P < 0.01) when the E-PRP samples were frozen at −20°C. However, no significant differences were observed for TGF-β1, VEGF-A, and fibronectin (P > 0.05). The concentration of GFs in the E-PRP did not necessarily correlate with the number of platelets.
Conclusions
Freezing the E-PRP for 3 months at −20°C increased the concentration of important proteins, such as PDGF-BB and EGF, and maintained the levels of others. These findings are essential because treatments, such as E-PRP, used by patients with ocular surface dysfunctions tend to prolong it in time. In addition, subsequent centrifugation of the E-PRP decreased the values of TFG-β1, but not the other GFs, which would allow adjusting the concentration of TFG-β1, as necessary. This procedure guarantees their correct conservation and viability.
Translational Relevance
This work demonstrates how clinical application can be improved by starting from basic research. The quantification of GFs and fibronectin in platelet-rich plasma (PRP) helps to clarify which is the best mode of preparation and preservation of PRP for clinical applications. This allows to optimize the product that is delivered to the patients as a treatment for the dysfunctions of the ocular surface, guaranteeing that the conservation does not affect at all the quality of the PRP that it is going to be used.
Keywords: autologous, platelet rich plasma, PRP, E-PRP, eye drops, growth factors, plasma, cornea
Introduction
There are different circumstances in which the ocular surface is altered. From a mild form, such as dry eye or environmental exposure, to a very severe one that may even compromise the patient's vision. Conventional treatments are sometimes not effective in solving the pathological condition. In this respect, the administration of blood products as a therapeutic option has acquired great prominence because of its biological composition and its ability to induce the regeneration of affected tissues.1–3 There are an immense variety of systems for obtaining autologous blood derivatives, with variable amounts of platelets and growth factors (GFs).
The application of platelet-rich plasma in ophthalmology (E-PRP) is a blood derivative used for the treatment of different affections of the ocular surface for a long time with excellent results. It was applied for the first time, either in the form of eye drops or as E-PRP clot, to corneal ulcers that did not cure adequately, and in the most severe cases together with amniotic membrane.4 Then it was used in patients with ocular surface dysfunction after LASIK,5–7 and also in patients with different severity of dry eye.8–10 It was subsequently used as a clot beneath bovine pericardium patches (Tutopatch; Tutogen Medical GmbH, Neunkirchen, Germany) in severe corneal ulcers or corneal perforations.11,12 Because of its great versatility, and in a further step toward the concept of fully autologous regeneration, E-PRP clots were used in the same type of patients but under an autologous fibrin membrane.13,14 The patches of bovine pericardium, such as the autologous fibrin membrane, were sutured to the conjunctiva and then a partial tarsorrhaphy was performed.14,15
Generally, the pathologies that involve ocular surface alterations require medium- or long-term treatments to obtain any significant improvement. Therefore it is necessary to know the viability and stability of the autologous preparation during that period to guarantee its correct composition and efficacy. Although E-PRP is currently used in the clinic with particularly good outcomes, there is not enough information regarding concentrations of major GFs and plasma proteins. No specific studies have been performed regarding their storage and conservation for the subsequent use as a treatment for different conditions of the ocular surface.
The aim of this study was to quantify the presence of GFs in E-PRP and to determine the storage procedures, which provided a higher concentration of them. For that objective, we compared the concentration of platelet-derived growth factor BB (PDGF-BB), transforming growth factor β1 (TGF-β1), epidermal growth factor (EGF), vascular endothelial growth factor A (VEGF-A), and fibronectin of fresh E-PRP and frozen E-PRP for 3 months at −20°C, with and without posterior centrifugation. The choice of these GFs was because of the beneficial effects observed in the maintenance of tissue homeostasis and the wound healing of the ocular surface because they affect cell proliferation, migration, differentiation, and survival.
Materials and Methods
This was an experimental study in which all the tests were performed in patients of Vissum Alicante, Spain. The study was conducted in accordance with the ethical standards set forth in the Declaration of Helsinki16 and with the approval by the Institute Ophthalmologic of Alicante Ethical Committee.
Volunteers
The consent form of all the volunteers was obtained before starting of the study.
The confidentiality of the data for the presentation of the outcomes was guaranteed, complying with the current General Data Protection Regulation (Regulation EU 2016/679).
Six healthy individuals (n = 6) were included in this study. Fifty percent of the cases were women, and the other 50% were men. Inclusion criteria to select normal volunteers for the study were subjects aged ≥18 years, not pregnant or breastfeeding women, with no systemic or ocular pathology, including dry eye, and any other ocular inflammatory disease.
To fulfill this last requirement, all the volunteers completed the Ocular Surface Disease Index (OSDI) questionnaire for ocular symptoms.17 The OSDI score is presented on a scale from 0 to 100, in which the high values are correlated with a greater disability of the ocular surface. This questionnaire is useful to discriminate normal subjects from subjects with some degree of alteration in the ocular surface. In this case, the index should be in the range considered as normal, OSDI ≤12.
Preparation of E-PRP
Autologous E-PRP was prepared according to the protocol described by our group in a previous study.8 Briefly, the blood of the volunteers was obtained by venipuncture in the arm in which Multyfly 21G (Sarstedt, Nümbrecht, Germany) and 9 mL vacuum tubes were used with 1 mL of 3.8% sodium citrate as anticoagulant (Monovette, Sarstedt, Nümbrecht, Germany). The blood cells, red cells, leukocytes, and platelets were counted in duplicate in the whole blood (before centrifugation), using the digital hematological counter Mythic 18 (Orphée, Montpellier, France). This procedure was also performed in duplicate after the E-PRP preparation. The means of the duplicate measurements were used for the subsequent analysis. The whole blood tubes were centrifuged at 340g (1400 rpm for 10 minutes) at 5°C in the Digicen 20 centrifuge (Orto-Alresa, Madrid, Spain) for the purpose of obtaining the E-PRP. This temperature allows decrease the platelet activation and increases its viability.18 After the centrifugation, each tube had three distinct phases. The lower, red, with the vast majority of the red cells in the sample, and the upper yellow representing the E-PRP. Between both, there was a thin white layer consisting of white cells. Manipulation of the plasma fraction, E-PRP, was performed under strict sterility conditions working inside the laminar flow cabin (Faster BHG 2004, Richmond Scientific Ltd, Great Britain) with sterile disposable materials.
All the E-PRP obtained avoiding the white layer were placed in disposable tubes of 50 mL. Then the final product was aliquoted in 1.5 mL conic tubes for further implementation of the four treatments with the following established parameters: (1) fresh; (2) fresh and spin at 5800g (6000 rpm for 5 minutes); (3) frozen at −20°C; and (4) frozen at −20°C and spin at 5800g before the enzyme-linked immunosorbent assay (ELISA) tests. “Fresh” means that no conservation or treatment was applied to these samples, and the tests were performed the same day of the blood extraction. Frozen samples were stored for 3 months at −20°C.
Determination of GFs in the E-PRP
To quantify the concentration of the most important GFs for ocular surface wound healing, such as EGF, PDGF-BB, TGF-β1, VEGF-A, and the plasma protein fibronectin, the sandwich ELISA immunoassay was performed. In the study the ELISA kit (eBioscience, Bender MedSystems GmbH, Vienna, Austria) was employed, which included 96 wells coated with a known amount of capture antibody and reagents necessary to do the experiment. The test was performed following indications of the manufacturers in the four groups of samples. Finally, a colored product was obtained whose absorbance was measured at a wavelength of 450 nm with the ELISA plate reader Tecan Sunrise-Basic (Tecan Sunrise Basic, Tecan Group Ltd, Männedorf, Switzerland) and the Maguellan software (Maguellan, Tecan Group Ltd, Männedorf, Switzerland). Antigen concentrations were calculated in the samples with the absorbance obtained. All samples were analyzed in duplicate because the specifications of the technique recommended it, not being necessary to do it in triplicate.
Sterility Control
To check product sterility, 1 mL of E-PRP sample subjected to four different treatments applied was cultured. We used thioglycolate broth and chocolate agar to qualitatively detect the presence of aerobic, anaerobic, and microaerophilic microorganisms. All samples were processed within the laminar flow hood. After inoculation, they were incubated at 35°C, the chocolate agar plate for 5 days, and thioglycolate broth for 21 days. All the cultures were monitored daily.
Statistical Analyses
The Kruskal-Wallis test was used to compare and to establish significant differences between GFs PDGF-BB, TFG-β, EGF, VEGF-A, and plasma protein fibronectin in E-PRP samples. This first analysis was performed with the SPSS software Version 15.0 (SPSS Inc., Chicago, IL) . In cases in which the Kruskal-Wallis was significant (when concentrations between treatments were different), multiple post hoc comparisons were performed using the Tukey test. This analysis was performed with the program RStudio 1.0.143 (RStudio, Boston, MA), Windows Vista/7/8/10. To determine whether the sample size was enough to answer our research question, we carried out a power analysis using the obtained results (mean differences between groups). In other words, we calculated how many participants per group we would need to achieve at least a power of 80% or 99%.19
In addition, a correlation analysis was performed between the concentration of the different GFs and fibronectin, and the blood cells present in the E-PRP (platelets, red cells, and leukocytes). Initially, it was performed for each of the GFs and the fibronectin globally, and then subsequently for each procedure applied to each GF separately. The results gave the Spearman correlation coefficient (r) along with the P value of the contrast of this coefficient. The significance level for all the contrasts of the analysis was 5% (P ≤ 0.05).
Results
E-PRP was obtained from six healthy individuals, aged 23 to 46 years (mean age ± standard deviation [SD], 32.5 ± 7.7). OSDI results of the donors were absolutely normal. The mean score ± SD obtained was 7.5 ± 4.3 and the values were ranged between 2.08 and 11.36, range considered as normal.17 Sterility tests carried out in E-PRP samples for each patient showed no microbial growth after the time established for each type of culture.
Table 1 shows the values of these cells in the whole blood (before centrifugation) and after obtaining the E-PRP. The results show that the platelets were concentrated almost twice, according to the concentration index obtained from 1.89. The red blood cells obtained in the E-PRP were almost insignificant compared with whole blood. The reduction observed was greater than 99%. Leukocyte counts in E-PRP were particularly low compared with whole blood, with a reduction of 82% in regard to baseline values. The increase of platelets and reduction of red and white blood cells in the E-PRP was statistically significant in regard to the baseline (P ≤ 0.001).
Table 1.
Blood Cell Count
| Platelets x103/µL | Red blood cells x106/µL | Leukocytes x103/µL | ||||
|---|---|---|---|---|---|---|
| WB | E-PRP | WB | E-PRP | WB | E-PRP | |
| Mean | 265.17 | 495.00* | 4.11 | 0.04* | 5.43 | 0.97* |
| SD | 69.75 | 95.14 | 0.32 | 0.01 | 0.70 | 0.30 |
SD, standard deviation; WB, whole blood.
P ≤ 0.001.
GFs and fibronectin concentrations in E-PRP were analyzed in samples on the day of the collection (fresh samples), and frozen at –20˚C after being stored for 3 months with or without posterior centrifugation. All the concentrations of the GFs increased considerably in the fresh or frozen E-PRP with respect to the basal plasmatic levels (Table 2). The exception was the fibronectin in which values increased slightly in the fresh or frozen E-PRP, with respect to the basal plasma levels, and EGF in which the value was similar to baseline in fresh E-PRP. The results obtained with each molecule for each of four treatments are shown in the Table 3. To determine whether the sample size was enough, we carried out a power analysis using the mean differences between groups to calculate how many participants per group we would need to achieve at least a power of 80%. These results are observed at the end of Table 3. Significant differences (P < 0.05) of PDGF-BB, TGF-β1, and EGF were obtained after performing the Kruskal-Wallis statistical test (Table 3). However, this statistical test did not specify which treatments were different, therefore the Mann-Whitney U test was performed. The concentration of PDGF-BB and EGF was significantly higher (P < 0.05) in treatment 3 (frozen at −20°C) and treatment 4 (frozen at −20°C and spin) over treatments 1 (fresh) and 2 (fresh and spin) (Figs. 1, 2). The concentration levels reached by TGF-β1 were significantly higher (P < 0.05) with treatment 1 (fresh) and 3 (frozen at −20°C) over treatments 2 (fresh and spin) and 4 (frozen at −20°C and spin) (Fig. 3). However, no significant differences were observed among the treatments for VEGF-A and fibronectin protein (P > 0.05) (Figs. 4, 5).
Table 2.
Comparison of Levels of GFs in Plasma and in Fresh and Frozen E-PRP
| Molecules | Plasmaa | Fresh E-PRPb | Frozen E-PRPb |
|---|---|---|---|
| PDGF-BB | <2000 pg/mL | 5065.83 pg/mL | 11,961.67 pg/mL |
| TGF-β 1 | 1725 pg/mL | 84,165.71 pg/mL | 74,710.36 pg/mL |
| EGF | 110–350 pg/mL | 338.24 pg/mL | 672.25 pg/mL |
| VEGF -A | 47.3 pg/mL | 274.25 pg/mL | 494.49 pg/mL |
| Fibronectin | 400 μg/mL | 544.10 μg/mL | 549.02 μg/mL |
Values obtained from the eBioscience Kit protocol.
Values obtained from our experiments.
Table 3.
Concentration of GFs and Fibronectin in the Four Treatments Applied to E-PRP
| E-PRP | PDGF-BB (pg/mL) | TGF-β1 (pg/mL) | EGF (pg/mL) | VEGF-A (pg/mL) | Fibronectin (µg/mL) |
|---|---|---|---|---|---|
| Fresh | |||||
| Mean | 5065.83 | 84165.71 | 338.24 | 274.25 | 544.10 |
| SD | 2028.96 | 1905.18 | 119.92 | 152.09 | 27.85 |
| Fresh spin | |||||
| Mean | 3186.67 | 7038.93 | 299.54 | 244.81 | 543.69 |
| SD | 2387.71 | 2084.48 | 130.49 | 212.89 | 17.14 |
| Frozen | |||||
| Mean | 11961.67 | 74710.36 | 672.25 | 494.49 | 549.02 |
| SD | 4287.07 | 7151.48 | 109.35 | 378.54 | 12.48 |
| Frozen spin | |||||
| Mean | 12855.4 | 35772.86 | 560.56 | 460.05 | 557.79 |
| SD | 4230.79 | 11292.15 | 99.03 | 354.05 | 11.27 |
| P value | 0.001 * | 0.000 * | 0.001 * | 0.198 | 0.471 |
| Sample size calculation (power = 80%) | 2 | 2 | 2 | 19 | 29 |
| Sample size calculation (power = 99%) | 4 | 2 | 4 | 41 | 62 |
SD, standard deviation.
Statistically significant differences between groups fresh, fresh spin, frozen, and frozen spin with the Kruskal-Wallis test (P ≤ 0.05).
Figure 1.
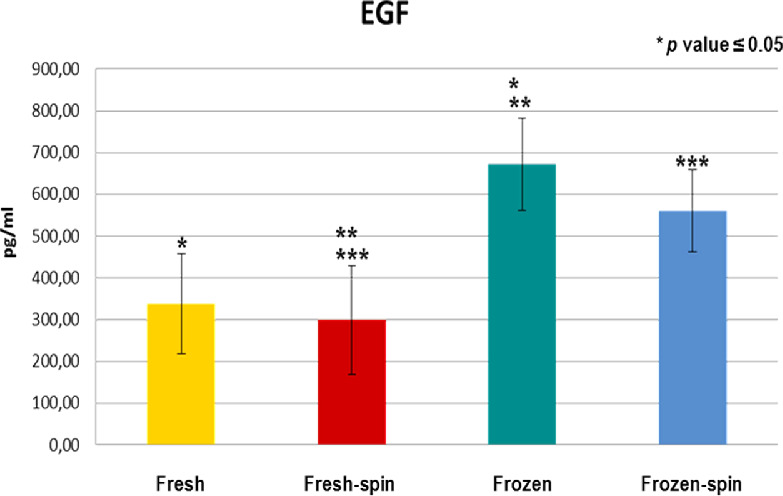
Concentration of EGF in E-PRP after the application of four procedures. **p ≤ 0.05 between the yellow group (fresh) and the blue (frozen-spin). ***p ≤ 0.05 between the red group (fresh-spin) and the green (frozen).
Figure 2.
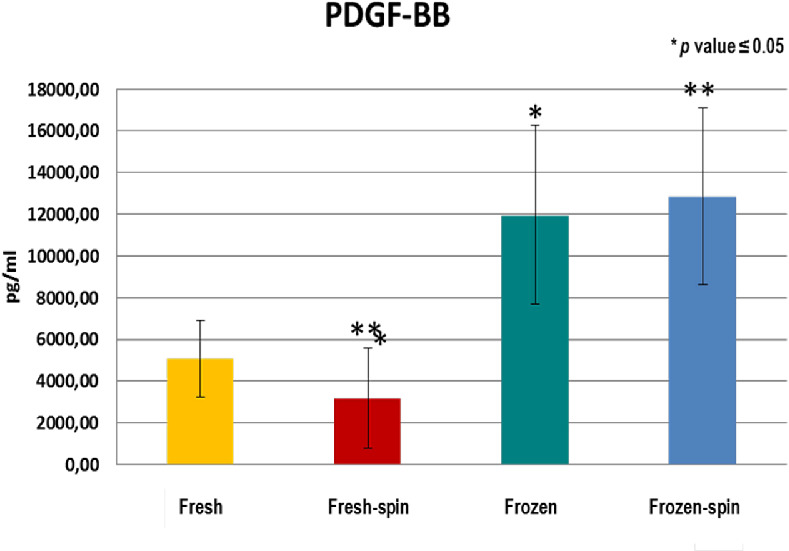
Concentration of PDGF-BB in E-PRP after the application of four procedures. **p ≤ 0.05 between the yellow group (fresh) and the blue (frozen-spin).
Figure 3.
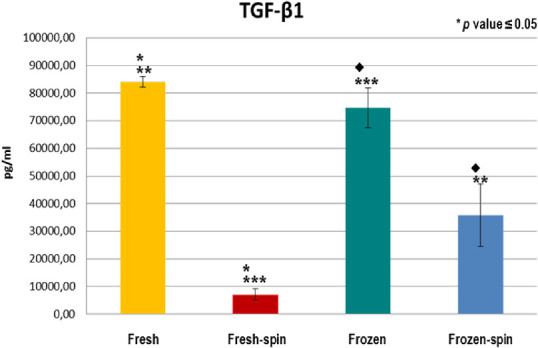
Concentration of TGF-β1 in E-PRP after the application of four procedures. **p ≤ 0.05 between the yellow group (fresh) and the blue (frozen-spin). ***p ≤ 0.05 between the red group (fresh-spin) and the green (frozen). ♦p ≤ 0.05 between the green group (frozen) and the blue (frozen-spin).
Figure 4.
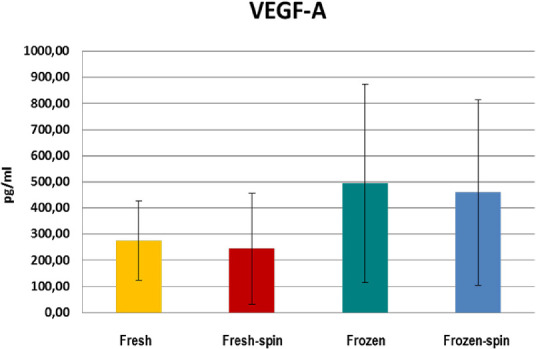
Concentration of VEGF-A in E-PRP after the application of four procedures.
Figure 5.
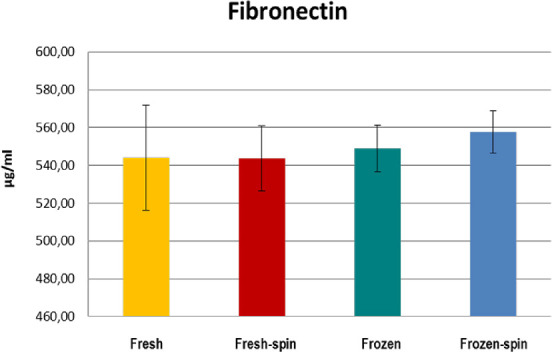
Concentration of the plasma protein fibronectin in E-PRP after the application of four procedures.
Statistical correlations were performed among GFs, fibronectin and blood cells present in the E-PRP samples subjected to the different treatments.
There was a positive and significant correlation between PDGF-BB in the fresh E-PRP and platelets (r = 0.841 and P = 0.036). On the contrary, no correlation was found between the platelet count and the rest of GFs or fibronectin (P > 0.05). In addition, there was also a strong and significant positive correlation between TGF-β1 and VEGF-A (r = 0.943 and P = 0.005), which shows a relationship between these two factors.
There was a significant and positive correlation between leukocytes and the concentration of TGF-β1 in the −20°C E-PRP (r = 0.932 and P = 0.007). This indicates that the higher concentration of leukocytes in the initial E-PRP (fresh) shows a higher concentration of TGF-β1 in the frozen E-PRP.
Discussion
The blood derivatives used in ophthalmology represent an immense variety of products, including autologous serum, traditional platelet-rich plasma (PRP), E-PRP, plasma rich in GFs (PRGF), platelet clot, or autologous fibrin membrane.2,20 Each of these products varies in their preparation, structure, composition, and concentration of GFs. The E-PRP has been used for the treatment of different affectations of the ocular surface, such as dry eye, post-LASIK, dormant corneal ulcers, or even as adjuvant in anterior segment reconstruction corneal surgeries for some time.7,10,14,15,21 Even more, recently E-PRP has been used intraocularly for an extreme case of hypotension.22 However, a detailed evaluation of the composition and biological stability of the preparation was still needed to confirm that the autologous E-PRP maintains its biological potential after preservation. The concentrations of platelets, leukocytes, and red blood cells in the whole blood and E-PRP were determined to achieve these objectives. The main GFs of the E-PRP and also fibronectin were quantified.
There are different opinions about which platelet concentration index is appropriate to use PRP as a therapeutic tool in the field of tissue regeneration. There are those who prefer high or very high concentration indices,23–25 who concentrate platelets moderately,8,15,26 or those who dilute it for clinical application.27,28 Finally, there are authors who prefer that their preparations do not contain platelets, either because they obtain autologous serum (AS),29–31 or those that prepare a plasma rich in platelets, but then activate it, to obtain PRGF.32–34 Both preparations, AS or PRGF, present variable concentrations of GF.32,35
In our study, the results obtained showed that the level of platelets in the E-PRP was almost twice as high as the basal values in the whole blood. Therefore the centrifugation speed used for the preparation of the E-PRP and the established time were adequate to ensure the optimal supply of GF for clinical uses.25,36 The red blood cells count in the E-PRP was almost insignificant, obtaining a reduction index greater than 99%. Leukocytes values found in the E-PRP were particularly low, showing a reduction of 82% in respect to the baseline. This concentration was comparable to that found by some authors in PRP25,37 or slightly higher than that described by others for PRGF.38,39 This small number of leukocytes found in the E-PRP do not classify the E-PRP as a blood derivative “with” leukocytes. PRP used in areas of medicine other than ophthalmology are prepared with high concentrations of white blood cells.39–44 Those PRPs used in ophthalmology incorporating the buffy coat would also fall into this category.25,27,28 As much as possible, leukocytes should be avoided in PRP preparations. Their presence increases the concentration of the proinflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α), and the metalloproteinases MMP-9 and MMP-13.45,46 However, it should be considered that non-leukocyte blood derivatives have significantly higher IL-1β than PRP with leukocytes.39 This is why, at the time of blood coagulation, the leukocytes become trapped in the clot releasing their content, including the ILs.
The EGF participates actively in the migration and proliferation of the corneal epithelium in tissue regeneration processes.47 The concentrations of EGF in the frozen E-PRP samples were higher than in fresh E-PRP, and higher than those found with PRGF,32,38 or with AS35 by another authors. The subsequent centrifugation did not modify the EGF concentration, probably because all of the EGF had been released after freezing. PDGF is a modulator of the chemotaxis and cellular mitosis of fibroblasts.48 Of the three different isoforms, PDGF-AA, PDGF-AB, PDGF-BB, we chose PDGF-BB because it has the highest chemotactic power among the three.49,50 PDGF-BB followed the same pattern as EGF, giving much higher values in frozen E-PRP. The concentrations obtained for PDGF-BB were comparable to those found by other authors,51,52 and much higher than those of other researchers.53 However, there are interesting studies that compare the GFs of samples of PRP, PRGF, and AS, but determine the PDGF-AB.32,35,38 The positive correlation between the platelets and the PDGF-BB was probably because of this GF that is released during the degranulation of platelets.54 Several authors have also found a positive correlation between them,55–57 whereas others have not found correlation.51,52,58 TGF-β1 is a multifunctional GF and one of the most important that intervene in the modulation of the behavior of ocular tissues59.59 Depending on the context, it may have proinflammatory or anti-inflammatory effects60.60 TGF-β1 values in the fresh and frozen E-PRP were high, but subsequent centrifugation drastically decreased its concentration. Then it could be presumed that platelets do not need to be activated to increase the TGF-β1 values. A great variability was found in the bibliography,61,62 but TGF-β1 values found in the E-PRP were higher than those obtained by others.32,63 However, it is known that TGF-β1 antagonizes epithelial migration stimulated by EGF, so it is better to have a lower concentration of this GF.64 The positive correlation found between TGF-β1 and the initial concentration of platelets by other authors56 did not occur in our study. A positive correlation was also found between leukocytes and TGF-β1 in the frozen E-PRP65.65 A strong and positive correlation was also found between TGF-β1 and VEGF-A. The presence of VEGF, dose-dependent, induces cell migration and cell proliferation, accompanied by upregulation of TGF-β1 mRNA.66 However, cytokines derived from leukocytes, together with the TGF-β1 released by platelets and plasma-derived factors, have the capacity to induce the expression of VEGF-A genes.67 In this way, VEGF-A and TGF-β1 cooperate to act in processes of cellular chemotaxis and neovascularization promoting the regeneration of tissues. VEGF-A is a potent angiogenic and endothelial cell mitogen that is present in platelets and endothelial cells, stimulating the proliferation of blood vessels.65 We did not find differences in VEGF-A between any of the treatments used, although a slightly higher concentration was found in the frozen samples, probably because the expression of VEGF-A is modulated by other GF, as well as by cytokines.68 Values in the fresh E-PRP were the same as those found by other authors in fresh PRGF, whereas the values of the frozen E-PRP turned out to be double those of the PRGF stored under the same conditions.32 In contrast, another study found that the highest levels of VEGF corresponded to PRGF.38 Although VEGF high values were found, they did not detect neovascularization in any of the patients treated with PRGF.33,69 Unlike the previous GFs, we would be interested that the VEGF values in the E-PRP were not very high because the overexpression of VEGF can lead to vascular alterations of the retina70 or maintenance of tumors71.71 Fibronectin is an adhesion protein, which is free in plasma, and is one of the main constituents of the extracellular matrix. It is one of the first to reach the place when there is a corneal wound, filling the bed of the lesion, which produces cell migration during the repair process of the corneal epithelium.72,73 The values obtained of this protein remained constant in the four types of procedures performed. This could be explained because fibronectin is a plasma protein and its presence does not depend on the activation of platelets. The possible limitations of this study could be related to the number of volunteers from whom the blood samples were obtained to perform the trial. However, because four different treatments had to be applied and five determinations were made in each one of them, it was not viable to increase the number of individuals. To draw relevant conclusions, a careful statistical analysis was carried out using the most restrictive tests for this type of study, including a power analysis. This analysis showed that between two and four samples were necessary to obtain significant results with a 99% power between groups.
Conclusions
The content of GFs in the E-PRP could be influenced by cells other than platelets, such as leukocytes, and there are other mechanisms of interaction, not dependent on platelets. In this study, different procedures applied to E-PRP have been evaluated to find out how they are affected in freezing, storage time, and centrifugation. According to the results obtained, we can conclude that freezing E-PRP at −20°C for 3 months provided higher levels of the GFs EGF and PDGF-BB, and maintained the concentrations of TGF-β1, VEGF-A, and fibronectin. These results are important because they make it possible to guarantee that the freezing of the E-PRP, as a preservation tool for this preparation, is adequate to maintain and even increase the GFs present in the E-PRP during the use of this treatment by patients. In addition, we observed that centrifugation after 5800g decreased TFG-β1 values, but not the other GFs. This aspect is particularly interesting because it would allow us to regulate the amount of TFG-β1 desired in the E-PRP in some way.
Acknowledgments
Network for Cooperative Research in Health “OFTARED” - Reference: RD16/0008/0012. Funded by Instituto de Salud Carlos III and co-funded by European Regional Development Fund (ERDF), “A way to make Europe”.
Disclosure: A.E. Rodriguez, None; S. Gisbert, None; A. Palazón, None; J.L. Alio, None
References
- 1. Garg A, Sheppard JD, Donnenfeld ED, Meyer D, Mehta CK. Ojo Seco Y Otros Trastornos de La Superficie Ocular: Diagnóstico Y Tratamiento En Xero-Dacriología. Madrid, España: Editorial Médica Panamericana, S.A.; 2008. [Google Scholar]
- 2. Nugent RB, Lee GA. Ophthalmic use of blood-derived products. Surv Ophthalmol. 2015; 60: 406–434. [DOI] [PubMed] [Google Scholar]
- 3. Vercesi A, Grande GA, Echevarría G, et al.. Evaluation of topical use of growth factors derived from platelets in the treatment of corneal ulcers in rabbits. Oftalmol Clin Exp. 2009; 3: 23–28. [Google Scholar]
- 4. Alio JL, Abad M, Artola A, Rodriguez-Prats JL, Pastor S, Ruiz-Colecha J. Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology. 2007; 114: 1286–1294. [DOI] [PubMed] [Google Scholar]
- 5. Alio JL, Pastor S, Ruiz-Colecha J, Rodriguez A, Artola A. Treatment of ocular surface syndrome after LASIK with autologous platelet-rich plasma. J Refract Surg. 2007; 23: 617–619. [DOI] [PubMed] [Google Scholar]
- 6. Javaloy J, Alió JL, Rodriguez AE, Vega A, Muñoz G. Effect of platelet-rich plasma in nerve regeneration after LASIK. J Refract Surg. 2013; 29: 213–219. [DOI] [PubMed] [Google Scholar]
- 7. Alio JL, Rodriguez AE, Abdelghany AA, Oliveira RF. Autologous platelet-rich plasma eye drops for the treatment of post-LASIK chronic ocular surface syndrome. J Ophthalmol. 2017; 2017: 2457620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alio J, Arnalich-Montiel F, Rodriguez A. The role of “eye platelet rich plasma” (E-PRP) for wound healing in ophthalmology. Curr Pharm Biotechnol. 2012; 13: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 9. Alio JL, Colecha JR, Pastor S, Rodriguez A, Artola A. Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res. 2007; 39: 124–129. [DOI] [PubMed] [Google Scholar]
- 10. Alio JL, Rodriguez AE, Ferreira-Oliveira R, Wróbel-Dudzińska D, Abdelghany AA. Treatment of dry eye disease with autologous platelet-rich plasma: a prospective, interventional, non-randomized study. Ophthalmol Ther. 2017; 6: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alio JL, Rodriguez AE, Martinez LM. Bovine pericardium membrane (Tutopatch) combined with solid platelet-rich plasma for the management of perforated corneal ulcers. Cornea. 2013; 32: 619–624. [DOI] [PubMed] [Google Scholar]
- 12. Ortuño-Prados VJ, Alio JL. Tratamiento de úlcera corneal neurotrófica con plasma rico en plaquetas y Tutopatch®. Arch Soc Esp Oftalmol. 2011; 86: 121–123. [DOI] [PubMed] [Google Scholar]
- 13. Alio JL, Rodriguez AE, Martinez LM, Rio AL. Autologous fibrin membrane combined with solid platelet-rich plasma in the management of perforated corneal ulcers. JAMA Ophthalmol. 2013; 131: 745–751. [DOI] [PubMed] [Google Scholar]
- 14. Arnalich F, Rodriguez AE, Luque-Rio A, Alio JL. Solid platelet rich plasma in corneal surgery. Ophthalmol Ther. 2016; 5: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alio JL, Rodriguez AE, WróbelDudzińska D. Eye platelet-rich plasma in the treatment of ocular surface disorders. Curr Opin Ophthalmol. 2015; 26: 325–332. [DOI] [PubMed] [Google Scholar]
- 16. General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014; 81: 14–18. [PubMed] [Google Scholar]
- 17. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000; 118: 615–621. [DOI] [PubMed] [Google Scholar]
- 18. Masoudi EA, Ribas J, Kaushik G, Leijten J, Khademhosseini A. Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Curr Stem Cell Rep. 2016; 2: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chow S, Wang H, Shao J. Sample Size Calculations in Clinical Research. 2nd ed New York, NY: Chapman & Hall/CRC; 2008. [Google Scholar]
- 20. De Pascale MR, Sommese L, Casamassimi A, Napoli C. Platelet derivatives in regenerative medicine: an update. Transfus Med Rev. 2015; 29: 52–61. [DOI] [PubMed] [Google Scholar]
- 21. Alio JL, Rodriguez AE, De Arriba P, Gisbert S, Abdelghany AA. Treatment with platelet-rich plasma of surgically related dormant corneal ulcers. Eur J Ophthalmol. 2018; 28: 515–520. [DOI] [PubMed] [Google Scholar]
- 22. Abdalrahman O, Rodriguez AE, Alio Del Barrio JL, Alio JL. Treatment of chronic and extreme ocular hypotension following glaucoma surgery with intraocular platelet-rich plasma: a case report. Eur J Ophthalmol. 2019; 29: NP9–NP12. [DOI] [PubMed] [Google Scholar]
- 23. Avila MY. Restoration of human lacrimal function following platelet-rich plasma injection. Cornea. 2014; 33: 18–21. [DOI] [PubMed] [Google Scholar]
- 24. Tanidir ST, Yuksel N, Altintas O, Yildiz DK, Sener E, Caglar Y. The effect of subconjunctival platelet-rich plasma on corneal epithelial wound healing. Cornea. 2010; 29: 664–669. [DOI] [PubMed] [Google Scholar]
- 25. Mazzocca AD. Platelet-rich plasma differs according to preparation method and human variability. J Bone Jt Surg. 2012; 94: 308. [DOI] [PubMed] [Google Scholar]
- 26. Figueroa MS, Govetto A, Arriba-Palomero Pd. Short-term results of platelet-rich plasma as adjuvant to 23-G vitrectomy in the treatment of high myopic macular holes. Eur J Ophthalmol. 2015; 26: 491–496. [DOI] [PubMed] [Google Scholar]
- 27. Kim KM, Shin Y-T, Kim HK. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn J Ophthalmol. 2012; 56: 544–550. [DOI] [PubMed] [Google Scholar]
- 28. Lee JH, Kim MJ, Ha SW, Kim HK. Autologous platelet-rich plasma eye drops in the treatment of recurrent corneal erosions. Korean J Ophthalmol. 2016; 30: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. López-García JS, García-Lozano I, Rivas L, Ramírez N, Raposo R, Méndez MT. Autologous serum eye drops diluted with sodium hyaluronate: clinical and experimental comparative study. Acta Ophthalmol. 2014; 92: 22–29. [DOI] [PubMed] [Google Scholar]
- 30. Urzua CA, Vasquez DH, Huidobro A, Hernandez H, Alfaro J. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Curr Eye Res. 2012; 37: 684–688. [DOI] [PubMed] [Google Scholar]
- 31. Celebi ARC, Ulusoy C, Mirza GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefe's Arch Clin Exp Ophthalmol. 2014; 252: 619–626. [DOI] [PubMed] [Google Scholar]
- 32. Anitua E, Muruzabal F, Pino A, Merayo-Lloves J, Orive G. Biological stability of plasma rich in growth factors eye drops after storage of 3 months. Cornea. 2013; 32: 1380–1386. [DOI] [PubMed] [Google Scholar]
- 33. Lopez-Plandolit S, Morales M-C, Freire V, Grau AE, Duran JA. Efficacy of plasma rich in growth factors for the treatment of dry eye. Cornea. 2011; 30: 1312–1317. [DOI] [PubMed] [Google Scholar]
- 34. Merayo-Lloves J, Sanchez-Avila RM, Riestra AC, et al.. Safety and efficacy of autologous plasma rich in growth factors eye drops for the treatment of evaporative dry eye. Ophthalmic Res. 2016; 56: 68–73. [DOI] [PubMed] [Google Scholar]
- 35. López-García JS, García-Lozano I, Rivas L, Ramírez N, Méndez MT, Raposo R. Stability of growth factors in autologous serum eyedrops after long-term storage. Curr Eye Res. 2015; 3683: 1–7. [DOI] [PubMed] [Google Scholar]
- 36. Stellos K, Kopf S, Paul A, et al.. Platelets in regeneration. Semin Thromb Hemost. 2010; 36: 175–184. [DOI] [PubMed] [Google Scholar]
- 37. Pochini AC, Antonioli E, Bucci DZ, et al.. Analysis of cytokine profile and growth factors in platelet-rich plasma obtained by open systems and commercial columns. Einstein (São Paulo). 2016; 14: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anitua E, de la Fuente M, Muruzabal F, Riestra A, Merayo-Lloves J, Orive G. Plasma rich in growth factors (PRGF) eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Exp Eye Res. 2015; 135: 118–126. [DOI] [PubMed] [Google Scholar]
- 39. Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015; 473: 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anitua E, Zalduendo MM, Prado R, Alkhraisat MH, Orive G. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015; 103: 1011–1020. [DOI] [PubMed] [Google Scholar]
- 41. Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS One. 2015; 10: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beca T, Hernandez G, Morante S, Bascones A. Plasma rico en plaquetas. Una revisión bibliográfica. Av Periodoncia. 2007; 19: 39–52. [Google Scholar]
- 43. Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutan Aesthet Surg. 2014; 7: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhong W, Sumita Y, Ohba S, et al.. In vivo comparison of the bone regeneration capability of human bone marrow concentrates vs. platelet-rich plasma. PLoS One. 2012; 7: e40833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Jt Surg Am. 2012; 94: 1–8. [DOI] [PubMed] [Google Scholar]
- 46. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011; 39: 2135–2140. [DOI] [PubMed] [Google Scholar]
- 47. Wang L, Wu X, Shi T, Lu L. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor κB subtype-regulated CCCTC binding factor (CTCF) activation. J Biol Chem. 2013; 288: 24363–24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006; 81: 1241–1257. [DOI] [PubMed] [Google Scholar]
- 49. Kim WJ, Mohan RR, Mohan RR, Wilson SE. Effect of PDGF, IL-alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest Ophthalmol Vis Sci. 1999; 40: 1364–1372. [PubMed] [Google Scholar]
- 50. Colciago A, Celotti F, Casati L, et al.. In vitro effects of PDGF isoforms (AA, BB, AB and CC) on migration and proliferation of SaOS-2 osteoblasts and on migration of human osteoblasts. Int J Biomed Sci. 2009; 5: 380–389. [PMC free article] [PubMed] [Google Scholar]
- 51. González M, Arteaga-vizcaíno M, Ruiz A, et al.. Niveles del factor de crecimiento derivado de plaquetas en el plasma rico en plaquetas antes y despues de antiagregantes plaquetarios (PDGF levels in platelet-rich plasma before and after anti platelets drugs). Av en Biomed. 2013; 2: 127–136. [Google Scholar]
- 52. Weibrich G, Kleis WKG, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002; 30: 97–102. [DOI] [PubMed] [Google Scholar]
- 53. Ronci C, Ferraro AS, Lanti A, et al.. Platelet-rich plasma as treatment for persistent ocular epithelial defects. Transfus Apher Sci. 2015; 52: 300–304. [DOI] [PubMed] [Google Scholar]
- 54. González M, Arteaga-Vizcaíno M, Benito M, Benito M. Application of platelet rich plasma (PRP) and its derivatives in dental implantologie and plastic surgery. Invest Clin. 2012; 53: 408–418. [PubMed] [Google Scholar]
- 55. Kobayashi Y, Saita Y, Nishio H, et al.. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J Orthop Sci. 2016; 21: 683–689. [DOI] [PubMed] [Google Scholar]
- 56. Yuan N, Wang C, Wang Y, et al.. Preparation of autologous platelet-rich gel for diabetic refractory dermal ulcer and growth factors analysis from it. Chinese J Reparative Reconstr Surg. 2008; 22: 468–471. [PubMed] [Google Scholar]
- 57. Zimmermann R, Jakubietz R, Jakubietz M, et al.. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001; 41: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 58. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004; 114: 1502–1508. [DOI] [PubMed] [Google Scholar]
- 59. Saika S. TGFbeta pathobiology in the eye. Lab Invest. 2006; 86: 106–115. [DOI] [PubMed] [Google Scholar]
- 60. Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopinski P. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol. 2016; 41: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011; 39: 266–271. [DOI] [PubMed] [Google Scholar]
- 62. Grainger DJ, Mosedale DE, Metcalfe JC. TGF-beta in blood: a complex problem. Cytokine Growth Factor Rev. 2000; 11: 133–145. [DOI] [PubMed] [Google Scholar]
- 63. Stenwall PA, Bergström M, Seiron P, et al.. Improving the anti-inflammatory effect of serum eye drops using allogeneic serum permissive for regulatory T cell induction. Acta Ophthalmol. 2015; 93: 654–657. [DOI] [PubMed] [Google Scholar]
- 64. Mishima H1, Nakamura M, Murakami J, Nishida T, Otori T. Transforming growth factor-beta modulates effects of epidermal growth factor on corneal epithelial cells. Curr Eye Res. 1992; 11: 691–696. [DOI] [PubMed] [Google Scholar]
- 65. De La Mata J. Plasma rico en plaquetas: ¿un nuevo tratamiento para el reumatologo? Reumatol Clin. 2013; 9: 166–171.22902984 [Google Scholar]
- 66. Li ZD, Bork JP, Krueger B, et al.. VEGF induces proliferation, migration, and TGF-beta1 expression in mouse glomerular endothelial cells via mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Biochem Biophys Res Commun. 2005; 334: 1049–1060. [DOI] [PubMed] [Google Scholar]
- 67. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004; 56: 549–580. [DOI] [PubMed] [Google Scholar]
- 68. Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001; 26: 25–35. [DOI] [PubMed] [Google Scholar]
- 69. López-Plandolit S, Morales MC, Freire V, Etxebarría J, Durán JA. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea. 2010; 29: 843–848. [DOI] [PubMed] [Google Scholar]
- 70. Duffy AM, Bouchier-Hayes DJ, Harmey JH. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. Austin, TX: Landes Bioscience; 2004. [Google Scholar]
- 71. Stevenson W, Cheng SF, Dastjerdi MH, Ferrari G, Dana R. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin). Ocul Surf. 2012; 10: 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pastar I, Stojadinovic O, Yin NC, et al.. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014; 3: 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015; 49: 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


