Abstract
Purpose
Although the expression of microRNAs (miRNAs) in retinal pigment epithelial (RPE) cells undergoing epithelial-mesenchymal transition (EMT) is involved in the pathogenesis of proliferative vitreoretinopathy (PVR), its expression in the vitreous of patients with primary retinal detachment (RD) and different PVR grading has not yet been investigated. We assessed the expression of miRNAs in the vitreous humor (VH) of patients diagnosed with RD and different grading of PVR.
Methods
The VH was extracted from the core of the vitreous chamber in patients who had undergone standard vitrectomy for primary RD. RNA was extracted and TaqMan Low-Density Arrays (TLDAs) were used for miRNA profiling that was performed by single TaqMan assays. A gene ontology (GO) analysis was performed on the differentially expressed miRNAs.
Results
A total of 15 eyes with RD, 3 eyes for each grade of PVR (A, B, C, and D) and 3 from unaffected individuals, were enrolled in this prospective comparative study. Twenty miRNAs were altered in the comparison among pathological groups. Interestingly, the expression of miR-143-3p, miR-224-5p, miR-361-5p, miR-452-5p, miR-486-3p, and miR-891a-5p increased with the worsening of PVR grading. We also identified 34 miRNAs showing differential expression in PVR compared to control vitreous samples. GO analysis showed that the deregulated miRNAs participate in processes previously associated with PVR pathogenesis.
Conclusions
The present pilot study suggested that dysregulated vitreal miRNAs may be considered as a biomarker of PVR and associated with the PVR-related complications in patients with RD.
Translational Relevance
The correlation between vitreal miRNAs and the pathological phenotypes are essential to identify the novel miRNA-based mechanisms underlying the PVR disease that would improve the diagnosis and treatment of the condition.
Keywords: microRNA, profiling, proliferative vitreoretinopathy, retinal detachment
Introduction
Proliferative vitreoretinopathy (PVR) is a multifactorial and complex clinical syndrome common to a variety of clinical disorders, including retinal detachment (RD).1 The frequency of PVR remains largely unchanged in primary RD, with the incidence ranging from 5.1% to 11.7% of all rhegmatogenous RDs, and it is believed to be the leading cause of RD surgery failure accounting for 75% of retinal redetachment surgeries.1,2 PVR is characterized by pre-, sub-, or intra-retinal fibrosis (scarring) that grows on the membrane surface of the detached retina and posterior hyaloids causing foreshortening of the retina, traction, and recurrent detachment mostly within the first 6 to 8 weeks after surgery.1 Typically, PVR with recurrent RDs requires additional surgical interventions and is associated with poor visual recovery.2–8
Although the pathogenesis is not elucidated,8–10 previous studies suggested that the epithelial-mesenchymal transition (EMT)11–13 of the retinal pigment epithelial (RPE) cells and the inflammatory response-associated pathways might be involved in the pathogenesis underlying PVR.13–18 However, to date, there are no effective medications for the prevention and treatment of PVR and an urgent approach is demanded.19–22
MicroRNAs (miRNAs) are small endogenous noncoding RNAs that negatively regulate gene expression within all cell types. The miRNAs play a key role in cellular physiology and various biological pathways in specific cell types and tissues.23–25 Previously, abnormal miRNA expression has been reported in cellular and extracellular compartments with respect to cancers and other diseases, such as cardiac, neurological, and ocular,26–32 and previous studies have shown that specific miRNAs induce/inhibit EMT in other fibroblast-like cells.33–35
The aberrant expression of miRNAs in RPE cells undergoing EMT is involved in the pathogenesis of PVR.36–44 Takayama et al. demonstrated the involvement of miR-148a-3p in the regulation of migration ability of RPE cells,36 and the same function was reported by Wang et al. for miR-182.44 Nevertheless, the characteristics and the distinct role of miRNAs in PVR and their expression in the vitreous of patients with primary RD with different PVR grading have been poorly investigated, with only few papers published.37,45
In this pilot study, 754 miRNAs were subjected to real-time PCR expression profiling in order to identify the differentially expressed miRNAs in the vitreous of patients diagnosed with primary RD and a different grading of PVR.
Materials and Methods
This prospective pilot study included consecutive eyes undergoing pars plana vitrectomy for the treatment of primary RD with and without PVR.
All surgery procedures were performed by the same surgeon at the Department of General Ophthalmology, Medical University of Lublin (Poland) between January and June 2018.
The exclusion criteria were as follows: patients with diabetes mellitus, known rheumatic and autoimmune diseases, systemic treatments involving corticosteroids or immunomodulatory drugs, vitreous hemorrhage, uveitis, glaucoma, or any concomitant retinal pathology, a previous ocular trauma, a diagnosed eye tumor, or who had undergone intraocular surgery or treatment within 6 months after the diagnosis of RD. These systemic or ocular comorbidities might influence the mechanisms underlying ocular fibrosis.
The present patient study was approved by the Ethics Committee of the Medical University of Lublin (KE-0254/277/2019) in compliance with the Declaration of Helsinki. Written informed consent was obtained from each participant allowing the use of their biological materials and clinical data.
PVR Grading and Patient Grouping
Based on the severity of the PVR, the patients were classified into four stages: A (minimal), B, C, and D (massive) according to the “Retina Society Terminology Committee.”46
As proposed by Zandi et al.,18 in the current study, the risk of developing postoperative PVR in patients with RD with low PVR severity (grades A or B) or without PVR was found to be similar. Thus, PVR grade C was included until three quadrants were seen with visible PVR membrane formation. However, the severity was grade D if all four quadrants were affected.
Because advanced PVR is challenging for accurate grading, all patients underwent indirect fundus ophthalmoscopy with scleral indentation prior to surgery. Two masked expert retinal specialists (M.D.T. and K.N.) investigated the fundus and assigned the PVR score; the discrepancies were resolved by a third investigator (R.R.).
Control vitreous samples (CTRL) were obtained from patients who underwent vitrectomy for primary symptomatic idiopathic floaters.
Handling of Vitreous Fluid Samples
A 3-port 23-gauge vitrectomy was performed on all the patients while they were under local anesthesia. The Resight 700 (Carl Zeiss Meditec AG, Jena, Germany) wide-angle viewing system or the Binocular Indirect Ophthalmo-Microscope wide-angle viewing system (BIOM; Oculus Inc., Wetzlar, Germany) was used. Sclerotomy was carried out at 3.5 mm parallel to the limbus at 30°.33 Then, 2 mL vitreous sample extracted from the core of the vitreous cavity before vitrectomy was subjected to centrifugation at 700 × g for 10 minutes to exclude any circulating cells or debris. The pellets were stored at −80°C until further analysis.
miRNA Expression Profiling in Vitreous Humor by TLDAs
Total RNA was isolated from 400 µL vitreous humor (VH) using miRNeasy Mini Kit (Qiagen), according to the manufacturer's protocol. The amount and purity of RNA were assessed using NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). The expression of 754 miRNAs was evaluated by real-time PCR using the TaqMan Low-Density Arrays (TLDAs) from 15 VH samples (3 patients for each grade of the disease and 3 unaffected individuals). About 30 ng of RNA was transcribed using TaqMan MicroRNA Reverse Transcription Kit and Megaplex RT Primers Human Pool A version 2.1 and Pool B version 3.0 (Thermo Fisher Scientific) and pre-amplified by TaqMan PreAmp Master Mix Kit and Megaplex PreAmp Primers using the Human Pool A version 2.1 and Pool B version 3.0 (Thermo Fisher Scientific). The products were loaded in TaqMan Human MicroRNA Array version 3.0 A and B (Thermo Fisher Scientific), and the real-time PCR reactions were carried out on a 7900 HT Fast Real-Time PCR System (Applied Biosystems) using TaqMan Universal Master Mix II without uracil-DNA N-glycosylase UNG (Thermo Fisher Scientific), according to the manufacturer's instructions. Expression fold changes of differentially expressed (DE) miRNAs were calculated by applying the 2−ΔΔCt method.47
Statistical Analysis
The expression data were subjected to significance analysis of microarrays (SAMs), computed by Multi Experiment Viewer version 4.8.1 (http://mev.tm4.org) using the multiclass tests and 1-way analysis of variance (ANOVA) test (P < 0.05) among ΔCts. The endogenous control was selected based on the global median normalization method, which allowed us to identify the miRNAs with the most stable expression in the samples.24 We used three different endogenous controls for each TLDA panel (comparisons among PVR stages: panel A: miR-197, U6, and median of ΔCts; panel B: miR-1285, U6, and median of ΔCts; PVR versus CTRL: panel A: median of ΔCts, miR-146a-5p, and miR-28-3p; panel B: median of ΔCts, miR-625-3p, and miR-30e-3p; A + B versus CTRL: panel A: miR-29a-3p, miR-20b-5p, and miR-24-3p; panel B: miR-30-3p, median of ΔCts, and miR-30a-5p; C + D versus CTRL: panel A: miR-17-5p, miR-320a, and miR-28-3p; panel B: U6, median of ΔCts, and miR-30e-3p), considering differentially expressed only those miRNAs that were deregulated according to two endogenous control. Gene ontology (GO) analysis was performed on the DE miRNAs through DIANA-miRPath version 3.0 (http://snf-515788.vm.okeanos.grnet.gr/).48
Results
miRNA Expression Profile in the VH of Patients With PVR
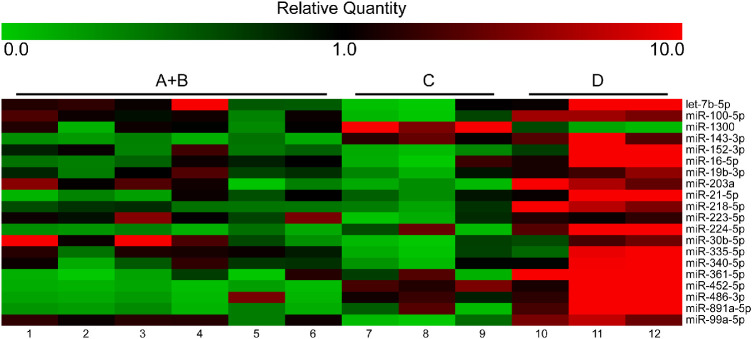
The expression of 754 miRNAs in the VH of 15 patients, including 3 patients for each grade of the disease (A, B, C, and D) and 3 unaffected individuals, was analyzed by TLDA profiling. When comparing the different disease stages, statistical analysis of profiling results was performed by grouping A and B samples, characterized by absent or minimal proliferation. We identified 20 miRNAs with altered expression in one or more pathological groups, according to at least two out of three endogenous controls. Specifically, let-7b-5p, miR-100-5p, miR-1300, miR-143-3p, miR-152-3p, miR-16-5p, miR-19b-3p, miR-203a, miR-21-5p, miR-218-5p, miR-223-5p, miR-224-5p, miR-30b-5p, miR-335-5p, miR-340-5p, miR-361-5p, miR-452-5p, miR-486-3p, miR-891a-5p, and miR-99a-5p showed differential expression in different comparisons (Table 1, Fig. 1).
Table 1.
TLDA Profiling Showed the Differential Expression of 20 MiRNAs in the Different Comparisons
| ANOVA | C Versus A + B | D Versus A + B | D Versus C | ||||
|---|---|---|---|---|---|---|---|
| miRNA | P Value | FC | P Value | FC | P Value | FC | P Value |
| let-7b-5p | 0.022 | −16.85 | 0.021 | 3.76 | 0.33 | 28.76 | 0.009 |
| miR-100-5p | 0.003 | −13.21 | 0.008 | 4.9 | 0.06 | 24.76 | 0.001 |
| miR-1300 | 0.003 | 12.59 | 0.005 | −3.45 | 0.1 | −23.53 | 0.001 |
| miR-143-3p | < 0.0001 | 9.93 | 0.0005 | 26.41 | < 0.0001 | 2.65 | 0.08 |
| miR-152-3p | 0.01 | −5.28 | 0.1 | 12.03 | 0.024 | 33.68 | 0.003 |
| miR-16-5p | 0.026 | −4.94 | 0.22 | 16.77 | 0.035 | 32.68 | 0.009 |
| miR-19b-3p | 0.019 | −2.79 | 0.1 | 3.59 | 0.049 | 10.03 | 0.006 |
| miR-203a | 0.033 | −5.64 | 0.13 | 8.95 | 0.07 | 30.51 | 0.011 |
| miR-21-5p | 0.011 | 1.19 | 0.83 | 22.15 | 0.004 | 18.56 | 0.013 |
| miR-218-5p | < 0.0001 | −1.45 | 0.27 | 15.28 | < 0.0001 | 22.3 | < 0.0001 |
| miR-223-5p | 0.016 | −11.65 | 0.008 | 1.14 | 0.85 | 13.3 | 0.013 |
| miR-224-5p | 0.003 | 2.46 | 0.38 | 36.26 | 0.001 | 28.97 | 0.01 |
| miR-30b-5p | 0.039 | −26.91 | 0.017 | −1.02 | 0.98 | 26.33 | 0.034 |
| miR-335-5p | 0.025 | −8.72 | 0.033 | 3.09 | 0.22 | 26.99 | 0.009 |
| miR-340-5p | 0.031 | −2.91 | 0.25 | 8.36 | 0.039 | 24.38 | 0.012 |
| miR-361-5p | 0.003 | 4.82 | 0.26 | 36.81 | 0.001 | 18.78 | 0.014 |
| miR-452-5p | 0.0002 | 19.17 | 0.001 | 33.38 | < 0.0001 | 5.6 | 0.07 |
| miR-486-3p | 0.011 | 6.06 | 0.08 | 32.98 | 0.004 | 5.43 | 0.14 |
| miR-891a-5p | 0.002 | 2.46 | 0.4 | 35.38 | 0.0007 | 21.01 | 0.005 |
| miR-99a-5p | 0.001 | −21.67 | 0.002 | 3.82 | 0.1 | 32.84 | 0.0006 |
The average FC and the P value derived from multiple comparisons for each miRNA are shown. The P value of the ANOVA test between all groups is also shown. Significant P values are highlighted in bold.
FC, fold change.
Figure 1.
Heatmap showing the expression of miRNAs analyzed through TLDA profiling. The miRNA expression is represented as relative quantity (RQ), calculated with respect to the average of ΔCts of all the analyzed samples.
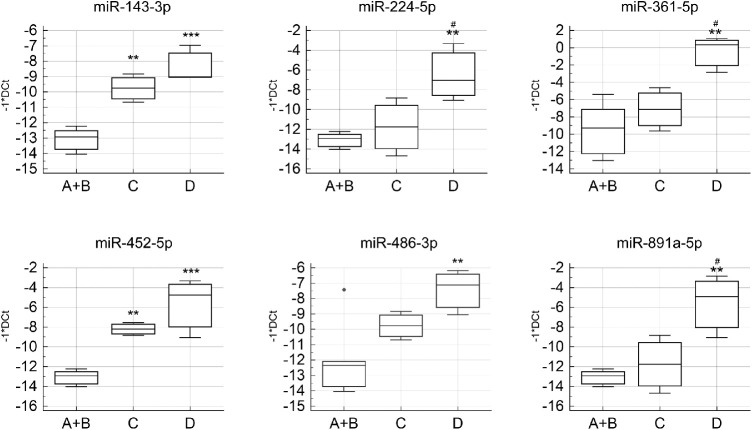
Interestingly, miR-143-3p, miR-224-5p, miR-361-5p, miR-452-5p, miR-486-3p, and miR-891a-5p expression aggravated the disease, suggesting a possible application of these miRNAs as biomarkers for PVR (Fig. 2).
Figure 2.
Boxplots showing the expression of miR-143-3p, miR-224-5p, miR-361-5p, miR-452-5p, miR-486-3p, and miR-891a-5p in the three pathological groups. **P value versus A + B < 0.005; ***P value versus A + B < 0.0005; #P value versus C < 0.05.
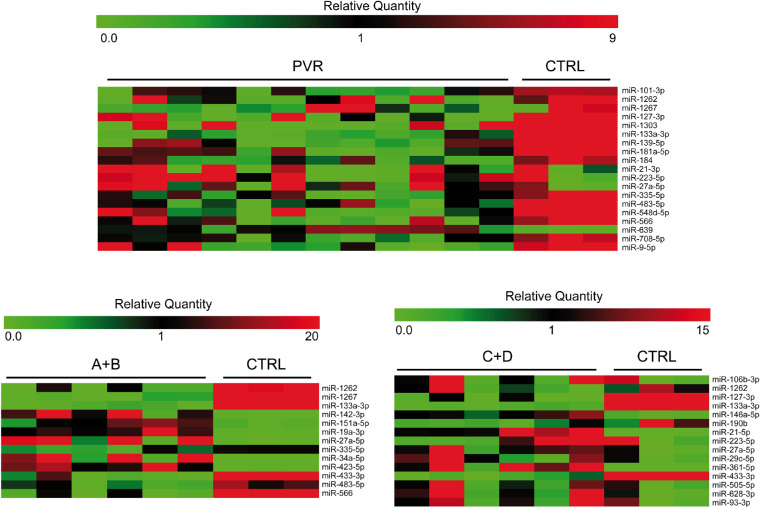
In addition, we compared the vitreous of patients with PVR to unaffected individuals. This analysis was performed in three steps, grouping all PVR samples, samples from stages A and B, and samples from stages C and D, respectively. This additional analysis showed the deregulation of different sets of miRNAs in each comparison (Fig. 3, Tables 2, 3, 4).
Figure 3.
Heatmap showing the expression of miRNAs analyzed through TLDA profiling in the comparisons of patients with PVR compared to unaffected individuals. The miRNA expression is represented as relative quantity (RQ), calculated with respect to the average of ΔCts of all the analyzed samples.
Table 2.
The miRNAs Showing Altered Expression in all Patients With PVR Compared to Unaffected Individuals
| PVR versus CTRL | ||
|---|---|---|
| miRNA | FC | P Value |
| miR-101-3p | −6.94 | 0.001 |
| miR-1262 | −24.41 | 3.00E-05 |
| miR-1267 | −54.44 | 0.001 |
| miR-127-3p | −49.79 | 6.00E-05 |
| miR-1303 | −45.25 | 2.00E-05 |
| miR-133a-3p | −61.18 | 1.00E-05 |
| miR-139-5p | −23.79 | 1.00E-04 |
| miR-181a-5p | −13.55 | 0.002 |
| miR-184 | −7.37 | 2.00E-04 |
| miR-21-3p | 22.23 | 0.001 |
| miR-223-5p | 57.22 | 1.00E-06 |
| miR-27a-5p | 53.55 | 0.001 |
| miR-335-5p | −10.61 | 0.002 |
| miR-483-5p | −15.59 | 2.00E-04 |
| miR-548d-5p | −13.3 | 7.00E-04 |
| miR-566 | −33.43 | 1.00E-05 |
| miR-639 | 13.24 | 7.00E-06 |
| miR-708-5p | −7.68 | 4.00E-04 |
| miR-9-5p | −13.95 | 0.001 |
The average FC and the P value derived from the t-test are shown for each miRNA.
FC, fold change.
Table 3.
The MiRNAs Showing Altered Expression in Stage A + B Patients Compared to Unaffected Individuals
| A + B versus CTRL | ||
|---|---|---|
| miRNA | FC | P Value |
| miR-1262 | −27.56 | 0.003 |
| miR-1267 | −59.32 | 0.001 |
| miR-133a-3p | −63.19 | 1.00E-05 |
| miR-142-3p | 32.66 | 0.001 |
| miR-151a-5p | 18.31 | 1.00E-03 |
| miR-19a-3p | 25.32 | 1.00E-04 |
| miR-27a-5p | 48.41 | 0.003 |
| miR-335-5p | −5.75 | 0.007 |
| miR-34a-5p | 38.92 | 0.003 |
| miR-423-5p | 45.42 | 6.00E-05 |
| miR-433-3p | −41.76 | 0.002 |
| miR-483-5p | −10.67 | 0.003 |
| miR-566 | −38.37 | 0.002 |
The average FC and the P value derived from the t-test are shown for each miRNA.
FC, fold change.
Table 4.
The MiRNAs Showing Altered Expression in Stage C + D Patients Compared to Unaffected Individuals
| C+D vs CTRL | ||
|---|---|---|
| miRNA | FC | P-Value |
| miR-106b-3p | 42.68 | 0.001 |
| miR-1262 | −21.96 | 0.008 |
| miR-127-3p | −56.47 | 0.003 |
| miR-133a-3p | −45.23 | 6.00E-06 |
| miR-146a-5p | 11.88 | 0.005 |
| miR-190b | −24.04 | 7.00E-05 |
| miR-21-5p | 43.92 | 6.00E-04 |
| miR-223-5p | 47.08 | 0.003 |
| miR-27a-5p | 53.79 | 0.001 |
| miR-29c-5p | 28.51 | 0.001 |
| miR-361-5p | 52.31 | 0.002 |
| miR-433-3p | −44.76 | 0.005 |
| miR-505-5p | 28.64 | 0.002 |
| miR-628-3p | 31.01 | 0.001 |
| miR-93-3p | 39.46 | 0.001 |
The average FC and the P value derived from the t-test are shown for each miRNA.
FC, fold change.
GO analysis
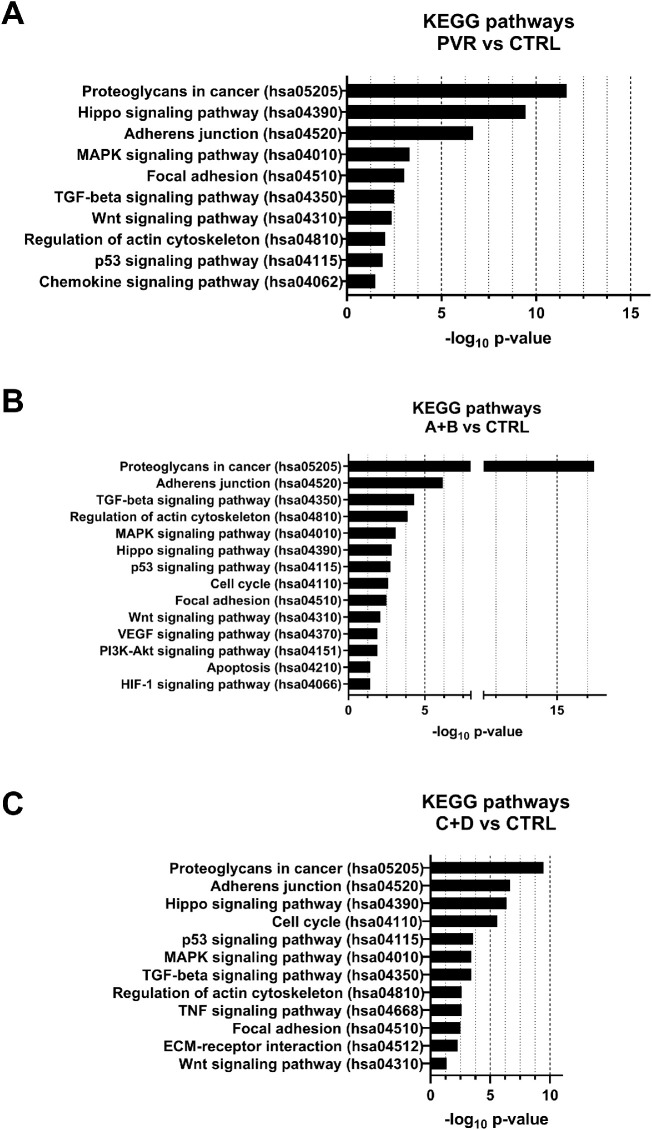
GO analysis was performed on the six miRNAs showing increased expression with PVR worsening. GO results showed that miR-143-3p, miR-224-5p, miR-361-5p, miR-452-5p, miR-486-3p, and miR-891a-5p participate in the biological processes involved in PVR pathogenesis, such as cell cycle regulation, adhesion to the extracellular matrix (ECM), and regulation of actin cytoskeleton10,49–51 (Fig. 4).
Figure 4.
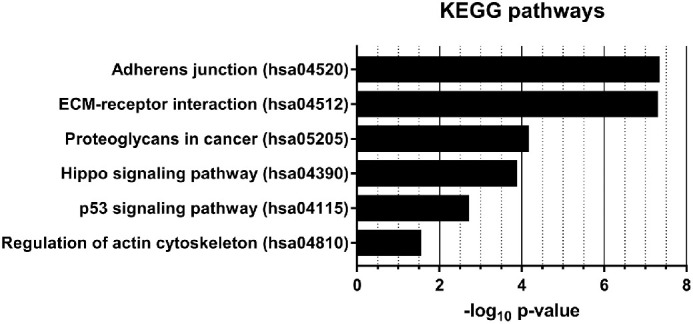
PVR-related Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways regulated by miR-143-3p, miR-224-5p, miR-361-5p, miR-452-5p, miR-486-3p, and miR-891a-5p. The X-axis represents the -log10 of the P value for each pathway.
Similarly, GO analysis was performed on miRNAs showing altered expression in the three comparisons with respect to unaffected individuals. Again, results showed the involvement of DE miRNAs in biological processes previously associated with PVR pathogenesis10,49–51 (Fig. 5).
Figure 5.
PVR-related Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways regulated by miRNAs showing altered expression in the comparisons PVR versus CTRL, A + B versus CTRL, and C + D versus CTRL. The X-axis represents the -log10 of the P value for each pathway.
Discussion
The current pilot study identified altered expression of 20 miRNAs in one or more pathological groups of the VH of patients with primary RD and a different grading of PVR. The analysis revealed that the expression of six miRNAs (miR-143-3p, miR-224-5p, miR-361-5p, miR-452-5p, miR-486-3p, and miR-891a-5p) increased with the worsening of the disease; similarly, expression profiles of vitreal miRNAs in patients with PVR were compared with unaffected individuals, showing that several miRNAs may be used as molecular biomarkers for the disease. According to the GO analysis, these miRNAs participated in the biological processes involved in PVR pathogenesis, such as cell cycle regulation, adhesion to ECM, and regulation of actin cytoskeleton.
PVR is the main cause of retinal surgical failure.52 The PVR is primarily treated using vitrectomy, systematic peeling and dissecting epiretinal membranes, and retinal tamponade with silicone oil or gas.53–55 However, recurrent traction proliferation causes postsurgical retinal re-detachment.52
Currently, the adjuvant therapy for the treatment of PVR includes agents, such as anti-inflammatory drugs, growth factor inhibitors, and antioxidants.2,22 Recently, a series of low-dose methotrexate injections seem to be beneficial for treating complex retinal detachment caused by PVR,56,57 however, all these therapies are quite elusive and an urgent approach is demanded. Thus, additional studies are essential to elucidate the mechanisms regulating the early phases and development of PVR.
The development and progression of fibrotic lesions, including proliferative diabetic retinopathy (PDR) and PVR are caused by EMT. In addition, wound healing and stimulation of inflammatory cytokines lead to EMT, thereby forming pre- or subretinal fibrous membranes.1
Importantly, RPE cells play a vital role in the development of fibrosis on the retina and constitute the largest cellular component of epiretinal membranes in addition to hyalocytes, retinal Müller glial cells, fibroblasts, and macrophages.1 RPE cells are usually quiescent in healthy condition. Interestingly, trauma or intraocular diseases damage the RPE or cause retinal detachment. The subsequent repair triggers the loss of cell-cell contact in RPE cells, and, also, the epithelial cells are stimulated to proliferate into motile fibroblast-like cells.1
Initially, the transforming growth factor-β (TGF-β), promotes various types of fibrotic diseases, including PVR and PDR.1,54,55,58–64 Subsequently, trans-differentiated RPE cells migrate into the inner retinal layers or vitreous body, produce ECM components, and transform into fibroblast-like cells. This phenomenon results in the formation of epiretinal membranes that contract and cause retinal detachment as well as visual impairment.1,54,55,58–63
The miRNAs regulate the complex physiological and pathological processes, such as embryogenesis, organ development, oncogenesis, and angiogenesis.65,66
Intriguingly, miRNAs are positive or negative regulators of EMT that target the multiple components of the EMT machinery and exacerbate their critical roles in TGF-β2-induced EMT in human RPE cells.37,67 In addition, miRNAs regulate fibrosis in several organs.68
Although the role of miRNAs in PVR is not yet clarified, no study has investigated their expression in the vitreous of patients with RD with different PVR grades. Nevertheless, most of the miRNAs showing altered expression in the inter-stage comparisons has been previously associated with other ocular diseases, as shown in Table 5.31,37,69–88
Table 5.
Previous Studies Reporting the Association of DE miRNAs Deregulated in Inter Stage Comparisons With Ocular Diseases
| miRNA | Disease | Reference |
|---|---|---|
| let-7b-5p | Diabetic retinopathy, age-related cataract | 69 , 70 |
| miR-143-3p | Glaucoma, human subconjunctival fibrosis, oxygen-induced retinopathy | 71 – 73 |
| miR-152-3p | Diabetic retinopathy, age-related macular degeneration, oxygen-induced retinopathy | 73 – 75 |
| miR-16-5p | Proliferative vitreoretinopathy, glaucoma, age-related cataract | 37 , 76 , 77 |
| miR-19b-3p | Idiopathic epiretinal membrane and macular hole | 31 |
| miR-21-5p | Proliferative vitreoretinopathy, ischemic retina, diabetic retinopathy | 37 , 78 , 79 |
| miR-223-5p | Noninfectious uveitis | 80 |
| miR-224-5p | Uveal melanoma | 81 |
| miR-30b-5p | Diabetic retinopathy | 82 |
| miR-335-5p | Age-related macular degeneration | 83 |
| miR-340-5p | UVB-mediated retinal pigment epithelium cell damage | 84 |
| miR-361-5p | Age-related macular degeneration, retinoblastoma | 85 , 86 |
| miR-452-5p | Cataract | 87 |
| miR-486-3p | Retinoblastoma | 88 |
The only study that assessed the miRNA expression in the VH of patients with PVR diseases, including PDR, was performed by Usui-Ouchi and co-workers.37 These authors used quantitative real time (qRT)-PCR to identify miR-21-5p in the VH as a potential disease-modifying agent. Furthermore, the expression of miR-21-5p is enhanced by the disease-associated expression of TGF-β2 and/or high glucose conditions, which could be crucial in the fibroproliferative response of RPE cells during the development of retinal fibrotic disorders. In addition, the cell migration and proliferation of RPE cells was increased markedly. Also, the level of miR-16-5p was upregulated in the vitreous of the same eyes. Consistent with this report, the current data showed an increased expression of miR-21-5p and miR-16-5p in vitreous patients with PVR.
Among the miRNAs previously associated with EMT, miR-223-5p was shown to be upregulated after TGF-β treatment of RPE cells, whereas all the other differentially expressed miRNAs (except for miR-1300 and miR-891a-5p) regulate the EMT in cancer.40 Further, several studies reported the involvement of differentially expressed miRNAs in angiogenesis in eye-associated diseases (let-7b-5p, miR-152-3p, miR-21-5p, miR-218-5p, and miR-30b-5p)37,69,74,78,79,81,82,89 or various cancer models (miR-891a-5p).90 Similarly, among differentially expressed miRNAs, 12 out of 20 (let-7b-5p, miR-152-3p, miR-16-5p, miR-19b-3p, miR-203a, miR-21-5p, miR-224-5p, miR-335-5p, miR-340-5p, miR-486-3p, miR-891a-5p, and miR-99a-5p) are associated with fibrosis regulation.91–102
The present pilot study, which we believe has been done for the first time, described a putative correlation between miRNAs and fibrotic phenomena in patients with PVR following RD. Nevertheless, the present study has some limitations: the low number of samples and the modality of grading of PVR. The grading of PVR, even if assigned based on the classification system established by the “Retina Society Terminology Committee,”46 is still conducive to a subjective clinical choice of the retinal disease specialists and not on an objective diagnostic method. Despite the small number of patients enrolled in this pilot study, our results suggest that vitreal miRNAs could be considered PVR biomarkers. Needless to say, further studies on larger cohorts, possibly including vitreous samples from unaffected individuals, will be useful to validate miRNAs as disease biomarkers.
Conclusions
In conclusion, a set of DE miRNAs were identified in PVR conditions. Despite the small cohort, our study suggests that specific miRNAs could be considered good candidates as biomarkers for PVR. Furthermore, elucidating the role of other miRNAs in EMT in RPE cells in vitro and in PVR in vivo would provide an in-depth insight into the EMT-related gene expression. Thus, additional studies on the correlation between vitreal miRNAs and the pathological phenotypes are essential to identify the novel miRNA-based mechanisms underlying the PVR disease that would improve the diagnosis and treatment of the condition.
Acknowledgments
M.D.T. and C.Ba. were responsible for the writing, review, and editing, data handling, formal analysis, investigation, and revision. M.Re., C.B., and M.R. were responsible for the conceptualization, methodology, and revision. R.R. and K.N. were responsible for the investigation. A.T., S.S., R.R., and C.P. were responsible for the supervision and validation of the study. S.T. was responsible for the visualization. M.D.T. and R.R. were responsible for the funding acquisition. M.D.T. was responsible for the project administration. All authors reviewed and approved the manuscript for submission toward publication.
The authors alone are responsible for the content and writing of the paper.
The present study was approved by the Ethics Committee of the Medical University of Lublin (KE-0254/277/2019) in compliance with the Declaration of Helsinki.
Disclosure: M.D. Toro, None; M. Reibaldi, None; T. Avitabile, None; C. Bucolo, None; S. Salomone, None; R. Rejdak, None; K. Nowomiejska, None; S. Tripodi, None; C. Posarelli, None; M. Ragusa, None; C. Barbagallo, None
References
- 1. Pastor JC, Rojas J, Pastor-Idoate S, Di Lauro S, Gonzalez-Buendia L, Delgado-Tirado S. Proliferative vitreoretinopathy: a new concept of disease pathogenesis and practical consequences, Prog Retin Eye Res. 2016; 51: 125–155. [DOI] [PubMed] [Google Scholar]
- 2. Gagliano C, Toro MD, Avitabile T, Stella S, Uva MG. Intravitreal steroids for the prevention of PVR after surgery for retinal detachment. Curr Pharm Des. 2015; 21: 4698–4702. [DOI] [PubMed] [Google Scholar]
- 3. Yao J, Hu LL, Li XM, et al.. Comprehensive circular RNA profiling of proliferative vitreoretinopathy and its clinical significance. Biomed Pharmacother. 2019; 111: 548–554. [DOI] [PubMed] [Google Scholar]
- 4. Cardillo JA, Stout JT, LaBree L. Post-traumatic proliferative vitreoretinopathy. The epidemiologic profile, onset, risk factors, and visual outcome. Ophthalmology. 1997; 104: 1166–1173. [DOI] [PubMed] [Google Scholar]
- 5. Tseng W, Cortez RT, Ramirez G, Stinnett S, Jaffe GJ. Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. Am J Ophthalmol. 2004; 137: 1105–1115. [DOI] [PubMed] [Google Scholar]
- 6. Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998; 43: 3–18. [DOI] [PubMed] [Google Scholar]
- 7. Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy developments in adjunctive treatment and retinal pathology. Eye (Lond). 2002; 16: 369–374. [DOI] [PubMed] [Google Scholar]
- 8. Jusufbegovic D, Tamiya S, Kaplan HJ. Risk factors and prevention of proliferative vitreoretinopathy. Expert Rev Ophthalmol. 2015; 10: 431–440. [Google Scholar]
- 9. Pastor JC, De la Rúa ER, Martıń F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002; 21: 127–144. [DOI] [PubMed] [Google Scholar]
- 10. Tosi GM, Marigliani D, Romeo N, Toti P. Disease pathways in proliferative vitreoretinopathy: an ongoing challenge. J Cell Physiol. 2014; 229: 1577–1583. [DOI] [PubMed] [Google Scholar]
- 11. Casaroli-Marano RP, Pagan R, Vilaro S. Epithelial-mesenchymal transition in proliferative vitreoretinopathy: intermediate filament protein expression in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999; 40: 2062–2072. [PubMed] [Google Scholar]
- 12. Tamiya S, Kaplan HJ.. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp Eye Res. 2016; 142: 26–31. [DOI] [PubMed] [Google Scholar]
- 13. Pennock S, Haddock LJ, Eliott D, Mukai S, Kazlauskas A. Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res. 2014; 40: 16–34. [DOI] [PubMed] [Google Scholar]
- 14. Limb GA, Little BC, Meager A, et al.. Cytokines in proliferative vitreoretinopathy. Eye. 1991; 6: 686–693. [DOI] [PubMed] [Google Scholar]
- 15. El-Ghrably IA, Dua HS, Orr GM, Fischer D, Tighe PJ. Intravitreal invading cells contribute to vitreal cytokine milieu in proliferative vitreoretinopathy. Br J Ophthalmol. 2001; 85: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garweg JG, Tappeiner C, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv Ophthalmol. 2013; 58: 321–329. [DOI] [PubMed] [Google Scholar]
- 17. Limb GA, Alam A, Earley O, Green W, Chignell AH, Dumonde DC. Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr Eye Res. 1994; 13: 791–798. [DOI] [PubMed] [Google Scholar]
- 18. Zandi S, Pfister IB, Traine PG, et al.. Biomarkers for PVR in rhegmatogenous retinal detachment. PLoS One. 2019; 14: e0214674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmadieh H, Feghhi M, Tabatabaei H, Shoeibi N, Ramezani A, Mohebbi MR. Triamcinolone acetonide in silicone-filled eyes as adjunctive treatment for proliferative vitreoretinopathy: a randomized clinical trial. Ophthalmology. 2008; 115: 1938–1943. [DOI] [PubMed] [Google Scholar]
- 20. Yamakiri K, Sakamoto T, Noda Y. Oneyear results of a multicenter controlled clinical trial of triamcinolone in pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dehghan MH, Ahmadieh H, Soheilian M. Effect of oral prednisolone on visual outcomes and complications after scleral buckling. Eur J Ophthalmol. 2009; 20: 419–423. [DOI] [PubMed] [Google Scholar]
- 22. Kaneko H, Terasaki H.. Biological involvement of microRNAs in proliferative vitreoretinopathy. Transl Vis Sci Technol. 2017; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu N, Olson EN.. MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010; 18: 510–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salzman J. Circular RNA expression: its potential regulation and function, trends. Genet. 2016; 32: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ebbesen KK, Hansen TB, Kjems J. Insights into circular RNA biology. RNA Biol. 2017; 14: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ragusa M, Caltabiano R, Russo A, et al.. MicroRNAs in vitreus humor from patients with ocular diseases. Mol Vis. 2013; 19: 430–440. [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang SJ, Chen X, Li CP, et al.. Identification and characterization of circular RNAs as a new class of putative biomarkers in diabetes retinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 6500–6509. [DOI] [PubMed] [Google Scholar]
- 28. Wang K, B. Long F, Liu JX, et al.. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016; 37: 2602–2611. [DOI] [PubMed] [Google Scholar]
- 29. Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017; 466: 167–171. [DOI] [PubMed] [Google Scholar]
- 30. Lukiw WJ. Circular RNA (circRNA) in Alzheimer's disease (AD). Front Genet. 2013; 4: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russo A, Ragusa M, Barbagallo C, et al.. MiRNAs in the vitreous humor of patients affected by idiopathic epiretinal membrane and macular hole. PLoS One. 2017; 12: e0176618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ragusa M, Barbagallo C, Statello L, et al.. MiRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: pathological and diagnostic implications. Cancer Biol Ther. 2015; 16: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carew RM, Wang B, Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res. 2012; 347: 103–116. [DOI] [PubMed] [Google Scholar]
- 34. Vettori S, Gay S, Distler O. Role of microRNAs in fibrosis. Open Rheumatol J. 2012; 6: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castilla MA, Moreno-Bueno G, Romero-Pérez L. Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol. 2011; 223: 72–80. [DOI] [PubMed] [Google Scholar]
- 36. Takayama K, Kaneko H, Hwang S-J. Increased ocular levels of microrna-148a in cases of retinal detachment promote epithelial-mesenchymal transition microRNA-148 in retinal detachment. Invest Ophthalmol Vis Sci. 2016; 57: 2699–2705. [DOI] [PubMed] [Google Scholar]
- 37. Usui-Ouchi A, Ouchi Y, Kiyokawa M, Sakuma T, Ito R, Ebihara N. Upregulation of Mir-21 levels in the vitreous humor is associated with development of proliferative vitreoretinal disease. PloS One. 2016; 28: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jun J.H, Joo CK.. MicroRNA-124 controls transforming growth factor b1-induced epithelial-mesenchymal transition in the retinal pigment epithelium by targeting rhoG. Invest Ophthalmol Vis Sci. 2016; 57: 12–22. [DOI] [PubMed] [Google Scholar]
- 39. Adijanto J, Castorino JJ, Wang Z-X, Maminishkis A, Grunwald GB, Philp NJ. Microphthalmia associated transcription factor (MITF) promotes differentiation of human retinal pigment epithelium (RPE) by regulating microRNAs-204/211 expression. J Biol Chem. 2012; 287: 20491–20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen X, Ye S, Xiao W, Luo L, Liu Y. Differentially expressed microRNAs in TGFb2- induced epithelial-mesenchymal transition in retinal pigment epithelium cells. Int J Mol Med. 2014; 33: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 41. Hou Q, Zhou L, Tang J. LGR4 is a direct target of microRNA-34a and modulates the proliferation and migration of retinal pigment epithelial ARPE-19 cells. PLoS One. 2016; 11: e0168320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li M, Li H, Liu X, Xu D, Wang F. MicroRNA- 29b regulates TGF-b1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells by targeting AKT2. Exp Cell Res. 2016; 345: 115–124. [DOI] [PubMed] [Google Scholar]
- 43. Wang FE, Zhang C, Maminishkis A. MicroRNA-204/211 alters epithelial physiology. FASEB J. 2010; 24: 1552–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang L, Dong F, Reinach PS. MicroRNA-182 suppresses HGF/SF-induced increases in retinal pigment epithelial cell proliferation and migration through targeting c-Met. PLoS One. 2016; 11: e0167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsunekawa T, Kaneko H, Takayama K, et al.. Correlation between miR-148 expression in vitreous and severity of rhegmatogenous retinal detachment. Biomed Res Int. 2017; 2017: 3427319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hilton G, Machemer R, Michels R, Okun E, Schepens C, Schwartz A. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983; 90: 121–125. [DOI] [PubMed] [Google Scholar]
- 47. Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 48. Vlachos IS, Zagganas K, Paraskevopoulou MD, et al.. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015; 43: W460–W466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Du Y, Chen Q, Huang L, et al.. VEGFR2 and VEGF-C suppresses the epithelial-mesenchymal transition via YAP in retinal pigment epithelial cells. Curr Mol Med. 2018; 18: 273–286. [DOI] [PubMed] [Google Scholar]
- 50. Yang S, Li H, Li M, Wang F. Mechanisms of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Discov Med. 2015; 20: 207–217. [PubMed] [Google Scholar]
- 51. Moysidis SN, Thanos A, Vavvas DG. Mechanisms of inflammation in proliferative vitreoretinopathy: from bench to bedside. Mediators Inflamm. 2012; 2012: 815937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schwartz SG, Flynn Jr HW, Mieler WF. Update on retinal detachment surgery. Curr OpinOphthalmol. 2013; 24: 255–261. [DOI] [PubMed] [Google Scholar]
- 53. Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy developments in adjunctive treatment and retinal pathology. Eye (Lond). 2002; 16: 369–374. [DOI] [PubMed] [Google Scholar]
- 54. Enaida H, Hata Y, Ueno A, et al.. Possible benefits of triamcinolone-assisted pars plana vitrectomy for retinal diseases. Retina. 2003; 23: 764–770. [DOI] [PubMed] [Google Scholar]
- 55. Reibaldi M, Longo A, Avitabile T, et al.. Transconjunctival nonvitrectomizing vitreous surgery versus 25-gauge vitrectomy in patients with epiretinal membrane: a prospective randomized study. Retina. 2015; 35: 873–879. [DOI] [PubMed] [Google Scholar]
- 56. Amarnani D, Machuca-Parra AI, Wong LL, et al.. Effect of methotrexate on an in vitro patient-derived model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 3940–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Benner JD, Dao D, Butler JW, Hamill KI. Intravitreal methotrexate for the treatment of proliferative vitreoretinopathy. BMJ Open Ophthalmol. 2019; 4: e000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saika S. TGFb pathobiology in the eye. Lab Invest. 2006; 86: 106–115. [DOI] [PubMed] [Google Scholar]
- 59. Zhang L, Lei W, Wang X, Tang Y, Song J. Glucocorticoid induces mesenchymal-to-epithelial transition and inhibits TGF-b1-induced epithelial-to-mesenchymal transition and cell migration. FEBS Lett. 2010; 584: 4646–4654. [DOI] [PubMed] [Google Scholar]
- 60. Hoerster R, Muether PS, Vierkotten S, Hermann MM, Kirchhof B, Fauser S. Upregulation of TGF-ss1 in experimental proliferative vitreoretinopathy is accompanied by epithelial to mesenchymal transition. Graefes Arch Clin Exp Ophthalmol. 2014; 252: 11–16. [DOI] [PubMed] [Google Scholar]
- 61. Cao Y, Feng B, Chen S, Chu Y, Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Invest Ophthalmol Vis Sci. 2014; 55: 7321–7331. [DOI] [PubMed] [Google Scholar]
- 62. Winkler J, Hoerauf H.. TGF-ss and RPE-derived cells in taut subretinal strands from patients with proliferative vitreoretinopathy. Eur J Ophthalmol. 2011; 21: 422–426. [DOI] [PubMed] [Google Scholar]
- 63. Mony S, Lee SJ, Harper JF, Barwe SP, Langhans SA. Regulation of Na, K-ATPase beta1-subunit in TGF-beta2-mediated epithelial-to-mesenchymal transition in human retinal pigmented epithelial cells. Exp Eye Res. 2013; 115: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Romano GL, Platania CBM, Drago F, et al.. Retinal and circulating miRNAs in age-related macular degeneration: an in vivo animal and human study. Front Pharmacol. 2017; 8: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011; 67: 129–139. [DOI] [PubMed] [Google Scholar]
- 66. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010; 11: 597–610. [DOI] [PubMed] [Google Scholar]
- 67. Allegra A, Alonci A, Campo S, et al.. Circulating microRNAs: new biomarkers in diagnosis, prognosis and treatment of cancer (review). Int J Oncol. 2012; 41: 1897–1912. [DOI] [PubMed] [Google Scholar]
- 68. Eissa MG, Artlett CM.. The MicroRNA miR-155 is essential in fibrosis. Noncoding RNA. 2019; 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhou Q, Frost RJ, Anderson C, et al.. Let-7 contributes to diabetic retinopathy but represses pathological ocular angiogenesis. Mol Cell Biol. 2017; 37: e00001–e000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dong Y, Zheng Y, Xiao J, Zhu C, Zhao M. MicroRNA let-7b induces lens epithelial cell apoptosis by targeting leucine-rich repeat containing G protein-coupled receptor 4 (Lgr4) in age-related cataract. Exp Eye Res. 2016; 147: 98–104. [DOI] [PubMed] [Google Scholar]
- 71. Li X, Zhao F, Xin M, et al.. Regulation of intraocular pressure by microRNA cluster miR-143/145. Sci Rep. 2017; 7: 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hwang YH, Jung SA, Lyu J, Kim YY, Lee JH. Transforming growth factor-β1-induced human subconjunctival fibrosis is mediated by microRNA 143/145 expression. Invest Ophthalmol Vis Sci. 2019; 60: 2064–2071. [DOI] [PubMed] [Google Scholar]
- 73. Desjarlais M, Rivera JC, Lahaie I, et al.. MicroRNA expression profile in retina and choroid in oxygen-induced retinopathy model. PLoS One. 2019; 14: e0218282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Haque R, Hur EH, Farrell AN, Iuvone PM, Howell JC. MicroRNA-152 represses VEGF and TGFβ1 expressions through post-transcriptional inhibition of (Pro) renin receptor in human retinal endothelial cells. Mol Vision. 2015; 21: 224. [PMC free article] [PubMed] [Google Scholar]
- 75. Ménard C, Rezende FA, Miloudi K, et al.. MicroRNA signatures in vitreous humour and plasma of patients with exudative AMD. Oncotarget. 2016; 7: 19171–19184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu Y, Chen Y, Wang Y, et al.. microRNA profiling in glaucoma eyes with varying degrees of optic neuropathy by using next-generation sequencing. Invest Ophthalmol Vis Sci. 2018; 59: 2955–2966. [DOI] [PubMed] [Google Scholar]
- 77. Li Y, Liu S, Zhang F, Jiang P, Wu X, Liang Y. Expression of the microRNAs hsa-miR-15a and hsa-miR-16-1 in lens epithelial cells of patients with age-related cataract. Int J Clin Exp Med. 2015; 8: 2405–2410. [PMC free article] [PubMed] [Google Scholar]
- 78. Gutsaeva DR, Thounaojam M, Rajpurohit S, et al.. STAT3-mediated activation of miR-21 is involved in down-regulation of TIMP3 and neovascularization in the ischemic retina. Oncotarget. 2017; 8: 103568–103580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen Q, Qiu F, Zhou K, et al.. Pathogenic role of microRNA-21 in diabetic retinopathy through downregulation of PPARα. Diabetes. 2017; 66: 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Verhagen FH, Bekker CPJ, Rossato M, et al.. A disease-associated microRNA cluster links inflammatory pathways and an altered composition of leukocyte subsets to noninfectious uveitis. Invest Ophthalmol Vis Sci. 2018; 59: 878–888. [DOI] [PubMed] [Google Scholar]
- 81. Falzone L, Romano GL, Salemi R. Prognostic significance of deregulated microRNAs in uveal melanomas. Mol Med Reports. 2019; 19: 2599–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mazzeo A, Lopatina T, Gai C, Trento M, Porta M, Beltramo E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp. Eye Res. 2019; 184: 56–63. [DOI] [PubMed] [Google Scholar]
- 83. Ertekin S, Yıldırım O, Dinç E, Ayaz L, Fidancı SB, Tamer L. Evaluation of circulating miRNAs in wet age-related macular degeneration. Mol Vis. 2014; 20: 1057–1066. [PMC free article] [PubMed] [Google Scholar]
- 84. Yan J, Qin Y, Yu J, Peng Q, Chen X. MiR-340/iASPP axis affects UVB-mediated retinal pigment epithelium (RPE) cell damage. J Photochem Photobiol B. 2018; 186: 9–16. [DOI] [PubMed] [Google Scholar]
- 85. Grassmann F, Schoenberger PG, Brandl C, et al.. A circulating microRNA profile is associated with late-stage neovascular age-related macular degeneration. PLoS One. 2014; 9: e107461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu B, Lu B, Wang X, Jiang H, Kuang W. MiR-361-5p inhibits cell proliferation and induces cell apoptosis in retinoblastoma by negatively regulating CLDN8. Childs Nerv Syst. 2019; 35: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 87. Dunmire JJ, Lagouros E, Bouhenni RA, Jones M, Edward DP. MicroRNA in aqueous humor from patients with cataract. Exp Eye Res. 2013; 108: 68–71. [DOI] [PubMed] [Google Scholar]
- 88. Venkatesan N, Deepa PR, Khetan V, Krishnakumar S. Computational and in vitro investigation of miRNA-gene regulations in retinoblastoma pathogenesis: miRNA mimics strategy. Bioinform Biol Insights. 2015; 9: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Han S, Kong YC, Sun B, Han QH, Chen Y, Wang YC. MicroRNA-218 inhibits oxygen-induced retinal neovascularization via reducing the expression of roundabout 1. Chin Medical J. 2016; 129: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yao S, Hu M, Hao T, et al.. MiRNA-891a-5p mediates HIV-1 Tat and KSHV Orf-K1 synergistic induction of angiogenesis by activating NF-κB signaling. Nucleic Acids Res. 2015; 43: 9362–9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li L, Zhang L, Zhao X, Cao J, Li J, Chu G. Downregulation of miR-152 contributes to the progression of liver fibrosis via targeting Gli3 in vivo and in vitro. Exp Ther Med. 2019; 18: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xiao B, Zhu Y, Huang J, Wang T, Wang F, Sun S. Exosomal transfer of bone marrow mesenchymal stem cell-derived miR-340 attenuates endometrial fibrosis. Biol Open. 2019; 8:pii: bio039958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lan T, Li C, Yang G, et al.. Sphingosine kinase 1 promotes liver fibrosis by preventing miR‐19b‐3p‐mediated inhibition of CCR2. Hepatol. 2018; 68: 1070–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mamdouh S, Khorshed F, Aboushousha T, et al.. Evaluation of mir-224, mir-215 and mir-143 as serum biomarkers for HCV associated hepatocellular carcinoma. Asian Pac J Cancer Prev. 2017; 18: 3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tang N, Wu Y, Cao W, et al.. Lentivirus-mediated over-expression of let-7b microRNA suppresses hepatic fibrosis in the mouse infected with Schistosoma japonicum. Exp Parasitol. 2017; 182: 45–53. [DOI] [PubMed] [Google Scholar]
- 96. Davoodian P, Ravanshad M, Hosseini SY, et al.. Effect of TGF-β/smad signaling pathway blocking on expression profiles of miR-335, miR-150, miR-194, miR-27a, and miR-199a of hepatic stellate cells (HSCs). Gastroenterol Hepatol Bed Bench. 2017; 10: 112–117. [PMC free article] [PubMed] [Google Scholar]
- 97. He Q, Wang CM, Qin JY, et al.. Effect of miR-203 expression on myocardial fibrosis. Eur Rev Med Pharmacol Sci. 2017; 21: 837–842. [PubMed] [Google Scholar]
- 98. Li Q, Xie J, Wang B, et al.. Overexpression of microRNA-99a attenuates cardiac hypertrophy. PLoS One. 2016; 11: e0148480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yao S, Hu M, Hao T, et al.. MiRNA-891a-5p mediates HIV-1 Tat and KSHV Orf-K1 synergistic induction of angiogenesis by activating NF-κB signaling. Nucleic Acids Res. 2015; 43: 9362–9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang Q, Xu M, Qu Y, et al.. Analysis of the differential expression of circulating microRNAs during the progression of hepatic fibrosis in patients with chronic hepatitis B virus infection. Mol Med Reports. 2015; 12: 5647–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huang Y, He Y, Li J. MicroRNA-21: a central regulator of fibrotic diseases via various targets. Curr Pharm Des. 2015; 21: 2236–2242. [DOI] [PubMed] [Google Scholar]
- 102. Xie T, Liang J, Guo R, Liu N, Noble PW, Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genom. 2011; 43: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]