Abstract

Potassium hydroxide-impregnated char sorbents (KOH/char) prepared via an ultrasonic-assisted method were used for SO2 removal from flue gas. The desulfurization experiment was analyzed using a fixed-bed reactor under 40–150 °C temperature range, using simulated flue gas. X-ray diffraction (XRD), Fourier-transform infrared spectroscopy, and scanning electron microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS) were used to analyze both the chemical and physical characteristics of the sorbents. The analyzed results exposed that the complete elimination of SO2 from flue gas was achieved when using the char/KOH sorbent with a mass ratio of char to KOH of 11:1. It was noted that temperature had a substantial influence on the desulfurization performance with sulfur capacity maximized at 100 °C. Experimental results also revealed that a small amount of O2 present in the solvent could improve the SO2 removal efficiency of the sorbent. The analyzed XRD patterns showed that K2SO4 was the main desulfurization product, which was consistent with the SEM/EDS analysis. The experimental results were well-described with the Lagergren first-order adsorption kinetics model with the activation energy (Ea) of the SO2 adsorption by KOH/char sorbent of 20.25 kJ/mol.
1. Introduction
Sulfur dioxide (SO2) is a major pollutant formed in the combustion of fossil fuels. The direct release of SO2 to the atmosphere can result in severe environmental issues such as the formation of acid rain. Coal-fired power plants are the principal basis for power production in China and donated as the primary contributor to atmospheric SO2 pollution. Therefore, it is of high importance to efficiently and economically remove SO2 from flue gas in order to curtail the environmental unfriendly SO2 emissions. Wet flue gas desulfurization (FGD) technologies such as ammonia scrubbing, Ca/Mg-based processes, wet oxidation-absorption, and Mn-based sorbents processes were considered as the primary technology for desulfurization (de-SOx) of flue gas.1−4 However, these wet de-SOx technologies are still facing several disadvantages, including high capital costs, high water consumption, and secondary pollution. As a result, the development of dry de-SOx methods offers a promising method to remove SO2 while overcoming the issues mentioned above. Ca/Mg-based materials are predominantly used for SO2 removal in dry FGD technologies.5 However, the process are still facing secondary pollution.6,7 Research on replacing calcium-based sorbents with more versatile and practical materials is always one of the hottest topics in the field of desulfurization of flue gas. Activated carbon (AC) materials such as char can be used as an adsorbent, catalyst, or catalyst support because of their relatively large BET surface area, amenability to surface functionalization, and stability in acidic media.1,8 In the cause of using AC as a catalyst support, the reactivity of the activated components is often improved significantly.9 Liu and co-workers used an AC-supported V2O5 adsorbent for simultaneous SO2 and NO removal.10 Their results indicated that the modification of the functional groups containing oxygen on the surface char could change the chemical and physical properties of AC, hence increase the corresponding desulfurization activity. Ma et al.11 investigated the application of the palm shell activated carbon (PSAC)-supported CeO2 material for the eradication of both SO2 and NOx from flue gas and revealed that the decent desulfurization properties were a result of enhanced physicochemical characteristics from interactions between AC support and CeO2.12 Alkali metals, such as potassium and sodium, can significantly enhance the catalytic activity of the AC when it is used as a catalyst. There is a good chance that an additional alkali metal into AC material can enhance SO2 adsorption from flue gas. Microporous AC obtained by mixing anthracite with KOH or NaOH was featured with a high surface area, which makes it a promising desulfurization sorbent.13 Moreover, the desulfurization products are activated char-supported alkali sulfates (K2SO4, Na2SO4, etc.), which can be used as soil conditioners or slow-release fertilizers, eliminating the need for sorbent regeneration.14 Therefore, the dry desulfurization process using AC-based sorbents possesses several advantages over other desulfurization methods, including lower capital cost, smaller site area, no water consumption, and low sorbent regeneration cost.
In this study, we are going to explore the possibility of using a new type of char material to act as a sorbent during the dry desulfurization operation. Lignite char impregnated with KOH was used as sorbents to eliminate SO2 from flue gas in a fixed-bed reactor, and the effect of reaction parameters, including temperature, KOH content, steam, and oxygen concentration, toward the removal efficiency of SO2 was systematically studied. Also, the surface properties and crystalline structures of the designed sorbents before and after desulfurization were analyzed by scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier-transform infrared (FT-IR) spectroscopy to rationalize the desulfurization performance of the sorbents.
2. Results and Discussion
2.1. Influence of Temperature on the Desulfurization Performance of K12 Sorbent
Figure 2a shows the SO2 removal efficiency and breakthrough sulfur capacities of K12 as a function of temperature. As shown in Figure 2a, complete removal of SO2 by the K12 sorbent was achieved at the initial stage for all operating temperatures. The breakthrough time of SO2 by the K12 sorbent increases with the increase of operating temperature with temperatures between 40 and 100 °C. The SO2 removal efficiency of the K12 sorbent reached the breakthrough point at approximately 185 min at 40 °C and increased to 341 min at 100 °C. The breakthrough time of SO2 by the K12 sorbent decreased significantly with increasing temperature when the operating temperature is higher than 120 °C. The breakthrough time of the K12 sorbent is only 68 min when increasing the operating temperature to 150 °C. The breakthrough sulfur capabilities of K12 at various temperatures are shown in Figure 2b. It was noted that the relationship between temperature and breakthrough sulfur capacity of K12 showed a similar trend to that of the breakthrough time of SO2. In Figure 2b, the breakthrough sulfur capacity of K12 increased from 180.65 mg S/g sorbent at 40 °C to 342.20 mg S/g sorbent at 100 °C and decreased to 60.86 mg S/g with further increasing the desulfurization temperature to 150 °C. This peculiarity can be linked to the reduction in the adsorption ability of the char and the reactivity of KOH at elevated temperatures. SO2 was both physically and chemically adsorbed on K12, and its chemical adsorption was mainly credited to the reaction between KOH and SO2 on the char surface. However, the physical adsorption may possibly be as a result of van der Waals forces between the SO2 molecules and the hydroxyl groups on the char surface. Furthermore, the physical adsorption process was strongly affected by temperature because of the breaking of van der Waals forces under temperatures higher than 100 °C, which led to the release of the adsorbed SO2 from the char surface hence reducing the breakthrough sulfur capacity. Thus, it can be deduced that 100 °C was the optimal temperature for SO2 removal by K12.
Figure 2.
Desulfurization performance of char-supported K12 sorbent in 10% steam and 5% O2 at different temperatures: (a) removal efficiency of SO2 and (b) breakthrough sulfur capacity of sorbent, respectively.
2.2. Impact of K Content on the Desulfurization Performance of Potassium Hydroxide Impregnated Char Sorbents
Figure 3a,b compare the SO2 removal efficiencies and sulfur capacities of various char/KOH sorbents at 100 °C. Figure 3a shows that the shortest breakthrough time of SO2 by char was 104 min, indicating that impregnating with KOH is an efficient technique to improve the desulfurization performance of the char sorbent. Figure 3a also shows that the breakthrough time increased from 104 to 341 min when the KOH content was increased from 0 to 12%, and then decreased to 197 and 85 min when the KOH content increased to 15 and 20%, respectively. In addition, the desulfurization efficiency of all char sorbent with different KOH concentrations remained at 100% before the breakthrough. The SO2 breakthrough time indicated that the optimal KOH content was 12% for SO2 removal using the char adsorbent. This result indicated that excessive KOH content was, in fact, detrimental to the desulfurization reaction, which might cause KOH aggregation and reduced the amount of available active sites for SO2 adsorption.
Figure 3.
Desulfurization performance of char sorbents with variations of KOH-loading contents in 10% steam and 5% O2 at 100 °C: (a) removal efficiency of SO2 and (b) breakthrough sulfur capacity of sorbent.
The sulfur capacities of char sorbents with different KOH contents are shown in Figure 3b. The analyzed experimental results reviewed that the sulfur capacity was initially enhanced and then decreased at KOH contents higher than 12%, which was also coherent with the trend in the desulfurization curves in Figure 3a. The maximum sulfur capacity of K12 in Figure 3b was found to be 342.20 mg S/g sorbent.
Table 1 lists the breakthrough sulfur capacities, breakthrough times, and conversions for each sorbent. The theoretical sulfur capacity was influenced by the amount of KOH impregnated into the char, assuming a 1–2 ration between SO2 and KOH. The sorbent conversion was the ratio of the breakthrough sulfur capacity to the theoretical sulfur capacity. As discussed above, the highest breakthrough sulfur capacity was achieved by K12 at 100 °C. The theoretical sulfur capacity linearly increased from 205.10 mg S/g sorbent to 820.50 mg S/g sorbent, which is proportional to the KOH content in char given in Table 1. Furthermore, the conversion of K12 (69.51%) was higher than the other sorbents, which might be attributed to its physical properties (e.g., pore size). The molecular diameter of SO2 is 0.28 nm, which is sufficiently small to pass through the char micropores and react with the active component (potassium hydroxide).16
Table 1. Properties of Potassium Hydroxide Impregnated Char Sorbents with Different K Contents.
| sorbent | breakthrough time (min) | breakthrough sulfur capacity (mg S per g sorbent) | theoretical sulfur capacity (mg S per g sorbent) | conversion of sorbent (%) |
| Char | 118 | 114.20 | ||
| K5 | 104 | 124.22 | 205.10 | 56.18 |
| K10 | 185 | 206.01 | 410.30 | 27.44 |
| K12 | 341 | 342.20 | 492.30 | 69.51 |
| K15 | 197 | 217.12 | 615.40 | 21.08 |
| K20 | 85 | 68.83 | 820.50 | 11.90 |
The SO2 removal efficiencies of char, char/K, and K12 under 100 °C, shown in Figure 4, remained above 90% at 118, 216, and 341 min, respectively. The sulfur capacity of char/K was the highest because of its high specific surface area and large amount of surface functional groups. Potassium was assumed to improve the char pore volume and surface activity, which resulted in increased SO2 adsorption performance. Moreover, many alkaline functional groups were formed on the char surface after potassium addition, which promoted SO2 adsorption by char and increased the sulfur capacity of char/K.16,17 However, the desulfurization properties of K12, including the breakthrough time and the sulfur capacity, were higher than that of char/K, suggesting that KOH can effectively activate and promote the desulfurization performance of char.
Figure 4.

Desulfurization properties of char, char/K, and K12 sorbents in 10% steam and 5% O2 at 100 °C.
2.3. Effect of Steam on the Desulfurization Performance of K12
The SO2 removal efficiency profiles and breakthrough sulfur capacities of K12 with a steam concentration of 0 to 15% and 5% O2 under 100 °C are shown in Figure 5. As shown in Figure 5a that the SO2 removal efficiency of K12 remained above 90% for approximately 20 min in the absence of steam, but the breakthrough time significantly increased from 104 to 341 min when the steam concentration was increased from 5 to 10%. According to the literature, the presence of steam in low concentration in flue gas is beneficial to desulfurization by char because of the natural affinity between polar molecules such as H2O and SO2.18 Water has an unusual high adsorption capacity over a range of conditions that results in relatively strong physical adsorption of SO2 on the char surface. In addition, SO2 can be converted to H2SO3 when dissolved in water, which is then oxidized by O2 to form H2SO4, subsequently reacting with KOH to form K2SO4. The reaction between SO2 and KOH is mainly surface-controlled according to the open literature.19 The chemical adsorption of SO2 by KOH-impregnated char throughout these chemical reactions significantly improved the desulfurization properties of K12.20
Figure 5.
Desulfurization properties of K12 sorbent in 5% O2 at 100 °C with different steam contents: (a) removal efficiency of SO2 and (b) sulfur capacity of sorbent.
Figure 5b shows the sulfur adsorption capacity at the breakthrough point of K12 sorbents when operating at different steam contents. The breakthrough sulfur capacity of K12 increased from 9.69 to 340.87 mg S/g sorbent when the steam concentration was increased from 0 to 10%. On further increasing the stream content, the breakthrough sulfur capacity of K12 decreased significantly with breakthrough sulfur capacity reduced to 203.10 mg S/g sorbent upon 15% steam. These results agree with the desulfurization results in Figure 5a, which indicate an optimum steam concentration of 10% for K12. Therefore, it is believed that high steam concentration in the flue gas made it difficult for the desulfurization process to take place in the char-supported KOH sorbents. The inhibition of SO2 adsorption with steam is possibly caused by water molecules occupying the active sites and blocked the pores of the char sorbent. The inhibition of SO2 adsorption with high steam concentration is possibly caused by water molecules occupying the active sites and blocked the pores of the char sorbent.1 Therefore, it is believed that high steam concentration in the flue gas made it difficult for the desulfurization process to take place in the char-supported KOH sorbents.
2.4. Impact of O2 Concentration on the Desulfurization Efficiency of KOH Impregnated Char Sorbents
Oxygen performed a crucial role in SO2 removal by char-based sorbents. It is well-accepted that the oxidization of SO2 by O2 can significantly increase the removal efficiency of SO2. Figure 6 compares the desulfurization properties of K12 at various O2 contents in 10% steam under a desulfurization temperature of 100 °C. As shown in Figure 6a, the breakthrough time of K12 during desulfurization significantly increased from 50 to 341 min when the oxygen concentration was increased from 0 to 5%. However, the breakthrough time of K12 significantly decreased to 210 and 20 min when the O2 content was further increased to 8 and 10%, respectively. In Figure 6b, the breakthrough sulfur capabilities of K12 at different O2 contents displayed a similar trend to the breakthrough points of K12. In Figure 6b, the breakthrough sulfur capacity of K12 was 340.87 mg S/g sorbent at 5% O2. However, in the absence of O2, the breakthrough sulfur capacity of K12 was 30.08 mg S/g sorbent. The adsorbed O2 can dissociate and convert to active lattice oxygen, that has the ability to catalytically oxidize SO2 resulting in the enhanced desulfurization efficiency of K12. This also suggests that the oxygen in flue gas can significantly enhance the SO2 removal performance of the KOH-impregnated char sorbents.
Figure 6.
Desulfurization performance of char-supported K12 sorbent with different O2 concentrations in 10% steam at 100 °C: (a) removal efficiency of SO2 and (b) sulfur capacity.
2.5. XRD Spectra of K12 Sorbent before and after Desulfurization as a Function of Temperature
Figure 7 shows the XRD patterns of the K12 sorbent before and after the desulfurization reaction at various temperatures. There are no diffraction peaks for KOH, as revealed in the XRD patterns of the K12 sorbent before desulfurization, indicating that KOH was well-dispersed on the lignite char and only existed as ions rather than crystals. After desulfurization, diffraction peaks for K2SO4 can be observed, indicating the fact that KOH was converted into K2SO4 during the desulfurization process. Subsequently, the peak intensity of K2SO4 gradually increased with increasing temperature, suggesting that large K2SO4 particles were formed because of its high crystallinity.21
Figure 7.
XRD spectra of K12 sorbent before and after desulfurization at different temperatures.
2.6. FTIR Spectra of the K12 Sorbent before and after Desulfurization as a Functional Temperature
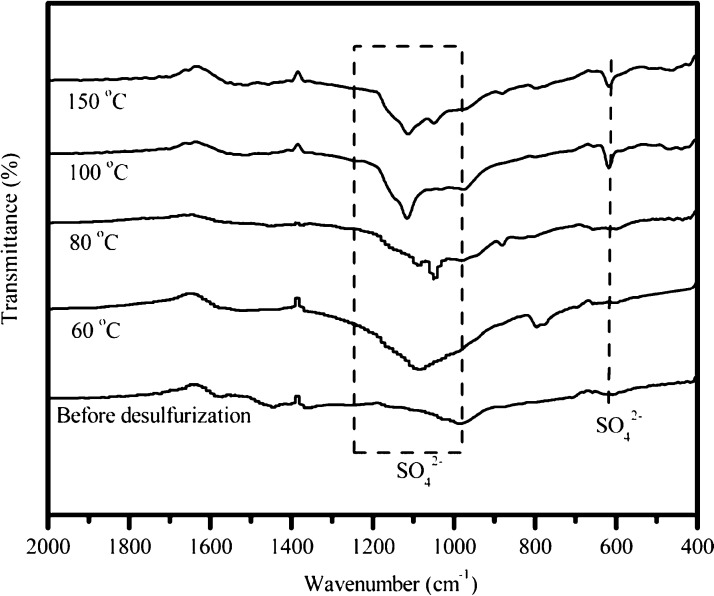
Figure 8 shows the FTIR spectra of the raw and spent K12 sorbent after desulfurization at different temperatures. The peaks at 1610, 1545–1570, 1460–1490, and 700–900 cm–1, can be assigned to aromatic carbon, aromatic C=C stretching vibrations, aromatic ring stretch vibrations, and out-of-plane aromatic C–H vibrations, respectively.22,23 The peaks at 1047 and 600 cm–1 corresponding to the vibrations of S–O and S=O in either SO42– or H2SO4.24 It can be seen in Figure 8 that SO42– was formed after the sulfidation reaction, suggesting that catalytic oxidation occurred during SO2 adsorption as well. This result further confirms the validity of the proposed SO2 adsorption mechanism into char-based material from XRD results.
Figure 8.
FT-IR spectra of K12 sorbent before and after desulfurization at different temperatures.
2.7. SEM Spectra of the K12 Sorbent after Desulfurization
Figure 9 shows the SEM images of the K12 sorbent under 100 °C desulfurization temperature. As observed in Figure 9, the potassium ions were well-dispersed in the char pores, and KOH primarily existed as nanoparticles. energy-dispersive X-ray spectroscopy (EDS) analysis showed that O (Figure 9c) and S (Figure 9d) were closely associated with K (Figure 9b), indicating that the desulfurization product was a compound that contained K, S, and O elements. This is also consistent with the XRD and FTIR results. As a dry FGD process, potassium hydroxide impregnated char sorbents can effectively remove the SO2 from flue gas. This desulfurization process has such advantages of lower capital cost, smaller site area, and no water consumption in comparison with other desulfurization processes. Moreover, the final desulfurization product, which is lignite char along with K2SO4, which can be used as either soil conditioner or fertilizer and avoids the cost of sorbent regeneration and loss. Further research for characterizing char-supported K2SO4 is required prior to applying the potassium hydroxide-impregnated char sorbents.
Figure 9.

SEM images of a K12 sorbent particle after sulfidation experiments at 100 °C (a); K mapping (b); O mapping (c); and S mapping (d).
2.8. Kinetic Mechanism of KOH-Impregnated Char Sorbents for the Removal of SO2
Three models kinetic equations; (1) Lagergren first-order adsorption kinetic model, (2) McKay second-order adsorption kinetic model, and (3) Boyd quasi-level movement mechanical model, were applied in the experimental data to determine an optimal kinetics model for SO2 removal using KOH-impregnated char sorbents.
2.8.1. Lagergren First-Order Adsorption Kinetic Model25
The Lagergren first-order adsorption kinetic model is given as
| 1 |
with k1, qe, qt representing the apparent rate constant of the Lagergren first-order adsorption model (min–1); the time at which the sorbent reaches saturation (min); and the total SO2 adsorbed per unit mass of sorbents at time t (mg/g), and qe represents the equilibrium adsorption capacity (mg/g). Equation 2 was derived from the definite integration when qt = 0 at t = 0 and qt = qt at t = t.
| 2 |
As shown in Figure 10a, plotting ln(qe – qt) versus t produced straight lines, implying that the surface adsorption was a first order and the rate-controlling step. The R-value of fitting a straight line under each condition is shown in Table 2 and also confirms that the Lagergren first-order adsorption kinetic model is a good fit for the experimental data.
Figure 10.
Kinetic model of potassium hydroxide-impregnated char sorbents for FGD: (a) Lagergren model; (b) McKay model; and (c) Boyd model.
Table 2. Correlation Coefficients of Kinetic Models.
| temperature (°C) | Lagergren model | McKay model | Boyd model |
|---|---|---|---|
| 40 | 0.9995 | 0.9996 | 0.9995 |
| 60 | 0.9996 | 0.9995 | 0.9997 |
| 80 | 0.9998 | 0.9998 | 0.9998 |
| 100 | 0.9998 | 0.9998 | 0.9998 |
| 120 | 0.9995 | 0.9980 | 0.9970 |
| 150 | 0.9988 | 0.8650 | 0.8520 |
2.8.2. McKay Second-Order Adsorption Kinetic Model26
The absorption rate equation of McKay two-level adsorption kinetic model is shown as follows
| 3 |
where k2 represents the McKay second-order adsorption rate constant (g·(mg·min)−1). Equation 4 was derived from the definite integration when qt = 0 at t = 0 and qt = qt at t = t.
| 4 |
Figure 10b shows the plot of 1/(qe – qt) versus time t obtained by fitting the experimental data using the McKay model. Figure 10b shows that the experimental data was not simulated accurately by the McKay model at higher temperatures. Additionally, Table 2 shows that the McKay model was not suitable to fit the experimental results in the initial stage, confirming that the model is not suitable for simulating SO2 adsorption by KOH-impregnated char sorbents.
2.8.3. Bangham Kinetics Model26
The Bangham kinetics model can be expressed by eq 5
| 5 |
where k3 is the apparent adsorption rate parameter.
The simulation of the Bangham kinetics model and the experimental results are compared and shown in Figure 10c. As shown, the model did not fit the experimental data accurately during initial stages or under higher temperatures. The results in Table 2 also suggest that the Bangham kinetics model can not work as an ideal model to describe the adsorption of SO2 into KOH-impregnated char sorbents.
2.8.4. Activation Energy
The Arrhenius equation (eq 6) was used to obtain the activation energy
| 6 |
where k, A, R, and T symbolizes the rate constant, the frequency factor, the universal gas constant (R = 8.314 J k–1 mol–1), and the absolute temperature (K); and the activation energy (Ea) is the slope of ln k versus 1/T. As shown in Figure 11, the Ea was found to be 20.25 kJ/mol. This result indicates that the SO2 in the flue gas was adsorbed onto KOH-char mainly by adsorption at the low temperature through chemical reactions.
Figure 11.
Relation of ln k with 1/T for K12 sorbent for the FGD reaction.
3. Conclusions
KOH-impregnated char sorbents were made by an ultrasonic impregnation process and used in the removal of sulfur from the flue gas under desulfurization temperature of 40–150 °C, using a vertical fixed-bed reactor. The KOH/char sorbents completely removed SO2 from flue gas at desulfurization temperatures between 40 and 150 °C. Temperature proved to have an extensive influence on the desulfurization performance, and the optimal desulfurization performance was achieved at 100 °C. The char-to-KOH ratio of 11:1 was found to be the best mass ratio for the desulfurization process. The oxygen and steam concentrations in the flue gas can also enhance the removal efficiency of SO2 by the KOH/char sorbents, with ideal concentrations in flue gas of 5% oxygen and 10% steam, respectively. The Lagergren first-order adsorption kinetic model presented the best fit to the SO2 adsorption data and revealed that the activation energy of SO2 adsorption was 20.25 kJ/mol.
4. Experimental Section
4.1. Sample Preparation
Inner Mongolia Chinese lignite exhibiting an ultimate analysis of 45.12% fixed carbon; 19.96% moisture content; 30.89% volatile matter; and 4.03% ash content was used in this study. The raw lignite was separated to a particle size range of 1–3 mm after crushing. The coal samples were then acid-washed using 0.4 N HCl aqueous solution for 12 h. After that, it was dried for 12 h in nitrogen at 105 °C. Gasification experiments were carried out in a vertical fixed-bed tubular quartz reactor with an internal diameter of 2.0 cm heated by an electric furnace. About 2.0 g of sample was used and before the start of each experiment, the reactor was purged with N2 at a 100 mL/min flow rate for 5 min to ensure an inlet atmosphere is achieved. When the furnace reached the desired temperature (700 °C), steam was then introduced (15% vol balanced with nitrogen) for 30 min. After the gasification, the reactor was taken out of furnace and cooled down in room temperature. The lignite char samples were then impregnated by a KOH solution at different concentrations under the assistance of ultrasonication for 5 h at 50 °C.9 KOH solutions with concentrations of 5, 10, 12, 15, and 20% were used to impregnate the char, which were labeled as K5, K10, K12, K15, and K20, respectively. The K12 samples were washed with deionized H2O until the pH of the filtrate was 7. The obtained sample was named as char/K.
4.2. Samples Characterization
The functional groups of KOH-impregnated char sorbents before and after desulfurization were studied with a Nicolet 6700 spectrometer (Thermo Electron. Co., USA). The samples were mixed with pulverized KBr and pelletized before analysis. The FTIR scanning region range was from 4000 to 400 cm–1, and the FTIR resolution was confirmed to be 4 cm–1. The crystal structure of the samples was determined by using an XRD (XRD 7000, Shimadzu) in a 2θ range of 10 to 80° having a step size of 0.03°. The changes in the sample morphologies were observed using SEM (JMF-7500F, Japan) equipped with an EDS detector functioning at 20 kV and 80 mA.
4.3. Desulfurization Experiments
The desulfurization experiments were conducted by means of a vertical fixed-bed tubular quartz reactor with an inside diameter of 20 mm. The reaction temperature (40–150 °C) was achieved by heating the samples using an electric furnace (Figure 1) functioning at a space velocity of 1000 h–1. The simulated flue gas consisted of 0.10% SO2, O2, steam, and balanced with N2 gas. The feed gas was supplied from gas cylinders, and mass flow controllers controlled the gas flow rates. The steam content varied from 0 to 15% and was added into the flue gas by adjusting an evaporator. Approximately 10 g of sample was fed into the quartz reactor. An online flue gas analyzer (MRU MGA5, Germany) was used to investigate the composition of the gas at both the inlet and outlet of the reactor. The online flue gas analyzer had a detecting range of ±2 ppm, and the experimental runs were repeated for at least three times for result accuracy.
Figure 1.
Schematic representation of the FGD experiment setup.
The desulfurization efficiency of SO2 was determined using eq 7.
| 7 |
where C0 and Ct represent both the inlet and outlet concentrations of SO2 (ppmv), respectively. The desulfurization breakthrough time was established when the outlet concentration of SO2 decrease below 50 ppmv, corresponding to a 95% SO2 removal efficiency.1 The breakthrough sulfur capacity of the sorbent (qt, mg/g) was calculated using eq 8.15
| 8 |
with Q, t, m representing the volume flow rate of SO2 (m3/min), the breakthrough time of sorbent (min), and the weight of sorbent (g), respectively.
References
Acknowledgments
The authors appreciate the financial support from the Excellent Talents Training Program of the University of Science and Technology Liaoning (2019RC12), the Key R&D Funding Scheme of Liaoning Province (2017308008), and the Liaoning high-level innovation team overseas training project (2018LNGXGJWPY-YB010) is also acknowledged.
The authors declare no competing financial interest.
References
- Zhao Y.; Dou J.; Duan X.; Chai H.; Oliveira J.; Yu J. Adverse Effects of Inherent CaO in Coconut Shell-Derived Activated Carbon on Its Performance during Flue Gas Desulfurization. Environ. Sci. Technol. 2020, 54, 1973–1981. 10.1021/acs.est.9b06689. [DOI] [PubMed] [Google Scholar]
- Xiao Z.; Li D.; Wang F.; Sun Z.; Lin Z. Simultaneous removal of NO and SO2 with a new recycling micro-nano bubble oxidation-absorption process based on HA-Na. Sep. Purif. Technol. 2020, 242, 116788. 10.1016/j.seppur.2020.116788. [DOI] [Google Scholar]
- Hao R.; Yang S.; Zhao Y.; Zhang Y.; Yuan B.; Mao X. Follow-up research of ultraviolet catalyzing vaporized H2O2 for simultaneous removal of SO2 and NO: Absorption of NO2 and NO by Na-based WFGD byproduct (Na2SO3). Fuel Process. Technol. 2017, 160, 64–69. 10.1016/j.fuproc.2017.02.021. [DOI] [Google Scholar]
- Xuan Y.; Yu Q.; Gao H.; Wang K.; Duan W. Modular manganese/diatomite-Santa Barbara Amorphous-15 sorbent for moderate-temperature flue gas desulfurization. Chem. Eng. J. 2020, 395, 124984. 10.1016/j.cej.2020.124984. [DOI] [Google Scholar]
- Chen Y.; Li J.; Li Y.; Cao X.; Tang S.; Wang S.; Chang S.; Ye W.; Li W. Comparative Study of the Flue Gas Desulfurization Using Different-Grade Natural Manganese Oxides. Chem. Eng. Technol. 2020, 43, 337–342. 10.1002/ceat.201900266. [DOI] [Google Scholar]
- Li X.; Dong Z.; Dou J.; Yu J.; Tahmasebi A. Catalytic reduction of NO using iron oxide impregnated biomass and lignite char for flue gas treatment. Fuel Process. Technol. 2016, 148, 91–98. 10.1016/j.fuproc.2016.02.030. [DOI] [Google Scholar]
- Liu X.-L.; Guo J.-X.; Chu Y.-H.; Luo D.-M.; Yin H.-Q.; Sun M.-C.; Yavuz R. Desulfurization performance of iron supported on activated carbon. Fuel 2014, 123, 93–100. 10.1016/j.fuel.2014.01.068. [DOI] [Google Scholar]
- Rambabu N.; Azargohar R.; Dalai A. K.; Adjaye J. Evaluation and comparison of enrichment efficiency of physical/chemical activations and functionalized activated carbons derived from fluid petroleum coke for environmental applications. Fuel Process. Technol. 2013, 106, 501–510. 10.1016/j.fuproc.2012.09.019. [DOI] [Google Scholar]
- Dou J.; Yu J.; Tahmasebi A.; Yin F.; Gupta S.; Li X.; Lucas J.; Na C.; Wall T. Ultrasonic-assisted preparation of highly reactive Fe–Zn sorbents supported on activated-char for desulfurization of COG. Fuel Process. Technol. 2015, 135, 187–194. 10.1016/j.fuproc.2015.01.035. [DOI] [Google Scholar]
- Liu Q.; Liu Z. Carbon supported vanadia for multi-pollutants removal from flue gas. Fuel 2013, 108, 149–158. 10.1016/j.fuel.2011.05.015. [DOI] [Google Scholar]
- Ma J.; Liu Z.; Liu S.; Zhu Z. A regenerable Fe/AC desulfurizer for SO2 adsorption at low temperatures. Appl. Catal., B 2003, 45, 301–309. 10.1016/s0926-3373(03)00176-0. [DOI] [Google Scholar]
- Arcibar-Orozco J. A.; Rangel-Mendez J. R.; Bandosz T. J. Reactive adsorption of SO2 on activated carbons with deposited iron nanoparticles. J. Hazard. Mater. 2013, 246–247, 300–309. 10.1016/j.jhazmat.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Dou J.; Zhao Y.; Tahmasebi A.; Yu J. Sulfidation and regeneration of iron-based sorbents supported on activated-chars prepared by pressurized impregnation for coke oven gas desulfurization. Korean J. Chem. Eng. 2016, 33, 2849–2857. 10.1007/s11814-016-0148-9. [DOI] [Google Scholar]
- Yin F.; Yu J.; Dou J.; Gupta S.; Moghtaderi B.; Lucas J. Sulfidation of iron-based sorbents supported on activated chars during the desulfurization of coke oven gases: Effects of Mo and Ce addition. Energy Fuels 2014, 28, 2481–2489. 10.1021/ef5004842. [DOI] [Google Scholar]
- Sumathi S.; Bhatia S.; Lee K. T.; Mohamed A. R. Performance of palm shell activated carbon impregnated with CeO2 and V2O5 catalyst in simultaneous removal of SO2 and NO. J. Appl. Sci. 2010, 10, 1052–1059. 10.3923/jas.2010.1052.1059. [DOI] [Google Scholar]
- Lillo-Ródenas M. A.; Lozano-Castelló D.; Cazorla-Amorós D.; Linares-Solano A. Preparation of activated carbons from Spanish anthracite. Carbon 2001, 39, 751–759. 10.1016/s0008-6223(00)00186-x. [DOI] [Google Scholar]
- Lillo-Ródenas M. A.; Cazorla-Amorós D.; Linares-Solano A. Understanding chemical reactions between carbons and NaOH and KOH. Carbon 2003, 41, 267–275. 10.1016/s0008-6223(02)00279-8. [DOI] [Google Scholar]
- Liu G.; Huang Z.-H.; Kang F. Preparation of ZnO/SiO2 gel composites and their performance of H2S removal at room temperature. J. Hazard. Mater. 2012, 215–216, 166–172. 10.1016/j.jhazmat.2012.02.050. [DOI] [PubMed] [Google Scholar]
- Lisovskii A.; Semiat R.; Aharoni C. Adsorption of sulfur dioxide by active carbon treated by nitric acid: I. Effect of the treatment on adsorption of SO2 and extractability of the acid formed. Carbon 1997, 35, 1639–1643. 10.1016/s0008-6223(97)00129-2. [DOI] [Google Scholar]
- Barrera A.; Montoya J. A.; Viniegra M.; Navarrete J.; Espinosa G.; Vargas A.; Del Angel P.; Pérez G. Isomerization of n-hexane over mono-and bimetallic Pd-Pt catalysts supported on ZrO2-Al2O3-WOx prepared by sol-gel. Appl. Catal., A 2005, 290, 97–109. 10.1016/j.apcata.2005.05.011. [DOI] [Google Scholar]
- Wu X.; Hong X.; Luo Z.; Hui K. S.; Chen H.; Wu J.; Hui K. N.; Li L.; Nan J.; Zhang Q. The effects of surface modification on the supercapacitive behaviors of novel mesoporous carbon derived from rod-like hydroxyapatite template. Electrochim. Acta 2013, 89, 400–406. 10.1016/j.electacta.2012.11.067. [DOI] [Google Scholar]
- Sabio E.; González E.; González J. F.; González-García C. M.; Ramiro A.; Gañan J. Thermal regeneration of activated carbon saturated with p-nitrophenol. Carbon 2004, 42, 2285–2293. 10.1016/j.carbon.2004.05.007. [DOI] [Google Scholar]
- He Q.; Zhang Z.; Xiong J.; Xiong Y.; Xiao H. A novel biomaterial-Fe3O4: TiO2 core-shell nano particle with magnetic performance and high visible light photocatalytic activity. Opt. Mater. 2008, 31, 380–384. 10.1016/j.optmat.2008.05.011. [DOI] [Google Scholar]
- Aksu Z. Biosorption of reactive dyes by dried activated sludge: equilibrium and kinetic modelling. Biochem. Eng. J. 2001, 7, 79–84. 10.1016/s1369-703x(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Ho Y. S.; McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. 10.1016/s0032-9592(98)00112-5. [DOI] [Google Scholar]
- Boyd G. E.; Adamson A. W.; Myers L. S. Jr The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. 10.1021/ja01203a066. [DOI] [PubMed] [Google Scholar]