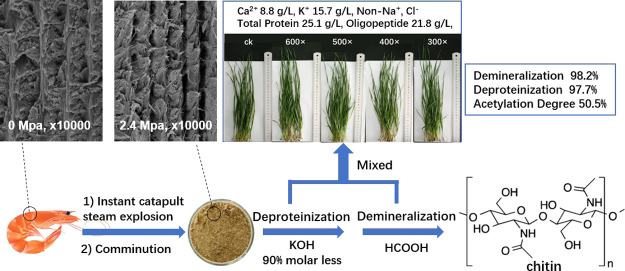
Abstract
An environmentally friendly approach for the comprehensive utilization of shrimp shell waste was reported. Instant catapult steam explosion (ICSE) was employed for shrimp shell waste pretreatment. After ICSE, lower crystallinity and greater surface areas of shrimp shells were achieved, which significantly enhanced the extraction of chitin. Compared to the traditional method, weaker organic acid (HCOOH) and much lower dosages of KOH (90% molar less) were used, and chitin with a high demineralization rate (98.2%) and deproteinization rate (97.7%) was obtained. The wastewater was neutralized by simply intermixing, and it was recycled as a potential plant fertilizer because it contained more oligopeptides, calcium, and potassium, but it was less salty and therefore non-toxic to plants. The whole process produced less solid waste and no waste water. The obtained chitin also showed a low degree of acetylation (50.5%), which demonstrates the potential for environmentally friendly preparation of chitosan in dilute alkali through ICSE.
Introduction
Shrimp shell waste is one of the major sources used for the preparation of chitin, whose content is approximately 15–40% on dry weight of shrimp shell.1,2 However, conventional chemical processes for chitin extraction are well known to be wasteful and costly and to produce high levels of pollution.3 To remove calcium carbonate and protein in the shell, large dosages of hydrochloric acid (HCl) and sodium hydroxide (NaOH) are usually used. Meanwhile, the production of 1 ton of chitin produces more than 400 m3 of wastewater with high ammonia, high salinity, and a high chemical oxygen demand, which is very difficult for subsequent water treatment.4,5 New eco-friendly technologies have been applied for the comprehensive utilization of shrimp waste, especially, biotechnologies such as fermentation and enzyme catalysis.3,6−8 However, these processes are usually expensive, time-consuming, and difficult to operate continuously on a large scale and may still require additional chemical methods.
On the other hand, protein (20–40%) and calcium carbonate (20–40%) in shrimp shells are typically wasted because of their high recovery cost and low value,9 and protein is easily rendered useless under the severe conditions used during chitin extraction. Actually, calcium and protein, together with protein derivatives such as polypeptides and amino acids, could be desirable ingredients for environmentally friendly plant fertilizers.10,11 However, during the conventional extraction of chitin from shrimp waste, the wastewater produced by the conventional demineralization (DM) and deproteinization (DP) processes contains suitable amounts of calcium and protein. Unfortunately, this wastewater is rarely used because of its high salinity and corrosiveness, and the large amounts of sodium and chloride ions in the wastewater have negative effects on both the growth and physiological activity of the plant.12 In order to reduce the usage of strong acid/alkali, an alternative treatment that can disrupt the crystalline structure is desirable,13−16 especially the newly developed HOW-CA process to extract high value chitin from the crustacean shell using hot water for DP and carbonic acid for DM, which was superior to the conventional method, offering both economic and environmental benefits.17
Steam explosion, an environmentally friendly physicochemical technology, is often used to destroy biomass structures via superheated steam and a sudden pressure release,18 but the use of steam explosion for shrimp shell waste treatment has rarely been investigated. Instant catapult steam explosion (ICSE) is an innovative steam explosion technology with a maximum explosion speed of 0.00875 s.19 It is proven to effectively destabilize crystals and intermolecular forces within cellulose in minutes, without causing substantial damage to the polymer molecular chains.20 The powerful seepage force of the steam during ICSE could provide a unique opportunity to destroy the interactions among chitin, minerals, and proteins in shells and break their crystalline structures, thereby increasing the reactive area and enhancing the extraction process.21−23 Therefore, it could be possible to remove more proteins and minerals from shrimp shells using less acid and base, or weaker acids such as organic acids, which would allow the environmentally friendly production of chitin, as well as easier recovery of calcium and protein.
Herein, we demonstrated an approach for the pretreatment of shrimp waste through ICSE, and potassium hydroxide and formic acid were used to remove protein and calcium carbonate from the shells. After chitin extraction, the basic and acidic solutions were neutralized by mixing to serve as a potential liquid fertilizer, containing essential elements for plant growth including calcium ions, potassium ions, and proteinaceous materials. To evaluate the viability of our design, the effects of ICSE on both shrimp shell structure and chitin extraction were investigated, and the obtained chitin and solution were analyzed. A preliminary research on the effect of the obtained solution on the growth of wheat seedlings was also conducted.
Materials and Methods
Materials
Dry shrimp shells were obtained from Guanfa Pharmaceutical Co. Ltd. (Dalian, China). It was confirmed that all shells were from a single species of shrimp Fenneropenaeus chinensis. All chemicals used in this study were of analytical grade and purchased from Aladdin Industrial Co. Ltd. (Shanghai, China). The water used was prepared using Millipore Elix System (Millipore, MA).
ICSE Treatment of Shrimp Shells
Before the test, take 2500 g of shells was moistened by 2500 g of water for 3 h. Five treatment groups were set, each with a capacity of 500 g and a treatment pressure of 1.6, 1.8, 2.0, 2.2, and 2.4 MPa for 3 min. Five the ICSE treatment was carried out on the QB-300 ICSE device from Gentle Science & Technology Co. Ltd. (Henan, China).
Microstructure Characterization of Shrimp Shells
Scanning electron microscopy (SEM) images were obtained with a su8020 scanning electron microscope (Hitachi, Japan). The voltage was 3.0 kV, and working distance was 9600 μm. Gas sorption isotherms were measured using ASAP 2460 surface area and a pore-size analyzer (Micrometrics, USA). X-ray diffraction (XRD) was performed on a Bruker D8 ADVANCE X-ray powder diffractometer (Bruker, Germany). Generator intensity was 40 kV, and generator current was 40 mA. The sample was scanned from 2θ = 5 to 70°, in step of 0.02°. Crystallinity index (CrI) determination for chitin was done using the equation24
| 1 |
where I110 is the maximum intensity at 22° and I020 is the maximum intensity at 10°. The intensity of amorphous diffraction, Iam, was obtained at around 16°.
Chitin Extraction and Waste Solution Recovery
The shrimp shells after ICSE treatment were broken into coarse powder using an ordinary kitchen blender (Midea, Guangdong, China). The contents of chitin, CaCO3, and protein in the shells were quantified using a literature method.25 Total nitrogen of shell and chitin nitrogen contents were measured by elemental analysis (Vario EL cube, Elementar, Germany).26 Corrected protein concentrations were obtained by subtracting chitin nitrogen from total nitrogen and multiplying it by 6.25. Ash content was determined by heating it to 600 °C for 4 h. Analyses showed that the shrimp shell consisted of 18.1% chitin, 33.3% CaCO3, and 38.6% protein.
Note that in the following experiments, the dosages of shrimp shells as the starting material were 10 g for each group. The temperature and reaction time used referenced the optimum parameters in a previous literature.27 To remove protein in the shell, 50 mL of KOH solution with the concentrations ranged from 1 to 8% wt was used, and the reaction was carried out at a temperature of 85 °C for 150 min. The base filter liquor was kept, and the deproteinated shells were collected in a 150 μm sieve and directly proceeded to the process of DM. Dilute HCOOH (60 mL) was used to remove the calcium carbonate, and the concentrations (v/v) ranged from 2.5 to 20%. The reaction was conducted at room temperature for 60 min. At the end, the material was filtrated, and the acid filter liquor was kept. The solid fraction was washed to neutrality with water and then dried at 80 °C, and chitin was obtained as a light-yellow solid.
Deproteination (DP) was expressed as percentages and calculated using the following equation28
| 2 |
where Po and PR are protein contents (g/g) before and after reaction, while O and R represent the masses (g) of original sample and obtained product, respectively.
DM was calculated using the following equation28
| 3 |
where Ao and AR are ash contents (g/g) before and after reaction, and O and R are masses (g) of the original sample and obtained product, respectively.
The alkaline solution after the process of DP and acid solution after the process of DM were neutralized by combining them together. The solution was filtered to remove the precipitate. The obtained solution contained protein and calcium ions, and this solution was denoted as Pr&CaS (protein and Ca2+ solution). The concentrations of calcium ions and potassium ions were measured by inductively coupled plasma optical emission spectrometry (ICP-OES) using an ICP emission spectrometer 730 (Agilent, United States), and total nitrogen content was measured using a Hanon K9840 automatic Kjeldahl apparatus (Hanon, China). The pH value of the solution was determined using an Orion Star A111 pH meter (Thermo Fisher Scientific, USA).
Analysis of the Chitin Products and Pr&CaS
Fourier transform infrared (FTIR) was performed on Nicolet iS10 FT-IR spectrometer instruments (Nicolet, USA) with the wave number range of 400–4000 cm–1. The resolution ratio was 4 cm–1, and scan time was 32 times. The DA was evaluated by the infrared spectroscopy method adapted from previous report, calculating using the following equations29
| 4 |
Solid-state 13C NMR spectra were recorded at 600 MHz on a Jeol JNM-ECZ600R spectrometer (JEOL Ltd., Tokyo, Japan). The degree of acetylation (DA) is calculated by measuring the integral of the carbonyl or methyl group (ICH3) divided by the integral of all the carbon atoms (IC1 + IC2 + IC3 + IC4 + IC5 + IC6) in the backbone30
| 5 |
The content of amino acids in Pr&CaS was analyzed using a GmbH A300 amino acid analyzer (MembraPure, Germany). Molecular weight of protein was collected by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS (ultrafleXtreme, Bruker, Germany).
Wheat seeds (Jingdong 22) were obtained from China Agricultural University (Beijing, China). The seeds were placed on cotton-cushioned Petri dishes with 10 mL of Pr&CaS of different concentrations (300×, 400×, 500×, 600×) for 12 h and DI water as control (ck). Then, they were grown in sterilized soil in a growth chamber (GXZ-SMART, Ningbo Jiangnan Instrument Co., China) at 25 °C, and 5 mL of the Pr&CaS solutions and DI water were added into the corresponding soil of each group every two days, and the plants were grown for 14 days. At 7th day, the plant heights were measured. At 14th day, they were removed from the soil, and average lengths and fresh and dry weights of the shoots were determined at the end of the experiment.
Results and Discussion
Microstructure Change of Shrimp Shells by ICSE Treatment
The shell of crustacean is formed through templating of mineral growth by an organic scaffold which consists of chitin fibers. The Bouligand structure in the crustacean cuticle is a complex hierarchical composite stacking of helicoidal aligned planar fiber layers, where long chains of the polysaccharide a-chitin and protein were assembled into chitin–protein nanofibers and mineralized at the nanoscale with crystalline mineral.13 This architecture was clearly observed in a SEM image of a fracture surface of shrimp shells (Figure 1a). The composite structure of the cuticle provides the stiffness, strength, and hardness of shrimp shells, but they also became structural barriers for the removal of protein and CaCO3 during chitin extraction. However, after ICSE treatment, interstices between the layers appeared in the fracture surface, and the planar sheets were more easily identified (Figure 1b), maybe as a result of the steam penetration produced by ICSE. The steam penetrated the structure of the shell and quickly expanded when decompression; thus, the internal structure was disrupted by a mechanical shearing force. This change could increase the surface area of shells, thus making protein and CaCO3 more accessible and available for interactions with enzymes or chemical reagents. Also, the structure of helicoidal organization became ambiguous, and the nanofibers seemed to be more amorphous and with less stiffness.
Figure 1.
SEM photos of a thin section of untreated [(a) ×10,000] and ICSE-pretreated shrimp shells [(b) ×10,000] and the nitrogen adsorption–desorption curves of untreated (c) and ICSE-pretreated shrimp shells (d).
The change in surface area was confirmed statistically through Brunauer–Emmett–Teller (BET) analysis. Figure 1c,d shows nitrogen adsorption isotherm curves of a raw shell sample and an ICSE-treated shell sample, respectively. Their adsorption cumulative pore volume curves are provided in Supporting Information. Both of samples exhibited type IV isotherms and H3 hysteresis loops, which could point to the presence of slit-shaped pores. The raw shell sample had a BET surface area of 1.72 m2/g, and the treated sample had a BET surface area of 2.11 m2/g. The pore volume also increased from 0.0097 to 0.0141 cm3/g after ICSE. In agreement with the enlarged, visible cracks in the SEM images above, the increase in the pore volume and surface area is likely caused by the powerful seepage force of the steam that deconstructed the shell microstructure and expanded the narrow cracks.
XRD patterns of both raw shrimp shell (0 MPa) and shell after ICSE pre-treatment (2.4 MPa) areshown in Figure 2 and were used to analyze the crystallinity of CaCO3 and chitin. The CaCO3 peaks were located at 2θ values of 29.2 and 39.6°, while the chitin peaks were observed at 9.5, 19.2, 23.4, and 26.4°.29 After ICSE, the intensities of the XRD peaks were much lower than that for the raw material, and these peak intensities are correlated with the degree of crystallization, suggesting that ICSE decreased the degree of chitin and CaCO3 crystallization. This is also supported by the CrI of chitin. Calculated using the eq 1, the CrI110 values for raw shells and that after ICSE were 87.1 and 71.4%, respectively, and the CrI020 values for raw and ICSE-treated shell were 79.6 and 57.5%, respectively. The maximum decreases in the CrI110 and CrI020 were 15.7 and 24.5%, respectively, which suggested deformation of the crystalline structure in the shell. Besides, several XRD peaks occur or became more obvious at 2θ equal to 22.7 (0 1 2), 29.2 (1 0 4), 36.6 (1 1 0), and 50.1 (1 1 8), which can be attributed to the diffraction peaks of CaCO3.31 This is probably because CaCO3 crystals inside the shrimp shell became easier to absorb X-rays after the deformation of organic crystalline structures. The increase in surface area (Figure 1) and decrease in crystallinity of the shrimp shells were expected to increase the reagent-solid contact area and enhance the extraction of chitin, and these hypotheses are investigated below.
Figure 2.

The XRD patterns of raw (0 MPa) and ICSE-treated shrimp shells (2.4 MPa).
Chitin Extraction and Waste Solution Recovery
After the ICSE pretreatment, the shrimp shell waste was subjected to DP and DM processes for chitin extraction. Considering the potential use of the protein and Ca contents as a fertilizer, potassium hydroxide (KOH) was used to remove the protein instead of NaOH, as potassium ions are an essential element for plant growth,32 whereas excessive sodium ions are harmful to plants. Formic acid (HCOOH), a weak organic acid, was used to decompose the calcium carbonate in the shrimp shells instead of HCl, as HCl is strongly corrosive and chloride ions are disadvantageous for plants. More importantly, formate ions are widespread in the roots and leaves of plants33,34 and have antibacterial properties that are important to inhibit deterioration of the protein-rich Pr&CaS.35
To avoid wasting soluble substances during the clean process and to reduce the consumption of water, we attempted to eliminate the first cleaning process, which meant that the solid fraction that remained after step one was directly moved to the next step, without cleaning with water. In addition, for the obtained neutralized solution, Pr&CaS, to avoid the formation of Ca(OH)2 at basic pH or cultivating microorganisms at neutral pH, a weakly acidic pH is favorable. Therefore, a slight excess of acid was used, whose molar amount was higher than that of alkali and two estimated equivalents of CaCO3 combined.
After ICSE treatment at 1.6, 1.8, 2.0, 2.2, or 2.4 MPa, shrimp wastes (10 g for each group) were deprotonated with KOH (2 g in 50 mL water, w/v 4%). The reaction was conducted at 85 °C for 150 min. Then, the remaining solid after filtration was subjected to 60 mL of an HCOOH aqueous solution (v/v 10%) for DM. As a control, raw shrimp wastes also underwent the same process. As illustrated in Figure 3a, the DP extent of the raw shrimp shells under these conditions was only 78.9%, but the extent reached 91.8% after ICSE treatment at 1.6 MPa and increased with increasing ICSE pressure (calculated using eq 2). The DP rate was 94.7% at 2.2 MPa and was 95.3% at 2.4 MPa. Analysis of the ash content revealed that the DM increased similarly (Figure 3b), which was calculated using eq 3. The DM of the chitin obtained from raw shrimp was low at 85.2%, but it increased to 96.4% at 2.2 MPa and 95.9% at 2.4 MPa. It is notable that the doses of base used here are much lower than traditional methods. With lower dosages of base in this study, the extent of DP (i.e., 95.3%) has reached relatively high levels compared to previous studies,27 which can be attributed to the destruction of the shrimp shell’s tightly packed structures.
Figure 3.
Effect of pressure of ICSE on extent of DP (a) and DM (b).
The basic solution after DP process was a murky liquid containing large amounts of shrimp protein. In the conventional process, when adjusting alkaline waste using acid waste to neutral or weakly acidic pH, protein will precipitate in large amounts,36 and this solid protein is hardly absorbable or useable by plants. In our study, we found that the ICSE pressure had an obvious effect on the amount of protein precipitation, which decreased with increasing pressure (Figure 4a). The weight of the precipitated protein in the control group was 1.33 g, but this was reduced to 1.11 g with a pressure of 1.6 MPa, and the minimum amount of precipitate was 0.76 g at 2.4 MPa. This decrease in precipitation minimized the generation of solid waste.
Figure 4.
(a) Effect of pressure of ICSE on the amount of precipitation after mixture; (b–d) effect of pressure of ICSE on Ca2+ concentration (b), pH value (c), and total protein concentration (d).
Pr&CaS after filtration was a brown transparent liquid. The concentration of calcium ions, an important element in our fertilizer, was determined by ICP-OES. Figure 4b illustrates the effect of ICSE pressure on the calcium ion concentration and shows a positive correlation generally. The calcium ion content in the control group is approximately 5.17 g/L, but it ranged from 7.69 to 8.64 g/L after ICSE, which can be attributed to the deconstruction of the shrimp shells and increased CaCO3 digestion. With the same dosage of KOH and HCOOH, the pH values of Pr&CaS showed a slight increase with increasing pressure (Figure 4c), probably due to greater consumption of HCOOH by CaCO3 at higher pressures, which is consistent with the previously measured higher DM rates. The final pH ranged from 3.52 to 3.69, which meets the fertilizer acidity requirements.
To validate the total concentration of soluble shrimp protein after ICSE treatment (including derivative polypeptides and amino acids), the total nitrogen content of the solutions was measured via a Kjeldahl determination. As expected, Figure 4d indicates that higher pressure causes an increase in the total nitrogen content in Pr&CaS. The concentration of total protein increased from 1.29% for raw shells to 2.39% after 2.4 MPa ICSE, which means that ICSE can almost double the total nitrogen content with the same reagent consumption and same process. Combined with the change in DP rate and precipitated protein weight measured above, these data indicate that after ICSE, more protein was removed from shrimp shells, but less was precipitated, meaning more protein remains dissolved in solution.
According to the data above, the optimal results were obtained from pretreatment by ICSE at higher pressure (2.4 and 2.2 MPa), likely due to the increased power of the steam; thus, the shell structure was disrupted more. However, because 2.4 MPa is the maximum operational load and can damage the equipment over time in production, the method at 2.2 MPa was used for the following experiments.
Dose Optimization
The dosages of KOH and HCOOH were optimized, and in order to obtain a constant final pH of Pr&CaS, they were coadjusted. The concentrations of KOH (wt) were 1, 2, 4, 6, and 8%, and the corresponding HCOOH concentrations (v/v) were 2.5, 5, 10, 15, and 20%. The DP extent for a series chitin (Figure 5a) increased with the reagent input. The largest extents of DP were 97.7 and 97.7% when 6 and 8% of KOH were used, respectively. The DM rate did not change significantly when the HCOOH concentration exceeded 10% (Figure 5b). The highest DM was 98.2% when 20% of HCOOH was used, and the ash content was 2.68%. These DM rates are generally superior to those obtained by biological methods, and the remaining ash content can be easily removed by HCl if high-purity chitin is required.
Figure 5.
(a) Effect of concentration of KOH on extent of DP; (b) effect of concentration of HCOOH on extent of DM.
In consideration of cost-efficiency for the integrated utilization of shrimp waste, an ICSE pretreatment was selected at 2.2 MPa with a chemical dosage of 6% of KOH (3 g, 53.6 mmol) and 15% HCOOH as the optimized conditions to process 10 g of shrimp shells. In a traditional method for chitin extraction, an NaOH solution of >2 M concentration and solid/solvent ratio (g/mL) of 1/25 are needed, which means that more than 500 mmol NaOH is needed to treat 10 g of shrimp shell.27 In contrast, our process reduced the alkaline molar dosage by approximately 90% but obtained a similar DP rate. The molar dosage of HCOOH was similar to that of HCl, but the acid strength was weaker. The water consumption was reduced by half by excluding the first cleaning process. This is also a rapid and efficient treatment method, because the pretreatment of shrimp shells by ICSE is finished within minutes, and the whole process can be completed within hours.
The ICSE device used in this method, QB-300, is an industrial equipment which could be performed on a medium scale with the capacity of treatment of 100 kg per day. The device’s price is about 100 thousand USD, and its service life can reach 15 years. Based on its treatment capacity, the current method with optimized dosage can save the costs of KOH of 270 USD per day roughly (270 kg KOH is saved, 1 USD per kg of KOH), and the cost of acid is similar to that of traditional method. After the deduction of energy costs (∼15 USD per day), the accumulative saved cost can reach about 51,000 USD a year (at 200 working days per year), and thus, it can cover the price of device within about two years. More importantly, the expenditure of waste treatment, which is a major part of costs for traditional chitin production, can be greatly saved with this method.
Chitin Characterization
The chitin samples extracted from ICSE-treated shrimp shell under the optimized condition (2.2 MPa, 6% KOH + 15% HCOOH) was analyzed using FT-IR spectroscopy (Figure 6a), and all of them appeared quite similar to commercial chitin (Figure 6b). The FT-IR spectrum of the obtained chitin displays two narrow absorption bands at 1659 and 1625 cm–1, which corresponds to a typical amide I stretching band of C=O in α-chitin and can be attributed to the two types of H-bonds formed by amide groups in the antiparallel alignment present in crystalline regions of α-chitin. The band at 1557 cm–1 corresponds to the N–H of the deformed amide II, and the peak at 1416 cm–1 corresponds to a symmetrical deformation of the CH3 group. The band at 1378 cm–1 was assigned to the stretching vibration of C–O. The NH stretching bands at 3264 and 3108 cm–1 and the OH out-of-plane bending at 701 cm–1 are also visible, which is typical for α-chitin. The FT-IR spectrum demonstrated that the extracted material is α-chitin.
Figure 6.

FT-IR spectra of (a) chitin prepared from ICSE-treated shrimp shell and (b) commercial chitin.
The DA values were calculated using eq 4 and were found to be 50.5% for the prepared chitin and 72.1% for commercial chitin. To get more reliable values, 13C solid-state NMR was also used to evaluate the acetyl content. The 13C solid-state NMR spectra and the table of DAs are provided in Supporting Information (Figure S1 and Table S1). The DAs measured by eq 5 were 72.4 and 87.8% for the prepared chitin and commercial chitin, respectively, which were higher than the values measured by IR. However, both of the two detection methods showed that the DA of the prepared chitin was lower than the commercial chitin. In a recent study of us,37 the effect of ICSE on the chitin has been systematically investigated, which showed that DA of chitin was less affected by ICSE. In the present study, however, during the DP process, chitin with much lower CrI could be more susceptible to base, causing increased deacetylation reactions to occur. Note that the base concentration used was much lower than that in the conventional method. Chitosan is usually prepared from chitin by deacetylation at high temperatures in highly alkaline environments (>40 wt % concentration). This approach represents a potential for an improved method to obtain chitosan in a more environmentally friendly way with less consumption of strong bases and energy.38
Analysis of Pr&CaS
The free amino acids and soluble proteins in Pr&CaS were evaluated, and those obtained from the raw shrimp shells were also analyzed as a control. Amino acid analysis (Table 1) showed that the total content of free amino acids was 3.3 g/L after ICSE at 2.2 MPa, which was unexpectedly lower than the control group (5.7 g/L). In addition, some amino acids such as contents of glycine, β-alanine, tryptophan, and cysteine were reduced, and some others especially of glutamic acid, citrulline, alanine, and arginine were increased significantly. The possible reason is that ICSE treatment causes partial protein aggregates to dissociate while forming new protein aggregates,39 and protein aggregates containing certain amino acids such as cysteine were hydrolyzed by formic acid to soluble peptides, while protein aggregates containing others such as citrulline were more likely to be fully hydrolyzed into free amino acids by KOH.
Table 1. Amino Acid Composition and Content of the Mixed Solution (Pr&CaS) from Raw Shrimp Shells (0 MPa) and ICSE-Treated (2.2 MPa) Shrimp Shellsa.
| amino acid | 0 MPa | 2.2 MPa |
|---|---|---|
| phosphoserine | 17.88 | 29.80 |
| taurine | 26.93 | 22.06 |
| aspartic acid | 1.15 | 62.76 |
| threonine | 1.28 | 15.36 |
| serine | 23.02 | 95.85 |
| asparagine | 2.10 | 0.00 |
| glutamic acid | 24.69 | 580.80 |
| α-aminoadipic acid | 73.73 | 21.42 |
| glycine | 2034.85 | 57.40 |
| alanine | 1.58 | 219.46 |
| citrulline | 5.87 | 398.85 |
| α-aminobutyric acid | 1.57 | 13.58 |
| valine | 1.79 | 0.00 |
| cystine | 663.27 | 0.00 |
| cystha | 498.63 | 101.65 |
| methionine | 25.74 | 13.85 |
| isoleucine | 11.12 | 0.00 |
| leucine | 37.86 | 18.77 |
| norleucine | 3.47 | 48.00 |
| tyrosine | 7.04 | 65.58 |
| phenylalanine | 5.61 | 0.00 |
| homocystine | 24.33 | 0.00 |
| β-alanine | 939.84 | 167.77 |
| β-aminoisobutyric acid | 0.80 | 0.00 |
| γ-aminobutyric acid | 218.48 | 262.85 |
| histidine | 316.30 | 290.93 |
| 3-methylhistidine | 3.29 | 243.09 |
| 1-methylhistidine | 11.34 | 4.50 |
| tryptophan | 151.54 | 0.00 |
| anserine | 14.84 | 1.59 |
| hydroxylysine | 80.50 | 0.00 |
| ornithine | 239.45 | 0.00 |
| lysine | 154.73 | 74.33 |
| ethanolamine | 1.60 | 11.75 |
| arginine | 20.92 | 452.64 |
| hydroxyproline | 4.51 | 23.14 |
| proline | 23.36 | 3.39 |
| total | 5675.96 | 3309.25 |
Unit: mg/L.
Additionally, the concentrations of Ca2+ and total protein were measured to be 8.8 and 25.1 g/L, respectively, which were much higher than the control group (Table S2). K+ concentrations, pH values, and estimated peptide concentrations of Pr&CaS are also listed in Table S2. The solution underwent no deterioration when preserving it several months at room temperature; it may be due to weak acid pH and the existence of formate ion.
Because a higher total nitrogen content but fewer free amino acids existed in Pr&CaS, the concentration of soluble protein could be much higher than the control group. MALDI-TOF MS spectra supported this hypothesis, wherein under the same test conditions, the intensities of the m/z peeks of the ICSE sample (Figure 7b) were much higher than those of the control group (Figure 7a). Importantly, the m/z signals of the ICSE sample were mainly distributed from 400 to 600, whereas those of the control group were at 492.8 and 603.1, with some in the range of 1000–1400. The molecular weight distributions were also confirmed by gel permeation chromatography (GPC) (Figure S2).
Figure 7.
MALDI-TOF spectrum of Pr&CaS from raw shrimp shells (a) and ICSE-treated shrimp shells (b); (c) representative plates of control seedlings (ck) and seedlings treated with Pr&CaS with various dilute proportions (300×, 400×, 500×, 600×).
These results suggest that the soluble proteins in Pr&CaS were actually oligopeptide, and most of them were tetrapeptides or pentapeptide as estimated by the molecular weight. This also explains the higher concentration of soluble protein and less insoluble precipitation after ICSE, as shown in Figure 4a,d. Actually, ICSE treatment itself should not cause substantial damage to the protein chain.21 Instead, the completion of release of high density energy at extremely short time during ICSE could provide enough force to disrupt and unfold the compact structure of proteins and the proteoglycan complex. This dramatically increases the chemical accessibility and extraction of protein and thus promotes its degradation at higher temperatures in the alkali environment during the DP process. Amounts of oligopeptide that existed in Pr&CaS can likely be easily digested by plants and can act as readily available nutrition for plant growth. Furthermore, there is now compelling evidence that small polypeptides are also involved in many plant biological processes, which provides an opportunity for oligopeptides from shrimp protein to have a role in regulating plant growth, not just as nitrogen reserve or nutrition.40,41
A preliminary research on the effect on the growth of wheat seedlings and seed germination was conducted. The wheat seeds were grown in sterilized soil and treated with DI water (ck) and Pr&CaS with various dilute proportion (300×, 400×, 500×, 600×). The growth of seedlings experiments (14 days) showed that Pr&CaS may not have strong toxic or inhibiting effects on the growth of the plants (Figure 7c) and seed germination. Instead, we observed some positive impacts on germination rate, plant height, fresh, and dry weights (Table S3), which may be due to N, K, and Ca fertilization in plants grown. Interestingly, we also noted that at the 7th day, Pr&CaS at a relatively high concentration (300×) showed inhibition to the wheat plants (possibly because of high osmotic pressure caused by high concentration of K and Ca salt), but at the 14th day, some individual seeds were notably above the average in height (Figure 7c, 300×). This phenomenon could due to a stimulating effect of oligopeptides that promotes plants growth, which will be further studied in our subsequent work.
Conclusions
In summary, we developed a new approach for environmental comprehensive utilization of shrimp shell waste. Enlarged surface areas and decreased crystallinity were achieved in shrimp shells via steam with a powerful bursting force produced by ICSE. This treatment significantly enhanced the chitin extraction. Formic acid and much lower dosages of KOH were used in the chitin extraction process, and this process consumed less water. Chitin was obtained with high levels of DM and DP and low DA. The obtained solution was rich in oligopeptides, calcium, and potassium but contained no sodium and chloride, which implied its potential for environmentally friendly plant fertilizer. Compared with the control group, significantly more protein and calcium in shells were transferred to the solution and less was precipitated. The whole process was very simple and could be achieved on a medium or large scale. Considering the possible function of small polypeptides in regulating many plant biological processes, the effect of our potential fertilizer on plant growth will be investigated in detail in our subsequent study.
Acknowledgments
This work was supported by the Marine Economic Innovation & Development Project of Beihai (Bhsfs008) and the Research & Develop Project of ACADEMY OF AGRICULTURAL PLANNING ANG ENGINEERING MARA (2018ZZYF0102).
Glossary
Abbreviation
- GPC
gel permeation chromatography
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02705.
DA determined by 13C solid-state NMR, content analysis of Pr&CaS, effects of Pr&CaS on wheat growth, analysis of 13C solid-state NMR spectra, and GPC-profile of Pr&CaS (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yan N.; Chen X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. 10.1038/524155a. [DOI] [PubMed] [Google Scholar]
- Kannan S.; Gariepy Y.; Raghavan G. S. V. Conventional Hydrothermal Carbonization of Shrimp Waste. Energy Fuels 2018, 32, 3532–3542. 10.1021/acs.energyfuels.7b03997. [DOI] [Google Scholar]
- Mao X.; Guo N.; Sun J.; Xue C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Cleaner Prod. 2017, 143, 814–823. 10.1016/j.jclepro.2016.12.042. [DOI] [Google Scholar]
- Anh P. T.; My Dieu T. T.; Mol A. P. J.; Kroeze C.; Bush S. R. Towards eco-agro industrial clusters in aquatic production: the case of shrimp processing industry in Vietnam. J. Cleaner Prod. 2011, 19, 2107–2118. 10.1016/j.jclepro.2011.06.002. [DOI] [Google Scholar]
- Kandra P.; Challa M. M.; Kalangi Padma Jyothi H. Efficient use of shrimp waste: present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. 10.1007/s00253-011-3651-2. [DOI] [PubMed] [Google Scholar]
- Devi R.; Dhamodharan R. Pretreatment in Hot Glycerol for Facile and Green Separation of Chitin from Prawn Shell Waste. ACS Sustainable Chem. Eng. 2018, 6, 846–853. 10.1021/acssuschemeng.7b03195. [DOI] [Google Scholar]
- Lopes C.; Antelo L. T.; Franco-Uría A.; Alonso A. A.; Pérez-Martín R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Cleaner Prod. 2018, 172, 4140–4151. 10.1016/j.jclepro.2017.01.082. [DOI] [Google Scholar]
- Chakravarty J.; Yang C.-L.; Palmer J.; Brigham C. J. Chitin Extraction from Lobster Shell Waste using Microbial Culture-based Methods. Appl. Food Biotechnol. 2018, 5, 141–154. 10.22037/afb.v5i3.20787. [DOI] [Google Scholar]
- Chen X.; Li C.; Ji X.; Zhong Z.; Li P. Recovery of protein from discharged wastewater during the production of chitin. Bioresour. Technol. 2008, 99, 570–574. 10.1016/j.biortech.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Hepler P. K. Calcium: A Central Regulator of Plant Growth and Development. Plant Cell 2005, 17, 2142–2155. 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øvsthus I.; Breland T. A.; Hagen S. F.; Brandt K.; Wold A.-B.; Bengtsson G. B.; SeljÅsen R. Effects of Organic and Waste-Derived Fertilizers on Yield, Nitrogen and Glucosinolate Contents, and Sensory Quality of Broccoli (Brassica oleracea L. var. italica). J. Agric. Food Chem. 2015, 63, 10757–10767. 10.1021/acs.jafc.5b04631. [DOI] [PubMed] [Google Scholar]
- Flowers T. J.; Flowers S. A. Why does salinity pose such a difficult problem for plant breeders?. Agric. Water Manag. 2005, 78, 15–24. 10.1016/j.agwat.2005.04.015. [DOI] [Google Scholar]
- Chen X.; Yang H.; Zhong Z.; Yan N. Base-catalysed, one-step mechanochemical conversion of chitin and shrimp shells into low molecular weight chitosan. Green Chem. 2017, 19, 2783–2792. 10.1039/c7gc00089h. [DOI] [Google Scholar]
- Chang F.-S.; Chin H. Y.; Tsai M.-L. Preparation of chitin with puffing pretreatment. Res. Chem. Intermed. 2018, 44, 4939–4955. 10.1007/s11164-018-3346-8. [DOI] [Google Scholar]
- Tasaki K. A novel thermal hydrolysis process for extraction of keratin from hog hair for commercial applications. Waste Manage. 2020, 104, 33–41. 10.1016/j.wasman.2019.12.042. [DOI] [PubMed] [Google Scholar]
- Toan N. V.; Ng C.-H.; Aye K. N.; Trang T. S.; Stevens W. F. Production of high-quality chitin and chitosan from preconditioned shrimp shells. J. Chem. Technol. Biotechnol. 2006, 81, 1113–1118. 10.1002/jctb.1437. [DOI] [Google Scholar]
- Yang H.; Gözaydın G.; Nasaruddin R. R.; Har J. R. G.; Chen X.; Wang X.; Yan N. Towards the shell biorefinery: processing crustacean shell waste using hot water and carbonic acid. ACS Sustainable Chem. Eng. 2019, 7, 5532–5542. 10.1021/acssuschemeng.8b06853. [DOI] [Google Scholar]
- Anglès M. N.; Ferrando F.; Farriol X.; Salvadó J. Suitability of steam exploded residual softwood for the production of binderless panels. Effect of the pre-treatment severity and lignin addition. Biomass Bioenergy 2001, 21, 211–224. 10.1016/s0961-9534(01)00031-9. [DOI] [Google Scholar]
- Yu Z.; Zhang B.; Yu F.; Xu G.; Song A. A real explosion: the requirement of steam explosion pretreatment. Bioresour. Technol. 2012, 121, 335–341. 10.1016/j.biortech.2012.06.055. [DOI] [PubMed] [Google Scholar]
- Lizasoain J.; Trulea A.; Gittinger J.; Kral I.; Piringer G.; Schedl A.; Nilsen P. J.; Potthast A.; Gronauer A.; Bauer A. Corn stover for biogas production: Effect of steam explosion pretreatment on the gas yields and on the biodegradation kinetics of the primary structural compounds. Bioresour. Technol. 2017, 244, 949–956. 10.1016/j.biortech.2017.08.042. [DOI] [PubMed] [Google Scholar]
- Liu C.-G.; Liu L.-Y.; Zi L.-H.; Zhao X.-Q.; Xu Y.-H.; Bai F.-W. Assessment and regression analysis on instant catapult steam explosion pretreatment of corn stover. Bioresour. Technol. 2014, 166, 368–372. 10.1016/j.biortech.2014.05.069. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gong X.; Hu X.; Zhou N. Lignin monomer in steam explosion assist chemical treated cotton stalk affects sugar release. Bioresour. Technol. 2019, 276, 343–348. 10.1016/j.biortech.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Cubas-Cano E.; González-Fernández C.; Ballesteros I.; Tomás-Pejó E. Efficient utilization of hydrolysates from steam-exploded gardening residues for lactic acid production by optimization of enzyme addition and pH control. Waste Manage. 2020, 107, 235–243. 10.1016/j.wasman.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Tan T. S.; Chin H. Y.; Tsai M.-L.; Liu C.-L. Structural alterations, pore generation, and deacetylation of alpha- and beta-chitin submitted to steam explosion. Carbohydr. Polym. 2015, 122, 321–328. 10.1016/j.carbpol.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Shahidi F.; Synowiecki J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem. 1991, 39, 1527–1532. 10.1021/jf00008a032. [DOI] [Google Scholar]
- Percot A.; Viton C.; Domard A. Characterization of Shrimp Shell Deproteinization. Biomacromolecules 2003, 4, 1380–1385. 10.1021/bm034115h. [DOI] [PubMed] [Google Scholar]
- Benhabiles M. S.; Abdi N.; Drouiche N.; Lounici H.; Pauss A.; Goosen M. F. A.; Mameri N. Protein recovery by ultrafiltration during isolation of chitin from shrimp shells Parapenaeus longirostris. Food Hydrocolloids 2013, 32, 28–34. 10.1016/j.foodhyd.2012.11.035. [DOI] [Google Scholar]
- Ghorbel-Bellaaj O.; Younes I.; Maâlej H.; Hajji S.; Nasri M. Chitin extraction from shrimp shell waste using Bacillus bacteria. Int. J. Biol. Macromol. 2012, 51, 1196–1201. 10.1016/j.ijbiomac.2012.08.034. [DOI] [PubMed] [Google Scholar]
- Gbenebor O. P.; Adeosun S. O.; Lawal G. I.; Jun S.; Olaleye S. A. Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Int. J. Eng. Sci. 2017, 20, 1155–1165. 10.1016/j.jestch.2017.05.002. [DOI] [Google Scholar]
- Raymond L.; Morin F. G.; Marchessault R. H. Degree of Deacetylation of Chitosan Using Conductometric Titration and Solid-State NMR. Carbohydr. Res. 1993, 246, 331–336. 10.1016/0008-6215(93)84044-7. [DOI] [Google Scholar]
- Tangboriboon N.; Kunanuruksapong R.; Sirivat A. Preparation and properties of calcium oxide from eggshells via calcination. Mater. Sci.-Pol. 2012, 30, 313–322. 10.2478/s13536-012-0055-7. [DOI] [Google Scholar]
- Shabala S.; Cuin T. A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- Roschek B.; Fink R. C.; McMichael M.; Alberte R. S. Nettle extract (Urtica dioica) affects key receptors and enzymes associated with allergic rhinitis. Phytother. Res. 2009, 23, 920–926. 10.1002/ptr.2763. [DOI] [PubMed] [Google Scholar]
- Liu F.; He Y.; Wang L.; Sun G. Detection of Organic Acids and pH of Fruit Vinegars Using Near-Infrared Spectroscopy and Multivariate Calibration. Food Bioprocess Technol. 2011, 4, 1331–1340. 10.1007/s11947-009-0240-9. [DOI] [Google Scholar]
- Thompson J. L.; Hinton M. Antibacterial activity of formic and propionic acids in the diet of hens on salmonellas in the crop. Br. Poult. Sci. 1997, 38, 59–65. 10.1080/00071669708417941. [DOI] [PubMed] [Google Scholar]
- El-Beltagy A. E.; El-Sayed S. M. Functional and nutritional characteristics of protein recovered during isolation of chitin from shrimp waste. Food Bioprod. Process. 2012, 90, 633–638. 10.1016/j.fbp.2012.06.004. [DOI] [Google Scholar]
- Tian Z.; Wang S.; Hu X.; Zhang Z.; Liang L. Crystalline reduction, surface area enlargement and pore generation of chitin by instant catapult steam explosion. Carbohyd. Polym. 2018, 200, 255–261. 10.1016/j.carbpol.2018.07.075. [DOI] [PubMed] [Google Scholar]
- Wang W.; Du Y.; Qiu Y.; Wang X.; Hu Y.; Yang J.; Cai J.; Kennedy J. F. A new green technology for direct production of low molecular weight chitosan. Carbohydr. Polym. 2008, 74, 127–132. 10.1016/j.carbpol.2008.01.025. [DOI] [Google Scholar]
- Zhang Y.; Yang R.; Hua X.; Zhao W.; Zhang W.; Liu Z.; Zhang Y. Effect of steam flash-explosion on protein structure of high-temperature soybean meal using gel electrophoresis method. China Oils Fats 2013, 38, 76–79. [Google Scholar]
- Calvo P.; Nelson L.; Kloepper J. W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. 10.1007/s11104-014-2131-8. [DOI] [Google Scholar]
- Veselá M.; Friedrich J. Amino acid and soluble protein cocktail from waste keratin hydrolysed by a fungal keratinase of Paecilomyces marquandii. Biotechnol. Bioprocess Eng. 2009, 14, 84–90. 10.1007/s12257-008-0083-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.