Abstract
The endoplasmic reticulum (ER) engages mitochondria at specialized ER domains known as mitochondria-associated membranes (MAMs). Here, we used three-dimensional high-resolution imaging to investigate the formation of pleomorphic “megamitochondria” with altered MAMs in brown adipocytes lacking the Sel1L-Hrd1 protein complex of ER-associated protein degradation (ERAD). Mice with ERAD deficiency in brown adipocytes were cold sensitive and exhibited mitochondrial dysfunction. ERAD deficiency affected ER-mitochondria contacts and mitochondrial dynamics, at least in part, by regulating the turnover of the MAM protein, sigma receptor 1 (SigmaR1). Thus, our study provides molecular insights into ER-mitochondrial crosstalk and expands our understanding of the physiological importance of Sel1L-Hrd1 ERAD.
One Sentence Summary:
This study reveals Sel1L-Hrd1 ERAD as a new regulatory mechanism underlying ER-mitochondrial communication in a physiological setting.
Membrane contact sites mediate interorganellar communication and are key to organelle homeostasis and organismal health (1–3). Endoplasmic reticulum (ER)-derived mitochondria-associated membranes (MAMs) are indispensable for mitochondrial dynamics and function. MAMs, characterized by intimate contact between the ER and mitochondria (~10–25 nm apart) (4), mark sites for mitochondrial DNA synthesis and fission (5–7), and for calcium exchange and lipid biosynthesis (1, 8, 9). During mitochondrial fission, ER tubules envelop and constrict mitochondria, and the activated dynamin-related GTPase protein (Drp1) aggregates to cleave the organelle (5, 10–13). The mechanism underlying interorganellar communication and physiological consequences of miscommunication remain largely unclear.
ER-associated degradation (ERAD) is a conserved quality control mechanism to recruit ER proteins for cytosolic proteasomal degradation (14–19). The Sel1L-Hrd1 protein complex comprises the most conserved form of ERAD from yeast to humans. Sel1L resides on the ER membrane and controls the stability of the E3 ligase Hrd1 (16–18, 20–22). Sel1L-Hrd1 ERAD is indispensable for fundamental physiological processes in vivo such as lipid metabolism, water balance, food intake, and systemic energy homeostasis (15, 19, 23–31).
Formation of megamitochondria in Sel1L−/− brown adipocytes
Serendipitously, we observed elongated mitochondria in Sel1L−/− primary brown preadipocytes, resembling those in Drp1−/− preadipocytes but distinct from those in pro-fusion Opa1−/− cells (Fig. S1A–B). These findings prompted us to explore a possible role of Sel1L in mitochondria-rich brown adipose tissue (BAT), a potential therapeutic target for obesity (32). Acute cold challenge induces mitochondrial fission in BAT to enhance respiration and maintain body temperature (33), while having no impact on the expression of ERAD genes (Fig. S2A–B).
We generated adipocyte-specific Sel1L-deficient mice (Sel1LAdipCre) by crossing Sel1Lf/f mice to the adiponectin promoter-driven Cre mice (25). Sel1L was deleted specifically in both white adipose tissue (WAT) and BAT, resulting in lower Hrd1 expression (Fig. S2C). We next performed transmission electron microscopy (TEM) to visualize mitochondria in BAT from mice housed at room temperature (22°C), or 4°C for 6 hr. There was no difference in mitochondrial morphology between Sel1Lf/f and Sel1LAdipCre BAT at 22°C (Fig. 1A–B). Acute cold challenge led to smaller mitochondria in Sel1Lf/f BAT due to increased fission (Fig. 1A and 1C). By contrast, most mitochondria in Sel1LAdipCre brown adipocytes were enlarged and pleomorphic, with abnormal cristae architecture (yellow arrows, Fig. 1B–C and S3A–B). Consequently, mitochondrial density per cell was reduced in cold-stimulated Sel1LAdipCre mice compared to control (Fig. S3C).
Figure 1. Sel1L regulates mitochondrial morphology in BAT during cold exposure.

(A-C) Representative TEM images of BAT from Sel1Lf/f (A) and Sel1LAdipCre mice (B) at 22°C (left) or 4°C (right) for 6 hr, with quantitation of mitochondrial size shown in C (n=870, 601 mitochondria from 3 Sel1Lf/f mice each at 22°C and 4°C; 624, 821 from 3 Sel1LAdipCre mice each at 22°C and 4°C, one-way ANOVA). Yellow arrows, megamitochondria. M, mitochondrion; LD, lipid droplet. (D-E) Representative TEM images of BAT from Sel1LUcp1Cre mice at 4°C for 6 hr, with quantitation of mitochondrial size shown in E (n=676, 618 mitochondria for 3 Sel1Lf/f mice each at 22°C and 4°C; 582, 674 for 3 Sel1LUcp1Cre mice each at 22°C and 4°C, one-way ANOVA). (F-H) Representative SBF-SEM images (F) and 3D reconstruction (G) of mitochondria from BAT of Sel1Lf/f and Sel1LUcp1Cre mice at 4°C for 6 hr. Mitochondria in pseudo colors were reconstructed from a set of 150 SBF-SEM image stacks (65 nm/slice). (H) Quantitation of 3D volume of mitochondria (n=61 and 63, Sel1Lf/f and Sel1LUcp1Cre mice, Student’s t-test). All experiments have been repeated three times. Data are mean ± SEM. *, p< 0.05; **, p< 0.01; ***, p< 0.001; n.s., not significant.
To exclude a possible contribution from WAT, we generated brown adipocyte-specific Sel1L-deficient mice (Sel1LUcp1Cre) using Ucp1 promoter-driven Cre mice (Fig. S2D). Consistent with Sel1LAdipCre mice, mitochondria in Sel1LUcp1Cre BAT were dramatically enlarged and pleomorphic upon acute cold exposure (yellow arrows, Fig. 1D–E and S3D). This observation was confirmed using confocal microscopy, with BAT immunolabeled for translocase of outer mitochondrial membrane 20 (Tomm20) and matrix protein pyruvate dehydrogenase (PDH) (Fig. S3E).
To gain further insight into mitochondrial morphology at the three-dimensional (3D) level, we performed serial block-face scanning electron microscopy (SBF-SEM) (Fig. S4A), which allows imaging of a large field (over 10 cells) at 3D planes with nanometer resolution (Fig. S4B–C) (34). In cold-exposed Sel1Lf/f BAT, mitochondria were predominantly spherical or ovoid, with an average volume of ~1.7 μm3 (Fig. S4B and 1F–H). Mitochondrial volume was increased five-fold in Sel1L−/− BAT (Fig. 1F–H and S4C). Moreover, three-dimensional reconstruction analyses revealed pleomorphic megamitochondria, e.g. branched, or chair- or cup-shaped (Fig. 1G and Movies S1–2). Thus, Sel1L deficiency in brown adipocytes triggers the formation of pleomorphic megamitochondria within hours of cold exposure.
Sel1L controls ER-mitochondria contacts
We next explored whether Sel1L deficiency affected MAMs. In Sel1Lf/f BAT, MAMs were identified as thin sheets aligned with mitochondria, with the distance between the two organelles averaging 10–20 nm (cyan arrows, Fig. 2A, 2C–D and S5A). By contrast, MAMs of Sel1L−/− BAT formed tubules located closer to mitochondria, with an average distance of 5–10 nm (cyan arrows, Fig. 2B–D and S5B). Moreover, the number of MAMs per mitochondrion was increased in Sel1L−/− BAT following cold exposure (Fig. 2E). These peri-mitochondrial tubular structures were verified as ER by BiP-specific immunoelectron microscopy (Fig. 2F): pleomorphic megamitochondria appeared to “grow” around ER tubules in cold-stimulated Sel1L−/− BAT.
Figure 2. Sel1L controls ER-mitochondria contacts in cold-stimulated brown adipocytes.

(A-B) Representative TEM images of BAT from Sel1Lf/f (A) and Sel1LUcp1Cre (B) mice at 4°C for 6 hr. M, mitochondrion; Cyan arrows, MAMs; red lines, mitochondrial membranes; the ER, green. (C-E) Representative TEM images of the MAMs in BAT from Sel1Lf/f and Sel1LUcp1Cre mice at 4°C for 6 hr, with quantitation of ER-mitochondrion distance (D) and abundance of MAM per mitochondrion (E). n=35 and 40 MAMs (D) and n=450 and 572 mitochondria (E) for Sel1Lf/f and Sel1LUcp1Cre. (F) Representative TEM images of BiP-specific immunogold labeling in BAT from Sel1LAdipCre mice at 4°C for 6 hr. Red dotted line outlines one megamitochondrion; cyan arrows, BiP-positive ER tubule(s). (G) Representative FIB-SEM (left) and the 3D tomography images (300 and 170 slices, 5 nm/slice) of mitochondria in BAT from Sel1Lf/f and Sel1LUcp1Cre mice at 4°C for 6 hr. Magenta, mitochondria; green, ER. Cyan arrows, MAMs; orange arrows, MAMs going through the concave surface of a mitochondrion. All experiments have been repeated two to three times. Data are mean ± SEM. ***, p< 0.001 by Student’s t-test.
We next performed focused ion-beam SEM (FIB-SEM) to reconstruct mitochondria with MAMs at the 3D level. In comparison to SBF-SEM, FIB-SEM offers higher resolution at the z plane (35) with a resolution of 5 nm isotropic voxels (Fig. S6). Three-dimensional reconstruction revealed more intimate interactions between the ER (green) and mitochondria (purple) in Sel1LUcp1Cre BAT vs. a Sel1Lf/f mitochondria (pink) (Fig. 2G). In many U-shaped mitochondria, several ER tubules were found along the entire concavity of the organelle (orange arrows, Fig. 2G and Movie S3).
Formation of mitochondria-perforating ER tubules in the absence of Sel1L
In many U- or dumbbell-shaped megamitochondria, the outer membrane folded back on itself, with less than 10 nm between membranes (green arrows, Fig. 3A and S7A), which we speculated may fuse to embed ER tubules within the mitochondria. Indeed, there were many tubular structures embedded within mitochondrial profiles in Sel1L−/− BAT (red arrows, Fig. 3B–C, S7B–C). In some, double- or triple- membrane structures were noted. Moreover, these tubular structures served as a “hub” from which cristae folds radiated (Fig. 3B–C and S7B–D). To determine whether these peculiar structures inside mitochondria were ER, we performed BiP-specific immunoelectron microscopy. Clusters of BiP-positive signal were detected both perimitochondrially (cyan arrows) and within mitochondrial profiles (red arrows, Fig. 3D and S7E). We then performed SBF- and FIB-SEM to reconstruct whole mitochondria with perforating ER tubules. For example, we present 12 consecutive images of one megamitochondrion with two parallel penetrating ER tubules (Fig. S8 and 3E–F). Two ER tubules (arrows) perforated the mitochondrial profiles in parallel, with a distance of 1–1.3 μm apart, with radiating and interconnecting cristae folds, resembling wagon wheel spokes. Similarly, independent FIB-SEM analyses showed two parallel ER tubules (0.7–1 μm apart) penetrating another megamitochondrion in Sel1LUcp1Cre BAT upon cold (Fig. S9 and Movie S4). Thus, Sel1L deficiency leads to the formation of pleomorphic megamitochondria with perforating ER tubule(s) in brown adipocytes upon cold challenge.
Figure 3. Sel1L deficiency leads to the formation of megamitochondria with perforating ER tubules.
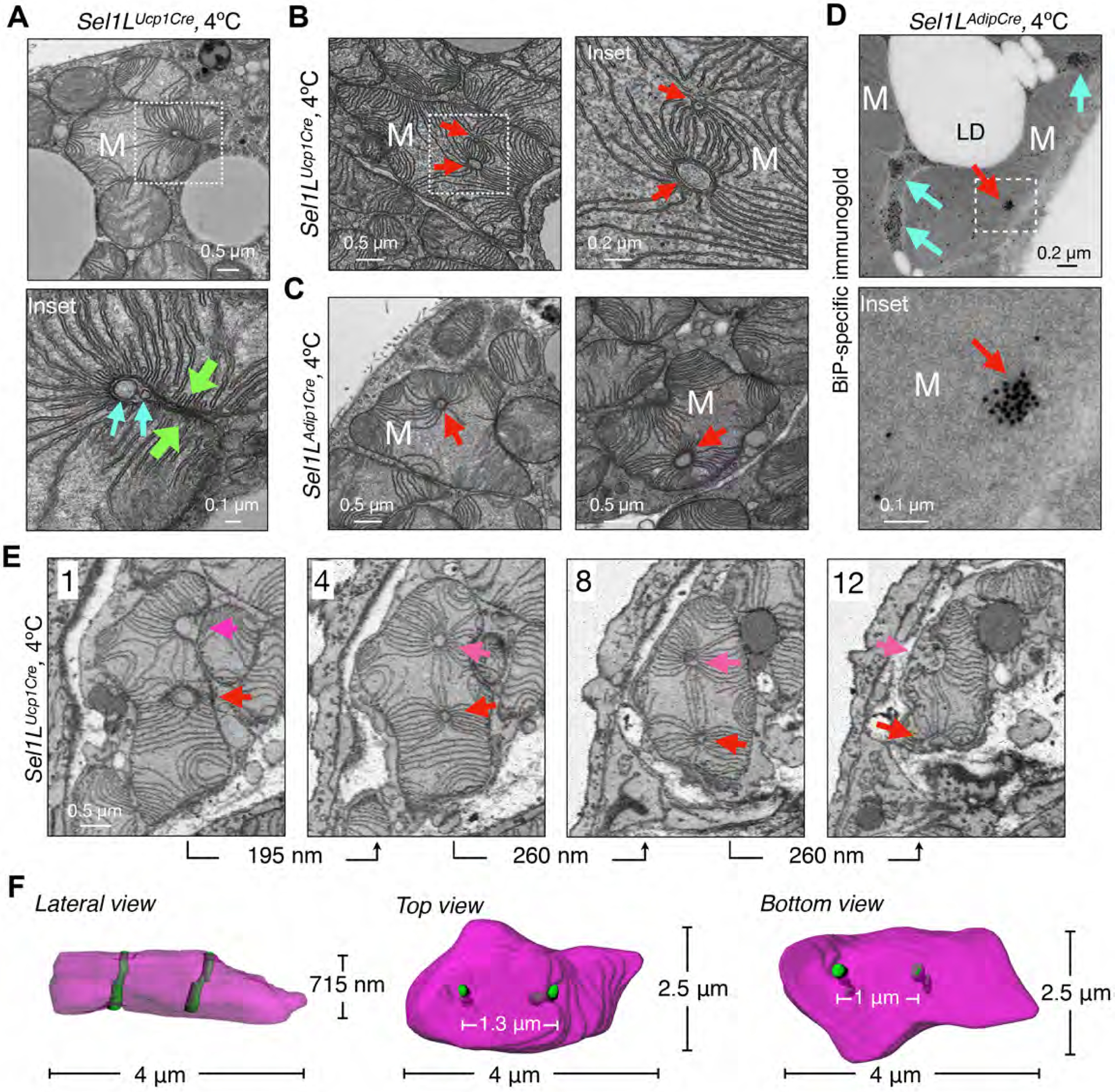
(A) Representative TEM images of BAT in Sel1LUcp1Cre mice housed at 4°C for 6 hr, showing a megamitochondrion wrapping around the tubular structures (cyan arrows). Green arrows, two opposite sides of a mitochondrion. (B-C) Representative TEM images of BAT from Sel1LUcp1Cre (B) and Sel1LAdipCre (C) mice at 4°C for 6 hr, showing megamitochondria with tubular structures (red arrows). (D) Representative BiP-immunogold TEM images of BAT in Sel1LAdipCre mice at 4°C for 6 hr. Red and cyan arrows, mitochondria-perforating ER tubules and peri-mitochondria ER tubules. (E-F) Representative SBF-SEM images (E) and 3D reconstruction (F) in BAT of Sel1LUcp1Cre mice at 4°C for 6 hr, showing four different slices of a megamitochondrion with two parallel perforating ER tubules (red and magenta arrows). All 12 slices (65 nm/slice) are shown in Fig. S8. All experiments have been repeated two to three times.
Impaired mitochondrial function and thermogenic response in Sel1L−/− mice
Next, we asked how Sel1L deficiency affected mitochondrial function and thermogenesis. Purified mitochondria from cold-exposed Sel1LAdipCre mice had reduced oxygen consumption rate (OCR) from oxidation of pyruvate and malate compared to those from Sel1Lf/f mice (Fig. 4A). In addition, differentiated Sel1L−/− brown adipocytes showed defective respiration in response to adrenergic receptor agonist norepinephrine (NE) stimulation (Fig. 4B). Mitochondrial functional defects were further confirmed using a targeted metabolomics analysis of 119 intracellular metabolites in NE-treated differentiated Sel1Lf/f vs. Sel1L−/− brown adipocytes (Fig. S10). Pathway analysis of the 30 significantly altered metabolites revealed three main pathways altered in Sel1L−/− adipocytes: TCA cycle, pyrimidine and purine metabolism (Fig. 4C), all of which are associated with mitochondrial function.
Figure 4. Sel1L in brown adipocytes regulates mitochondrial function and cold-induced thermogenesis.

(A-B) Oxygen consumption rate (OCR) of purified mitochondria from BAT of cold-exposed mice (A) and differentiated brown adipocytes (B) with the addition of various stimuli as indicated. NE, norepinephrine; Oligo, oligomycin; Pyr/Mal, pyruvate/malate; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; Anti, antimycin; Rot, Rotenone. (C) Metaboanalyst pathway enrichment analysis for the metabolites in Sel1Lf/f and Sel1L−/− brown adipocytes treated with 1 μM NE for 1 hr (n=3/group). (D) Rectal temperature of 8- to 10- week-old mice housed at 22°C or 4°C for 6 hr (n=7–10 mice each, one-way ANOVA). (E-F) Representative H&E (E) and Perilipin1 (F) staining of BAT from Sel1Lf/f and Sel1LAdipCre mice housed at 22°C or 4°C for 6 hr. All experiments have been repeated three times except panel C (3 samples per group). Data are mean ± SEM. ***, p< 0.001.
Both Sel1LAdipCre and Sel1LUcp1Cre mice appeared normal and grew comparably to their Sel1Lf/f littermates up to 22 weeks on low-fat diet at room temperature (Fig. S11A). While core body temperatures (~37°C) were similar between cohorts when housed at room temperature, Sel1LAdipCre mice were cold sensitive and dropped core body temperature to 26°C within 6 hr of cold exposure (Fig. 4D, vs. 33°C in Sel1Lf/f). Histological examination of BAT revealed that, while largely indistinguishable at room temperature, Sel1LAdipCre BAT exhibited larger lipid droplets upon cold exposure compared to Sel1Lf/f mice (Fig. 4E), pointing to defects in lipid mobilization. This was confirmed using immunostaining of lipid droplet binding protein Perilipin 1 (Fig. 4F). Similar observations were made with Sel1LUcp1Cre mice (Fig. 4D and S11B-C). Phosphorylation of hormone-sensitive lipase (HSL) was not affected in response to β3-adrenergic receptor agonists (Fig. S11D–E), thereby uncoupling β-adrenergic signaling from the thermogenic defect of Sel1L−/− mice.
Sel1L effect on mitochondria is mediated through Hrd1 ERAD
Sel1L may have an Hrd1-independent function, and vice versa (25, 36, 37). To determine whether Sel1L and Hrd1 act similarly to regulate mitochondria in BAT, we generated brown adipocyte-specific Hrd1-deficient (Hrd1Ucp1Cre) mice (Fig. S12A). Deletion of Hrd1 stabilized Sel1L protein (Fig. S12A), in line with the notion that Sel1L is a substrate of Hrd1. Hrd1Ucp1Cre mice grew normally (Fig. S12B), but were cold sensitive (Fig. 5A), with more lipid droplets in cold-challenged BAT compared to Hrd1f/f mice (Fig. S12C). Moreover, Hrd1Ucp1Cre BAT demonstrated enlarged mitochondrial, some with penetrating ER tubule(s), upon cold stimulation (Fig. 5B–D, S12D).
Figure 5. Hrd1, but not Ire1α of UPR, controls mitochondrial morphology and thermogenesis in brown adipocytes.

(A) Rectal temperature of 8- to 10- week-old Hrd1f/f and Hrd1Ucp1Cre mice housed at 22°C or 4°C for 6 hr, n=4/group (one-way ANOVA). (B-D) Representative TEM images of BAT from Hrd1f/f and Hrd1Ucp1Cre mice housed at 22°C or 4°C for 6 hr with quantitation shown in C. n= 543, 581 mitochondria for 22°C and 4°C, Student’s t-test. Yellow arrows, megamitochondria; red arrows, ER tubules within mitochondrial profiles. (E) RT-PCR analysis of Xbp1 mRNA splicing in BAT at 22°C or 4°C for 6 hr with quantitation of the ratio of spliced Xbp1 (s) to total Xbp1 (spliced + unspliced (u)) shown below the gel (n = 6 mice per group). BAT from wildtype mice injected with vehicle or tunicamycin (Tuni, 1 mg/kg) included as controls. (F-G) Representative TEM pictures of BAT from Ire1aAdipCre mice at 4°C for 6 hr with quantitation shown in G (n=500, 665 from 2 mice each, Student’s t-test). All experiments have been repeated two to three times. Data are mean ± SEM. **, p< 0.01; ***, p< 0.001.
Because ER stress has been linked to mild mitochondrial elongation (38, 39), we next asked whether the effect of ERAD deficiency on mitochondria was mediated through ER stress. Basal Xbp1 mRNA splicing was slightly higher in Sel1LAdipCre BAT compared to Sel1Lf/f BAT at room temperature; however, cold exposure reduced Xbp1 mRNA splicing in Sel1LAdipCre BAT (Fig. 5E). There was no detectable cell death in Sel1LAdipCre BAT (Fig. S13A). To study a possible impact of ER stress signaling on mitochondria in vivo, we generated mice lacking Ire1α, a key sensor of UPR, specifically in adipocytes (Ire1aAdipCre). These knockout mice grew normally and were not cold-sensitive (Fig. S13B–D). Mitochondria from Ire1aAdipCre exhibited normal morphology, with reduced size at 4°C (Fig. 5F–G). Thus, the effect of Sel1L on mitochondria is mediated by ERAD, independent of ER stress or cell death.
Sel1L-Hrd1 ERAD regulates ER-mitochondria contacts and dynamics via SigmaR1
We next explored how mitochondrial dynamics were affected by ERAD dysfunction. While total protein levels of mitochondrial respiratory proteins and Ucp1 were largely comparable between the cohorts (Fig. S14A), cold-induced phosphorylation of Drp1 at Ser616, a key activating event in mitochondrial division (11, 40) was reduced in Sel1LAdipCre BAT compared to Sel1Lf/f BAT (Fig. 6A). Opa1 processing to its shorter form upon cold exposure did not occur in Sel1LAdipCre BAT (Fig. 6A). Protein levels of outer mitochondrial membrane Drp1 receptors Fis1 and Mff were unchanged (Fig. S14A). Similar observations were obtained in Hrd1−/− BAT (Fig. S14B), but not in Ire1α−/− BAT (Fig. S13E). Furthermore, in Sel1L−/− BAT, cold exposure increased oligomerization of key fusion factor Mfn2 into high molecular weight (HMW) complexes as revealed by blue-native gels and sucrose gradient fractionation (Fig. S14C–D). Indeed, the Mfn2 HMW complex promotes mitochondrial fusion (41–43). We then tested the protein levels of several known MAM proteins including Mfn2, Vapb, Bap31 and SigmaR1. Only SigmaR1 levels were increased in Sel1L- and Hrd1-deficient BAT (Fig. 6B and S15A–B), but not in Ire1α−/− BAT (Fig. S13E). However, SigmaR1 mRNA level was comparable in Sel1L−/− vs. WT BAT in response to cold (Fig. S15C), pointing to a post-transcriptional regulation of SigmaR1 protein.
Figure 6. Sel1L-Hrd1 ERAD regulates MAM and mitochondrial dynamics via SigmaR1.

(A) Immunoblot analysis of mitochondrial dynamic proteins in BAT from Sel1Lf/f and Sel1LAdipCre mice at 22°C or 4°C for 6 hr with quantitation of phos-/total Drp1 and large (L)-/small (S)-Opa1 shown below the gel (n = 7 per group). (B) Immunoblot analysis of MAM proteins in BAT of Sel1Lf/f and Sel1LAdipCre mice with quantitation of SigmaR1/Hsp90 shown below the gel (n = 7 per group). (C) Immunoblot analysis of endogenous SigmaR1 in WT and Hrd1-deficient (Hrd1−/−) HEK293T cells with or without CHX and MG132 treatment. (D) Immunoblot analysis of GFP or endogenous SigmaR1 immunoprecipitates in HEK293T (with or without MG132 treatment), showing Hrd1-mediated SigmaR1 ubiquitination. (E) Immunoblot analysis of Flag immunoprecipitates in HEK293T cells transfected with a combination of plasmids, showing a dose-dependent interaction between SigmaR1-Flag and Mfn2-EGFP. (F) Immunoblot analysis of Mfn2 oligomers or -containing high molecular weight (HMW) complexes in brown adipocytes treated with or without NE (1 μM, 1 hr) in blue native (BN)-PAGE and regular SDS-PAGE. (G-H) Representative images of mito-DsRed- and mEmerald-Sec61b- expressing pre-adipocytes. DAPI (blue) with quantitation of ER-mitochondrial colocalization using Manders’ overlap coefficient (50 cells each) shown in H, one-way ANOVA. (I-J) Confocal images showing the spread and decay of mitochondria-targeted photoactivated GFP (mito-PAGFP) in brown adipocytes 60 min following NE stimulation and photoactivation. Active mitochondrial were stained with TMRE (red). Quantitation of changes of GFP signal intensity over time shown in J. (n=13, 15, and 10 for Sel1Lf/f, Sel1L−/− and Sel1L−/−;SigmaR1−/−, one-way ANOVA). All were repeated two to three times. Data are mean ± SEM. **, p< 0.01; ***, p< 0.001.
SigmaR1 is a single-span ER-resident protein residing at the MAMs where it may regulate ER-mitochondria contacts and mitochondrial dynamics (44, 45), although a detailed mechanism remains vague. SigmaR1 protein was degraded by the proteasome with a half-life of approximately 6 hr (Fig. 6C), interacted with the E3 ligase Hrd1 (Fig. S15D–E) and was ubiquitinated in a largely Hrd1-dependent manner (Fig. 6D). In the absence of Sel1L or Hrd1, SigmaR1 protein was stabilized (Fig. 6C) and accumulated in the ER (Fig. S15F). SigmaR1 interacted with Mfn2 as well as other MAM proteins Bap31 and Vapb, but not Ip3r (Fig. 6E and S15G), and were localized at very close proximity to mitochondria in Sel1L−/− cells (Fig. S16H). Genetic deletion of SigmaR1 reduced cold-induced Mfn2 HMW complexes in Sel1L−/− brown adipocytes (Fig. 6F and S16A). Deletion of SigmaR1 reversed mitochondrial elongation (Fig. S16B–C) and reduced ER-mitochondrial contacts in Sel1L−/− cells (Fig. 6G–H). In a mitochondrial fusion assay (33) using mitochondria-targeted photoactivated GFP (mito-PAGFP), deletion of SigmaR1 attenuated the spread and decay of GFP intensity seen in Sel1L−/− brown adipocytes upon NE treatment (Fig. 6I–J). Thus, SigmaR1 mediates mitochondrial hyperfusion in Sel1L-deficient cells, thereby linking ERAD to ER-mitochondrial contacts and mitochondrial dynamics in brown adipocytes.
Discussion
Here, we found a critical role for Sel1L-Hrd1 ERAD in regulating ER-mitochondria contacts and mitochondrial function. We observed profound changes in mitochondrial morphology, beyond mere elongation or swelling that is commonly associated with mitochondrial dysfunction or ER stress. Using 3D imaging techniques, we reconstructed these pleomorphic mitochondria and their associated MAMs. We speculate that the ER tubules embedded within profiles of pleomorphic megamitochondria represent halted fission and/or accelerated fusion intermediates. Our mechanistic studies identified MAM protein SigmaR1 as an ERAD substrate, which, when deleted, reduced ER-mitochondria contacts and rescued abnormal mitochondrial dynamics and morphology in Sel1L−/− cells. SigmaR1 may regulate mitochondrial dynamics by interacting with Mfn2 as well as other MAM proteins and promoting Mfn2 oligomerization in an unknown manner.
MAMs are disturbed in many diseases such as obesity (46), cardiac disease (47), and neurodegeneration (48); however, to date, the nature of this disruption in disease pathogenesis remains vague. Our data suggest that Sel1L-Hrd1 ERAD may regulate the dynamics of ER-mitochondria interactions via modulation of MAMs. Indeed, an inverse correlation between Sel1L and SigmaR1 expression levels has been reported in patients with neurodegeneration (49–51). Considering the recent identification of Sel1L mutants in progressive early-onset cerebellar ataxia in canines (52) and various cancers (53, 54), this study may have implications not only for interorganelle communication, but also for the pathophysiological role of Sel1L-Hrd1 ERAD.
Supplementary Material
ACKNOWLEDGEMENT
We thank Drs. Deyu Fang, Linda M. Hendershot, David Lombard, Jiandie Lin, Scott Soleimanpour, Tsung-Ping Su and Richard Wojcikiewicz for providing reagents, Fengbiao Mao for data analysis, Peter Arvan, Liangyou Rui, Yuguang Shi, Shengyi Sun, and Ormond MacDougald for constructive comments on the manuscript; other members in the Arvan/Qi laboratories for technical assistance and insightful discussions.
Funding:
This work is supported by 1R01GM123266, R01GM130695 (H.Sesaki.), 1R01DK107583 (J.W.), R01DK110439 and P20GM121176 (M.L.), R01NS086819 and R01NS091242 (T.H.S.), UL1TR000433, F32CA228328, and P30DK034933 (C.J.H), 1R01GM113188, 1R01DK105393, 1R01DK120047 and 1R35GM130292 (L.Q.). Z.Z. is supported by ADA Postdoctoral Fellowship (1-19-PDF-093). M.T. was supported in part by the Pew Latin American Postdoctoral Fellowship. C.A.L. was supported by a 2017 AACR NextGen Grant for Transformative Cancer Research (17-20-01-LYSS) and an ACS Research Scholar Grant (RSG-18-186-01).
Footnotes
Competing interests:
The authors declare no conflict of interest.
Data and materials availability:
The microarray data have been deposited into the GEO datasets with accession number GSE145895.
REFERENCES AND NOTES
- 1.Wu H, Carvalho P, Voeltz GK, Here, there, and everywhere: The importance of ER membrane contact sites. Science 361, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowland AA, Voeltz GK, Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13, 607–625 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornmann B et al. , An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325, 477–481 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csordas G et al. , Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174, 915–921 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman JR et al. , ER tubules mark sites of mitochondrial division. Science 334, 358–362 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murley A et al. , ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. eLife 2, e00422 (2013).23682313 [Google Scholar]
- 7.Lewis SC, Uchiyama LF, Nunnari J, ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 353, aaf5549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvador-Gallego R, Hoyer MJ, Voeltz GK, SnapShot: Functions of Endoplasmic Reticulum Membrane Contact Sites. Cell 171, 1224–1224 e1221 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Lee KS et al. , Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc Natl Acad Sci U S A 115, E8844–E8853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji WK et al. , Receptor-mediated Drp1 oligomerization on endoplasmic reticulum. J Cell Biol 216, 4123–4139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smirnova E, Griparic L, Shurland DL, van der Bliek AM, Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12, 2245–2256 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM, elegans C dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell 4, 815–826 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Bleazard W et al. , The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol 1, 298–304 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang J, Qi L, Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem Sci 43, 593–605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi L, Tsai B, Arvan P, New Insights into the Physiological Role of Endoplasmic Reticulum-Associated Degradation. Trends Cell Biol 27, 430–440 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho P, Goder V, Rapoport TA, Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126, 361–373 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Gardner RG et al. , Endoplasmic reticulum degradation requires lumen to cytosol signaling. Transmembrane control of Hrd1p by Hrd3p. J Cell Biol 151, 69–82 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampton RY, Gardner RG, Rine J, Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol Biol Cell 7, 2029–2044 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya A, Qi L, ER-associated degradation in health and disease - from substrate to organism. J Cell Sci 132, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vashistha N, Neal SE, Singh A, Carroll SM, Hampton RY, Direct and essential function for Hrd3 in ER-associated degradation. Proc Natl Acad Sci U S A 113, 5934–5939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Rapoport TA, Mechanistic insights into ER-associated protein degradation. Curr Opin Cell Biol 53, 22–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoebel S et al. , Cryo-EM structure of the protein-conducting ERAD channel Hrd1 in complex with Hrd3. Nature 548, 352–355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim GH et al. , Hypothalamic ER-associated degradation regulates POMC maturation, feeding and age-associated obesity. J Clin Invest 128, 1125–1140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi G et al. , ER-associated degradation is required for vasopressin prohormone processing and systemic water homeostasis. J Clin Invest 127, 3897–3912 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sha H et al. , The ER-associated degradation adaptor protein Sel1L regulates LPL secretion and lipid metabolism. Cell Metab 20, 458–470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun S et al. , Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival. Proc Natl Acad Sci U S A 111, E582–591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya A et al. , Hepatic Sel1L-Hrd1 ER-associated degradation (ERAD) manages FGF21 levels and systemic metabolism via CREBH. EMBO J 37, e99277 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji Y et al. , The Sel1L-Hrd1 Endoplasmic Reticulum-Associated Degradation Complex Manages a Key Checkpoint in B Cell Development. Cell reports 16, 2630–2640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y et al. , The endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 controls a critical checkpoint in B cell development in mice. J Biol Chem 293, 12934–12944 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei J et al. , HRD1-ERAD controls production of the hepatokine FGF21 through CREBH polyubiquitination. EMBO J 37, e98942 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S et al. , Epithelial Sel1L is required for the maintenance of intestinal homeostasis. Mol Biol Cell 27, 483–490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cypess AM, Kahn CR, Brown fat as a therapy for obesity and diabetes. Current opinion in endocrinology, diabetes, and obesity 17, 143–149 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wikstrom JD et al. , Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33, 418–436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denk W, Horstmann H, Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol 2, e329 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knott G, Marchman H, Wall D, Lich B, Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J Neurosci 28, 2959–2964 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz J et al. , Conserved cytoplasmic domains promote Hrd1 ubiquitin ligase complex formation for ER-associated degradation (ERAD). J Cell Sci 130, 3322–3335 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christianson JC et al. , Defining human ERAD networks through an integrative mapping strategy. Nat Cell Biol 14, 93–105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balsa E et al. , ER and Nutrient Stress Promote Assembly of Respiratory Chain Supercomplexes through the PERK-eIF2alpha Axis. Mol Cell, doi: 10.1016/j.molcel.2019.1003.1031. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lebeau J et al. , The PERK Arm of the Unfolded Protein Response Regulates Mitochondrial Morphology during Acute Endoplasmic Reticulum Stress. Cell reports 22, 2827–2836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM, A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143, 351–358 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steffen J et al. , Rapid degradation of mutant SLC25A46 by the ubiquitin-proteasome system results in MFN1/2-mediated hyperfusion of mitochondria. Mol Biol Cell 28, 600–612 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLelland GL et al. , Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy. eLife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiura A et al. , MITOL regulates endoplasmic reticulum-mitochondria contacts via Mitofusin2. Mol Cell 51, 20–34 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Gregianin E et al. , Loss-of-function mutations in the SIGMAR1 gene cause distal hereditary motor neuropathy by impairing ER-mitochondria tethering and Ca2+ signalling. Hum Mol Genet 25, 3741–3753 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Bernard-Marissal N, Medard JJ, Azzedine H, Chrast R, Dysfunction in endoplasmic reticulum-mitochondria crosstalk underlies SIGMAR1 loss of function mediated motor neuron degeneration. Brain 138, 875–890 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Arruda AP et al. , Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 20, 1427–1435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H, Wang S, Hu S, Chen Y, Ren J, ER-Mitochondria Microdomains in Cardiac Ischemia-Reperfusion Injury: A Fresh Perspective. Front Physiol 9, 755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paillusson S et al. , There’s Something Wrong with my MAM; the ER-Mitochondria Axis and Neurodegenerative Diseases. Trends Neurosci 39, 146–157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montibeller L, de Belleroche J, Amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD) are characterised by differential activation of ER stress pathways: focus on UPR target genes. Cell Stress Chaperones 23, 897–912 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prause J et al. , Altered localization, abnormal modification and loss of function of Sigma receptor-1 in amyotrophic lateral sclerosis. Hum Mol Genet 22, 1581–1600 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Mori T, Hayashi T, Su TP, Compromising sigma-1 receptors at the endoplasmic reticulum render cytotoxicity to physiologically relevant concentrations of dopamine in a nuclear factor-kappaB/Bcl-2-dependent mechanism: potential relevance to Parkinson’s disease. J Pharmacol Exp Ther 341, 663–671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyostila K et al. , A SEL1L mutation links a canine progressive early-onset cerebellar ataxia to the endoplasmic reticulum-associated protein degradation (ERAD) machinery. PLoS Genet 8, e1002759 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H, Bhattacharya A, Qi L, Endoplasmic reticulum quality control in cancer: Friend or foe. Semin Cancer Biol 33, 25–33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mellai M et al. , SEL1L SNP rs12435998, a predictor of glioblastoma survival and response to radio-chemotherapy. Oncotarget 6, 12452–12467 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu T et al. , Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev 28, 708–722 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwawaki T, Akai R, Yamanaka S, Kohno K, Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci USA 106, 16657–16662 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eguchi J et al. , Transcriptional control of adipose lipid handling by IRF4. Cell Metab 13, 249–259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada T et al. , Mitochondrial Stasis Reveals p62-Mediated Ubiquitination in Parkin-Independent Mitophagy and Mitigates Nonalcoholic Fatty Liver Disease. Cell Metab 28, 588–604 e585 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wittig I, Braun HP, Schägger H, Blue native PAGE. Nat Protoc 1, 418–428 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Sun S, Xia S, Ji Y, Kersten S, Qi L, The ATP-P2X7 Signaling Axis Is Dispensable for Obesity-Associated Inflammasome Activation in Adipose Tissue. Diabetes 61, 1471–1478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kikkert M et al. , Human HRD1 is an E3 ubiquitin ligase involved in degradation of proteins from the endoplasmic reticulum. J Biol Chem 279, 3525–3534 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Klein J, Fasshauer M, Klein HH, Benito M, Kahn CR, Novel adipocyte lines from brown fat: a model system for the study of differentiation, energy metabolism, and insulin action. Bioessays 24, 382–388 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Twig G et al. , Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. American journal of physiology. Cell physiology 291, C176–184 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Pike JA, Styles IB, Rappoport JZ, Heath JK, Quantifying receptor trafficking and colocalization with confocal microscopy. Methods 115, 42–54 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


