Abstract
Summary
Calcium and vitamin D intake and exercise are suboptimal among older adults. Following bone densitometry, a letter communicating individualized fracture risk accompanied by an educational brochure improved participants’ lifestyle—but no more than existing communication strategies—over 52 weeks. Simple communication strategies are insufficient for achieving optimal levels of bone health behaviors.
Purpose
The Patient Activation After DXA Result Notification (PAADRN) study was designed to evaluate whether a letter with individualized fracture risk and an educational brochure mailed to patients soon after their DXA might improve bone health behaviors (daily calcium intake, vitamin D supplementation, and weekly exercise sessions) compared to slower, less individualized communication characterizing usual care.
Methods
Participants ≥ 50 years were recruited, at three sites, following their DXA and randomized with 1:1 allocation to intervention and control (usual care only) groups. Data were collected at enrollment interview and by phone survey at 12 and 52 weeks thereafter. Intention-to-treat analyses were conducted on 7749 of the 20,397 eligible participants who enrolled. Changes in bone health behaviors were compared within and between study groups. Average treatment effects and heterogeneity of treatment effects were estimated with multivariable linear and logistic regression models.
Results
In unadjusted analyses, calcium intake, vitamin D supplementation, and weekly exercise sessions increased significantly over 52 weeks within both the intervention and control groups (all p < 0.001). In unadjusted analyses and multivariable models, increases in each behavior did not significantly differ between the intervention and control groups. Intervention group participants with a > 20% 10-year fracture risk at enrollment did, however, have a significantly greater increase in calcium intake compared to other study participants (p = 0.031).
Conclusions
Bone health behaviors improved, on average, over 52 weeks among all participants following a DXA. Receipt of the PAADRN letter and educational brochure did not directly improve bone health behaviors compared to usual care.
Trial registration
The Patient Activation after DXA Result Notification (PAADRN) Study is registered at ClinicalTrials.Gov: NCT01507662, https://clinicaltrials.gov/ct2/show/NCT01507662
Keywords: Osteoporosis, Fracture risk, Diet, Exercise, Patient-provider communication
Introduction
The evolving and increasingly accepted model of chronic disease care envisions that informed and activated patients are critical for monitoring and managing their condition outside their infrequent and brief medical office visits [1, 2]. Productive interactions between a healthcare system and patients reinforce patients’ understanding of the importance of practicing a recommended lifestyle regimen so that they ultimately obtain health benefits from effective self-management.
The National Osteoporosis Foundation (NOF) has published guidelines for self-management practices for maintaining good bone health and reducing the risk of osteoporotic fractures [3, 4]. Those guidelines recommend a daily minimum of 1000-mg calcium intake for women and men 50 years of age and older (increasing to 1200 mg for men over 70 years), 800–1000 IU vitamin D intake, and, depending on age and physical health, either moderate or vigorous physical activity (weight bearing and muscle strengthening). Clinical trials have demonstrated the benefits of increased calcium intake, vitamin D intake, and exercise on the preservation of or improvement in bone density, with subsequent reduction of fracture risk [5, 6].
Many older adults in the USA have suboptimal levels of calcium and vitamin D intake and exercise [7, 8]. Therefore, a challenge to healthcare systems is to identify and implement timely, low-cost, and effective strategies for translating clinical trial evidence and national recommendations into good bone health behaviors in large patient populations.
Patient-centered communication has been recommended as a strategy for improving productive interactions between patients and their healthcare system, leading to improved patient adherence to self-care practices. A review of the literature on trials of relatively low-cost, osteoporosis-related interventions for improving patient-centered communication (e.g., tailored messaging) yields mixed evidence on improving osteoporosis-related diet or physical activity behaviors at the population level. In trials of relatively short duration (i.e., 1–2 years), some studies have found favorable increases in calcium and/or vitamin D levels [9–11]. Other studies have found no meaningful increases [12–16]. Exercise frequency is rarely improved [9, 12, 13, 15, 16].
Understanding the cause-and-effect relationships in patient-directed interventions is challenging due to the potential moderating effects of levels of adherence to recommendations at enrollment in a study as well as baseline levels of patient readiness to change, engage in, or be activated toward behavioral change [11, 12, 17–19]. Behavioral theory and prior studies suggest that changes to osteoporosis-related lifestyle attitudes and behavior in response to an intervention might be moderated through intervening changes in factors such as osteoporosis-related knowledge or self-efficacy, or willingness to change [12, 14, 17–20].
The Patient Activation after DXA Result Notification (PAADRN) study is a double-blinded, parallel, pragmatic randomized controlled trial (RCT) designed to evaluate the effectiveness of rapid, low-cost, tailored, direct-to-patient mailed communication of fracture risk [21]. The primary end points of the PAADRN trial were as follows: guideline-concordant pharmacotherapy, daily calcium intake, proportion with vitamin D supplementation, and frequency of weight-bearing and strengthening exercises [22]. The letter provided an individualized fracture risk probability; and, an accompanying educational brochure described the importance of calcium and vitamin D intake and exercise (both weight bearing and muscle strengthening). The letter and brochure were developed during pre-trial formative studies to optimize participant acceptance and understanding of key behaviors for maintaining good bone health and reducing risk of fragility fracture [23–25]. The intervention assumed that rapid, clear, individualized feedback to patients of dual-energy X-ray absorptiometry (DXA) results would improve self-efficacy (i.e., patient knowledge, confidence, and competence) in initiating and maintaining treatment adherence compared to changes to bone health behaviors that might occur from the usual, generic feedback about DXA results at the participating trial sites.
The principal objective of this analysis was to evaluate the PAADRN intervention for: 1) average treatment effects (ATEs) on daily calcium intake, proportion with vitamin D supplementation, and weekly exercise sessions (weight bearing and strengthening) at 52 weeks following trial enrollment and 2) heterogeneity of treatment effects (HTEs) with respect to selected participant subgroups (baseline levels of each of these three behaviors and prior DXA or not [DXA-naïve]). Analyses of ATEs with respect to each of the three primary end points were based on the premise that receipt of both a letter with individualized fracture risk and an educational brochure would, on average, be more effective than usual care in motivating PAADRN intervention participants to engage in productive interactions with their healthcare teams, become more knowledgeable about the importance of good bone health behaviors and more competent in practice of those behaviors, and, therefore, achieve significant improvements in levels of these behaviors over a 52-week period. HTE analyses were based on the premise that the PAADRN intervention might differentially benefit participants according to baseline levels of recommended bone health behaviors, osteoporosis-related self-efficacy, and perceived or estimated fracture risk.
Methods
Study population
Patients ≥ 50 years old presenting for DXA between February 2012 and August 2014 at the University of Iowa (UI; the study coordinating center), University of Alabama at Birmingham, and Kaiser Permanente of Georgia were invited to participate. Patients were excluded if they were unable to read, speak, or understand English; were prisoners or unable to provide informed consent due to perceived cognitive disabilities; or did not have telephone access. PAADRN’s protocol was reviewed, approved, and monitored by the IRBs at each of the participating institutions.
Trial design and conduct
For guideline concordant pharmacotherapy, PAADRN was designed to achieve 91.3% power to detect a standardized effect size as small as 0.10 at p < 0.05 with attrition as high as 20% [21]. Recruitment was open from February 2012 to August 2014. Recruitment occurred from 28 days prior to or within 3 days following DXA completion. Pre-DXA recruitment occurred by mail and phone outreach queries of patients on DXA appointment schedules. “Same-day” recruitment was facilitated by waiting room posters and brochures and referral to research by the DXA technologist. For most participants, a face-to-face baseline interview occurred immediately following the DXA. At one study site, approximately 20% of baseline interviews were conducted within 2 days in order to accommodate the institution’s referral patterns.
Eligible patients who consented to participate completed a post-DXA baseline survey administered by the research assistants at each site. The baseline survey collected information related to participant sociodemographic characteristics (e.g., age, race/ethnicity, education), factors affecting fracture risk (e.g., height and weight for computation of body mass index), comorbidities, and osteoporosis-related knowledge, attitudes, and behaviors. Research assistants were blinded to allocation which occurred subsequently.
The survey, with a few baseline items omitted (e.g., sociodemographic variables), was repeated at 12 and 52 weeks. Follow-up surveys were conducted by telephone by trained data collectors at the UI Iowa Social Science Research Center. Follow-up data collectors were also blinded to treatment allocation. Data collection ended in August 2015.
A randomization algorithm at UI allocated all consenting patients by blocks of ordering providers to the intervention or control groups. Allocation was designed to yield a 1:1 intervention/usual care (control) group ratio. Allocation was unknown to research assistants, statisticians, and study investigators.
PAADRN’s intervention consisted of an individualized, one-page direct-to-patient letter accompanied by an educational brochure that was mailed 4 weeks post-DXA. The letter presented results of the participant’s DXA (lowest T-score [spine, pelvis, hip, femur] and interpretation [osteoporosis, osteopenia, or normal]), a graph of their 10-year probability of suffering a major osteoporotic fracture (calculated by FRAX® available at https://www.shef.ac.uk/FRAX/), and a bone health educational brochure [23–25]. The graph identified the participant’s estimated 10-year fracture risk in the context of a thermometer with green (< 10%), yellow (10–20%), and red (> 20%) zones. The educational brochure, which was not individualized, described the importance of calcium and vitamin D intake (providing examples of dietary and supplemental sources) and strengthening and weight-bearing exercises (providing examples of specific activities) for maintenance of good bone health.
In usual care, the letter conveying DXA results may vary widely in what information is conveyed, how fracture risk is expressed, and timing of its mailing (from days to weeks following a DXA). The letter is rarely accompanied by an educational brochure.
Measures
Primary end points
Daily calcium intake (mg/day), at baseline and 52 weeks, was estimated from responses to food sources (four items), calcium supplements (one item), and daily multiple vitamins (one item) [26]. Calcium from food sources was assessed by frequency (0–1, 2–3, 4–6, 7 or more units) per week for cups of milk, ounces of cheese, servings of yogurt, and cups of calcium-fortified beverages. Midpoints of response categories for < 7 days/week were used for intake estimation (e.g., if the respondent indicated “2–3,” then “2.5” was assumed as the “days per week”). The 7 or more category was scored as 7. Each amount was multiplied by the following quantities to obtain an estimated calcium intake: 300 mg/milk serving, 200 mg/oz cheese, 300 mg/yogurt serving, and 80 mg/cup serving of calcium-fortified beverage. The sum of these quantities was divided by 7 to get an average milligram-per-day estimate. Two other survey items assessed calcium from supplements: “Do you take calcium supplements?” and “Do you take a daily multiple vitamin?” To the milligram-per-day estimate from food sources, a “Yes” response to these items added 250 and 75 mg/day, respectively.
Vitamin D supplementation was assessed by the item regarding multiple vitamin (previous section), assuming all multiple vitamins have vitamin D, and the item “Do you take any supplemental vitamin D either alone or included in your calcium supplements (please do not include your daily multiple vitamin)?” An affirmative response to either item was considered to indicate use of supplemental vitamin D at the time of the survey.
Weekly exercise sessions at baseline and at 52 weeks were assessed from two items: “In the past 30 days, how many times per week were you engaged in aerobic activity?” and “In the past 30 days, how many times per week were you engaged in strength training?” Examples of aerobic activity and strength training were provided. Response categories were none, 1–2, 3–4, or 5 or more times per week. These categories were weighted 0 (“none”), 1.5 (midpoint of 1–2), 3.5 (midpoint of 3–4), and 5, respectively. A combined exercise score was the sum of the weighted values and resulted in possible scores of 0, 1.5, 3, 3.5, 5, 6.5, 7, 8.5, and 10. This score represents the relative number of sessions per week during which the recommended activities occurred.
Independent variable
The independent variable was whether the patient was in the intervention or control (usual care only) group.
Treatment effect modifiers
Four measures were evaluated for their potential to modify the response of participants to the PAADRN intervention: levels of daily calcium intake, vitamin D, and weekly exercise frequency at study enrollment; osteoporosis self-efficacy at study enrollment; DXA history prior to the DXA at the time of study enrollment; and relative fracture risk from the result of the study DXA.
Daily calcium intake, vitamin D supplementation, and weekly exercise frequency at baseline
These variables were entered into the HTE models as continuous (calcium, exercise) or dichotomous (vitamin D supplementation) measures. PAADRN intervention group participants might respond differently depending on whether or not their behaviors at baseline were at or below recommended levels. For example, PAADRN intervention participants with behaviors below recommended levels might change behaviors over 52 weeks more than participants at or above recommended levels.
Osteoporosis self-efficacy at baseline
Osteoporosis self-efficacy (OSE) at baseline was measured with two subscales: exercise (OSE-exercise, 10 items, α = 0.97) and diet (OSE-diet, 11 items, α = 0.96) [27]. Items represent attitudes toward initiation, maintenance, and persistence of osteoporosis-related behaviors. Each item was scored from 1 (“not at all confident”) to 10 (“very confident”). The OSE-exercise and OSE-diet subscale scores were each computed as the mean of the component item response scores. PAADRN intervention participants with greater self-efficacy at study enrollment might be more confident and prepared to modify their behaviors over 52 weeks and achieve, on average, levels of recommended care than those with lower levels of self-efficacy.
Prior DXA vs. DXA-naïve status at baseline
DXA status at enrollment was assessed by the survey item “Have you ever had a bone density test prior to this most recent DXA?” Participants with a prior DXA have a context for assessing current vs. historic bone health that is not available to DXA-naïve participants. In this context, prior DXA participants might change, on average, their behavior differently than DXA-naïve participants.
Fracture risk at baseline
Fracture risk at study enrollment was assessed by FRAX® scores based on the DXA qualifying the patient for study enrollment and classified into high (> 20% 10-year fracture risk), moderate (10–20%), and low (< 10%) [28]. These categories corresponded with the red, yellow, and green conveyance of fracture risk, respectively, in the individualized PAADRN intervention letter [23, 24]. Participants with a “high fracture risk” alert, for example, might be more attentive to addressing lifestyle changes to maintain or improve bone health compared to those with low fracture risk.
Statistical analysis
All analyses are based on intention-to-treat principles with multiple imputations when the primary end points were missing at 12 or 52 weeks. Initially, unadjusted comparisons of daily calcium intake, vitamin D supplementation, and weekly exercise sessions between the intervention and control groups at baseline and 52 weeks were conducted. Changes in daily calcium intake, vitamin D supplementation, and weekly exercise sessions from baseline to 52 weeks within the intervention and control groups were then computed and tested for significance of change against a null hypothesis of no change. Finally, the 52-week change between the intervention and control groups on each of the three measures was tested for evaluation of a null hypothesis of no difference.
Multivariable models were estimated to test for ATEs and HTEs: linear random effect regressions for daily calcium intake and weekly exercise sessions at 52 weeks and logistic random effect regression for vitamin D supplementation at 52 weeks. All ATE and HTE multivariable models included a random effect term to account for possible correlation among participants seen by the same ordering provider.
The ATE model specifications for each primary end point were:
model 1: independent variable (PAADRN intervention vs. control), baseline level of the primary end point, OSE at baseline (OSE-diet for daily calcium intake and vitamin D supplementation, OSE-exercise for weekly exercise sessions), prior DXA vs. DXA-naïve, fracture risk at enrollment, and participant covariates (listed in supplemental material, Table S1)
Multivariable HTE model specifications were similar to ATE model specifications but included an interaction term for the independent variable and one of several possible treatment effect modifiers:
model 2: independent variable (PAADRN intervention vs. control), baseline level of the specific primary end point, and a term for intervention vs. control interacted with baseline level of the primary end point
model 3: independent variable (PAADRN intervention vs. control), OSE at baseline (OSE-diet for calcium and vitamin D, OSE-exercise for exercise frequency), and a term for intervention vs. control interacted with baseline OSE
model 4: independent variable (PAADRN intervention vs. control), prior DXA or DXA-naïve status at baseline, and a term for intervention vs. control interacted with baseline DXA status
model 5: independent variable (PAADRN intervention vs. control), fracture risk based on FRAX estimate (high, moderate, low) from baseline DXA, and a term for intervention vs. control interacted baseline fracture risk category
The multivariable models for HTE assessment included the other modifier variables as main effects as well as the patient covariates.
A secondary analysis of ATEs was conducted on participants with “suboptimal” behaviors at baseline. Suboptimal behavior was defined as the lowest quartile of average daily calcium intake among all participants (< 675 mg/day), no vitamin D supplementation, or no strengthening or weight-bearing exercise sessions per week. The rationale for undertaking this secondary analysis was to assess if behavior change might be achieved in these subgroups through the individualized PAADRN letter vs. usual care.
Data management for calculation of measures and statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Participant enrollment
There were 20,397 potentially eligible patients, of whom 7749 agreed to participate, were interviewed at baseline, and were properly randomized to either the intervention or control group (Fig. 1). Of these, 6749 (87.1%) completed the 12-week and 5781 (74.6%) completed the 52-week follow-up interviews. Multiple imputations were used to include patients with missing outcomes, so that all 7749 patients were included in the intention-to-treat analysis. The intervention and control groups were well-matched on demographic and socioeconomic characteristics (Supplemental Materials, Table S1).
Fig. 1.
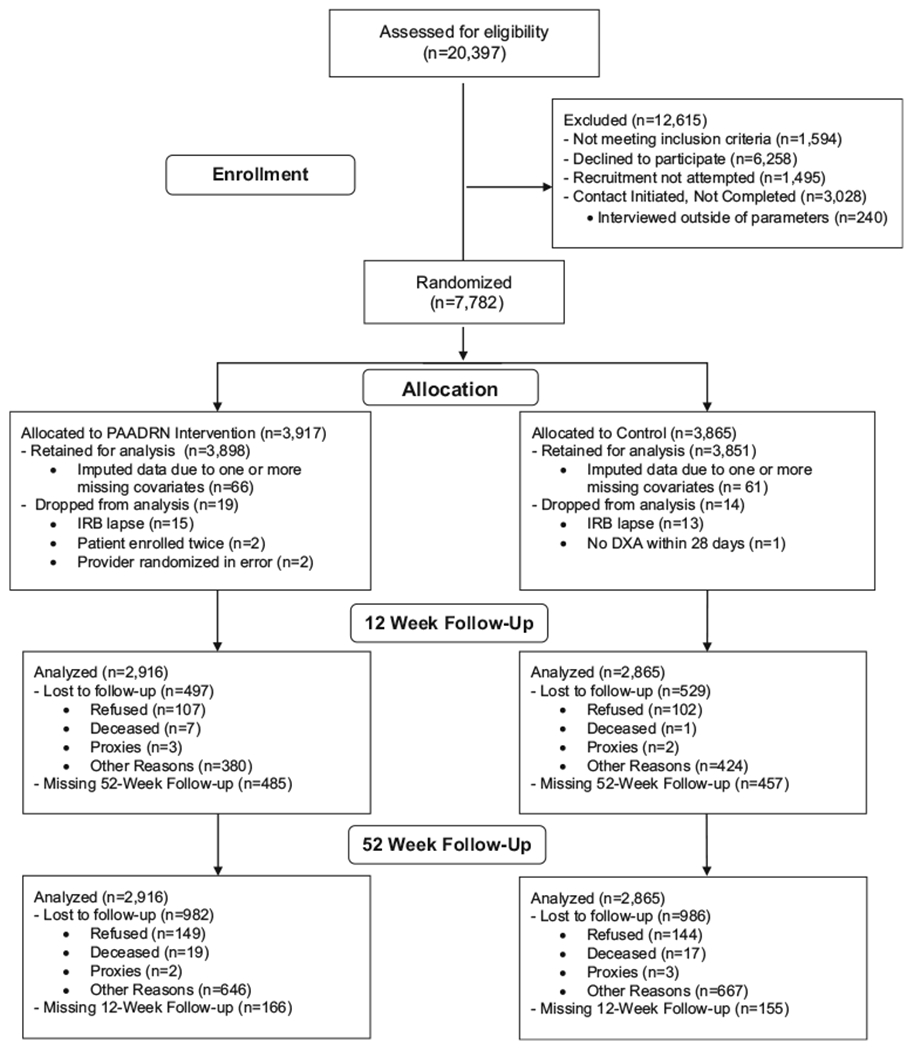
CONSORT flow diagram
Unadjusted average treatment effects
At baseline, overall average daily calcium intake among intervention participants was 954 mg/day and 970 mg/day among control participants (Table 1). These averages are consistent with US national estimates for comparable age groups [29–31]. Baseline average daily calcium intake was lower in the intervention group than in the control group (p = 0.043; Table 1). Slightly more than one half of participants in each group had vitamin D supplementation, and there were 3.3, on average, weekly exercise sessions. The proportion of participants with vitamin D supplementation and average weekly exercise sessions did not significantly differ between the intervention and control groups.
Table 1.
Unadjusted between- and within-group comparisons on bone health behaviors
| Intervention (letter + brochure) | Control | p value (intervention vs. control) | ||
|---|---|---|---|---|
| No. of participants | 3898 | 3851 | – | |
| Daily calcium intake | Baseline | 954.38 (343.09) | 970.13 (342.13) | 0.043 |
| 52 weeks | 1047.81 (332.75) | 1060.17330.18) | 0.118 | |
| Change from baseline to 52 weeks | 93.43 (294.59) | 90.04 (302.04) | 0.638 | |
| p value (change from baseline to 52 weeks) | < 0.001 | < 0.001 | – | |
| Proportion with vitamin D supplementation | Baseline | 0.55 (0.50) | 0.56 (0.50) | 0.824 |
| 52 weeks | 0.60 (0.49) | 0.60 (0.49) | 0.975 | |
| Change from baseline to 52 weeks | 0.05 (0.43) | 0.04 (0.42) | 0.833 | |
| p value (change from baseline to 52 weeks) | < 0.001 | < 0.001 | – | |
| Weekly exercise sessions | Baseline | 3.30 (2.89) | 3.33 (2.89) | 0.945 |
| 52 weeks | 3.92 (2.93) | 3.86 (2.93) | 0.346 | |
| Change from baseline to 52 weeks | 0.63 (2.78) | 0.53 (2.88) | 0.141 | |
| p value (change from baseline to 52 weeks) | < 0.001 | < 0.001 | – |
Italics indicate statistical significance (p < 0.05)
At 52 weeks following recruitment, significant increases occurred in average daily calcium intake, proportion with vitamin D supplementation, and average weekly exercise sessions within both the intervention and control groups (Table 1; all p < 0.001). For example, daily calcium intake increased 93 mg/day in the intervention group and 90 mg/day in the control group. The changes between baseline and 52 weeks in average daily calcium intake, proportion with vitamin D supplementation, and average weekly exercise sessions, however, were equivalent between the intervention and control groups (p = 0.638, 0.833, and 0.141, respectively; Table 1).
Average treatment effects adjusted for patient covariates
For the most part, randomization balanced covariate means between treatment groups (Table S1), but there were some differences between groups. Because of the overall balance between groups, we did not expect covariate-adjusted ATE and HTE models to alter the results from the unadjusted comparisons.
Table 2 displays estimates of the PAADRN intervention effects on average daily calcium intake (linear effect), proportion with vitamin D supplementation (odds ratio), and weekly exercise sessions (linear effect). The adjusted ATEs indicated that the PAADRN intervention did not independently contribute to the 52-week improvements in any of the three behaviors with statistical significance (i.e., p < 0.05). For example, in model 1, the intervention effect was an average increase of 0.145 mg/day calcium change (p = 0.982), an increase in the odds of vitamin D supplementation of 1.007 (p = 0.923), and an average increase of 0.092 exercise sessions/week (p = 0.139).
Table 2.
Evaluation of average treatment effects on primary end points: adjusted for patient covariates
| Behavior | Effect | Effect estimate | 95% CI | p value | |
|---|---|---|---|---|---|
| Daily calcium intake at 52 weeksa | Model 1 | Daily calcium intake at baseline | 0.552 | (0.532, 0.573) | < 0.001 |
| Intervention vs. control | 0.145 | (− 12.508, 12.798) | 0.982 | ||
| OSE-diet at baseline | 16.245 | (11.958, 20.531) | < 0.001 | ||
| Prior DXA vs. DXA-naïve | − 11.491 | (− 29.887, 6.905) | 0.214 | ||
| High fracture risk at baseline | 23.353 | (− 0.069, 46.774) | 0.051 | ||
| Moderate fracture risk at baseline | 42.801 | (26.211, 59.391) | < 0.001 | ||
| Proportion with vitamin D supplementation at 52 weeksb | Model 1 | Vitamin D supplementation at baseline | 20.516 | (18.088, 23.27) | < 0.001 |
| Intervention vs. control | 1.007 | (0.882, 1.149) | 0.923 | ||
| OSE-diet at baseline | 1.042 | (0.990, 1.095) | 0.110 | ||
| Prior DXA vs. DXA-naïve | 0.971 | (0.833, 1.131) | 0.704 | ||
| High fracture risk at baseline | 1.023 | (0.809, 1.294) | 0.846 | ||
| Moderate fracture risk at baseline | 1.028 | (0.846, 1.249) | 0.776 | ||
| Weekly exercise sessions at 52 weeksa | Model 1 | Weekly exercise sessions at baseline | 0.430 | (0.405, 0.455) | < 0.001 |
| Intervention vs. control | 0.092 | (− 0.03, 0.214) | 0.139 | ||
| OSE-exercise at baseline | 0.188 | (0.153, 0.222) | < 0.001 | ||
| Prior DXA vs. DXA-naïve | 0.042 | (− 0.121, 0.204) | 0.606 | ||
| High fracture risk at baseline | 0.014 | (− 0.191, 0.218) | 0.894 | ||
| Moderate fracture risk at baseline | 0.028 | (− 0.143, 0.200) | 0.741 |
Italics indicate statistical significance (p < 0.05). High fracture risk is > 20% 10-year fracture risk estimated from the baseline FRAX® score; moderate risk is 10–20% 10-year fracture risk
DXA dual-energy X-ray absorptiometry, OP osteoporosis
For the daily calcium intake and weekly exercise session regressions, the effect estimates represent an adjusted linear effect
For the vitamin D supplementation regression, the effect estimate represents an adjusted odds ratio
The strongest association with any one of the behaviors at 52 weeks was the baseline level of the behavior. For example, the effect estimate for baseline daily calcium intake in model 1 was 0.552 (p < 0.001; Table 2) indicating that on average, daily calcium intake at 52 weeks was significantly, proportionally associated with daily calcium intake at baseline.
Heterogeneity of treatment effects adjusted for patient covariates
Table 3 displays estimates of the joint effect of the PAADRN intervention with respect to several key measures on average daily calcium intake (linear effect), proportion with vitamin D supplementation (odds ratio), and weekly exercise sessions (linear effect). The only statistically significant joint effect estimate was for the interaction of “high fracture risk” with the PAADRN intervention on 52-week average daily calcium intake (39.927, p = 0.031, model 5). The interaction between the prior DXA and PAADRN intervention was marginally significant for 52-week average daily calcium intake (25.658, p = 0.052). These interactions suggest that the PAADRN intervention had a greater effect on improving average daily calcium intake among participants who had: 1) a high fracture risk (which was highlighted in the PAADRN intervention letter) and 2) possibly a DXA prior to their study DXA.
Table 3.
Evaluation of heterogeneity of treatment effects on primary end points: adjusted for patient covariates
| Behavior | Effect | Effect estimate | 95% CI | p value | |
|---|---|---|---|---|---|
| Daily calcium intake at 52 weeksa | Model 2 | Daily calcium intake at baseline | 0.554 | (0.526, 0.581) | < 0.001 |
| Intervention vs. control | − 28.707 | (− 65.081, 7.667) | 0.122 | ||
| Daily calcium intake at baseline × intervention | 0.029 | (− 0.006, 0.064) | 0.104 | ||
| Model 3 | OSE-diet at baseline | 15.941 | (10.477, 21.404) | < 0.001 | |
| Intervention vs. control | − 4.931 | (− 67.875, 58.014) | 0.878 | ||
| OSE-diet at baseline × intervention | 0.592 | (− 6.587, 7.770) | 0.872 | ||
| Model 4 | Prior DXA at baseline | − 27.055 | (− 48.834, − 5.276) | 0.015 | |
| Intervention vs. control | − 18.054 | (− 39.947, 3.839) | 0.106 | ||
| Prior DXA at baseline × intervention | 25.658 | (− 0.266, 51.582) | 0.052 | ||
| Model 5 | High fracture risk at baseline | 2.939 | (− 27.091, 32.969) | 0.847 | |
| Moderate fracture risk at baseline | 31.713 | (10.821, 52.606) | 0.003 | ||
| Intervention vs. control | − 14.155 | (− 31.128, 2.817) | 0.102 | ||
| High fracture risk at baseline × intervention | 39.927 | (3.623, 76.231) | 0.031 | ||
| Moderate fracture risk at baseline × intervention | 21.461 | (− 5.326, 48.248) | 0.116 | ||
| Proportion with vitamin D supplementation at 52 weeksb | Model 2 | Vitamin D supplementation at baseline | 22.356 | (18.297, 27.315) | < 0.001 |
| Intervention vs. control | 1.070 | (0.890, 1.287) | 0.464 | ||
| Vitamin D supplementation at baseline × intervention | 0.857 | (0.655, 1.122) | 0.259 | ||
| Model 3 | OSE-diet at baseline | 1.040 | (0.974, 1.112) | 0.233 | |
| Intervention vs. control | 0.988 | (0.521, 1.873) | 0.971 | ||
| OSE-diet at baseline × intervention | 1.002 | (0.930, 1.080) | 0.955 | ||
| Model 4 | Prior DXA at baseline | 1.024 | (0.841, 1.249) | 0.811 | |
| Intervention vs. control | 1.077 | (0.869, 1.333) | 0.499 | ||
| Prior DXA at baseline × intervention | 0.898 | (0.697, 1.156) | 0.403 | ||
| Model 5 | High fracture risk at baseline | 1.008 | (0.761, 1.334) | 0.958 | |
| Moderate fracture risk at baseline | 0.981 | (0.784, 1.227) | 0.867 | ||
| Intervention vs. control | 0.969 | (0.801, 1.173) | 0.744 | ||
| High fracture risk at baseline × intervention | 1.028 | (0.700, 1.508) | 0.889 | ||
| Moderate fracture risk at baseline × intervention | 1.100 | (0.834, 1.452) | 0.498 | ||
| Weekly exercise sessions at 52 weeksa | Model 2 | Weekly exercise sessions at baseline | 0.463 | (0.429, 0.496) | < 0.001 |
| Intervention vs. control | 0.006 | (− 0.212, 0.224) | 0.958 | ||
| Weekly exercise sessions at baseline × intervention | 0.025 | (− 0.028, 0.077) | 0.340 | ||
| Model 3 | OSE-exercise at baseline | 0.214 | (0.174, 0.253) | < 0.001 | |
| Intervention vs. control | 0.496 | (0.056, 0.936) | 0.027 | ||
| OSE-Exercise at baseline × intervention | − 0.052 | (− 0.107, 0.002) | 0.060 | ||
| Model 4 | Prior DXA at baseline | 0.011 | (− 0.182, 0.205) | 0.908 | |
| Intervention vs. control | 0.057 | (− 0.149, 0.264) | 0.584 | ||
| Prior DXA at baseline × intervention | 0.046 | (− 0.193, 0.284) | 0.707 | ||
| Model 5 | High fracture risk at baseline | − 0.054 | (− 0.326, 0.218) | 0.697 | |
| Moderate fracture risk at baseline | 0.016 | (− 0.204, 0.236) | 0.886 | ||
| Intervention vs. control | 0.067 | (− 0.125, 0.259) | 0.483 | ||
| High fracture risk at baseline × intervention | 0.101 | (− 0.302, 0.503) | 0.614 | ||
| Moderate fracture risk at baseline × intervention | 0.015 | (− 0.247, 0.277) | 0.911 |
Italics indicate statistical significance (p < 0.05). High fracture risk is > 20% 10-year fracture risk estimated from the baseline FRAX® score; moderate risk is 10–20% 10-year fracture risk
DXA dual-energy X-ray absorptiometry, OP osteoporosis
For the daily calcium intake and weekly exercise session regressions, the effect estimates represent an adjusted linear effect
For the vitamin D supplementation regression, the effect estimate represents an adjusted odds ratio
Secondary analysis of treatment effects among patients with suboptimal behavior at baseline
Suboptimal behavior with respect to good bone health behaviors was defined as calcium intake in the lower quartile of the distribution at baseline of all PAADRN study participants—< 675 mg/day (25.6% of the sample)—and frequency of strengthening and weight-bearing exercises—“0” sessions per week (28.4% of the sample). Absence of vitamin D supplementation at baseline (44.4% of the sample) was also considered to be suboptimal behavior.
Univariate and multivariable results in this secondary analysis were consistent with ATE findings in the entire PAADRN sample. For each of these subgroups of participants, both the letter and usual-care groups improved average levels of daily calcium intake, vitamin D supplementation, and weekly exercise sessions—but equally so (Table S2). In assessment of ATEs, the intervention effect was not significant (Table S3). In assessment of HTEs, neither the significant interaction of intervention with low fracture risk on daily calcium intake nor the marginally significant interaction of the intervention with prior DXA were observed (Table S4).
Discussion
The PAADRN intervention—which was a rapid, low-cost, direct-to-patient mailing of a letter with individualized fracture risk information and an educational brochure—had no significant effect on improving daily calcium intake, vitamin D supplementation, or the frequency of weight-bearing or strengthening exercise after 52 weeks compared to the less timely, generally untailored communication of DXA results to patients that characterizes usual care. The lack of significant findings on lifestyle change is disappointing since pre-trial formative work was undertaken to improve “patient-centeredness” of the PAADRN intervention materials [26–28].
The strongest association of osteoporosis-related self-management behaviors at 52 weeks post-enrollment was the level of those behaviors at baseline. Participants who had higher levels of daily calcium intake, vitamin D supplementation, and weekly exercise sessions at baseline also had higher levels 52 weeks later and vice versa. Simply put, over the 52-week period, adult lifestyle remained relatively persistent with respect to a “silent” asymptomatic disease like osteoporosis.
Health behaviors in later life are habitual, and an adult’s changing lifestyle may depend on intrinsic and extrinsic motivating factors [32, 33]. Intrinsic factors are quite powerful and reinforcing in symptomatic diseases like congestive heart failure where non-adherence to a recommended lifestyle regimen can rapidly lead to bothersome symptoms and acute events. Intrinsic factors are less salient in asymptomatic diseases like osteoporosis. Extrinsic motivating factors are more important for maintaining a recommended lifestyle regimen that might not yield short-term, tangible changes to perceived quality of life.
The PAADRN intervention letter and brochure were designed to improve participant knowledge of and competence in the practice of good bone health behaviors. The intervention goal was to motivate change in osteoporosis-related attitudes and behavior extrinsically, and in the short-term (i.e., 52 weeks), it appears to have been too weak of an intervention to change a sufficiently large number of adults’ daily calcium intake, vitamin D supplementation, and weekly exercise sessions. This conclusion is consistent with the lack of a statistical improvement in guideline-concordant osteoporosis pharmacotherapy among PAADRN participants, an outcome for which the study was sufficiently powered statistically [22]. Though the letter was individualized to a participant’s fracture risk, it might not have been sufficiently tailored to a participant’s other circumstances (e.g., baseline levels of bone health behaviors) to motivate behavioral change.
Though not statistically significant, might the 52-week changes in daily calcium intake, vitamin D supplementation, or exercise sessions have been clinically meaningful? Assuming that a ratio of change to the standard deviation which is at least 0.5 establishes a “minimally important difference,” few changes (Tables 1 and 2) come even close to this criterion [34, 35]. For example, among intervention participants, the standardized increase in daily calcium intake is only about 0.3 (i.e., 93/295) and the standardized increase in weekly exercise sessions is only about 0.2 (i.e., 0.63/2.78; Table 1).
The limited number of other RCTs that have evaluated simple, direct-to-patient communication of DXA results (including fracture risk) or osteoporosis-related self-management behaviors has concluded that a single or limited numbers of direct-to-patient contacts are unlikely to be effective in activating large numbers of patients to self-manage their dietary intake or exercise [9, 13–16]. One strength of the PAADRN intervention is that it did engage patients shortly after a teachable moment—the receipt of a DXA screening for bone density assessment—an important first step in developing a productive patient-healthcare system relationship [1, 2]. Modifications to patient-centered communication intended to motivate good bone health have been proposed:
repeated, periodic reminders potentially with adaptive tailoring [36, 37],
simultaneous messaging of results to both patient and provider [38–42], and
framing of messages with respect to quality of life gained (“gain framing”) or to quality of life lost (“loss framing”) [43].
These more complex strategies for addressing motivation for lifestyle change through tailored patient communications, however, would increase the intervention costs and might decrease the likelihood that the intervention would be sustained by an organization over time as competing priorities arise.
Another issue to be considered in future interventions is the mode of message delivery. PAADRN relied on written communication. A multimodal strategy might have improved the intervention response [44, 45]. A growing segment of patients prefers delivery modes other than direct postal mailings—telephone (including interactive voice recognition or text-messaging systems), electronic mail, and nurse contact. Future interventions might consider a multimodal approach tailored to patient preferences for receiving healthcare messages.
The PAADRN trial has limitations. It was conducted at only three sites in the USA. Our assessments of daily calcium intake, supplemental vitamin D, and exercise levels were based on patient report. Self-reported measures are subject to biases such as recall and social desirability. Though we followed generally acceptable methods of estimating behaviors (e.g., daily calcium intake), there remain diverse methodological approaches [42, 43]. Improvements in intermediate attitudinal and behavioral changes with respect to self-management of bone health were achieved within the context of a research study, in which participants were volunteers and were presumably interested in and motivated to improve their health. Only about one in three eligible participants actually consented. The extensive baseline and follow-up interviews administered to both PAADRN intervention and control participants may have brought attention to concerns about good bone health behaviors in both groups of motivated volunteers and diminished the effect of the PAADRN intervention. The high proportion of participants with low fracture risk (Table S1) may have weakened the intervention effect. Baseline levels of calcium intake were high, and this might have attenuated behavioral change on this endpoint. Finally, post-intervention interviews with participants were not conducted, so whether or not participants understood the information in the intervention letter and educational brochure and what actions they took to change behaviors are not known.
In summary, the PAADRN intervention improved the timing of communication of DXA results in an individualized direct-to-patient mailing compared to prevailing communication of DXA results. At 52 weeks, however, PAADRN’s intervention did not generally produce statistically significant improvements in daily calcium intake, vitamin D supplementation, or weekly exercise sessions compared to usual care. The intervention appears, however, to have motivated marginal increases in daily calcium intake among participants with high and moderate fracture risks. Compared to usual care, the intervention did not significantly improve bone health behaviors among those with low daily calcium intake, no vitamin D supplementation, or no weekly exercise sessions at baseline—those most likely to benefit from changed behavior.
Supplementary Material
Acknowledgements
We thank Rebecca Burmeister, MPH (UI); Mollie Giller, MPH (UI); April Miller RT (UI), CBDT; Amna Rizvi-Toner, BA, BS (UI); Kara Wessels, BA (UI); Brandi Robinson (KP); Akeba Mitchell (KP); Aimee Khamar (KP); and Roslin Nelson (KP) and all of the staff at the Iowa Social Science Research Center for recruiting and interviewing all study participants. All except Ms. Miller were compensated from grant funds for their time. We also thank Ryan Outman, MS (UAB), for coordinating and facilitating the recruitment of study participants. We also thank Thuy Nguyen, MS (UI), for managing the trial data. Finally, we thank the 7749 patients who participated in PAADRN.
Funding information This work was supported by R01 AG033035 to Dr. Cram and Dr. Wolinsky from the NIA at the NIH. Dr. Cram is also supported by a K24 AR062133 award from the NIAMS at the NIH.
Conflicts of interest F. D. Wolinsky, Y. Lou, S. W. Edmonds, S. F. Hall, M. P. Jones, P. Cram, and D. W. Roblin have no conflicts of interest. N. C. Wright has received unrestricted grant support from Amgen for work unrelated to this project. K. G. Saag has received grants from Amgen, Eli Lilly, and Merck and has served as a paid consultant to Amgen, Eli Lilly, and Merck unrelated to this project. The NIA, NIAMS, and NIH had no role in the (a) design and conduct of the study; (b) collection, management, analysis, and interpretation of the data; (c) preparation, review, or approval of the manuscript; or (d) decision to submit the manuscript for publication.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11657-017-0402-8) contains supplementary material, which is available to authorized users.
References
- 1.Wagner EH, Austin BT, Von Korff M (1996) Organizing care for patients with chronic illness. Milbank Q 74(4):511–544. 10.2307/3350391 [DOI] [PubMed] [Google Scholar]
- 2.Holman H, Lorig K (2004) Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep 119(3):239–243. 10.1016/j.phr.2004.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Osteoporosis Foundation. Calcium and vitamin D: what you need to know www.nof.org/calcium. Accessed 4/20/2015
- 4.National Osteoporosis Foundation. Exercise for strong bones. www.nof.org/exercise. Accessed 4/20/2015
- 5.Welten DC, Kemper HC, Post GB, van Staveren WA (1995) A meta-analysis of the effect of calcium intake on bone mass in young and middle aged females and males. J Nutr 125(11):2802–2813 [DOI] [PubMed] [Google Scholar]
- 6.Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW (1999) The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 9(1):1–12. 10.1007/s001980050109 [DOI] [PubMed] [Google Scholar]
- 7.Ma J, Johns RA, Stafford RS (2007) Americans are not meeting current calcium recommendations. Am J Clin Nutr 85(5):1361–1366 [DOI] [PubMed] [Google Scholar]
- 8.Troiano RP, Berrigan D, Dodd KW et al. (2008) Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40(1):181–188. 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 9.Blalock SJ, DeVellis BM, Patterson CC, Campbell MK, Orenstein DR, Dooley MA (2002) Effects of an osteoporosis prevention program incorporating tailored educational materials. Am J Health Promot 16(3):146–156. 10.4278/0890-1171-163.146 [DOI] [PubMed] [Google Scholar]
- 10.Nahm E-S, Resnick B, Brown C, Zhu S, Magaziner J, Bellantoni M, Brennan PF, Charters K, Brown J, Rietschel M, An M, Park BK (2017) The effects of an online theory-based bone health program for older adults. J Appl Gerontol 36(9):1117–1144. 10.1177/0733464815617284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan P, Maierle D, Csuka ME, Thomson A, Szabo A (2013) Computer-based intervention to enhance self-management of calcium and vitamin D intake in women. West J Nurs Res 35(8):986–1010. 10.1177/0193945913483369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaudoin C, Bessette L, Jean S, Ste-Marie L-G (2014) The impact of educational interventions on modifiable risk factors for osteoporosis after a fragility fracture. Osteoporos Int 25(7):1821–1830. 10.1007/s00198-014-2618-4 [DOI] [PubMed] [Google Scholar]
- 13.Drieling RL, Ma J, Thiyagarajan S, Stafford RS (2011) An Internet-based osteoporotic fracture risk program: effect on knowledge, attitudes, and behaviors. J Women’s Health 20(11):1895–1907. 10.1089/jwh.2010.2515 [DOI] [PubMed] [Google Scholar]
- 14.Evenson AL, Sanders GF (2016) Educational intervention impact on osteoporosis knowledge, health beliefs, self-efficacy, dietary calcium, and vitamin D intakes in young adults. Orthop Nurs 35(1): 30–36. 10.1097/NOR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 15.Solomon DH, Finkelstein JS, Polinski JM, Arnold M, Licari A, Cabral D, Canning C, Avorn J, Katz JN (2006) A randomized controlled trial of mailed osteoporosis education to older adults. Osteoporos Int 17(5):760–767. 10.1007/s00198-005-0049-y [DOI] [PubMed] [Google Scholar]
- 16.Winzenberg T, Oldenburg B, Frendin S, De Wit L, Riley M, Jones G (2006) The effect on behavior and bone mineral density of individualized bone mineral density feedback and educational interventions in premenopausal women: a randomized controlled trial [NCT00273260]. BMC Public Health 6(1):12 10.1186/1471-2458-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blalock SJ, DeVellis RF, Giorgino KB, DeVellis BM, Gold DT, Dooley MA, Anderson JJB, Smith SL (1996) Osteoporosis prevention in premenopausal women: using a stage model approach to examine the predictors of behavior. Health Psychol 15(2):84–93. 10.1037/0278-6133.15.2.84 [DOI] [PubMed] [Google Scholar]
- 18.Ryan P, Schlidt A, Ryan C (2013) The impact of osteoporosis prevention programs on calcium intake: a systematic review. Osteoporos Int 24(6):1791–1801. 10.1007/s00198-012-2259-4 [DOI] [PubMed] [Google Scholar]
- 19.Winzenberg TM, Oldenburg B, Frendin S, De Wit L, Jones G (2005) Effects of bone density feedback and group education on osteoporosis knowledge and osteoporosis self-efficacy in premenopausal women. J Clin Densitom 8(1):95–103. 10.1385/JCD:8:1:095 [DOI] [PubMed] [Google Scholar]
- 20.Wu F, Laslett LL, Wills K, Oldenburg B, Jones G, Winzenberg T (2014) Effects of individualized feedback and educational interventions on osteoporosis knowledge and self-efficacy: a 12-yr prospective trial. J Clin Densitom 17(4):466–472. 10.1016/j.jocd.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 21.Edmonds SW, Wolinsky FD, Christensen AJ, Lu X, Jones MP, Roblin DW, Saag KG, Cram P, PAADRN Investigators (2013) The PAADRN study: a design for a randomized controlled practical clinical trial to improve bone health. Contemp Clin Trials 34(1):90–100. 10.1016/jxct.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cram P, Wolinsky FD, Lou Y, Edmonds SW, Hall SF, Roblin DW, Wright NC, Jones MP, Saag KG, PAADRN Investigators (2016) Patient-activation and guideline-concordant pharmacological treatment after bone density testing: the PAADRN randomized controlled trial. Osteoporos Int 27(12):3513–3524. 10.1007/s00198-016-3681-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmonds SW, Solimeo SL, Lu X, Roblin DW, Saag KG, Cram P (2014) Developing a bone mineral density test result letter to send to patients: a mixed-methods study. Patient Prefer Adherence 8: 827–841. 10.2147/PPA.S60106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edmonds SW, Cram P, Lu X, Roblin DW, Wright NC, Saag KG, Solimeo SL, PAADRN Investigators (2014) Improving bone mineral density reporting to patients with an illustration of personal fracture risk. BMC Med Inform Dec Mak 14(101). 10.1186/s12911-014-0101-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edmonds SW, Solimeo SL, Nguyen V-T, Wright NC, Roblin DW, Saag KG, Cram P (2017) Understanding preferences for osteoporosis information to develop an osteoporosis patient education brochure. Perm J 21(1):16–24. 10.7812/TPP/16-024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Osteoporosis Foundation. Steps to estimate your calcium intake. https://www.nof.org/patients/treatment/calciumvitamin-d/steps-to-estimate-your-calcium-intake/. Accessed 11 July 2016
- 27.Horan ML, Kim KK, Gendler P, Froman RD, Patel MD (1998) Development and evaluation of the osteoporosis self-efficacy scale. Res Nurs Health 21(5):395–403. [DOI] [PubMed] [Google Scholar]
- 28.Leslie WD, Berger C, Langsetmo L et al. (2011) Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: results from the CaMos and Manitoba cohorts. Osteoporos Int 22(6):1873–1883. 10.1007/s00198-010-1445-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF (2010) Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 140(4):817–822. 10.3945/jn.109.118539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey RL, Fulgoni VL, Keast DR, Dwyer JT (2012) Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet 112(5):657–663. 10.1016/jjand.2012.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangano KM, Walsh SJ, Insogna KL, Kenny AM, Kerstetter JE (2011) Calcium intake in the United States from dietary and supplemental sources across adult age groups: new estimates from the National Health and Nutrition Examination Survey 2003–2006. J Am Diet Assoc 111 (5):687–695. 10.1016/j.jada.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandura A (1986) Social foundations of thought and action: asocial cognitive theory. Prentice-Hall, Upper Saddle River [Google Scholar]
- 33.Ryan RM, Deci EL (2000) Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 55(1):68–78. 10.1037/0003-066X.55.1.68 [DOI] [PubMed] [Google Scholar]
- 34.Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 41(5):582–592. 10.1097/01.MLR.0000062554.74615.4C [DOI] [PubMed] [Google Scholar]
- 35.Wyrwich KW, Wolinsky FD (2000) Identifying meaningful intra-individual change standards for health-related quality of life measures. J Eval Clin Pract 6(1):39–49. 10.1046/j.1365-2753.2000.00238.x [DOI] [PubMed] [Google Scholar]
- 36.Kingwell E, Prior JC, Ratner PA, Kennedy SM (2010) Direct-to-participant feedback and awareness of bone mineral density testing results in a population-based sample of mid-aged Canadians. Osteoporos Int 21(2):307–319. 10.1007/s00198-009-0966-2 [DOI] [PubMed] [Google Scholar]
- 37.Heimendinger J, O’Neill C, Marcus AC, Wolfe P, Julesburg K, Morra M, Allen A, Davis S, Mowad L, Perocchia RS, Ward JD, Strecher V, Warnecke R, Nowak M, Graf I, Fairclough D, Bryant L, Lipkus I (2005) Multiple tailored messages are effective in increasing fruit and vegetable consumption among callers to the Cancer Information Service. J Health Commun 10(Suppl 1):65–82. 10.1080/10810730500263646 [DOI] [PubMed] [Google Scholar]
- 38.Demark-Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, Peterson B, Macri JM, Rock CL, McBride CM, Kraus WE (2007) Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol 25(19): 2709–2718. 10.1200/JCO.2007.10.7094 [DOI] [PubMed] [Google Scholar]
- 39.Cranney A, Lam M, Ruhland L, Brison R, Godwin M, Harrison MM, Harrison MB, Anastassiades T, Grimshaw JM, Graham ID (2008) A multifaceted intervention to improve treatment of osteoporosis in postmenopausal women with wrist fractures: a cluster randomized trial. Osteoporos Int 19(12):1733–1740. 10.1007/s00198-008-0669-0 [DOI] [PubMed] [Google Scholar]
- 40.Davis JC, Guy P, Ashe MC, Liu-Ambrose T, Khan K (2007) HipWatch: osteoporosis investigation and treatment after a hip fracture: a 6-month randomized controlled trial. J Gerontol 62A(8): 888–891 [DOI] [PubMed] [Google Scholar]
- 41.Feldstein A, Elmer PJ, Smith DH, Herson M, Orwoll E, Chen C, Aickin M, Swain MC (2006) Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc 54(3):450–457. 10.1111/j.1532-5415.2005.00618.x [DOI] [PubMed] [Google Scholar]
- 42.Solomon DH, Katz JN, Finkelstein JS, Polinski JM, Stedman M, Brookhart MA, Arnold M, Gauthier S, Avorn J (2007) Osteoporosis improvement: a large-scale randomized controlled trial of patient and primary care physician education. J Bone Miner Res 22(11): 1808–1815. 10.1359/jbmr.070717 [DOI] [PubMed] [Google Scholar]
- 43.Jung ME, Martin Ginis KA, Phillips SM, Lordon CD (2011) Increasing calcium intake in young women through gain-framed targeted messages: a randomized controlled trial. Psychol Health 26(5):531–547. 10.1080/08870441003611544 [DOI] [PubMed] [Google Scholar]
- 44.Heyworth L, Kleinman K, Oddleifson S, Bernstein L, Frampton J, Lehrer M, Salvato K, Weiss TW, Simon SR, Connelly M (2014) Comparison of interactive voice response, patient mailing, and mailed registry to encourage screening for osteoporosis: a randomized controlled trial. Osteoporos Int 25(5):1519–1526. 10.1007/s00198-014-2629-1 [DOI] [PubMed] [Google Scholar]
- 45.Shultz SK, Wu R, Matelski JJ, Lu X, Cram P (2015) Patient preferences for test result notification. J Gen Intern Med 30(11):1651–1656. 10.1007/s11606-015-3344-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


