Abstract
Limited lower detection ranges associated with traditional immunoassay techniques have prevented the use of brain-specific proteins as blood biomarkers of stroke in the acute phase of care, as these proteins are often only present in circulation at low concentrations. Digital ELISA is a newly developed technique with allows for quantification of proteins in biofluids with up to 1000 times greater sensitivity than conventional ELISA techniques. The purpose of this study was to determine whether the extended lower limits of detection associated with digital ELISA could enable the use of brain-specific proteins as blood biomarkers of ischemic stroke during triage. Blood was sampled from ischemic stroke patients (n=14) at emergency department admission, as well as from neurologically normal controls matched in terms of risk factors for cardiovascular disease (n=33). Plasma levels of two brain-specific axonal proteins, neurofilament light chain (NfL) and Tau, were measured via digital ELISA, and receiver-operator characteristic analysis was used to determine their ability to discriminate between groups. Plasma levels of NfL and Tau were both significantly elevated in stroke patients versus controls, and could respectively discriminate between groups with 92.9% sensitivity and 84.9% specificity, and 85.7% sensitivity and 54.6% specificity. Furthermore, adjustment of measured NfL and Tau levels according to the lower-limits of detection associated with commercially-available conventional ELISA assays resulted in a dramatic and statistically significant decrease in diagnostic performance. Collectively, our results suggest that the increased sensitivity of digital ELISA could enable the use of brain-specific proteins as blood biomarkers of ischemic stroke during triage.
Keywords: Single molecule array, Simoa, Molecular diagnostics, Cerebrovascular disease, Cardiovascular disease, Biomarkers, Triage
Introduction
Stroke is currently the leading cause of permanent disability and the fifth leading of death in the United States [1]. It is well established that quick and accurate diagnosis of stroke improves the odds of positive outcome by increasing access to live-saving interventional therapies. While neuroradiological imaging is the gold standard for diagnosis, imaging techniques are often not available in the field and in-hospital setting during the earliest stages of care. Critical early decisions regarding patient transport, transfer, and referral are typically made by clinicians without extensive neurological expertise using symptom-based stroke recognition assessments such as the Cincinnati prehospital stroke scale. These symptom-based assessments have limited accuracy [2–5], and up to 35% of stroke patients are misdiagnosed at initial clinician contact, leading to devastating delays in care [6–8]. Thus, the identification of accurate blood biomarkers associated with stroke could lead to the development of point-of-care molecular diagnostics with the potential to aid in stroke recognition and better inform early triage decisions.
The proteomic composition of the brain is highly unique relative to other tissues; stroke-associated damage to neural tissue results in the release of brain-specific proteins into the extracellular environment and ultimately into peripheral circulation [9]. Therefore, the detection of such proteins in the blood could serve as a surrogate marker of stroke. However, the blood brain barrier limits the diffusion of brain-specific proteins into the blood [10], and they are often only present in circulation at low-picogram to sub-picogram concentrations; unfortunately, the lower limits of detection (LLOD) associated with conventional ELISA techniques traditionally used to assay blood are not robust enough to detect them at early enough time-points in stroke progression to make them clinically informative during triage [9].
However, recent advances in proteomic techniques with greater sensitivity may allow for detection of these markers earlier in pathology. Digital ELISA is an emerging immunoassay methodology which allows for femtogram-level detection of protein analytes in biofluids. First, antibody conjugated paramagnetic beads are used to capture single molecules of target protein, and protein-bead complexes are labeled with fluorophore conjugated detection antibody. Beads are then assessed for the presence or absence of target protein using a precision-fabricated microwell array capable of capturing one bead per well. This technique has been shown to be as much as 1,000 times more sensitive than traditional ELISA methods [11]. Thus, the extended detection limits associated with digital ELISA could allow for detection of brain-specific proteins in the blood at early enough time points to aid in stroke recognition in the acute phase of care.
Recently developed digital ELISA assays targeting two brain-specific axonal proteins, neurofilament light chain (NfL) and tau, have been successfully used to detect damage to neural tissue in an increasing number of acute and chronic neurological pathologies [12,13]. In the work presented here, we aimed to determine whether high sensitivity digital ELISA measures of these proteins can be used to diagnose ischemic stroke during triage, and compared the diagnostic accuracy of digital ELISA to conventional ELISA.
Results
Digital ELISA measures of circulating brain specific proteins in stroke
In order to determine the ability of digital ELISA measures of circulating brain specific proteins to diagnose stroke in the acute phase of care, peripheral blood samples were collected from a group of acute ischemic stroke patients at hospital admission (n=14), as well as from a group of neurologically normal control subjects with cardiovascular disease risk factors (n=33). Plasma concentrations of NfL and tau were assayed with high sensitivity digital ELISA, and receiver operator characteristic (ROC) analysis was used to determine the ability of measured levels to discriminate between groups.
The average age of the stroke group was approximately 10 years higher than that of the control group, however this difference was not statistically significant. Stroke patients were relatively well matched with controls in terms of sex and prevalence of risk factors for cardiovascular disease including hypertension, dyslipidemia, and diabetes. The stroke group exhibited and average NIHSS of 6.3±7.8, and the mean time from system onset to blood collection was 521±437 (Table 1).
Table 1.
Clinical and Demographic characteristics.
| Control (n=33) | Stroke (n=14) | p-value: | |
|---|---|---|---|
| aAge mean±SD: | 51.7±7.2 | 62.3±20.4 | 0.078 |
| bMale n(%): | 17 (51.5) | 6 (42.9) | 0.752 |
| bHypertension n(%): | 23 (69.7) | 11 (78.6) | 0.726 |
| bDyslipidemia n(%): | 13 (39.0) | 4 (28.6) | 0.529 |
| bDiabetes n(%): | 5 (15.2) | 2 (14.3) | 1.000 |
| Minutes from onset mean±SD: | - | 521±437 | - |
| NIHSS mean±SD: | - | 6.3±7.8 | - |
| Total anterior cerebral infarction n(%): | - | 2 (14.3) | - |
| Partial anterior cerebral infarction n(%): | - | 7 (50.0) | - |
| Posterior cerebral infarction n(%): | - | 3 (21.4) | - |
| Lacunar cerebral infarction n(%): | - | 2 (14.3) | - |
Means statistically compared using two-sample two-tailed t test
Proportions statistically compared using Fisher’s exact test
NIHSS, National Institutes of Health Stroke Scale
statistically significant.
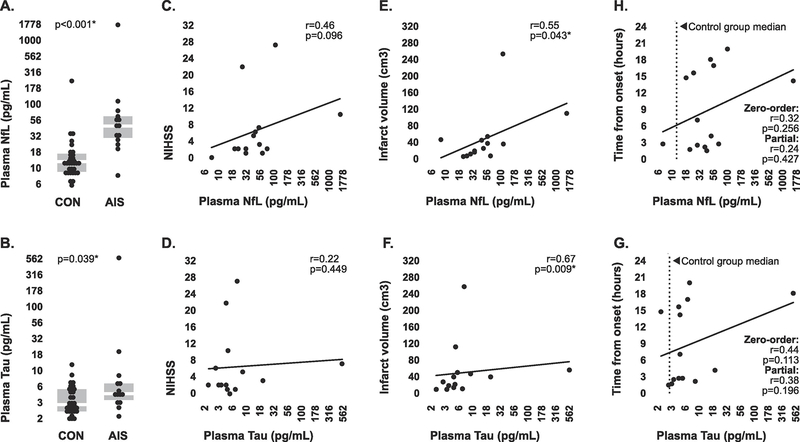
Plasma concentrations of NfL and tau measured with digital ELISA were above the manufacture-stated lower limit of detection for all samples. Both analytes exhibited significantly higher levels in plasma sampled from stroke patients than in plasma sampled from controls (Figure 1A, 1B). Plasma concentrations of NfL and Tau in stroke patients were positively correlated with stroke severity as assessed by NIHSS, however these relationships were not statistically significant (Figure 1C, 1D). However, Plasma concentrations of both analytes were significantly positively correlated with infarct volume (Figure 1E, 1F), providing some degree of evidence that the presence of both proteins in peripheral circulation is likely directly associated with neurological damage.
Figure 1. Circulating concentrations of brain-specific proteins in stroke patients and controls measured by digital ELISA.
(A-B) Median plasma concentrations of NfL and Tau in stroke patients and controls measured by digital ELISA. Intergroup comparisons were made via 2-tailed Mann-Whitney U test. Boxplots indicate interquartile range. (C-D) Associations between plasma concentrations of both brain-specific proteins and NIHSS within the stroke group. (E-F) Associations between plasma concentrations of both brain specific proteins and infarct volume within the stroke group. (H-G) Association between plasma concentrations of both brain-specific proteins and time from symptom onset within the stroke group, both before (zero-order) and after (partial) controlling for stroke severity. Strength of all correlations were tested using Spearman’s rho. *statistically significant. Plasma NfL and Tau concentrations are presented on a Log10 scale.
Plasma levels of both NfL and Tau in stroke patients were positively associated with the time from symptom onset to blood collection, however these relationships were not statistically significant, both before and after controlling for stroke severity using a composite score incorporating NIHSS and infarct volume. Furthermore, concentrations of both analytes in a majority of stroke samples which were collected under three hours from onset still fell above the median concentrations associated with control groups samples (Figure 1H, 1G), which was encouraging in terms of potential future use for detection of stroke early in pathology.
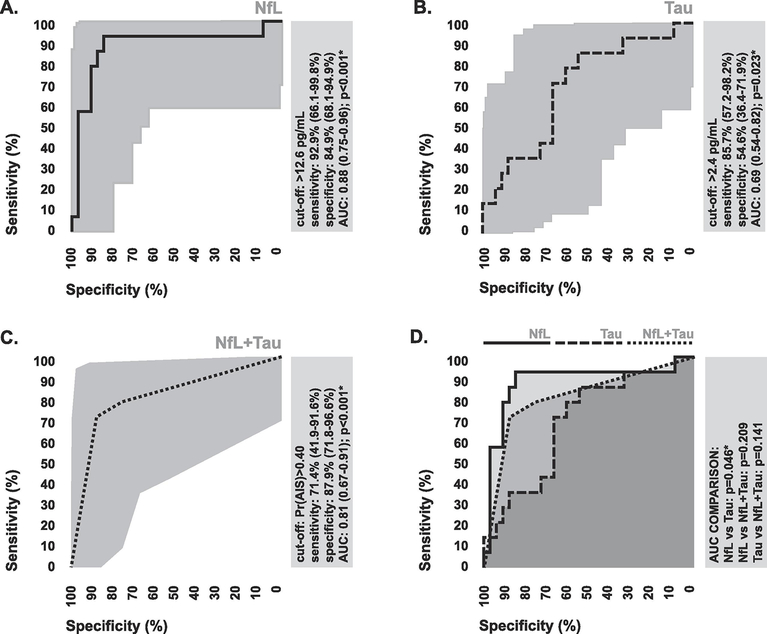
In terms of diagnostic performance, plasma levels of NfL measured with digital ELISA were able to discriminate between stroke patients and controls with 92.9% (0.95 CI: 66.1–99.8%) sensitivity and 84.9% (0.95 CI: 68.1–94.9%) specificity (Figure 2A), while plasma levels of Tau were able to discriminate between stroke patients and controls with 85.7% (0.95 CI: 57.2–98.2%) sensitivity and 54.6% (0.95 CI: 36.4–71.9%) specificity (Figure 2C). Based on comparison of areas under ROC curves, the difference in diagnostic performance between NfL and Tau was statistically significant (Figure 2D). The coordinate plasma levels of both NfL and Tau were able to collectively discriminate between stroke patients and controls with 71.4% (0.95 CI: 41.9–91.6%) sensitivity and 87.9% (0.95 CI: 71.8–96.6%) specificity (Figure 4C), suggesting that using a combined index of both markers for diagnosis has no advantage over using NfL alone.
Figure 2. Diagnostic performance of digital ELISA measures of brain specific-proteins.
(A-C) ROC curves depicting the ability of digital ELISA measures of plasma NfL, Tau, and their combination to discriminate between ischemic stroke patients and controls. To assess combined diagnostic performance, ROC curves were generated via k-nn prediction probabilities. The diagnostic cut-off with the highest combined sensitivity and specificity is indicated. (D) Comparison of the areas under ROC curves for digital ELISA measures of plasma NfL, Tau, and their combination. Areas were statistically compared using the DeLong method. 95% confidence intervals associated with all diagnostic statistics are indicated. AUC, area under curve; Pr, probability; *statistically significant.
Figure 4. Diagnostic comparison of digital ELISA measures and estimated conventional ELISA measures.
Comparison of the areas under ROC curves between digital ELISA measures and estimated conventional ELISA measures of plasma NfL, Tau, and their combination, with respect to their ability to discriminate between stroke patients and controls. Areas were statistically compared using the DeLong method. 95% confidence intervals associated with all diagnostic statistics are indicated. AUC, area under curve.
Comparison of digital ELISA and conventional ELISA
In order to compare the diagnostic performance of digital ELISA to conventional ELISA, the mean LLOD associated with commercially-available conventional ELISA kits for each biomarker were determined via a systematic search, and estimated conventional ELISA measures were generated by replacing all digital ELISA values below the mean LLOD with zero-values. ROC analysis was used to compare the diagnostic performance of the original digital ELISA values and these estimated conventional ELISA values.
Our systematic search identified 49 commercially available conventional ELISA assays for NfL, and 36 for tau, which met the inclusion criteria for analysis. The mean LLOD for conventional ELISA assays targeting NfL was 36.9 pg/mL, while the mean LLOD for conventional ELISA kits targeting Tau was 42.1 pg/mL (Table 2). Based on our measures with digital ELISA, only 7 out of 47 total samples (14.9%) exhibited plasma NfL concentrations above the mean LLOD for commercially available conventional ELISA assays. Furthermore, only 1 out of 47 total samples (2.1%) exhibited plasma tau concentrations above the mean LLOD for commercially available conventional ELISA assays (Table 3).
Table 2.
Characteristics of commercially-available conventional ELISA assays.
| NfL (n=49): | Tau (n=36): | |
|---|---|---|
| Immunoassay format: | ||
| Sandwich n (%): | 39 (79.6) | 35 (97.2) |
| Competitive n (%): | 8 (16.3) | 0 (0.0) |
| Unknown n (%): | 2 (4.1) | 1 (2.8) |
| Mode of detection: | ||
| Colormetric n (%): | 40 (81.6) | 28 (77.8) |
| Chemiluminesent n (%): | 5 (10.2) | 3 (8.3) |
| Flourescent n (%): | 0 (0.0) | 1 (2.8) |
| Unknown n (%): | 4 (8.2) | 4 (11.1) |
| LLOD mean (range): | 36.9 (12.4–156.0) pg/mL | 42.1 (6.3–500.0) pg/mL |
LLOD, lower limit of detection.
Table 3.
Number of samples above LLOD for digital ELISA versus conventional ELISA.
| Digital ELISA (actual): | Conventional ELISA (estimated): | ap-value: | |
|---|---|---|---|
| NfL: | |||
| Total samples above LLOD proportion (%): | 47/47 (100) | 7/47 (14.9) | <0.001* |
| Control samples above LLOD proportion (%): | 33/33 (100) | 1/33 (3.0) | <0.001* |
| AIS samples above LLOD proportion (%): | 14/14 (100) | 6/14 (42.9) | <0.001* |
| Tau: | |||
| Total samples above LLOD proportion (%): | 47/47 (100) | 1/47 (2.1) | <0.001* |
| Control samples above LLOD proportion (%): | 33/33 (100) | 0/33 (0.0) | <0.001* |
| AIS samples above LLOD proportion (%): | 14/14 (100) | 1/14 (7.1) | <0.001* |
Proportions statistically compared using Fisher’s exact test
LLOD, lower limit of detection
statistically significant.
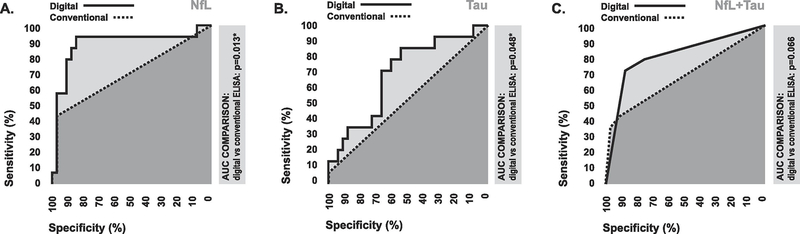
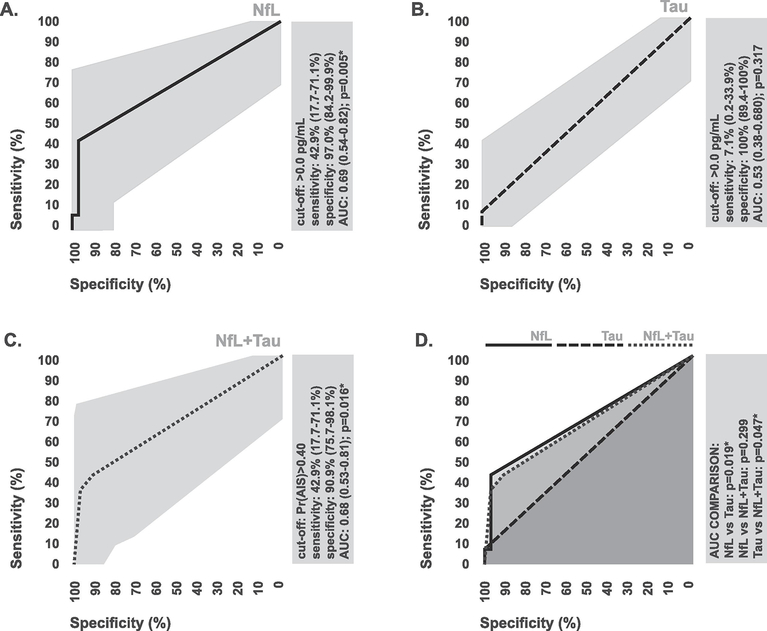
In terms of diagnostic performance, estimated conventional ELISA measures for NfL generated based on the mean LLOD for commercially available conventional ELISA assays could only discriminate between stroke patients and controls with 42.9% (0.95 CI: 17.7–71.1%) sensitivity and 97.0% (0.95 CI: 84.2–99.9%) specificity (Figure 3A). Estimated conventional ELISA measures for Tau could only discriminate between stroke patients and controls with 7.1% 0.95 CI: 0.2–33.9%) sensitivity and 100% (0.95 CI: 89.4–100%) specificity (Figure 3B). Using combined index of both estimated measures was not additive in terms of diagnostic performance (Figure 3C, 3D). Based on statistical comparison of areas under ROC curves, estimated conventional ELISA measures exhibited significantly lower levels of diagnostic accuracy in comparison to the original digital ELISA measures in the case of all markers (Figure 4A–4C), suggesting that the extended LLOD afforded by digital ELISA allows for improved diagnostic performance relative to conventional ELISA techniques.
Figure 3. Diagnostic performance of estimated conventional ELISA measures of brain specific-proteins.
(A-C) ROC curves depicting the ability of estimated conventional ELISA measures of plasma NfL, Tau, and their combination to discriminate between ischemic stroke patients and controls. To assess combined diagnostic performance, ROC curves were generated via k-nn prediction probabilities. The diagnostic cut-off with the highest combined sensitivity and specificity is indicated. (D) Comparison of the areas under ROC curves for estimated conventional ELISA measures of plasma NfL, Tau, and their combination. Areas were statistically compared using the DeLong method. 95% confidence intervals associated with all diagnostic statistics are indicated. AUC, area under curve; Pr, probability; *statistically significant.
Discussion
Limited lower detection ranges associated with traditional immunoassay techniques have prevented the use of brain-specific proteins as blood biomarkers of ischemic stroke in the acute phase of care, as these proteins are often only present in circulation at low concentrations. In this study, we aimed to determine is whether the extended LLOD afforded by digital ELISA over conventional ELISA techniques could enable the use of brain-specific proteins as a blood biomarkers of ischemic stroke during triage. In our analysis, digital ELISA measures of both NfL and tau exhibited dramatically higher levels of diagnostic accuracy relative to estimated conventional ELISA measures, and digital ELISA measures of NfL in particular showed high enough levels of discriminatory performance to suggest future clinical utility.
To our knowledge, this investigation is the first to explicitly compare digital ELISA to conventional ELISA for detection of circulating brain-specific proteins during stroke triage. Numerious prior studies have evaluated the diagnostic potential of circulating brain specific proteins including S100B, NSE, GFAP, and NfL for ischemic stroke recognition in the acute phase of care, and have often reported relatively poor levels of discriminatory ability [9,14–18]. However, these studies have almost universally used conventional ELISA techniques for biomarker quantification, and have often reported large numbers of samples under LLOD, which could have negatively impacted diagnostic performance. Consistent with these prior observations, our analysis suggested that only a small percentage of our plasma samples contained levels tau and NfL which would be detectable by conventional ELISA techniques. However, concentrations of NfL and tau measured with digital ELISA were above the LLOD in all samples, and digital ELISA measures exhibited dramatically higher levels of accuracy for stroke diagnosis relative to estimated conventional ELISA measures. This suggests that prior studies using conventional ELISA methods to evaluate the utility of brain-specific proteins as blood biomarkers in stroke may have underestimated their true diagnostic potential as a result of limited analytical sensitivity, and that future investigations should utilize digital ELISA methods where possible. Furthermore, these findings also suggest that future diagnostic meta-analyses investigating brain-specific proteins in ischemic stroke should be careful to account for differences between studies in the use of digital versus conventional ELISA techniques.
Our results, taken with those of other recent studies, suggest that the extended LLOD associated with digital ELISA may enable levels of diagnostic performance which could have clinical utility for stroke triage, especially in the case of NfL. We observed elevations in digital ELISA measures of circulating NfL in stroke patients at hospital admission compared to neurologically normal controls with cardiovascular disease risk factors, and these elevations were sufficient to discriminate between groups with 87% accuracy. These findings are consistent with a recent study by Tiedt et al. which described statistically significant elevations in digital ELISA measures of circulating levels in stroke patients at hospital admission relative to healthy controls using digital ELISA, however the authors reported no diagnostic statistics [19]. Our findings also concur with those of Onatsu et al., who reported that digital elisa measures of NfL taken 1–12 days following hospital admission are higher in stroke patients versus patients with transient ischemic attack, and could discriminate between groups with 74% accuracy [20]. Comparatively, the symptom-based stroke recognition tools currently used by emergency medical technicians, paramedics, nurses, and emergency department physicians during early triage have been shown to range between to range between 50–75% accuracy, depending on the type of clinician and levels of experience [2–5]. Thus, digital ELISA NfL measures may be able to add diagnostic value to the tools used to evaluate suspected stroke in the prehospital setting and early in-hospital setting. However, it is important to note that digital ELISA assays currently require extensive sample preparation and large equipment, thus limiting their immediate use for the type of stat blood testing required during stroke triage. However this limitation will likely be overcome, as promising new rapid point-of-care digital ELISA technologies are in development which could enable use in emergency medicine settings in the near future [21].
In addition to having potential diagnostic utility for stroke recognition, our results suggest that digital ELISA measures of brain-specific proteins could provide other clinically useful information regarding injury severity. Consistent with prior reports [14,19,20,22], measures of NfL and Tau were positively associated with infarct volume and NIHSS, suggesting that blood levels of these proteins may serve as fairly accurate surrogate measures of the extent of neuronal tissue damage. Thus, it is possible that they could have future utility to inform clinical management through use in monitoring secondary tissue injury, evaluating response to intervention, or predicting outcome.
While our results are exciting, it must be stated that this study is not without limitations. Most notably is the small sample size; while our sample size was adequate to support our conclusion that digital ELISA provides improved diagnostic performance over conventional ELISA, the true clinical accuracy associated with digital ELISA measures of these biomarkers will need to be determined in a larger patient population. Furthermore, patients in our control groups were neurologically normal; while they were well matched with stroke patients in terms of risk factors for cardiovascular disease, future studies should build upon this preliminary investigation and include patients with conditions that mimic stroke as controls, as this would better reflect the target-use scenario. Lastly, we did not directly measure biomarker levels with conventional ELISA in our comparison with digital ELISA, and instead generated estimated measures based on average manufacture-stated LLOD. However, because there is such a large number of conventional ELISA assays available for each marker, it would be imprudent to assume that if we directly tested one or even a small handful of these assays, that their performance characteristics would generalize in terms of the full complement of assays on the market. Thus, it could be argued that while this approach used estimated measures, it provides more comprehensive and generalizable results.
Conclusions
Collectively, our results suggest that the increased sensitivity of digital ELISA could enable the use of brain-specific proteins as blood biomarkers of ischemic stroke during triage. Due to these encouraging findings, future investigation using digital ELISA to evaluate the diagnostic performance of these markers in a larger and more translational clinical population is warranted.
Materials and Methods
Subjects
Acute ischemic stroke patients were recruited in the emergency department at Ruby Memorial Hospital, Morgantown, WV. All ischemic stroke patients displayed definitive radiographic evidence of vascular ischemic pathology on magnetic resonance imaging (MRI) or computed tomography (CT) according to the established criteria for diagnosis of acute ischemic cerebrovascular syndrome (AICS) [23]; all diagnoses were confirmed by an experienced vascular neurologist. Patients we excluded if they received a non-definitive diagnosis, reported a prior hospitalization within 30 days, were under 18 years of age, or were more than 24 hours past symptom onset. Time from symptom onset was determined by the time the patient was last known to be free of neurological symptoms. Injury severity was determined according to the National Institutes of Health Stroke Scale (NIHSS) at the time of blood draw. Control subjects were recruited as part of various different cardiovascular disease clinical investigations at Case Western Reserve University (Cleveland, OH), University Hospitals (Cleveland, OH), and West Virginia University. All procedures were approved by the institutional review boards of University Hospitals (IRB protocol# 20181112) and West Virginia University (IRB protocol# 1410450461R001). Written informed consent was obtained from all subjects or their authorized representatives prior to any study procedures.
Blood collection and processing
Blood for plasma specimens was drawn via venipuncture and collected in spray coated K2ETDA vacutainers (Becton Dickenson, Franklin Lakes, NJ). Blood was spun at 2,500*g for 10 minutes to separate plasma. Resultant plasma was aliquoted and stored at −80 C until analysis.
Neuroradiological Imaging
CT and MRI imaging was performed as soon as possible relative to hospital admission, and the BrainLAB iPlan Neuroradiology software package (BrainLAB, Westchester, Ill) was used to calculate infarct volume via manual tracing as described previously [24,25]. All traces were confirmed by an experienced neurologist. Type of cerebral infarction was classified via the four categories described by Bamford et al. [26] based solely on radiographic evidence of the following [27]: anterior circulation infarct with restricted cortical involvement (partial anterior cerebral infarction), large anterior circulation infarct with both cortical and subcortical involvement (total anterior cerebral infarction), small perforating artery infarction (lacunar cerebral infarction), vertebrobasilar territory infarction (posterior cerebral infarction).
Composite stroke severity score
A single composite score for total stroke severity incorporating both NIHSS and infarct volume was generated using principle components analysis via the prcomp() function of the ‘stats’ package for R (R project for statistical computing) [28]. Data were scaled and centered prior to analysis, and final composite values were generated via regression using the weights associated with first principle component (Supplemental Figure 1). This composite score was used to control for stroke severity in downstream partial correlation analysis
Digital ELISA
Absolute concentrations of NfL and Tau in plasma specimens were measured via the HD-1 Single Molecule Array (SiMoA) platform (Quanterix Corporation, Billerica, MA) using the technical methodology described by our group previously [29]. All measurements were performed by a technician blinded to clinical diagnosis. Total NfL and total Tau were measured via preconfigured assays (Catalog# 103186 and 101995) using manufacture recommended protocols. Plasma was diluted 4x with sample diluent for assays. Standard curves were generated via triplicate measurements of recombinant proteins (Quanterix catalog# 102255, 103221) and all specimens were assayed in duplicate. Intra-assay coefficient of variation (CV) values were calculated from technical replicate measures of specimens assayed within a single run, and fell below 10% for both proteins. Inter-assay CV values were calculated from technical replicate measures of high and low-range control specimens (Quanterix catalog# 102256, 102257, 103222, 103223) assayed across multiple runs, and fell below 15% for both proteins.
Comparison to conventional ELISA
Similar to the approach used by Akama et al. [30], and our group previously [31], a systematic search was used to aggregate the manufacture-stated LLODs associated with commercial conventional ELISA kits available for each of the two brain-specific proteins. The web search was performed via Google search engine using the search strings provided in Supplemental File 1. Assay specifications were recorded manually, and 20% of data were verified via a second extractor; the average interrater reliability was 98.7% across all recorded variables.
For each subject, estimated conventional ELISA measures for both brain-specific proteins were generated. To calculate these estimated measures, the concentrations of specimens with digital ELISA values below the mean manufacture-stated LLOD associated with available conventional ELISA assays were replaced with zero values. ROC analysis was used to compare the sensitivity and specificity of these estimated conventional ELISA measures to those of the original digital ELISA measures.
Statistics
Statistics were performed via R version 3.4. Fisher’s exact test was used for comparison of dichotomous variables, while T-test or Mann-Whitney U Test was used for comparison of continuous variables where appropriate. Strength of zero-order and partial correlations were assessed via Spearman’s rho using the ‘ppcor’ package. The diagnostic ability of binary classifiers was assessed via receiver operator characteristic (ROC) using the ‘pROC’ package. In the case of classification using a single variable, ROC curves were generated directly using the roc() function [32]. In the case of classification using multiple variables, classification was performed using k nearest neighbors (kNN) via the knn.cv() function of the ‘class’ package [33], and the resultant prediction probabilities were used for receiver operator characteristic (ROC) curve generation as described previously [34,35]. Statistical comparison of area under ROC curves was performed using roc.test() function according the non-parametric method described by DeLong et al. [36]. The null hypothesis was rejected when p<0.05. The parameters of all statistical tests performed are outlined in detail within the figure legends.
Supplementary Material
Highlights.
The limited sensitivity of conventional ELISA has prevented the use of circulating brain-specific proteins as stroke biomarkers.
We compared circulating measures of brain-specific proteins made using conventional ELISA and digital ELISA for stroke diagnosis.
Digital ELISA measures exhibited significantly higher levels of diagnostic performance than conventional ELISA measures.
Acknowledgements
The authors would like to thank Dr. Paul D. Chantler of West Virginia University, and Dr. Taura L. Barr, formally of West Virginia University, for providing access to stroke blood samples. The authors would like to further thank the SMART Center in the FPB School of Nursing at Case Western Reserve University for critical review of the manuscript and general research support.
Funding:
Research reported in this publication was supported by the National Institute of Nursing Research and the National Institute on Aging of the National Institutes of Health under Award Number P30NR015326 issued to SMM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by Case Western Reserve University FPB School of Nursing start-up funds issued to GCO.
Footnotes
Compliance with ethical standards:
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest:
The authors declare that they have no conflict of interest.
Data availability:
Data are available from the corresponding author upon reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosley I, Morphet J, Innes K, Braitberg G. Triage assessments and the activation of rapid care protocols for acute stroke patients. Australas Emerg Nurs J. 2013;16:4–9. [DOI] [PubMed] [Google Scholar]
- 3.Whiteley WN, Wardlaw JM, Dennis MS, Sandercock PAG. Clinical scores for the identification of stroke and transient ischaemic attack in the emergency department: a cross-sectional study. J Neurol Neurosurg Psychiatry. 2011;82:1006–10. [DOI] [PubMed] [Google Scholar]
- 4.Purrucker JC, Hametner C, Engelbrecht A, Bruckner T, Popp E, Poli S. Comparison of stroke recognition and stroke severity scores for stroke detection in a single cohort. J Neurol Neurosurg Psychiatry. 2015;86:1021–8. [DOI] [PubMed] [Google Scholar]
- 5.Studnek JR, Asimos A, Dodds J, Swanson D. Assessing the Validity of the Cincinnati Prehospital Stroke Scale and the Medic Prehospital Assessment for Code Stroke in an Urban Emergency Medical Services Agency. Prehosp Emerg Care. 2013;17:348–53. [DOI] [PubMed] [Google Scholar]
- 6.Arch AE, Weisman DC, Coca S, Nystrom KV, Wira CR, Schindler JL. Missed ischemic stroke diagnosis in the emergency department by emergency medicine and neurology services. Stroke. 2016;47:668–673. [DOI] [PubMed] [Google Scholar]
- 7.Lever NM, Nyström KV, Schindler JL, Halliday J, Wira C, Funk M. Missed Opportunities for Recognition of Ischemic Stroke in the Emergency Department. J Emerg Nurs. 2013;39:434–9. [DOI] [PubMed] [Google Scholar]
- 8.Jiang B, Ru X, Sun H, Liu H, Sun D, Liu Y, et al. Pre-hospital delay and its associated factors in first-ever stroke registered in communities from three cities in China. Sci Rep [Internet]. 2016. [cited 2018 May 3];6 Available from: http://www.nature.com/articles/srep29795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassbender K, Schmidt R, Schreiner A, Fatar M, Mühlhauser F, Daffertshofer M, et al. Leakage of brain-originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J Neurol Sci. 1997;148:101–5. [DOI] [PubMed] [Google Scholar]
- 10.Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, Mayberg MR, et al. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci. :13. [PMC free article] [PubMed] [Google Scholar]
- 11.Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28:595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–89. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Mielke MM. An Update on Blood-Based Markers of Alzheimer’s Disease Using the SiMoA Platform. Neurol Ther. 2019;8:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Marchis GM, Katan M, Barro C, Fladt J, Traenka C, Seiffge DJ, et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol. 2018;25:562–8. [DOI] [PubMed] [Google Scholar]
- 15.Rainer TH, Wong KS, Lam W, Lam NYL, Graham CA, Lo YMD. Comparison of plasma β-globin DNA and S-100 protein concentrations in acute stroke. Clin Chim Acta. 2007;376:190–6. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann-Ivol CG, Burkhard PR, Le Floch-Rohr J, Allard L, Hochstrasser DF, Sanchez J-C. Fatty Acid Binding Protein as a Serum Marker for the Early Diagnosis of Stroke: A Pilot Study. Mol Cell Proteomics. 2004;3:66–72. [DOI] [PubMed] [Google Scholar]
- 17.Altunayoglu Cakmak V, Gunduz A, Karaca Y, Alioglu Z, Mentese A, Topbas M. Diagnostic Significance of Ischemia-Modified Albumin, S100b, and Neuron-Specific Enolase in Acute Ischemic Stroke. J Acad Emerg Med [Internet]. 2014. [cited 2019 Oct 24]; Available from: http://www.akademikaciltip.com/sayilar/236/buyuk/1121.pdf
- 18.Ren C, Kobeissy F, Alawieh A, Li N, Li N, Zibara K, et al. Assessment of Serum UCH-L1 and GFAP in Acute Stroke Patients. Sci Rep [Internet]. 2016. [cited 2019 Oct 24];6 Available from: http://www.nature.com/articles/srep24588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, et al. Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018;91:e1338–47. [DOI] [PubMed] [Google Scholar]
- 20.Onatsu J, Vanninen R, Jäkälä P, Mustonen P, Pulkki K, Korhonen M, et al. Serum Neurofilament Light Chain Concentration Correlates with Infarct Volume but Not Prognosis in Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2019;28:2242–9. [DOI] [PubMed] [Google Scholar]
- 21.Yelleswarapu V, Buser JR, Haber M, Baron J, Inapuri E, Issadore D. Mobile platform for rapid sub–picogram-per-milliliter, multiplexed, digital droplet detection of proteins. Proc Natl Acad Sci. 2019;116:4489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen A, Stanne TM, Nilsson S, Klasson S, Rosengren L, Holmegaard L, et al. Circulating neurofilament light in ischemic stroke: temporal profile and outcome prediction. J Neurol. 2019;266:2796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidwell CS, Warach S. Acute Ischemic Cerebrovascular Syndrome: Diagnostic Criteria. Stroke. 2003;34:2995–8. [DOI] [PubMed] [Google Scholar]
- 24.O’Connell GC, Tennant CS, Lucke-Wold N, Kabbani Y, Tarabishy AR, Chantler PD, et al. Monocyte-lymphocyte cross-communication via soluble CD163 directly links innate immune system activation and adaptive immune system suppression following ischemic stroke. Sci Rep. 2017;7:12940–12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connell GC, Petrone AB, Tennant CS, Lucke-Wold N, Kabbani Y, Tarabishy AR, et al. Circulating extracellular DNA levels are acutely elevated in ischaemic stroke and associated with innate immune system activation. Brain Inj. 2017;31:1369–75. [DOI] [PubMed] [Google Scholar]
- 26.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet Lond Engl. 1991;337:1521–6. [DOI] [PubMed] [Google Scholar]
- 27.Lee LJ, Kidwell CS, Alger J, Starkman S, Saver JL. Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke. 2000;31:1081–9. [DOI] [PubMed] [Google Scholar]
- 28.Ross I, Gentleman Robert, Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 29.O’Connell GC, Alder ML, Webel AR, Moore SM . Neuro biomarker levels measured with high-sensitivity digital ELISA differ between serum and plasma. Bioanalysis. 2019;11:2087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akama K, Shirai K, Suzuki S. Highly sensitive multiplex protein detection by droplet-free digital ELISA: AKAMA et al et al. Electron Commun Jpn. 2019;102:43–7. [Google Scholar]
- 31.O’Connell GC, Alder ML, Smothers CG, Still CH, Webel AR, Moore SM. Use of high-sensitivity digital ELISA improves the diagnostic performance of circulating brain-specific proteins for detection of traumatic brain injury during triage. Neurol Res. 2020;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venables WN, Ripley BD. Modern Applied Statistics with S [Internet]. New York, NY: Springer New York; 2002. Available from: http://www.stats.ox.ac.uk/pub/MASS4 [Google Scholar]
- 34.O’Connell GC, Treadway MB, Petrone AB, Tennant CS, Lucke-Wold N, Chantler PD, et al. Leukocyte Dynamics Influence Reference Gene Stability in Whole Blood: Data-Driven qRT-PCR Normalization Is a Robust Alternative for Measurement of Transcriptional Biomarkers. Lab Med [Internet]. 2017;48 Available from: http://fdslive.oup.com/www.oup.com/pdf/production_in_progress.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell GC, Treadway MB, Tennant CS, Lucke-Wold N, Chantler PD, Barr TL. Shifts in Leukocyte Counts Drive the Differential Expression of Transcriptional Stroke Biomarkers in Whole Blood. Transl Stroke Res [Internet]. 2018. [cited 2018 Jun 14]; Available from: http://link.springer.com/10.1007/s12975-018-0623-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.