Abstract
Gram-negative bacteria of the human gastrointestinal (GI) tract microbiome: (i) are capable of generating a broad-spectrum of highly neurotoxic, pro-inflammatory and potentially pathogenic molecules; and (ii) these include a highly immunogenic class of amphipathic surface glycolipids known as lipopolysaccharide (LPS). Bacteroides fragilis (B. fragilis), a commensal, Gram negative, non-motile, non-spore forming obligatory anaerobic bacillus, and one of the most abundant bacteria found in the human GI tract, produces a particularly pro-inflammatory and neurotoxic LPS (BF-LPS). BF-LPS: (i) is known to be secreted from the B. fragilis outer membrane into the external-medium; (ii) can damage biophysiological barriers via cleavage of zonula adherens cell-cell adhesion proteins, thereby disrupting both the GI-tract barrier and the blood-brain barrier (BBB); (iii) is able to transit GI-tract barriers into the systemic circulation and cross the BBB into the human CNS; and (iv) accumulates within CNS neurons in neurodegenerative disorders such as Alzheimer’s disease (AD). This short communication provides evidence that the incubation of B. fragilis with aluminum sulfate [Al2(SO4)3] is a potent inducer of BF-LPS. The results suggest for the first time that the pro-inflammatory properties of aluminum may not only be propagated by aluminum itself, but by a stimulation in the production of microbiome-derived BF-LPS and other pro-inflammatory pathogenic microbial products normally secreted from human GI-tract-resident microorganisms.
GRAPHICAL ABSTRACT
Gram-negative bacteria such as B. fragilis of the human gastrointestinal (GI) tract microbiome generate pro-inflammatory glycolipids known as lipopolysaccharide (LPS) and other neurotoxic species, and are amongst the most pro-inflammatory molecules known. Incubation of aluminum sulfate [Al2(SO4)3] with B. fragilis in culture resulted in a significant increase in the release of LPS.
Keywords: aluminum, Alzheimer’s disease, B. fragilis, dysbiosis, fragilysin, BF-LPS, inflammation, lipopolysaccharide, microbiome
Overview
The human gastrointestinal (GI)-tract contains a complex and dynamic microbiome consisting primarily of anaerobic, Gram-negative bacteria, with archaea, fungi, microbial eukaryotes, protozoa, viruses, and other microorganisms making up the remainder [1–5]. Together with human host cells the GI-tract microbiome comprises an entire community of interacting biological entities referred to as the meta-organism where symbiotic associations and microbiome-host interactions are critical to human health and disease [5–12]. These disorders include lethal, progressive, age-related, inflammatory neurodegenerative disorders of the human CNS such as Alzheimer’s disease (AD) [3–13].
Of the 52 currently recognized divisions of bacteria, humans have co-evolved with just 2 dominant phyla: Bacteroidetes, representing about ~24% of all human GI-tract resident bacteria, and Firmicutes (~72%), with Actinobacteria (~3%), Proteobacteria (~1%) and Verrucomicrobia (~0.1%) making up the remainder [1–4,9]. These four major bacterial phyla represent the ‘bacterial-core’ of the human GI-tract microbiome [1–4,9–15]. The vast majority of all GI-tract microbiota consists of Gram-negative anaerobic bacteria, and Bacteroidetes species represent the most abundant Gram-negative anaerobes, outnumbering Escherichia coli (E.coli) in abundance by about 100 to 1 in some parts of the GI-tract [1–5,11–15]. Certain strains of Bacteroidetes species such as Bacteroides fragilis (B. fragilis), as a normal commensal microbe of the human GI-tract, are thought to be ordinarily beneficial to human health due to their multiple capabilities: (i) to biosynthesize useful metabolic co-factors and products such as polysaccharides, transport proteins, volatile fatty acids and other nutrients [9–11]; (ii) to cleave dietary fiber into digestible short-chain fatty acids (SCFAs) that include acetate, propionate, and butyrate [9,10,15]; (iii) to function in the maintenance, development and homeostasis of the host-immune system [15–19]; (iv) to support immunomodulation and protection against pathogens including potentially pathogenic GI-tract bacteria [9,15]; and (v) to support glucose homeostasis [9,20,21]. However, when stressed, B. fragilis release an extensive and complex array of highly neurotoxic, pro-inflammatory and potentially pathogenic molecules that promotes the establishment in the GI-tract microbiome of bacterial dysbiosis [3,6,7,23,24–29]. Secreted neurotoxins of B. fragilis comprise six major classes of secreted molecules and include, in order of abundance, lipopolysaccharide (LPS), lipooligosaccharide (LOS; consisting of smaller versions of full sized LPS), bacterial amyloids, endotoxins (such as fragilysin), exotoxins, and small non-coding RNA (sncRNA) [5–8,23–26] (Figure 1 and 2). The most prevalent non-spore forming, Gram-negative GI-tract bacterial phylum, Bacteroides, makes up around one quarter of the cells in a typical Western GI-tract microbiome; these cells harbor as much as ~250 mg of LPS, making BF-LPS one of the highest-abundance microbial-derived amphipathic, neurotoxic molecules in the human GI-tract [11,16,26–31]. LPS, also known as lipoglycan, bacterial endotoxin, bacterial sugar-lipid or glycolipid, are 50–100 kDa self-aggregating, thermostable components consisting of a lipid and a polysaccharide composed of an O-antigen, an outer core and an inner core covalently linked, and are the most densely-packed surface molecules found within the outer membrane of Gram-negative bacteria (Figure 1). Typically LPS stimulates the release of tumor necrosis factor alpha (TNFα), interleukin-1β (IL-1β), gamma interferon (IFNγ), interleukin 8 (IL-8), CXC ligand 8 (CXCL8) and other inflammatory cytokines and chemokines in various cell types, leading to an acute inflammatory response towards these bacterial molecular pathogens, which in the host orchestrates a robust anti-infectious, innate-immune response [22,25–33].
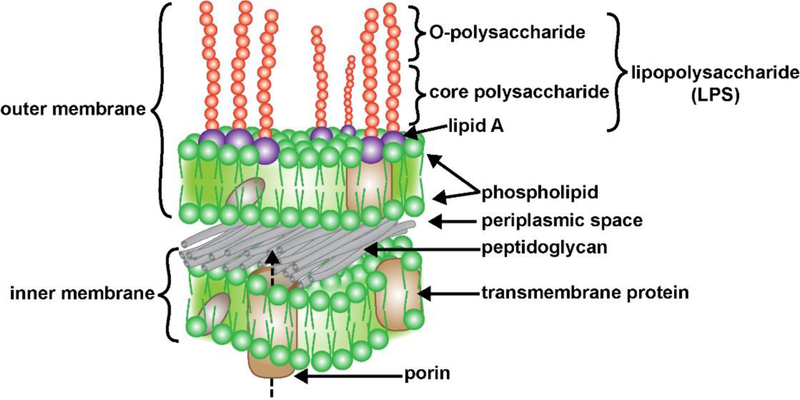
FIGURE 1 -.
Typical structure and organization of an anaerobic Gram-negative bacterial cell membrane and its containment of a lipopolysaccharide (LPS) coated surface (purple and red spheres); the two horizontal layers include an external (outer) and an internal (inner) membrane, both layers contain both integral (gray) and transmembrane (beige) globular proteins; the membranes are separated by an interwoven peptidoglycan layer and a periplasmic space; transmembrane protein complexes such as porin that transverse the inner membrane facilitate molecular communication and LPS transport between the bacterial cell interior to the bacterial outer membrane surface (dashed arrow) but the mechanisms are not well understood [20,47,57–59]. The outer surface of the external membrane contains a dense layer of LPS with lipids anchored in the membrane (purple spheres), and long core polysaccharide and O-polysaccharide side chains extending outward (red spheres); the externally facing LPS are highly thermostable, neurotoxic, pathogenic, extremely pro-inflammatory and a potent trigger of robust antigenic responses within the human immune system; LPS are constantly shed into the external environment where they may find their way past the GI-tract barrier into the systemic circulation and past the BBB into the brain parenchyma [1–5,9,15–18,39,50,57–62]; the mechanism of the induction of LPS and other GI-tract microbiome-derived neurotoxins by aluminum sulfate is not known; (source; figure adapted from https://www.dreamstime.com/stock-illustration-structure-gram-negative-bacteria-cell-wall-labeled-d-illustration-image84181743; last accessed 7 October 2019).
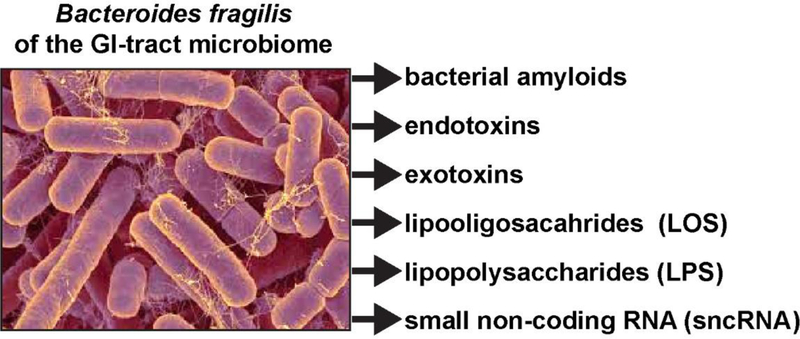
FIGURE 2 -.
Comparable to all other anaerobic Gram-negative bacilli, the gastrointestinal (GI) tract abundant Bacteroides fragilis is capable, when stressed, of releasing a broad spectrum of highly neurotoxic, pro-inflammatory and potentially pathogenic molecules; these comprise six major classes of secreted molecules and include bacterial amyloids, endotoxins, exotoxins, lipooligosacahride (LOS; consisting of smaller isoforms of LPS), lipopolysaccharide (LPS; in this photo yellowish filamentous structures associated with some B. fragilis bacillus rods) and small non-coding RNAs (sncRNA; some similar in size to microRNAs). For example, the human GI tract-abundant B. fragilis secretes the endotoxin-LPS B. fragilis LPS (BF-LPS) which has been shown to be strongly pro-inflammatory and extremely neurotoxic toward human CNS neurons in primary culture; BF-LPS may be the most pro-inflammatory bacterial-derived glycolipid known [5,6,26,51–53]. While the phyla Bacteriodetes (representing about ~24% of all GI-tract bacteria), Firmicutes (~72% of all GI-tract bacteria), Actinobacteria, Proteobacteria, and Verrumicrobia (together, typically ~4% of all GI-tract bacteria), are the most common microbes in the human GI tract microbiome it should be kept in mind that other microbes including fungi, protozoa, viruses, and other commensal microorganisms may also contribute neurotoxic exudates which are highly toxic, pro-inflammatory and detrimental to the homeostasis of CNS neurons; (micrograph of B. fragilis shown; the original photo is shown courtesy of Rosa Rubicondior; http://rosarubicondior.blogspot.com/2014/11/evolving-cooperation-but-for-who-or-what.html; last accessed 7 October 2019).
The neurobiological effects of environmentally abundant, neurotoxic metals on the growth and behavior of Gl-tract resident bacteria such as B. fragilis and their secreted lipopolysaccharides such as BF-LPS and other neurotoxic exudates are not well understood and incompletely characterized. In this communication we report for the first time the significant induction of BF-LPS by aluminum (sulfate). Interestingly, the pro-inflammatory effects of aluminum [26–28] may be supplemented via the actions of aluminum-induced neurotoxic glycolipids such as BF-LPS which along with fragilysin are known: (i) to disrupt normal bio-physiological barriers [26,30–33]; and (ii) to stimulate innate-immune signaling and support the pro-inflammatory neurodegeneration of central nervous system (CNS) tissues [26–30].
Bacteroides fragilis (B. fragilis; ATCC 23745; American Type Culture Collection, Manassas VA, USA) [26,2934] were propagated in ATCC® Medium 1490 (modified chopped meat medium; www.atcc.org/~/media/85260BB7A69A4640A5BB1042498807E4.ashx; https://www.atcc.org/~/media/EB141471E3D04ED9B6E940B3A505BE4C.ashx; last accessed 7 October 2019) under anaerobic conditions at 37°C (under Biosafety Level 2; BSL-2; https://www.vumc.org/safety/basics-biosafety-level-2; last accessed 7 October 2019) using either broth tubes or blood agar plates according to the supplier’s instructions [ATCC; ATCCusers/downloads/ 23745%20(1).pdf; last accessed 7 October 2019] and as previously described [26,31–35]; ATCC Medium 1490 was supplemented with degassed solutions containing metal sulfates [Na2SO4; MgSO4; In2(SO4)3; Ga2(SO4)3; or aluminum sulfate [Al2(SO4)3]; see details below] and made up to 0 (control), 50, 100 and 500 nM using sodium-, magnesium-, indium-, gallium- or aluminum-sulfate in ultrapure water (Invitrogen-ThermoFisher Scientific UltraPure™ DNase/RNase-Free Distilled Water; cat no. 10977015 or equivalent); these cultures were incubated anaerobically at 37°C for 48 hrs with or without metal sulfate additives; total fragilysin, LPS, bacterial amyloid and sncRNA were isolated as previously described, and/or characterized by our group or our collaborators, or were purchased from commercial sources for use as control markers [4–8,34–37]. Cultures of B. fragilis were incubated in parallel with aluminum sulfate [Al2(SO4)3; ultrapure reagent ~99.99 %; CAS Number 10043–01-3; Sigma-Aldrich 202614, St Louis MO, USA; https://www.sigmaaldrich.com/catalog/product/aldrich/202614?lang=en®ion=US&gclid=EAlalQobChMIIathY244wIV6R-Bh1niQ6DEAMYASAAEg JkffDBwE; last accessed 7 October 2019] or sodium sulfate (Na2SO4; anhydrous, granular, free-flowing, Redi-Dri™, ACS reagent, >99.9%; CAS Number 7757–82-6; Sigma Aldrich 1066370500); magnesium sulfate (MgSO4; anhydrous, ReagentPlus™, >99.5%; CAS Number 7487–88-9; Sigma Aldrich M7506); indium sulfate [In2(SO4)3; CAS Number 304655–87-6; Sigma Aldrich 288721]; gallium sulfate [Ga2(SO4)3; >99.9%; CAS Number 13494–91-2; Sigma Aldrich 254207; last accessed 7 October 2019] as metal sulfate controls. Stock solutions of 0.1 to 0.5 M of these metal sulfates were made up in ultrapure water and added to the ATCC® B. fragilis incubation Medium 1490 to a ambient concentration of 0 (control), 50, 100 and 500 nM; virtually identical results were obtained when stock metal sulfate solutions were directly added to B. fragilis cultures. LPS was extracted from B. fragilis according to established standard methods hot phenol-water methods or as previously described with some modifications [34–37]; alternately LPS was purchased from commercial sources and used according to the supplier’s instructions (LPS L8–274; Sigma Aldrich; https://www.sigmaaldrich.com/Graphics/COfAInfo/SigmaSAPQM/SPEC/L8/L8274/L8274-BULKSIG MA.pdf). All protein concentrations were quantified using a Qubit Fluorometric Protein Assay Kit (Cat Number Q33212; sensitivity 12.5 μg/ml to 5 mg/ml) and/or by using antibodies specific for LPS, fragilysin, bacterial amyloid and sncRNA according to the supplier’s instruction and as previously described [4–8,14,26,30–37].
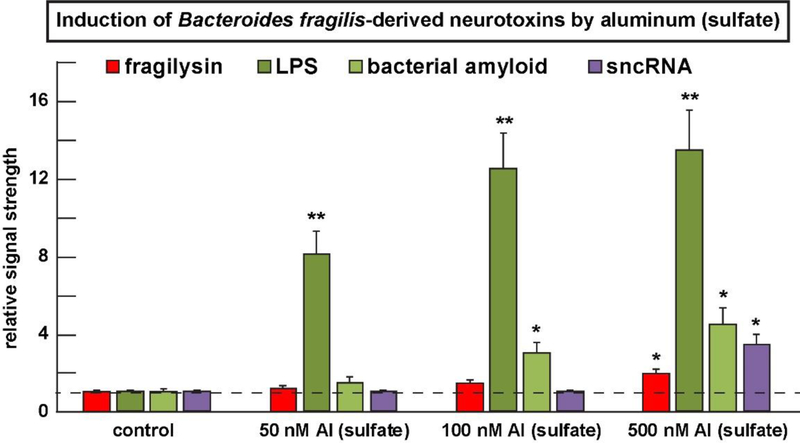
As mentioned earlier, the human Gl-tract microbiome-resident Gram-negative bacillus B. fragilis produces a notable assortment of soluble neurotoxins that are shed from the bacterial surface into their immediate environment (Figure 1 and 2); all of these Gl-tract microbiome-derived neurotoxins, and especially LPS, were found to be induced by nM levels of aluminum sulfate in B. fragilis cultures; for example in B. fragilis cultures just 50 nM aluminum sulfate after 24 hrs increased BF-LPS to levels 8.1-fold over control and the results are highly significant (Figure 3). Four other monovalent-, divalent- or trivalent-metal sulfates - sodium sulfate (Na2SO4), magnesium sulfate (MgSO4); indium sulfate [In2(SO4)3] or gallium sulfate [Ga2(SO4)3] displayed an inability to induce BF-LPS to the extent observed with aluminum sulfate [Al2(SO4)3] addition at any concentration tested. Interestingly: (i) the increase in BF-LPS abundance at 50 nM aluminum sulfate is not linearly proportionate to increases in LPS at 100 nM and 500 nM aluminum sulfate suggesting that the system may become rapidly saturated at the relatively lower concentrations of applied aluminum sulfate; (ii) an excess of aluminum sulfate added may be reacting with other components of the system under study; and (iii) at higher concentrations of aluminum sulfate (~100 nM and 500 nM) in this experimental test system other B. fragilis-secreted neurotoxins become induced above basal levels and the results are again highly significant (Figure 3). For example, at 500 nM [Al2(SO4)3] the neurotoxins LPS, fragilysin, bacterial amyloid and sncRNA were increased, respectively to 13.5-fold, 2.1-fold, 4.5-fold and 3.5-fold over control (Figure 3). While aluminum itself is pro-inflammatory, as measured by its ability to induce the pro-inflammatory transcription factor NF-kB (p50/p65) complex and up-regulate pathogenic microRNAs (miRNAs) such as miRNA-146a that support inflammation [25,28], aluminum-mediated induction of LPS and other neurotoxins such as LPS, fragilysin, bacterial amyloid and sncRNA may also contribute to the pro-inflammatory actions of aluminum-sulfate in the human GI-tract and the CNS [25–28]. Aluminum-induced up-regulation of microbiome-derived LPS may also contribute to systemic inflammation, a potential precursor to the development of AD [17,45,46,50], however this pathological mechanism is not well understood and requires additional study (Figure 3).
FIGURE 3 -.
The human GI-tract microbiome-resident Bacteroides fragilis (B. fragilis) produces an array of soluble neurotoxins (such as fragilysin, LOS, LPS, bacterial amyloid, sncRNA and others) that are secreted into their immediate environment (see text); many of these neurotoxins are known to cross both the GI-tract intestinal barrier into the systemic circulation and induce a systemic inflammation; some of these neurotoxins may cross the blood brain barrier (BBB) to access the brain parenchyma in the aging human BBB or in transgenic murine models for AD and other neurodegenerative disease states [39,51,57–62]. All of these GI-tract microbiome-derived neurotoxins and especially lipopolysaccharide (LPS) are induced by nM levels of aluminum sulfate in B. fragilis cultures; interestingly (i) the increase in LPS at 50 nM aluminum sulfate is not proportionate to increases in LPS at 100 nM and 500 nM aluminum sulfate suggesting that the system may become rapidly saturated at the relatively lower concentrations of applied aluminum sulfate; and (ii) at higher concentrations of aluminum sulfate (~500 nM) in this system other B. fragilis-secreted neurotoxins such as fragilysin, bacterial amyloid and sncRNA become induced above basal levels and the results are significant; while aluminum itself is pro-inflammatory as measured by its ability to induce the pro-inflammatory transcription factor NF-kB (p50/p65) complex [26–28], aluminum-mediated induction of LPS and other inflammation-supporting neurotoxins such as LPS may also contribute to the pro-inflammatory actions of aluminum-sulfate in both the human GI-tract and the human CNS [42,43,56]. Aluminum-induced up-regulation of microbiome-derived LPS may also contribute to systemic inflammation but this pathological mechanism is not well understood and requires further study; in Figure 3 a dashed horizontal line at 1.0 is included for ease of comparison; N=3 to 5 experiments per determination; data in the bar graph represents the mean and one standard deviation of that mean; *p<0.05; **p<0.001 (ANOVA).
One of the highest abundance Gram-negative bacteria-derived neurotoxins in the human microbiome, BF-LPS, is also the most abundant pro-inflammatory glycolipid in the human GI-tract [11,14,16,26,27,31,29,36–39] (Figure 1 and 2). Besides BF-LPS, B. fragilis also secretes neurotoxins in less abundance and these include the metalloproteinase fragilysin, a variety of different types of bacterial amyloid and sncRNA, as well as other as yet poorly characterized secreted microbial molecules [5–9,11,26,33,38], (Figure 2). Both BF-LPS shed from the exterior membrane surface of and the B. fragilis endotoxin fragilysin have been shown to cleave the zonula adherens protein, E-cadherin and thus disrupt normally homeostatic biophysiological membrane barriers [11–14,16–18,26,38,39]. When secreted neurotoxins of enterotoxigenic strains of B. fragilis leak through normally protective bio-physiological-mucosal barriers they can cause substantial inflammatory pathology systemically that can contribute to significant mortality and morbidity [38,39]. Dietary intake of fiber may have a determining role in regulating the composition, organization and stoichiometry of the GI-tract microbiome; for example Bacteroidetes species proliferate in porcine models fed high-fat diets that are deprived of sufficient dietary fiber and the presence of aluminum may potentiate these effects [38,40; unpublished observations]. Interestingly, based on the evolution of the NF-kB (p50/p65) pro-inflammatory transcription factor, BF-LPs has been recently shown to be the most inflammation-inducing glycolipid compared to TNFα, Aβ40 peptide, Aβ42 peptide, IL-1β, the combination of Ap42 peptide and IL-1β together or E. coli LPS (EC-LPS) [26]. Importantly, aluminum may represent an important ingested dietary factor capable of inducing pro-inflammatory signaling in the human GI-tract, systemic circulation and CNS via the initial up-regulation of neurotoxic glycolipids such as BF-LPS.
Lastly, aluminum is a pervasive neurotoxic element in our biosphere that is being increasingly mobilized both into our environment and into multiple aspects of our daily life through the air, the food we eat and the water we drink [42–45]. This is well above and beyond the contribution of alum [as KAl ] added to drinking water supplies worldwide to produce a clear, ‘finished’ water product [42–45]. Aluminum sulfate-induced up-regulation of LPS and other bacterial-derived neurotoxins may make a significant contribution: (i) via selective cadherin cleavage to biophysiological barrier disruption to allowing other neurotoxins access via systemic circulation into CNS compartments [11–14,16–19,39]; (ii) towards systemic inflammation, sometimes referred to as a precursor to inflammatory neurodegenerative diseases such as AD [45–51]; (iii) to the accumulation of pro-inflammatory LPS within the human brain parenchyma and neuronal cytoplasm [5–8,49–52]; and/or (iv) to the LPS-mediated disruption of homeostatic genetic activities involving brain gene transcription in CNS neurons [4–8;48–53]. Ingested aluminum particularly over the long term might contribute chronically to AD-type change and promote AD as a disease transformation rather than a disease state where epigenetics may play a role in both cause and eventual treatment [54–56]. If dietary aluminum crosses GI-tract barriers to access aluminum-sensitive Gram-negative bacterial species such as B. fragilis, to produce increased amounts of BF-LPS, aluminum may ultimately increase LPS abundance in the systemic circulation and eventually cross the BBB into the CNS (57–62). There is recent evidence that LPS: (i) can both disrupt and transverse the BBB to gain access to the brain parenchyma, associate with senile plaques (characteristic lesions of the AD brain) and interact with the nuclear envelope of neurons [6,8,48,49,52,60,61]; (ii) functions to increase blood-brain barrier permeability to Thioflavin-S (MW ~319), to 14C-sucrose (MW ~342) and to 99mTc-albumin (MW 66,500)’ in experimental mouse models [59,60]; and (iii) this suggests that LPS-mediated BBB-disruption may allow the entry of other pathogenic GI-tract-derived neurotoxins such as fragilysin (MW ~20,600), LOS (MW <10,000), bacterial amyloid (CsgA-His; MW~13,900), and/or sncRNA (MW~14,100) into the brain. We speculate that combinations of environmentally abundant metals or other ingested ‘dietary’ factors which stimulate the profusion of neurotoxins derived from anaerobic Gram-negative bacteria and other constituents of the GI-tract microbiome may significantly contribute to the initiation, development and/or propagation of inflammatory neurodegeneration and related neurological disease processes with an inflammatory component.
HIGHLIGHTS.
the human gastrointestinal (GI)-tract microbiome contains an abundance of Bacteroides fragilis;
Bacteroides fragilis generates pro-inflammatory glycolipid lipopolysaccharide (BF-LPS); aluminum sulfate [Al2(SO4)3] significantly induces the generation of BF-LPS;
this may contribute to human systemic inflammation and neuro-inflammatory disease.
Acknowledgments
The experimental research work in this ‘Short Communication’ was presented in part at the Vavilov Institute of General Genetics Autumn 2018 Seminar Series (MHCTMTyr o6êw reHeTMKM MMeHM BaBunoBa OceHb 2018 CeMMHap cepuu) in Moscow, Russia, October 2018, at the Society for Neuroscience (SFN) Annual Meeting, Chicago IL USA, October 2019. Sincere thanks are extended to L. Cong, F. Culicchia, C. Eicken, K. Navel, A.I. Pogue and W. Poon for helpful discussions in this research area, for short postmortem interval (PMI) human brain and retinal tissues or extracts, for initial bioinformatics and data interpretation, and to A.I. Pogue and D. Guillot for expert technical assistance and medical artwork. Research on microRNAs, neurotoxins, pro-inflammatory and pathogenic signaling in the Lukiw laboratory involving the microbiome, the innate-immune response, amyloidogenesis, synaptogenesis, and neuroinflammation in AD and other human neurological diseases was supported through an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness (RPB); the Louisiana Biotechnology Research Network (LBRN; Baton Rouge LA, USA), the Alzheimer Association Chicago IL, USA, National Institutes of Health grants (NIH, Bethesda MD, USA) NEI EY006311, NIA AG18031, and NIA AG038834 (WJL) and was not supported by any pro- or anti-aluminum lobby or private interest group. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Center for Research Resources, or the National Institutes of Health.
Abbreviations
- AD
Alzheimer’s disease
- BBB
blood brain barrier
- BF
Bacteroides fragilis (B. fragilis)
- BF-LPS
LPS from B. fragilis
- CNS
central nervous system
- GI
gastrointestinal
- LOS
lipooligosaccharide
- LPS
lipopolysaccharide
- miRNA
microRNA
- NF-kB (p50/p65)
nuclear factor for kappa B p50/p65 subunit
- sncRNA
small, non-coding RNA
Footnotes
Author Contributions
PNA, JMH and WJL performed all bacterial culture work and neurotoxin, including LPS and other neurotoxic protein extraction from B. fragilis, and antibody-based detection of B. fragilis endotoxins, exotoxins, lipooligosaccahride (LOS), lipopolysaccharide (LPS), bacterial amyloids, and small non-coding RNAs (sncRNA). PNA, JMH, YZ, TB, CT, MEP, WL, WJL collected, analyzed, performed bioinformatics analysis and summarized the data and reviewed the current neurologically-relevant miRNA literature; YZ, WL and WJL performed the experiments and were involved in data extraction and bioinformatics; WJL wrote the article.
Conflict of Interest/disclosure/ethics statement
Declaration of interest for all authors including financial and personal relationships with other people or organizations: none. We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. This work was reviewed and approved by the Institutional Biosafety Committee/Institutional Review Board (IBC/IRB) at the LSU Health Sciences Center, New Orleans LA 70112 USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Barko PC, McMichael MA, Swanson KS, Williams DA, The gastrointestinal microbiome: a review. J Vet Intern Med. (2018) 32: 9–25. doi: 10.1111/jvim.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blum HE, The human microbiome. Adv Med Sci. 62 (2017) 414–420. doi: 10.1016/j.advms.2017.04.005. [DOI] [PubMed] [Google Scholar]
- [3].Alkasir R, Li J, Li X, Jin M, Zhu B, Human gut microbiota: the links with dementia development. Protein Cell. 8 (2017) 90–102. doi: 10.1007/s13238-016-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bhattacharjee S, Lukiw WJ, Alzheimer’s disease and the microbiome. Front. Cell Neurosci. 7 (2013) 153 10.3389/fncel.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao Y, Lukiw WJ, Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer’s disease (AD). J Nat Sci. 1 (2015) 7: pii: e138. [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao Y, Lukiw WJ, Bacteroidetes neurotoxins and inflammatory neurodegeneration. Mol Neurobiol. 55 (2018) 9100–9107. doi: 10.1007/s12035-018-1015-y. [DOI] [PubMed] [Google Scholar]
- [7].Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR, Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87 (2016) 2324–2332. 10.1212/WNL.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhan W, Stamova B, Sharp FR, Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain. Front. Aging Neurosci. 10 (2018) 42 10.3389/fnagi.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Di Lorenzo F, De Castro C, Silipo A, Molinaro A, LPS structures of Gram-negative populations in the gut-microbiota and effects on host interactions. FEMS Microbiol Rev. (2019); doi: 10.1093/femsre/fuz002. [DOI] [PubMed] [Google Scholar]
- [10].Durack J, Lynch SV, The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med. 216 (2019) 20–40. doi: 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11] .Fathi P, Wu S, Isolation, detection, and characterization of enterotoxigenic Bacteroides fragilis in clinical samples. Open Microbiol. J. 10 (2016) 57–63. 10.2174/1874285801610010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Foster JA, Lyte M, Meyer E, Cryan JF, Gut microbiota and brain function: an evolving field in neuroscience. Int. J. Neuropsychopharmacol. (2016) 19 yv114. 10.1093/ijnp/pyv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sarkar RS, Banerjee S, Gut microbiota in neurodegenerative disorders. J Neuroimmunol. 328 (2019) 98–104. doi: 10.1016/j.jneuroim.2019.01.004. [DOI] [PubMed] [Google Scholar]
- [14].Sears CL, Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin. Microbiol. Rev. 22 (2009) 349–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seksik P, Landman C, Understanding microbiome data: a primer for clinicians. Dig. Dis. 33 (2015) 11–16. 10.1159/000437034. [DOI] [PubMed] [Google Scholar]
- [16].Sears CL, Geis AL, Housseau F, Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J Clin Invest. 124 (2014) 4166–4172. doi: 10.1172/JCI72334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ghaisas S, Maher J, Kanthasamy A, Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. 158 (2016) 52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18] .Kowalski K, Mulak A, Brain-gut-microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil. 25 (2019) 48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19] .Franceschi F, Ojetti V, Candelli M, Covino M, Cardone S, Potenza A, Simeoni B, Gabrielli M, Sabia L, Gasbarrini G, Lopetuso L, Scaldaferri F, Rossini PM, Gasbarrini A, Microbes and Alzheimer’ disease: lessons from H. pylori and GUT microbiota. Eur Rev Med Pharmacol Sci. 23 (2019) 426–430. doi: 10.26355/eurrev_201901_16791. [DOI] [PubMed] [Google Scholar]
- [20].Simpson HL, Campbell BJ, Review article: dietary fiber-microbiota interactions. Aliment Pharmacol Ther. 42 (2015) 158–79. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ticinesi A, Tana C, Nouvenne A, The intestinal microbiome and its relevance for functionality in older persons. Curr Opin Clin Nutr Metab Care. 22 (2019) 4–12. doi: 10.1097/MCO.0000000000000521. [DOI] [PubMed] [Google Scholar]
- [22].Iannone LF, Preda A, Blottière HM, Clarke G, Albani D, Belcastro V, et al M., Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev Neurother. (2019) doi: 10.1080/14737175.2019.1638763. [DOI] [PubMed] [Google Scholar]
- [23].Giau VV, Wu SY, Jamerlan A, An SSA, Kim SY, Hulme J, Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients. 10 (2018) pii: E1765. doi: 10.3390/nu10111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sampson TR, Mazmanian SK, Control of brain development, function, and behavior by the microbiome cell host microbe. Cell Host Microbe 17 (2016) 565–576. 10.1016/j.chom.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pogue AI, Percy ME, Cui JG, Li YY, Bhattacharjee S, Hill JM, Kruck TPA, Zhao Y, Lukiw WJ, Up-regulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal sulfate-stressed human astroglial (HAG) primary cell cultures. J Inorg Biochem. 105 (2011) 1434–1437. doi: 10.1016/j.jinorgbio.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lukiw WJ, Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s disease. Front Microbiol. 7 (2016) 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Campbell A, Yang EY, Tsai-Turton M, Bondy SC. Pro-inflammatory effects of aluminum in human glioblastoma cells. Brain Res. 933 (2002) 60–65. [DOI] [PubMed] [Google Scholar]
- [28].Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TPA, Percy ME, Tarr MA, et al. Characterization of an NF-kB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J Inorg Biochem. 103 (2009) 1591–1595. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- [29].Bulgari O, Dong X, Roca AL, Caroli AM, Loor JJ, Innate immune responses induced by lipopolysaccharide and lipoteichoic acid in primary goat mammary epithelial cells. J Anim Sci Biotechnol. 8 (2017) doi: 10.1186/s40104-017-0162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Poxton IR, Edmond DM, Biological activity of Bacteroides lipopolysaccharide — a reappraisal. Clin Infect Dis. 20 (1995) S149–53. [DOI] [PubMed] [Google Scholar]
- [31].Hofstad T, Sveen K, Dahlén G, Chemical composition, serological reactivity and endotoxicity of lipopolysaccharides extracted in different ways from Bacteroides fragilis, Bacteroides melaninogenicus and Bacteroides oralis. Acta Pathol Microbiol Scand B. 85 (1977) 262–270. [DOI] [PubMed] [Google Scholar]
- [32].Hashimoto M, Eguchi H, Tawaratsumida K, Kirikae T, Suda Y, Identification of a TLR2-stimulating lipoprotein in Bacteroides fragilis JCM 11019 Innate Immun. 19 (2013) 132–139. doi: 10.1177/1753425912454179. [DOI] [PubMed] [Google Scholar]
- [33].Holton J, Enterotoxigenic Bacteroides fragilis. Curr Infect Dis Rep. 10 (2008) 99–104. [DOI] [PubMed] [Google Scholar]
- [34].Dalland E, Hofstad T, Growth of Bacteroides fragilis in continuous culture and in batch cultures at controlled pH. Appl Microbiol. 28 (1974) 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bacic MK, Smith CJ, Laboratory maintenance and cultivation of Bacteroides species. Curr Protoc Microbiol. 13 (2008) doi: 10.1002/9780471729259.mc13c01s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rezania S, Amirmozaffari N, Tabarraei B, Jeddi-Tehrani M, Zarei O, Alizadeh R, Masjedian F, Zarnani AH, Extraction, purification and characterization of lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna J Merd Biotechnol. 3 (2011) 3–9. [PMC free article] [PubMed] [Google Scholar]
- [37].Davis MR Jr, Goldberg JB, Purification and visualization of lipopolysaccharide from Gram-negative bacteria by hot aqueous-phenol extraction. J Vis Exp. 63 (2012). pii: 3916. doi: 10.3791/3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].A Shiryaev S, Aleshin AE, Muranaka N, Kukreja M, Routenberg DA, G Remacle A, Liddington RC, Cieplak P, Kozlov IA, Strongin AY, Structural and functional diversity of metalloproteinases encoded by the Bacteroides fragilis pathogenicity island. FEBS J. 29 (2014) 2487–2502. doi: 10.1111/febs.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu S, Lim KC, Huang J, Saidi RF, Sears CL, Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci U S A. 95 (1998) 14979–14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Heinritz SN, Weiss E, Eklund M, Aumiller T, Heyer CM, Messner S, Rings A, Louis S, Bischoff SC, Mosenthin R, Impact of a high-fat or high-fiber diet on intestinal microbiota and metabolic markers in a pig model. Nutrients (2016) 8:E317 10.3390/nu8050317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Holscher HD, Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 8 (2017) 172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pogue AI, Lukiw WJ, The mobilization of aluminum into the biosphere. Front Neurol. 5 (2014) 262. doi: 10.3389/fneur.2014.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Walton JR, Chronic aluminum intake causes Alzheimer’s disease: applying Sir Austin Bradford Hill’s causality criteria. J Alzheimers Dis. 40 (2014) 765–838. doi: 10.3233/JAD-132204 [DOI] [PubMed] [Google Scholar]
- [44].Flaten TP, Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull. 55 (2001) 187–96. [DOI] [PubMed] [Google Scholar]
- [45].Russ TC, Killin LOJ, Hannah J, Batty GD, Deary IJ, Starr JM. Aluminium and fluoride in drinking water in relation to later dementia risk. Br J Psychiatry. 1–6 (2019) doi: 10.1192/bjp.2018.287. [DOI] [PubMed] [Google Scholar]
- [46].Walker KA, Ficek BN, Westbrook R, Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem Neurosci. (2019) doi: 10.1021/acschemneuro.9b00333. [DOI] [PubMed] [Google Scholar]
- [47].Giridharan VV, Masud F, Petronilho F, Dal-Pizzol F, Barichello T, Infection-induced systemic inflammation is a potential driver of Alzheimer’s disease progression. Front Aging Neurosci. 11 (2019) 122. doi: 10.3389/fnagi.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sheppard O, Coleman MP, Durrant CS, Lipopolysaccharide-induced neuroinflammation induces presynaptic disruption through a direct action on brain tissue involving microglia-derived interleukin 1 beta. J Neuroinflammation. 16 (2019) 106. doi: 10.1186/s12974-019-1490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhao Y, Cong L, Lukiw WJ, Lipopolysaccharide (LPS) accumulates in neocortical neurons of Alzheimer’s disease (AD) brain and impairs transcription in human neuronal-glial primary co-cultures. Front Aging Neurosci. 9 (2017) 407. doi: 10.3389/fnagi.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhao Y, Jaber VR, Lukiw WJ, Secretory products of the human GI-tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol. 7 (2017) v318. doi: 10.3389/fcimb.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rakic S, Hung YMA, Smith M, So D, Tayler HM, Varney W, Wild J, Harris S, Holmes C, Love S, Stewart W, Nicoll JAR, Boche D, Systemic infection modifies the neuro-inflammatory response in late stage Alzheimer’s disease. Acta Neuropathol Commun. 6 (2018) 88. doi: 10.1186/s40478-018-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hill JM, Bhattacharjee S, Pogue AI, Lukiw WJ, The gastrointestinal tract microbiome and potential link to Alzheimer’s disease. Front Neurol. 5 (2014) doi: 10.3389/fneur.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lukiw WJ, Cong L, Jaber VR, Zhao Y, Microbiome-derived lipopolysaccharide (LPS) selectively inhibits neurofilament light chain (NF-L) gene expression in human neuronal-glial (HNG) cells in primary culture. Front Neurosci. 12 (2018) 896. doi: 10.3389/fnins.2018.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Maloney B, Lahiri DK. Epigenetics of dementia: understanding the disease as a transformation rather than a state. Lancet Neurol. 15 (2016) 760–774. doi: 10.1016/S1474-4422(16)00065-X. [DOI] [PubMed] [Google Scholar]
- [55].Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H. Alzheimer’s disease and environmental exposure to lead: the epidemiologic evidence and potential role of epigenetics. Curr Alzheimer Res. 9 (2012) 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang Q, Li N, Jiao X, Qin X, Kaur R, Lu X, Song J, Wang L, Wang J, Niu Q. Caspase-3 short hairpin RNAs: a potential therapeutic agent in neurodegeneration of aluminum-exposed animal model. Curr Alzheimer Res. 11 (2014) 961–970. [DOI] [PubMed] [Google Scholar]
- [57].Oliveira J, Reygaert WC. Gram negative bacteria StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; (2019); http://www.ncbi.nlm.nih.gov/books/NBK538213/; last accessed 7 October 2019. [Google Scholar]
- [58].Erickson MA, Banks WA. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev. 70 (2018) 278–314. doi: 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Klein G, Raina S. Regulated assembly of LPS, its structural alterations and cellular response to LPS defects. Int J Mol Sci. 20 (2019) pii: E356. doi: 10.3390/ijms20020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Logsdon AF, Erickson MA, Chen X, Qiu J, Lim YP, Stonestreet BS, Banks WA. Inter-alpha inhibitor proteins attenuate lipopolysaccharide-induced blood-brain barrier disruption and downregulate circulating interleukin 6 in mice. J Cereb Blood Flow Metab. (2019) 271678X19859465. doi: 10.1177/0271678X19859465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Barton SM, Janve VA, McClure R, Anderson A, Matsubara JA, Gore JC, et al. Lipopolysaccharide induced opening of the blood brain barrier on aging 5XFAD mouse model. J. Alzheimers Dis. 67 (2019) 503–513. doi: 10.3233/JAD-180755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vargas-Caraveo A, Sayd A, Maus SR, Caso JR, Madrigal JLM, García-Bueno B, Leza JC. Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci Rep. 7 (2017) 13113. doi: 10.1038/s41598-017-13302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]