Using a combination of neuroimaging and behavioural studies, Abdelgabar et al. show that the cerebellum helps us perceive the actions of others. Disorders such as spinocerebellar ataxia type 6, which disrupt cerebellar functioning, impair our ability to perceive the kinematics of other people’s actions, with potential implications for social cognition.
Keywords: spincocerebellar ataxia, cerebellar function, social cognition, imaging methodology, movement disorders
Abstract
Our cerebellum has been proposed to generate prediction signals that may help us plan and execute our motor programmes. However, to what extent our cerebellum is also actively involved in perceiving the action of others remains to be elucidated. Using functional MRI, we show here that observing goal-directed hand actions of others bilaterally recruits lobules VI, VIIb and VIIIa in the cerebellar hemispheres. Moreover, whereas healthy subjects (n = 31) were found to be able to discriminate subtle differences in the kinematics of observed limb movements of others, patients suffering from spinocerebellar ataxia type 6 (SCA6; n = 21) were severely impaired in performing such tasks. Our data suggest that the human cerebellum is actively involved in perceiving the kinematics of the hand actions of others and that SCA6 patients’ deficits include a difficulty in perceiving the actions of other individuals. This finding alerts us to the fact that cerebellar disorders can alter social cognition.
Introduction
The ability to perceive hand actions of others plays a key role in our ability to learn fine motor skills from conspecifics and interact successfully with them in cooperative and competitive settings. Cerebral cortical regions involved in motor control, including the premotor cortex and inferior parietal cortex, where mirror neurons were found in the monkey (di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti et al., 1996; Kohler et al., 2002; Keysers et al., 2003; Fogassi et al., 2005; Rozzi et al., 2008), as well as the primary somatosensory cortex (SI) (Gazzola and Keysers, 2009; Caspers et al., 2010; Keysers et al., 2010), have all been shown to be necessary for extracting subtle information from the observed kinematics of hand actions (Urgesi et al., 2014; Keysers et al., 2018). A powerful task to reveal the impact of disturbing these cortical regions requires participants to judge the weight of an object lifted by another individual (Pobric and Hamilton, 2006; Hamilton et al., 2007; Valchev et al., 2017). This task depends on the ability to transform subtle kinematic cues into a weight estimate in that participants rely on the velocity of movement when the object is lifted from the table to determine the weight of the object (Hamilton et al., 2007). Perturbing activity in the premotor cortex and SI disrupts the ability to perceive the weight (Pobric and Hamilton, 2006; Valchev et al., 2017), suggesting a causal role of premotor and somatosensory region in action perception.
The cerebellum is a key partner of these neocortical brain regions during motor control, where its role is well established (Kelly and Strick, 2003; Gao et al., 2018). It is perhaps not surprising that some have speculated that the cerebellum may also play a role in the perception and prediction of the kinematics of observed hand actions. Specifically, it has been proposed that the cerebellum could leverage its forward models (i.e. neural computations that transform motor signals into expected sensory consequences) to predict the actions of others (Miall, 2003; Wolpert et al., 2003; Fuentes and Bastian, 2007; Gazzola and Keysers, 2009; Rizzolatti and Sinigaglia, 2010). Although this proposal is intuitively appealing, we still have little evidence for the cerebellum being a reliable and even necessary node of the action observation network (Sokolov et al., 2017). This is because functional MRI evidence for its recruitment during action observation is mixed, and very few neuromodulation or lesion studies have explored the impact of cerebellar disruptions on hand action observation.
With a few exceptions, imaging studies on action perception have typically focused on the involvement of the neocortex, leaving the information about cerebellar activity limited to what the field of view of functional MRI of these studies usually included, i.e. the dorsal cerebellum (Aziz-Zadeh, 2006; Gazzola et al., 2007a, b; Catmur et al., 2008; Gazzola and Keysers, 2009; Agnew et al., 2012; Brunner et al., 2014; Plata Bello et al., 2014; Di Cesare et al., 2015; Jelsone-Swain et al., 2015; Thomas et al., 2018). Several other experimental studies fail to observe cerebellar activation to hand action observation (Iacoboni et al., 1999, 2001; Buccino et al., 2004; Orr et al., 2008; Rocca and Filippi, 2010; Jastorff et al., 2012; Sasaki et al., 2012; Horan et al., 2014). This inconsistency is also reflected in meta-analyses of action observation studies, with some finding no (Caspers et al., 2010) or very limited cerebellar activations (Molenberghs et al., 2012), and others finding several clusters (Van Overwalle et al., 2014). In their extensive meta-analysis, Van Overwalle et al. found that only 28% of the reviewed studies investigating action observation report cerebellar activity. The degree to which these inconsistencies depend on data acquisition and data analysis pipelines not optimized for the cerebellum is difficult to estimate post hoc, and experiments that optimize methods for the cerebellum, assess the reliability of activations in individual participants, and assess replicability across studies are required. The first part of this manuscript will therefore present four functional MRI experiments that map and replicate the recruitment of cerebellar voxels during hand action observation using MRI acquisition and analysis methods optimized for the cerebellum. These studies highlight that lobules VI and VIII of the cerebellar hemispheres are consistently recruited by action observation.
However, to establish whether the cerebellum causally contributes to hand action observation, its activity must be perturbed and the impact on action perception measured. Unfortunately, only two studies have taken that route so far. First, Sokolov et al. (2010) showed that four patients with tumours in the left lateral cerebellum (but not those with lesions in the vermis) were impaired in their ability to detect whether a point-light walking motion was embedded in random dot motion of that locomotor activity. However, the motor control of routine walking and of skilled hand actions is fundamentally different, as demonstrated by the fact that lesioning the pyramidal tract that transmits the cortical output to the spinal cord leaves routine treadmill walking unaltered (Eidelberg and Yu, 1981), but severely impairs skilled hand actions (Forssberg et al., 1999; Duque et al., 2003; Hermsdörfer et al., 2003). Second, Cattaneo et al. (2012) tested the involvement of the cerebellum in the perception of action sequences. They showed eight participants affected by cerebellar ischaemia sets of four still photographs taken during an action (e.g. opening a bottle and pouring a glass of water). One of the four pictures did not fit the temporal sequence of the action, and the task was to identify which one was the intruder. They found the performance of five of the cerebellar patients to be below the range of the 16 healthy control subjects. While this study does not explore the processing of the subtle kinematic cues, it provides the first evidence that cerebellar impairments can affect the ability of participants to identify acts not belonging to a particular action sequence. However, while dozens of studies in hundreds of participants establish that premotor and parietal regions of the neocortex are necessary for the optimal perception of observed actions (Urgesi et al., 2014; Keysers et al., 2018), the necessary role of the cerebellum in hand action observation hinges on a single study with eight patients that does not directly test kinematics. In the second part of the study we therefore aimed to provide new evidence for a contribution of the cerebellum to action perception, and the first evidence for its role in processing subtle kinematic cues during hand action perception. To this aim, we tested the ability of 21 patients with spinocerebellar ataxia of subtype 6 (SCA6) to detect the weight of a box by observing the kinematics of a hand lifting the box in a video setting. SCA6 is a rare late-onset neurodegenerative disorder characterized by ataxia and associated with a loss of Purkinje cells in the cerebellum (Du et al., 2013). A voxel-based morphology study points to loss of grey matter in the hemispheres of lobule VI (Rentiya et al., 2017) as being the primary cause of the upper limb ataxia—adjacent to regions in which we found cerebellar activations to action observation in part one of our study. Task performance was compared with that of 31 age-matched control subjects. Participants were tested in (i) a condition in which a sleeve on the actor’s arm occluded muscle shape information, forcing participants to focus on the arm’s kinematics to judge the weight of the box (Sleeve); and in (ii) a condition in which the sleeve was removed to reveal information on the appearance of muscle contractions, which complements the arm’s kinematic information (NoSleeve). Comparing the two groups in the Sleeve condition will reveal whether the cerebellum is necessary for kinematic processing. Comparing the gain in performance across the two conditions (i.e. the NoSleeve − Sleeve performance difference) across groups will reveal whether the cerebellum is necessary to extract additional information from biological shape.
The two main aims of our work are therefore to establish: (i) whether and where hand action observation reliably activates the cerebellum; and (ii) whether perturbations of cerebellar functioning impair the ability to process the kinematic and/or shape of observed actions.
Materials and methods
Experiments and participants
See Table 1 for an overview. Experiment 1 was aimed at localizing cerebellar activity to action observation using different analysis pipelines, and at comparing the results between pipelines and those found in the literature. Experiments 2 and 3 tested the replicability of the results of Experiment 1 on two independent samples of participants, and on a different MRI scanner. Experiment 4 tested the impact of the weight discrimination task on the previously identified action observation network, and Experiment 5 was aimed at directly testing the involvement of the cerebellum in action perception by comparing the accuracy in weight estimation between SCA6 patients and matched controls.
Table 1.
Experiment overview
| Experiment | Number of subjects incl. (recruited), subject type | Mean age ± SD [range] | Gender F, M | Technique | Task | Experimental aim | Relevant figures and tables |
|---|---|---|---|---|---|---|---|
| Experiment 1, AO | 31 (35) healthy | 23 ± 4 [19–40] | 21, 10 | fMRI | Action observation | Localize cerebellar voxels responding to action observation | Fig. 2, Tables 2 and 3, Supplementary Figs 1, 2, 6 and 7, Supplementary Tables 1–4 |
| Experiment 2, AOrep1 | 25a (25) healthy | 25.2 ± 4 [19–32] | 13, 12 | fMRI | Action observation | Replicability of cerebellar activations to action observation | Fig. 2, Tables 2 and 3, Supplementary Figs 1, 2, 6 and Supplementary Tables 1–4 |
| Experiment 3, AOrep2 | 23 (23) healthy | 25.5 ± 3.6 [21–33] | 11, 12 | fMRI | Action observation | Replicability of cerebellar activations to action observation | Fig. 2, Tables 2 and 3, Supplementary Figs 1, 2, 6 and 7, Supplementary Tables 1–4 |
| Experiment 4, WD | 25a (25) healthy | 25.2 ± 4 [19–32] | 13, 12 | fMRI | Weight estimation | Localize cerebellar activations to the weight discrimination task and compare them with Experiments 1–3 | Fig. 3, Supplementary Table 4 and Supplementary Fig. 5 |
| Experiment 5, SCA6 | 19WD, and 17SARA (21) SCA6 | 62 ± 7 [49–80] 60.8 ± 7 [49–68] | WD 14, 5 SARA 12, 5 Total 15, 6 | Behav + eye tracking (n = 4) | Weight estimation | Investigate whether cerebellar deficits are reflected in decreased accuracy in perception | Fig. 4 and Supplementary Fig. 3 |
| 31 healthy | 61 ± 7 [43–74] | 15, 16 | Behav + eye tracking (n = 7) | Weight estimation | |||
| Experiment 6, EMG | 10 healthy | 32.5 ± 4.3 [29, 42] | 4, 6 | FDI EMG | Action observation | Identify whether stronger EMG activity during ActionOBS could explain brain activity in motor regions | Supplementary Fig. 4 |
| Experiment 7, fMRI | 7 (7) healthy | 26 ± 4.5 [21–33] | 5, 2 | fMRI | Action observation and eye movements | Test whether differences in eye movements between ActionOBS and CtrlOBS alone could explain the stronger activity for ActionOBS | Supplementary Fig. 6 |
All groups of participants are independent except athose in which the same 25 participants underwent both the passive observation and the weight estimation task in separate sessions. In Experiment 1, four participants were excluded from the statistical analysis: two due to excessive head motion (displacement of >3.5 mm voxel dimension), one reported sleepiness, and one because of image distortion. In Experiment 5, two participants were excluded from the weight lifting task because pre-symptomatic, and two more were excluded from the correlation with SARA because they did not have SARA scores. Experiments 6 and 7 are control experiments aimed at addressing some possible confounds. AO = action observation; AOrep = action observation replication; FDI = first dorsal interosseous; fMRI = functional MRI; WD = weight discrimination.
All tested healthy participants had a normal or corrected to normal vision, and none had a history of neurological conditions or treatments. The participants tested in the MRI also met MRI safety requirements.
The SCA6 patient group was recruited in collaboration with the Department of Neurology at the Erasmus MC Rotterdam (Supplementary material). The severity of disease progression was clinically assessed by a licensed neurologist using the Scale of the Assessment and Rating of Ataxia (SARA) (Schmitz-Hubsch et al., 2006; Saute et al., 2012). SARA includes eight items (gait, stance, sitting, speech disturbance, finger chase, nose-finger test, fast alternating hand movements and heel-shin slide) reflecting neurological manifestations of cerebellar ataxia (Weyer et al., 2007). SARA scores range from 0 to 40, with higher scores corresponding to higher progression. The average SARA score for our patients’ group (nSARA = 17) was 11.38 ± 5.75 [standard deviation (SD); range: 2 to 21.5]. The 31 healthy participants that were recruited as the control group, matched the SCA6 group for age [t(50) = 0.96, P = 0.34], handedness (SCA6: 19 right-handed and two left-handed, Controls: 27 right and four left-handed, Yates corrected χ2 = 0, P = 0.94) and gender (SCA6 15 female: 6 male, Controls 15 female: 16 male, Yates corrected χ2 = 1.86, P = 0.17). However, our patient group contained fewer males numerically, an issue that is addressed in the control analyses. Control subjects did not receive a clinical assessment.
All participants signed an informed consent in accordance with the Declaration of Helsinki. The functional MRI study protocols were approved by the medical ethical committee of the University of Groningen (METc2012/380), the ethics review board of the University of Amsterdam (2015-BC-4697), the Academic Medical Center of Amsterdam (W15_243#15.0288), and the clinical study protocol was approved by the Medical Ethical Committee of the Erasmus MC Rotterdam (MEC-2013-095).
Stimuli, tasks and paradigms
Action observation task
During the observation task participants watched 39 unique videos of a human right hand interacting with objects displayed on a table (ActionOBS) (Fig. 1A). The 39 control videos displayed a hand movement without a meaningful object interaction (CtrlOBS). Experiments 1 and 2 also contained a third static condition, in which the hand rested close to the object (Arnstein et al., 2011; Valchev et al., 2016). This static condition was not included in Experiment 3, and therefore not included in the group analyses. Conditions were randomized across participants and presented using the Presentation® software (Version 18.0, Neurobehavioral Systems, Inc., Berkeley, CA, www.neurobs.com) in a single functional MRI run. Participants were instructed to pay close attention to the videos shown.
Figure 1.
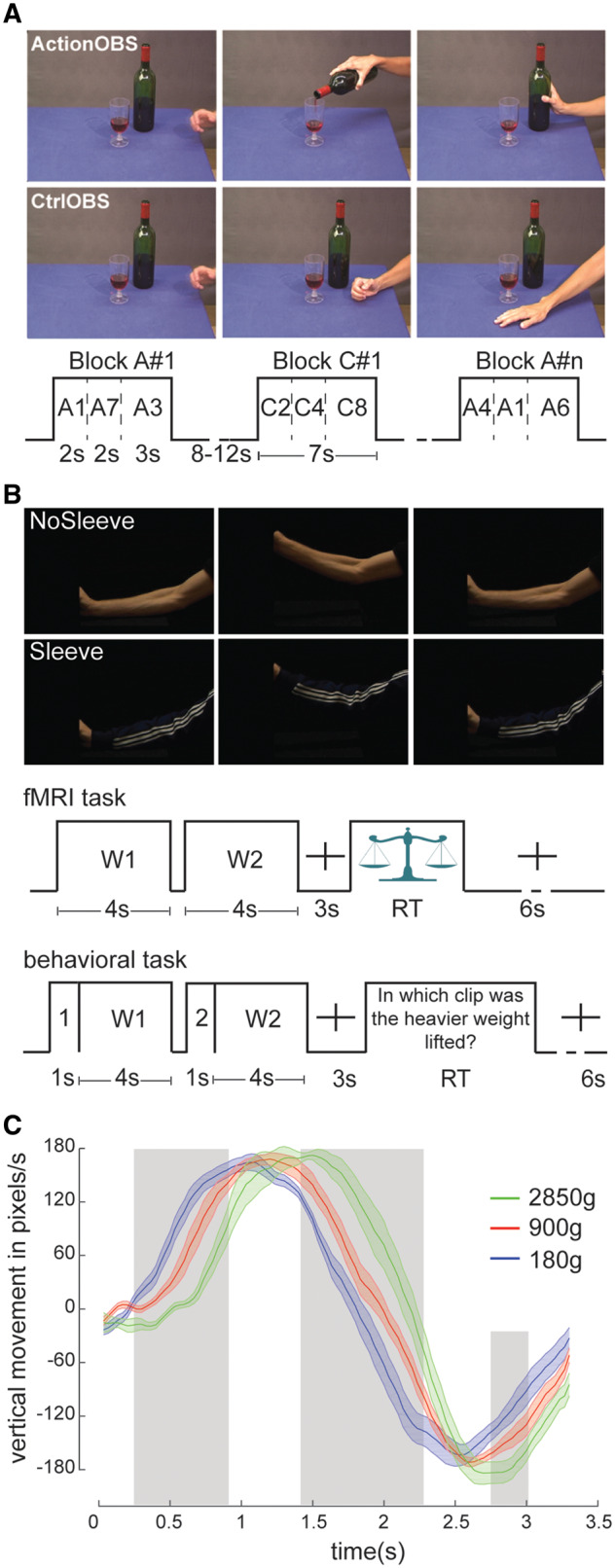
Experimental tasks. (A) Action observation task. Example of 1 of 39 possible actions and its control, followed by the task structure. A = action; C = control; Ctrl = control; OBS = observation. The ActionOBS and CtrlOBS videos were grouped in blocks of 7 s. Each block contained three actions from the same condition, with a total of 13 blocks for each condition. Blocks were separated by a fixation cross for a random period of 8–12 s, displayed on a background that was visually similar to the table. (B) Weight discrimination task. Frame extracted from the NoSleeve (top) and Sleeve (bottom) weight lifting condition, followed by the trial structure for the functional MRI (top) and behavioural experiments (bottom). In the functional MRI (fMRI) task the window of time participants were requested to answer was indicated by a weighing scale. In the behavioural task, clips were preceded by the number 1 or 2 denoting whether it was the first or second clip of the pair. The sentence following the video was translated from Dutch for illustration purposes. RT = participant’s reaction time. (C) Kinematic analysis of the weight-lifting videos. Mean ± standard error of the mean (SEM) of the vertical velocity of the forearm as a function of weight relative to the onset of the videos, averaged over the Sleeve and NoSleeve conditions. Moments in which velocity carries significant information about the weight are marked in grey, as revealed by a one-way ANOVA comparing velocity across the three weights at P < 0.01.
Weight discrimination task
Participants performed a two-alternative forced-choice task, in which at every trial, participants had to choose in which of the two presented videos the heavier object was lifted. The 4-s video clips showed a human arm lifting an object. To avoid participants deducing the weight from object movement only (e.g. differences in object shaking during the lifting phase), a black panel occluded both the object and the hand from vision. To disentangle whether the contribution of cerebellum mainly comes from computation of action kinematics or from arm shape information, two versions of the task were created: (i) in half of the trials, the arm lifting the object was sleeved thus making the kinematic of the arm the only information available to perform the task (Sleeve); and (ii) in the other half, the arm was uncovered thus allowing both kinematic and shape information to be used (NoSleeve) (Fig. 1B). During the video recording, the actor was instructed to lift one of three weights (2850 g, 900 g and 180 g) within 4 s. A metronome was used to time the lift, and a reference line was marked on the wall in front of the actor to help maintain the same lifting height throughout all videos. The actor was aware of the object weight to avoid hesitation in the lifting. Videos were recorded using a digital video camera (Sony DSRPDX10P) and edited using Adobe Premiere Pro (Version CS5, Adobe System Incorporated, San Jose, USA). As expected, the differences in weight lead to differences in the kinematic of the video-recorded actions that allow viewers to deduce the weight (Fig. 1C) (Hamilton et al., 2007). Clips showing the same lifted weight were never paired. In half of the trials the heaviest object was lifted first, in the other half as second. The order was randomized in Psychopy2 (Peirce, 2009). After the second clip, the task instruction was presented until the subject indicated his/her response. Before the beginning of the task participants performed four training trials.
Some minor task differences were present between Experiments 4 and 5 (Fig. 1B).
Experiment 5: Behaviour
Participants gave the response by pressing the arrow keys on a standard QWERTY keyboard using their right hand. Ninety-six trials were presented in total, and participants had the option to take a short voluntary break after the first half of the trials.
Experiment 4: Functional MRI
Participants indicated their responses by means of an MRI compatible button box. Participants used their left hand to select the first clip and their right hand to select the second. Stimuli were presented using Presentation® software. For the functional MRI experiment, a numerosity task was additionally introduced and intermixed with the weight discrimination task. Participants had to estimate and compare the number of moving dots shown in Videos 1 and 2 instead of weight. The movement of the dots followed the kinematic of the arm presented in the Sleeve and NoSleeve conditions, but the arm was not visible. As an error occurred in the randomization of this condition, and this task was not performed by the SCA6 group, the numerosity condition was not included in the group analyses. Seventy-two trials were presented in total (24 for each of the three conditions).
Functional MRI data acquisition
All MRI datasets included an anatomical scan. Experiment 1 included one functional scan of the action observation task. Experiments 2 and 3 aimed at comparing the effect of different numbers of simultaneous slice acquisition on task-based functional MRI, and included four and five functional scans of action observation, respectively. The results of this comparison are the subject of a separate manuscript (Bhandari et al., 2019). As participants of Experiment 1 only saw the videos once, we only included the first view of the action observation task, independently of the number of simultaneously acquired slices. Experiment 4 included two functional runs of the weight lifting task. These two runs were randomly presented between the four observation runs of Experiment 2. The scanning parameters were chosen to achieve a coverage of the entire cerebrum and cerebellum (Supplementary Table 1).
Localization of cerebellar activations, impact of different analysis pipelines and replicability
The impact of different pipelines on cerebellar task-based responses was analysed on data from Experiment 1. The four considered pipelines mainly differed in the order in which the preprocessing and first level subject statistics were computed, and in the normalization template. Because the comparison revealed a no clear advantage of using pipelines optimized for the cerebellum compared to the traditional one, the method and results of this comparison are presented in the Supplementary material, Supplementary Tables 2 and 3 and Supplementary Fig. 1.
All of the analyses included in the main text therefore follow the traditional approach that includes: slice-time correction, realignment of functional images to the computed mean, co-registration of the anatomical image to the mean, whole brain normalization to the MNI template (final voxel size: 2 × 2 × 2 mm) based on the parameter generated during the segmentation of the co-register anatomy, a smoothing with a 6 mm full-width at half-maximum Gaussian kernel followed by a general linear model (GLM). Analyses testing the possibility of activation leakage between the anterior cerebellum and the temporal cortex due to smoothing are reported in the Supplementary material.
For Experiments 1–3, the GLM included two standard box car predictors that modelled the ActionOBS and CtrlOBS video presentation. Experiments 1 and 2 also included a predictor modelling the static conditions. All predictors were convolved with the canonical haemodynamic response function (HRF). The last six regressors of no interest included the displacements and rotations along the three axes, determined during image realignment. The ActionOBS−CtrlOBS contrast was computed at the subject-level to generate action-specific activations for observation. Analyses of variance on the ActionOBS−CtrlOBS contrast values from Experiments 1–3 were also implemented to directly compare the results of the three experiments to each other (within-subjects ANOVA) as well as to baseline (one-way ANOVA).
All analyses were run in SPM8 and 12 (Wellcome Trust Centre for Neuroimaging, UCL, UK) using MATLAB 7.14 (The MathWorks Inc., Natick, USA) with a bounding box size-adjusted to include the entire cerebellum [−90 −126 −72; 91 91 109], complemented by custom MATLAB scripts. Unless specified otherwise, all analyses were estimated within the cerebellar mask using the cerebellar anatomical map from the Anatomy toolbox (http://www.fz-juelich.de/ime/spm_anatomy_toolbox) (Geyer et al., 1996, 1999, 2000; Amunts et al., 1999; Grefkes et al., 2001; Geyer, 2004; Eickhoff et al., 2005, 2006, 2007; Caspers et al., 2006; Choi et al., 2006). The Anatomy toolbox was also used to define regions of interest, and guide anatomical descriptions of clusters of activity.
Unless otherwise specified, all statistical maps were thresholded at PFWE < 0.05 with a minimal cluster size of 10 voxels. We chose peak-level familywise error (FWE) correction as we wished to (i) interpret activation of individual voxels, and, motivated by the inconsistencies of cerebellar activations in the literature; (ii) to limit the risks of type I errors.
To investigate the consistency in location of voxels responding to action observation between participants and studies, we computed consistency maps (Gazzola and Keysers, 2009) (Supplementary material). However, as the consistency maps cannot confirm that voxels responding to action observations are present in all participants, we counted the number of activated voxels within each participant. This counting was done separately for the four cerebellar anatomical regions of interest (left and right lobule VI, and VIIb/VIIIa) shown to be involved in the execution of complex actions (Schlerf et al., 2010), and for the cerebellum as a whole. Additionally, lobule V was used as a control region as it has been shown not to differentiate simple from complex actions. To compare the reliability of cerebellar activations with that of the cortex, the counting was done for three additional cortical regions, typically associated with the action observation network (Gazzola and Keysers, 2009; Caspers et al., 2010; Molenberghs et al., 2012): the premotor area [Brodmann area (BA) 44], the inferior parietal complex (PF) and the SI.
Localization of the weight discrimination task
The GLM of Experiment 4 included eight boxcar predictors: three modelled the video presentation (i.e. from the beginning of Video 1 to the end of Video 2) associated with the Sleeve, NoSleeve and numerosity conditions; two captured the participant’s responses at the time the weighting scale was presented separately for the left and right hand; one captured text information given to our participants at the beginning and the end of the each session; one included button presses that happened outside the response window; and one included the four videos used for training (only for the first session). The six head motion parameters were again added as co-variates of no interest. Analyses of variance were used to compare the Sleeve and NoSleeve conditions to each other (within-subjects ANOVA), and to baseline (one-way ANOVA). As for Experiments 1–3, unless otherwise specified, the ANOVAs were computed within the cerebellar mask, at PFWE < 0.05.
To test whether the videos used for the weight estimation task elicited activity in the areas to be found active for general action observation, an additional GLM was computed within a binary mask obtained by the global null conjunction of Experiments 1, 2 and 3 [Exp1ActionOBS-CtrlOBS OR Exp2ActionOBS-CtrlOBS OR Exp3ActionOBS-CtrlOBS] (tFWE = 2.06) from the one-way ANOVAs that included the ActionOBS–CtrlOBS from all three experiments. Results are shown at PFWE < 0.05.
Analyses of behavioural data
Task performance scores were calculated as proportion of correct responses. We checked their normality using the Lilliefors test. Performance for the Sleeve and the NoSleeve–Sleeve difference were normally distributed (both P > 0.12). The performance in the NoSleeve condition and the average score of Sleeve and NoSleeve violated normality (both P < 0.002). Accordingly, we used non-parametric tests as our main approach, and parametric analyses (ANOVAs and Bayesian analyses) were only used to supplement analyses for the Sleeve and NoSleeve–Sleeve difference.
Control experiments
To explore whether visual cerebellar activity reflects differential motor activity, we recorded EMG activity from the right hand while participants viewed ActionObs and ActionCtrl vidoes (Supplementary Fig. 4). To explore the effect of eye movements, we measured eye tracking data from four patients and seven healthy subjects for the weight discrimination task (Supplementary material) and also functional MRI activity while participants viewed the ActionObs and ActionCtrl videos while fixating a cross, and while performing eye movements without the action videos (Supplementary material and Supplementary Fig. 6).
Data availability
Data are available online at https://openeuro.org.
Results
Localization of action observation activations in the cerebellum and their reliability
Viewing goal-directed hand actions compared to control stimuli (ActionOBS–CtrlOBS) in Experiment 1 bilaterally recruits lobules VI, VIIb and VIIIa of the cerebellar hemispheres (Table 2, Fig. 2A, Supplementary Fig. 1 and Supplementary material).
Table 2.
Cerebellar activations to ActionOBS–CtrlOBS for Experiments 1–3
| Cluster size | Voxels in cyto | % Cluster | Hem | Cyto or anatomical description | % Area | Peak information | |||
|---|---|---|---|---|---|---|---|---|---|
| T | x | y | z | ||||||
| Experiment 1 ActionOBS–CtrlOBS PFWE < 0.05, t = 4.31 | |||||||||
| 655 | 523 | 79.8 | R | Lobule VI (Hem) | 29 | 9.06 | 28 | −54 | −26 |
| 7.72 | 20 | −70 | −22 | ||||||
| 61.8 | 9.4 | R | Area FG4 | 12.6 | 5.60 | 24 | −44 | −18 | |
| 14.4 | 2.2 | R | Lobule VIIa crusI (Hem) | 0.4 | |||||
| 11.9 | 1.8 | R | Area hOc3v [V3v] | 1.4 | |||||
| 11.9 | 1.8 | R | Area FG1 | 4.8 | |||||
| 340 | 328.6 | 96.7 | L | Lobule VI (Hem) | 17.5 | 10.84 | −28 | −54 | −26 |
| 6.20 | −20 | −68 | −24 | ||||||
| 249 | 103.4 | 41.5 | R | Lobule VIIIa (Hem) | 14.2 | 6.38 | 28 | −60 | −54 |
| 6.08 | 20 | −66 | −54 | ||||||
| 5.51 | 30 | −54 | −52 | ||||||
| 97.1 | 39 | R | Lobule VIIb (Hem) | 14.8 | 8.23 | 16 | −76 | −50 | |
| 24.1 | 9.7 | R | Lobule VIIIb (Hem) | 3.4 | |||||
| 12.9 | 5.2 | R | Lobule VIIIa (Verm) | 6.1 | |||||
| 162 | 85.9 | 53 | L | Lobule VIIIa (Hem) | 11.3 | 7.18 | −22 | −62 | −52 |
| 51.9 | 32 | L | Lobule VIIb (Hem) | 7.6 | 6.68 | −18 | −70 | −50 | |
| 6.59 | −16 | −74 | −48 | ||||||
| 22 | 13.6 | L | Lobule VIIIb (Hem) | 3.6 | |||||
| Experiment 2 ActionOBS–CtrlOBS PFWE < 0.05, t = 4.31 | |||||||||
| 398 | 131.3 | 33 | R | Lobule VIIIb (Hem) | 18.3 | 8.66 | 20 | −58 | −52 |
| 105.1 | 26.4 | R | Lobule VIIIa (Hem) | 14.5 | 8.10 | 12 | −70 | −48 | |
| 50.4 | 12.7 | R | Lobule IX (Hem) | 7.2 | |||||
| 38.4 | 9.6 | R | Lobule VIIb (Hem) | 5.9 | 8.28 | 16 | −72 | −52 | |
| 20.5 | 5.2 | R | Lobule VIIIa (Verm) | 9.8 | |||||
| 262 | 205.9 | 78.6 | R | Lobule VI (Hem) | 11.4 | 7.69 | 30 | −50 | −24 |
| 5.03 | 20 | −68 | −22 | ||||||
| 44.9 | 17.1 | R | Area FG4 | 9.2 | |||||
| 10.4 | 4 | R | Area FG3 | 1.6 | |||||
| 153 | 67.1 | 43.9 | L | Lobule VIIIa (Hem) | 8.8 | 7.53 | −16 | −66 | −48 |
| 4.78 | −24 | −52 | −50 | ||||||
| 53.4 | 34.9 | L | Lobule VIIIb (Hem) | 8.8 | 5.31 | −18 | −58 | −52 | |
| 126 | 94.8 | 75.2 | L | Lobule VI (Hem) | 5.1 | 7.01 | −30 | −48 | −22 |
| 5.40 | −26 | −56 | −18 | ||||||
| 23.4 | 18.6 | L | Area FG4 | 4 | |||||
| Experiment 3 ActionOBS–CtrlOBS PFWE < 0.05, t = 4.31 | |||||||||
| 514 | 433.8 | 84.4 | L | Lobule VI (Hem) | 23.2 | 8.45 | −26 | −52 | −18 |
| 6.72 | −18 | −68 | −22 | ||||||
| 43.3 | 8.4 | L | Area FG4 | 7.3 | |||||
| 15.8 | 3.1 | L | Lobule V (Hem) | 2.2 | |||||
| 12.9 | 2.5 | L | Area FG3 | 1.6 | |||||
| 452 | 372.8 | 82.5 | R | Lobule VI (Hem) | 20.7 | 6.89 | 28 | −52 | −22 |
| 5.96 | 18 | −70 | −22 | ||||||
| 5.88 | 20 | −68 | −24 | ||||||
| 61.3 | 13.6 | R | Area FG4 | 12.5 | |||||
| 402 | 139.9 | 34.8 | R | Lobule VIIIa (Hem) | 19.3 | 8.34 | 26 | −58 | −54 |
| 99.8 | 24.8 | R | Lobule VIIIb (Hem) | 13.9 | 5.32 | 18 | −52 | −50 | |
| 76.5 | 19 | R | Lobule VIIb (Hem) | 11.7 | 8.88 | 14 | −74 | −50 | |
| 22.6 | 5.6 | R | Lobule VIIIa (Verm) | 10.8 | |||||
| 85 | 47.8 | 56.2 | L | Lobule VIIIa (Hem) | 6.3 | 6.19 | −10 | −74 | −50 |
| 32.5 | 38.2 | L | Lobule VIIb (Hem) | 4.8 | |||||
| 75 | 35.1 | 44.5 | L | Lobule VIIIa (Hem) | 4.6 | 6.11 | −22 | −58 | −46 |
| 6.10 | −32 | −52 | −50 | ||||||
| 24.8 | 31.3 | L | Lobule VIIIb (Hem) | 4.1 | |||||
Regions with ActionOBS–CtrlOBS ≥ 4.31 labelled using SPM Anatomy Toolbox. Results are shown at PFWE < 0.05 with cluster size >10 voxels.
Cyto = cyto-architectonic area; Hem = hemisphere.
Figure 2.
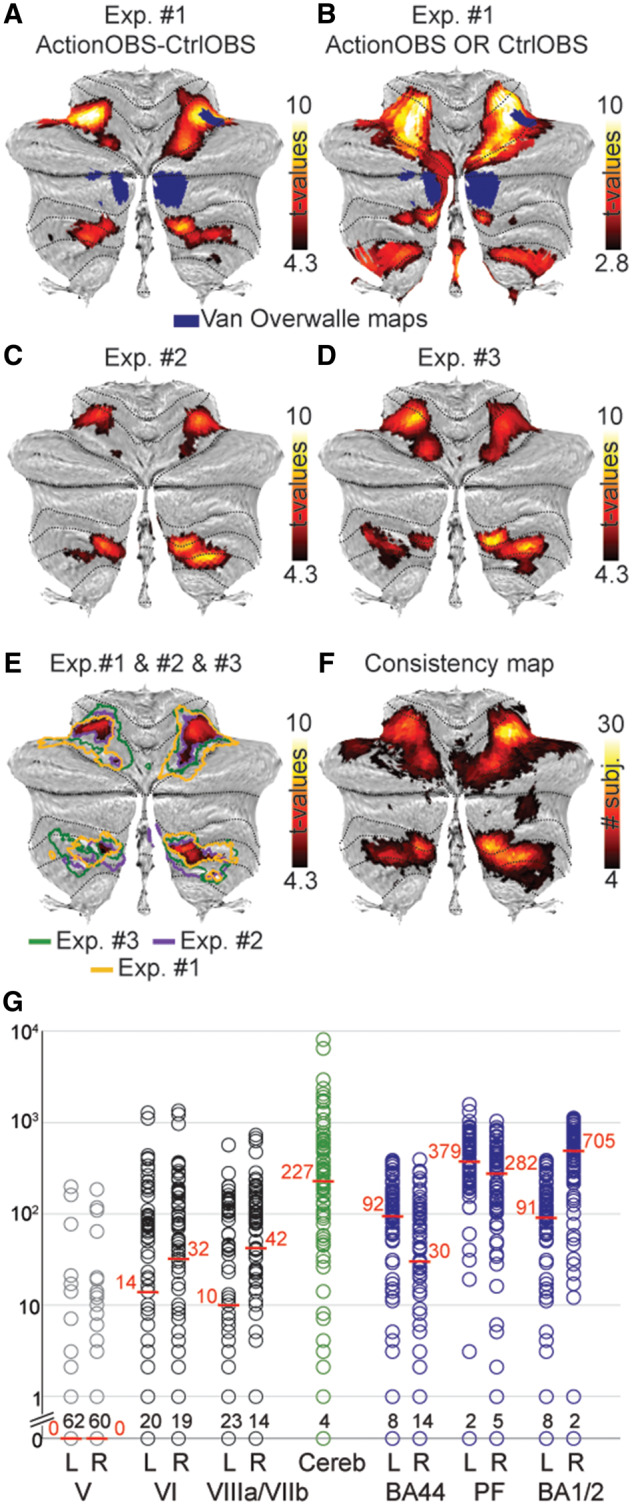
Reliability of cerebellar action observation activations. (A and B) In blue the maps presented by Van Overwalle et al. (2014), and the results of the ActionOBS–CtrlOBS contrast of Experiment 1 in the hot colour scale in (A) and of the global null conjunction ActionOBS OR CtrlOBS for Experiment 1 in (B), both at PFWE < 0.05. (C and D) ActioOBS–CtrlOBS related activity for Experiments 2 and 3, respectively. PFWE < 0.05, t = 4.3. (E) Activations common to Experiments 1–3. Yellow, blue and green contours indicate the borders of the clusters shown in A, C and D to facilitate the qualitative comparison. (F) Consistency map computed on the smoothed data for the ActionOBS–CtrlOBS (Punc < 0.001, t = 3.1) contrast across the three experiments. The hot scale indicates the number of participants for which a particular voxel was significantly activated by the ActionOBS–CtrlOBS contrast. (G) Circles indicate the number of significant voxels a given subject had in each of the four cerebellar clusters of interest (black), in the control lobule V (grey), in total in the cerebellum (green), and in three cortical regions also commonly activated by the ActionOBS–CtrlOBS contrast (blue). The median is indicated by the red lines and numbers. Data are presented on a logarithmic scale and the number of participants having no voxels in a particular cluster is indicated in black on the x-axis.
Overlapping our activations with action observation maps from the meta-analysis of Van Overwalle et al. (2014) (blue clusters of Fig. 2A) reveals only a small portion of the right lobule VI is common between the two maps. To test whether the limited overlap is due to subtracting our control condition, we overlapped the meta-analysis map with a global null conjunction of our conditions (i.e. ActionOBS OR CtrlOBS, PFWE < 0.05, t = 2.8). The overlap remains limited to right lobule VI (Fig. 2B).
Considering this inconsistency, we (i) replicated the experiment on a different scanner in two new groups of participants; and (ii) explored how many of our participants have activations in the cerebellum.
Replicating the analysis in new participants confirms the cerebellar recruitment, despite using different scanners and sequences (Fig. 2C–E and Tables 2 and 3).
Table 3.
Comparison between Experiments 1, 2 and 3 in number of voxels and peak distance per cluster of activity
| Number of voxels | Min Euclidean distance | ||||||
|---|---|---|---|---|---|---|---|
| Exp1 | Exp2 | Exp3 | Exp 1–3 | Exp1, Exp2 | Exp1, Exp3 | Exp2, Exp3 | |
| Lob VI R | 336 | 115 | 454 | 88 | 2.0 | 2.0 | 2.0 |
| Lob VI L | 537 | 216 | 391 | 202 | 7.5 | 2.8 | 4.0 |
| LobVIIIa/ VIIb R | 148 | 84 | 130 | 20 | 4.5 | 2.8 | 3.5 |
| LobVIIIa/ VIIb L | 223 | 198 | 265 | 120 | 4.9 | 6.3 | 7.2 |
| Outside ROIs | 179 | 344 | 299 | 56 | |||
For each of the four cerebellar clusters, and for each experiment separately, the number of voxels surviving PFWE < 0.05 for the contrast ActionOBS–CtrlOBS is reported. The fourth column reports the number of voxels counted within the conjunction of the three experiments. The last row indicates the number of cerebellar voxels not falling within the region of interest. Columns 5–7 indicate the minimum Euclidean distance between the activation-peaks identified belonging to the four clusters by the Anatomy toolbox for SPM. ROIs = regions of interest.
Looking at individual participants reveals that all but four (all from Experiment 1) of the 79 participants have significant activations to the ActionOBS–CtrlOBS contrast when tested at P < 0.001 (t = 3.1) within the cerebellum (green in Fig. 2G). The majority (68/79, 86.1%) additionally had >10 voxels activated (Fig. 2G and Supplementary Table 4) and most had at least 10 voxels in each of the cerebellar lobules identified in the group (regions of interest encompassing lobule VI or lobule VIIb+VIIIa) (black in Fig. 2G). A binomial distribution indicates that finding 10 or more voxels significant by chance at P = 0.001 in a region of interest of 2085 voxels (the largest region of interest we have) is highly unlikely (P < 2 × 10−5). To explore the spatial specificity of the activity in lobule VI further, we also performed this analysis for neighbouring lobule V, which harbours very few voxels responding in this contrast, with the majority of participants (78% for the left and 75% for the right lobule V) (grey in Fig. 2G and Supplementary Table 4) having none.
To compare the reliability of cerebellar activations with those of the cerebrum, we took three regions consistently associated with the action observation system, BA44, the PF complex and SI (Keysers and Gazzola, 2009; Caspers et al., 2010; Molenberghs et al., 2012), and counted activated voxels in these regions subject by subject (Supplementary Table 4). Chi2 tests comparing the proportion of participants with zero voxels activated in the four cerebellar and six cerebral regions using Fisher’s exact test in R indicates that for Experiments 1 and 2 the proportion with zero voxels activated is larger in the cerebellum (Exp1, P = 0.001; Exp2, P = 0.004; Exp3, P = 0.86). When combining all three experiments, the difference in proportion becomes highly significant (P < 0.001), with the cerebral regions of interest hosting significant voxels in a larger proportion of participants than the cerebellar regions of interest. Consistency maps indicate that the right lobule VI hosts the most consistently activated voxel, with 30 participants having significant activations in that specific voxel (Fig. 2F).
In addition to examining the contrast ActionOBS-CtrlOBS, we also extracted the average activity within our cerebellar regions of interest separately for ActionOBS and CtrlOBS (Supplementary Fig. 2).
To ensure that the observed cerebellar activity was not due to more imitative motor programs during ActionOBS than CtrlOBS, we collected EMG data while a new group of 10 participants watched the ActionOBS and CtrlOBS stimuli outside the scanner. Results show no difference in muscle activity across ActionOBS and CtrlOBS [F(1,9) = 1, P = 0.33, BF10 = 0.1] (Supplementary Fig. 4).
In summary, we found that our task reliably activates the cerebellum at the individual and group level, and across scanners and pipelines. In particular, we provide evidence for a consistent involvement of cerebellar lobules VI and VIIb and VIIIa in action observation, matching the involvement of these lobules during the execution of complex actions shown by Schlerf et al. (2010). Despite the replicability of our results across three experiments, we did find that cerebral activations remain more consistent than the cerebellar activity across individuals, possibly explaining why smaller studies in the past may have failed to emphasize cerebellar activity.
Cerebellar activation to the weight discrimination task
Observing an arm lifting an object to judge its weight activates several regions of the cerebellum (Fig. 3A and B and Supplementary Table 6) (PFWE < 0.05, t = 2.8). The responses to lifting movements overlap with the ALE meta-analysis maps (Van Overwalle et al., 2014) beyond lobule VI, in both left and right lobule VIIa of crus I. Computing the GLM of the weight discrimination experiment within the global null mask of the previous three experiments shows that all clusters observed in Experiments 1–3 were activated by the observation of lifting movement (Fig. 3C and D and Supplementary Table 6).
Figure 3.
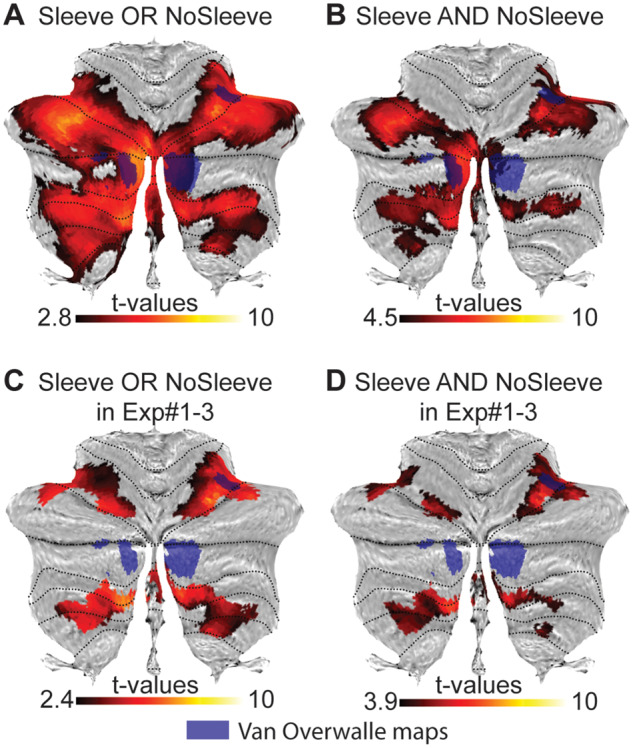
Functional MRI results of the weight discrimination task. (A) Voxels significantly activated by either the Sleeve (only kinematic information available) or the NoSleeve (both kinematic and shape information) condition (global null conjunction in SPM at PFWE < 0.05, t = 2.8, min 10 voxels). In blue the clusters identified by Van Overwalle et al. (2014), as responding to action perception. (B) Voxels activated by both (conjunction-conjunction in SPM) the NoSleeve and Sleeve conditions (PFWE < 0.05; t = 4.5, min 10 voxels). (C) Same as in A but within the clusters of activation found in Experiments 1–3 (Exp1 > 0 OR Exp2 > 0 OR Exp3 > 0). Results are shown at PFWE < 0.05, t = 2.8, min 10 voxel. (D) Same as in C but within the clusters of activation found in Experiments 1–3 (PFWE < 0.05; t = 3.9, min 10 voxels). All activations are shown on the flat map of the cerebellum offered by the SUIT toolbox.
What aspect of action observation is processed in the cerebellum? By disentangling the activity common to the Sleeve and NoSleeve conditions mentioned above (conjunction Sleeve and NoSleeve) from that specific to the NoSleeve condition (NoSleeve–Sleeve), we can attempt to identify regions involved in kinematic and shape processing, respectively. The eye-tracking maps from the control participants show that the two conditions are indeed explored differently (Supplementary Fig. 3A). When the arm was covered, participants focus similarly on the proximal and distal part of the arm [t(12) = 1.523, P = 0.154], but if no sleeve is present, participants focus significantly more on the proximal part of the arm [t(12) = −9.482, P < 0.001] that reveals shape information in the upper arm musculature. Results from the functional MRI data indicate that in contrast to the conjunction that revealed consistent cerebellar involvement for kinematic processing, at FWE correction at peak level nothing survives for both the Sleeve–NoSleeve and the NoSleeve–Sleeve contrast within the cerebellum (t = 4.42, P > 0.05), while 22 voxels in the fusiform area FG4 become apparent for the contrast NoSleeve–Sleeve when the analyses are run for the whole brain (t = 5.4, P < 0.05). Accordingly, the cerebellum is significantly recruited by the kinematic cues common to both conditions, but not by the differential shape cue that the NoSleeve–Sleeve contrast situates in the ventral visual stream instead.
Cerebellar contribution to action perception
The Mann-Whitney U-test on task performance reveals a significant difference between SCA6 and control subjects for the Sleeve condition (nSCA6 = 21; nctrl = 31; U = 199.5; P < 0.009), in which participants depend on the kinematic information (Fig. 4A). The same test reveals that the gain of performance in the NoSleeve compared to the Sleeve condition (i.e. NoSleeve performance – Sleeve performance) does not differ significantly across groups (nSCA6 = 21; nctrl = 31; U = 274.5; P > 0.34). Not surprisingly, the two groups therefore also differ when the total performance is considered, including both the Sleeve and NoSleeve trials (nSCA6 = 21; nctrl = 31; U = 183; P < 0.004). Using d’ instead of per cent correct leads to similar conclusions. To explore whether our pattern of findings, which includes a significant group difference for the Sleeve condition and a lack of significant group difference in the gain of performance, might indicate that the cerebellum contributes to kinematic but not shape processing, we performed a Bayesian t-test in JASP. The Bayes factors (BF) in favour of the alternative hypothesis Controls > SCA6 are BF = 14.7 (Sleeve) and BF = 0.19 (NoSleeve–Sleeve performance). Accordingly, we have strong evidence for a group difference in kinematic processing (Sleeve), and moderate evidence for a lack of difference for shape processing (NoSleeve–Sleeve).
Figure 4.
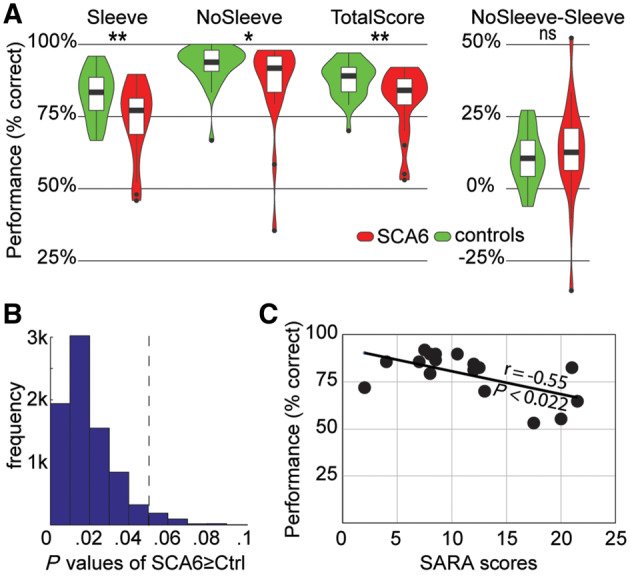
Behavioural results. (A) Violin plot of the performance (per cent correct responses) in the weight discrimination task for the 21 SCA6 patients (red) and 31 control subjects (green) for the different conditions. *P < 0.05, **P < 0.01 using Mann-Witney U tests to compare SCA6 versus controls group in each condition. (B) Distribution of P-values obtained from the 8008 possible subsamples of gender-matched control groups, again using the Mann-Whitney U-test to compare the total score (Sleeve and NoSleeve trials together) across groups. (C) The significant negative association between symptom severity (SARA) and total score in the weight perception task. The r-value reflects the non-parametric Spearman rank-order correlation. Higher SARA scores reflect more severe symptoms and predict more perceptual impairment. ns = not significant.
To explore if this group difference in the performance could be due to the less than ideal matching on gender, we carried out two further analyses. First, we performed a parametric ANOVA on the performance in the Sleeve condition with two groups (SCA6 versus Controls) × 2 genders. The interaction of Gender × Group was not significant [F(1,48) = 2.66, P = 0.11], suggesting that the group difference does not depend on gender. Second, we created control groups that were exactly matched in gender to the SCA6 group by subselecting six males out of the 16 available in the control group, keeping all the 15 females. There are 8008 ways to subsample six males out of 16, and for each of them, we calculated the P-value for the group difference in total performance using the Mann-Whitney U one-tailed test. The median P-value across the 8008 subsamples was P = 0.016, and 7675 of the 8008 (96%) have P < 0.05 (Fig. 4B). This confirmed that compared to the majority of randomly subsampled, gender-matched control groups, the SCA6 group shows impaired performance in our task.
To explore whether there is a significant association between the severity of the degenerative disorder and the performance in our task, we calculated the Spearman rank order correlations between the total performance score and the SARA score for the 17 patients for which we do have the SARA score (Fig. 4C). We found that the association is significant: R = −0.55, t(15) = −2.54, P < 0.022.
Finally, to explore whether the perceptual impairment we observe in SCA6 patients would also be visible in implicit measures, we added eye-tracking in our last participants (four SCA6 and seven control subjects), which do not show any significant group difference (Supplementary material). Thus, even though the small sample size might have biased us to find only large group differences, the qualitatively similar pattern in the two groups suggests that SCA6 did not severely alter how subjects explored the stimuli in space and time.
Discussion
Our primary aims were (i) to explore whether and where the cerebellum is robustly activated by the observation of hand actions of other individuals; and (ii) whether disrupting the cerebellum leads to significant impairments in hand action observation.
Regarding activations, using scanning parameters that include the entire cerebellum (both in terms of field of view during acquisition and bounding box during analysis) we found that across three studies and a total of 79 participants, the cerebellum was consistently recruited by the contrast between goal-directed hand actions and meaningless movements of the hand close to an object. Single subject analyses confirmed that the cerebellum was recruited in all but four participants. More specifically, we found that activity is reliably induced in the lateral hemispheres of lobule VI, and in a cluster encompassing lobules VIIb and VIIIa. All these activations are bilateral. Without using smoothing, it is apparent that the dorsal cluster in lobule VI is distinct from activity in the ventral visual pathway, and is thus not the result of bleeding of activity from visual neocortical regions. Each of these clusters were found to be activated in the majority of individual participants. Together these results provide strong evidence that the cerebellum is consistently recruited by hand action observation.
This raises the question of why former studies failed to consistently report cerebellar activations. Our comparison of pipelines identifies two potential reasons: (i) up to SPM8, the default bounding box for analyses prevented the identification of some of the cerebellar clusters; and (ii) most studies focusing on the cerebrum have to choose between a larger field of view (i.e. more spatial coverage) versus a shorter acquisition time (i.e. increased task sensitivity), which often ends in favouring a smaller field of view therefore cutting out the cerebellum in at least some participants. At the second level of analysis, if part of the cerebellum is missing in the field of view for some of the participants, this region is entirely removed from the search volume on which statistical analyses are computed across all subjects. This may have further reduced the consistency with which cerebellar activity is reported. Finally, a comparison between the number of participants activating our cerebellar regions of interest compared to classic cerebral regions of interest such as BA44 or PF, shows that the cerebellar regions of interest indeed are slightly less reliably recruited, providing an additional factor. Overall, our three studies provide clear evidence that with proper measurement procedures and analysis pipelines, cerebellar recruitment during hand action observation can be demonstrated. The finding that these same regions are also activated when using a different, weight judgement task shows that this consistency does not depend on a specific task.
It is interesting that one of our complex action foci (ActionObs–ActionCtrl) was localized in the anterior part of lobule VI, which is where Schlerf and colleagues found activity when participants performed complex but not simple motor actions (Grodd et al., 2001; Schlerf et al., 2010). Our second focus was in the posterior inferior lobule VIIb expanding into VIIIa, adjacent to the secondary sensorimotor finger map (Grodd et al., 2001; Schlerf et al., 2015). Lobule V, associated with less complex actions, however did not show consistent visual activation, be it in the contrast or while comparing each condition against baseline (Supplementary Fig. 2). This distinction is reminiscent of that in the cerebral cortex, where M1 is not consistently recruited by action observation, while the premotor cortex, involved in more complex motor control, is (Gazzola and Keysers, 2009). That regions involved in motor control become recruited during observation is in line with the notion that cerebro-cerebellar loops involved in fine kinematic control of hand actions may also serve as a valuable system to process fine kinematics of observed actions (Miall, 2003; Wolpert et al., 2003; Fuentes and Bastian, 2007; Gazzola and Keysers, 2009; Rizzolatti and Sinigaglia, 2010; Sokolov et al., 2017). Alternatively, cerebellar activity to action observation could reflect automatic imitation of complex actions more than the control stimuli, with the cerebellum simply executing imitative motor programs. That EMG recordings show no difference in muscle activity across ActionObs and CtrlObs speak against this interpretation.
To explore whether the cerebellum is necessary for extracting information from the kinematics of the hand actions of others, we tested whether patients with SCA6 are impaired in a weight-lifting task that has been shown to depend on precise processing of hand movement kinematics (Hamilton et al., 2007). Our results indicate that SCA6 patients are indeed impaired in their kinematic processing as borne out by a group difference in the Sleeve condition that impoverishes muscle shape information. This impairment was more pronounced in patients with more severe SCA6 symptoms. Interestingly, when we analysed the data of the stimuli without the sleeves, we found that muscle shape processing appears to be preserved, as Bayesian statistics confirm that the patients benefited from the additional muscle shape as much as the controls did. That both the SCA6 patients and their controls benefit from exposing the muscle shape in the NoSleeve condition speaks to the fact that our participants did use shape information. That they benefited equally suggests that shape information was not significantly influenced by SCA6, and fits with our interpretation that the SCA6 impairment in the sleeved condition could be explained by a perturbation of kinematic perception. These results complement the results of the only other study that has, to our knowledge, examined the impact of cerebellar damage in action observation (Cattaneo et al., 2012), in that the two studies probed different aspects of hand action observation. In the task of Cattaneo et al. (2012), participants viewed four still frames of an action, and had to decide which was not part of that action. Solving that task does not require fine kinematic analyses, but an understanding of whether a particular hand-object interaction would be appropriate to achieve a particular goal. In our task, all videos show a hand successfully lifting an object, and performance thus depends on analysis of kinematics. That SCA6 patients were impaired in the Sleeve condition, in which kinematics was the primary cue, but could benefit from additional muscle shape, highlights that cerebellar degeneration particularly impairs kinematic processing. Moreover, these findings dovetail with our functional MRI results, which show consistent cerebellar activity for the kinematic stimuli (Sleeve), but not for the additional shape information provided in the NoSleeve condition. Future experiments comparing performance in action perception and non-biological motion analysis will be needed to explore whether these processes rely on partially distinct cerebellar substrates, or whether the action observation deficit we observed is part of a more general visual motion deficit (Nawrot and Rizzo, 1995; Handel et al., 2009; Avanzino et al., 2015; Broersen et al., 2016).
As the cerebellum is involved in eye movement control, we were concerned that patients may be compromised in their ability to follow the movements of the arm with their gaze. However, our control data obtained from a small number of SCA6 patients do not suggest severe impairments in how our patients deploy their gaze. Future studies could include functional MRI of SCA6 patients to explore where in the cerebellum degeneration alters task-related activity, and whether this includes regions associated with gaze-control. A previous voxel-based morphometry study points to a loss of grey matter in the hemispheres of lobule VI as the primary cause of upper limb ataxia triggered by SCA6 (Rentiya et al., 2017), which is in close vicinity to and partly overlaps with regions in which we found cerebellar activations to action observation, but is lateral relative to the sections of lobule VI mostly associated with eye movements (Supplementary Fig. 5). Data from an additional control experiment further suggest that the differential cerebellar activity is unlikely to be due to differential eye movements (Supplementary Fig. 6).
Based on functional MRI data alone, in 2009 we hypothesized the ventral premotor cortex (vPM), SI and parietal region PF could, via the cerebellum, map visual input, from high level visual regions, onto the motor machinery involved in performing similar actions (Gazzola and Keysers, 2009). Beyond confirming the visual activation of these regions (Supplementary Fig. 7 and Supplementary Table 5), and more finely localizing cerebellar activity, we now show that disorders affecting the cerebellum disrupt action perception as measured by weight judgement. The same task is also disrupted by altering activity in the vPM (Pobric and Hamilton, 2006) or SI (Valchev et al., 2017). Measuring brain activity while perturbing SI, we showed altering activity in one of these nodes disrupts activity of all of those nodes (Valchev et al., 2016)—including the cerebellar lobule VI (Table 2 in Valchev et al., 2016). This suggests that much like action control (Wolpert and Ghahramani, 2000), action observation relies on a cortico-cerebellar loop that maps sensory input onto motor control structures (inverse models) and motor programs to expected sensory input (forward models). This loop brings descending information from our cortical network (includinf vPM, SI, PF, and inferior frontal gyrus/middle temporal gyrus) to the cerebellum (lobules VI and VIIb/VIIIa) and ascending information from the cerebellum back to the cerebral cortex (Fig. 5). Anatomical studies suggest the former occurs via the pons and the latter via the thalamus and interposed nucleus of the cerebellum (Teune et al., 2000). In line with the latter, we also found robust thalamic activity (Supplementary Fig. 8). Given the strong involvement of all these structures in kinematics rather than shape (Schmahmann and Pandya, 1993), we propose that this loop transforms subtle kinematic cues into reportable perceptions of observed actions. Asking what each brain region individually contributes to this perception/action loop is perhaps as ill posed as asking what each part of a gear-box contributes to torque conversion—function emerges from the interplay of parts. Our Sleeve–NoSleeve data additionally suggest when perception can draw from shape cues, ventral visual brain structures around the fusiform gyrus additionally come into play, but studies investigating the causal impact of these regions onto tasks such as weight discrimination are, to our knowledge, still lacking.
Figure 5.
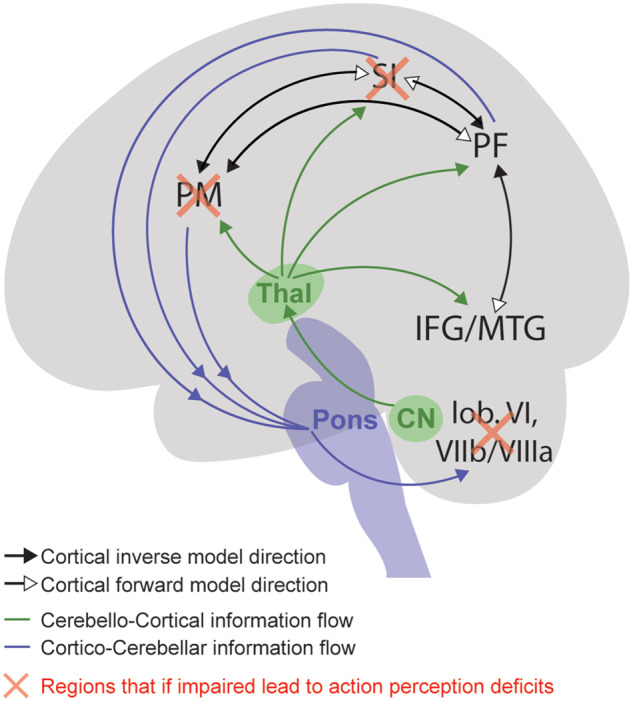
Information flow during action observation. Circuit we propose to be involved in the perception of other people’s actions. Cortical regions send information to the cerbellum through the pons (blue arrows), and information processed in the cerebellum flows to the cortex through the thalamus (Thal) via the cerebellar nuclei (CN, green arrows). The different colours of the arrows between cortical regions indicate the direction of the forward and inverse model. Red crosses indicate that a perturbation of the activity in those regions (either by non-invasive brain stimulation technique or degenerative deficits) influences action perception. IFG = inferior frontal gyrus; MTG = middle temporal gyrus; PF = parietal region PF; PM = premotor cortex.
In the light of our findings we believe that it is time to consider the cerebellum a reliable and necessary component of the network that allows us to process the kinematics of observed hand actions. Clinically, one of the core complaints of many stroke survivors and their spouses are impairments in social cognition (Hillis, 2014). These social sequelae are often not on the radar of neurological staff. We hope that by showing that SCA6 patients have deficits in perceiving the kinematics of the actions performed by other individuals—deficits that gets worse with the severity of the disease—our results contribute to an increased awareness that neurological disorders affecting the cerebellum could have consequences for social perception. Being impaired in perceiving what other individuals around us do is likely to impact the way we relate to others and thereby reduce our wellbeing.
Supplementary Material
Acknowledgements
We thank Filippo Migliorati and Rajat Thomas for their support during data analysis. We thank Marc Thioux, Anita Sibeijn-Kuiper, Judith Streurman and Teresa De Sanctis for help in acquiring the MRI data. We thank Jessica Willems, Esther Brusse, Henk-Jan Boele, Ruben van der Giessen, Cullen B. Owens for facilitating the experiments with SCA6 patients and performing neurological examinations. We thank the Spinoza Center Amsterdam and the Neuroimaging Center at the UMCG for contributing scan time for the functional MRI data acquisition. We thank Diederick Stoffers and Joris Coppens for helping with the eye-tracking set-up, Riccardo Paracampo for his help in collecting and analysing the EMG data, and Efe Soyman for helping with the analysis of the movement kinematic of the stimuli of Experiment 4. We thank Styliani Ioanna Gkavanozi and Laura Fornari for help with editing the stimuli of Experminet 7. We finally thank F. Van Overwalle for providing the mirror-maps from his meta-analysis (Van Overwalle et al., 2014).
Author contributions: Funding was obtained by V.G., C.K., C.I.D. and R.Bh. The experiment was conceived by V.G., C.I.D. and C.K. together with A.R.A. and J.S. FMRI data were collected by J.S. (Experiment 1) and R.Bh. (Experiments 24 and 7) with facilitation from V.G. Behavioural data on healthy volunteers and SCA6 patients were collected by A.R.A., R.Br. and S.P. with facilitation of C.I.D. EMG data were collected by R.Bh. and A.R.A. Data analysis was performed by J.S., R.Bh., A.R.A., R.Br and V.G., with suggestions from C.K. and C.I.D. The manuscript was written by J.S., A.R.A., R.Br, R.Bh and V.G., with extensive input from C.K. and comments from all the other authors.
Glossary
Abbreviations
- SARA =
Scale of the Assessment and Rating of Ataxia
- SCA6 =
spinocerebellar ataxia type 6
- SI =
primary somatosensory cortex
Funding
This work was supported by the Netherlands Organization for Scientific Research (056-13-017, NIHC to C.K., VENI 451-09-006 to V.G., VIDI 452-14-015 to V.G.), the Brain and Behavior Research Foundation (NARSAD young investigator 22453 to V.G.), the BIAL foundation (Research Project 255/16 to R.Bh.), the European Research Council of the European Commission (ERC-StG-312511 to C.K.; ERC-Adv and ERC-PoC C.I.D.), as well as the Dutch agencies for fundamental and medical research (NWO-ALW and Zon-Mw; C.I.D.).
Competing interests
The authors declare no competing interests.
References
- Agnew ZK, Wise RJS, Leech R. Dissociating object directed and non-object directed action in the human mirror system; implications for theories of motor simulation. PLoS One 2012; 7: e32517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 1999; 412: 319–41. [DOI] [PubMed] [Google Scholar]
- Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J Neurosci 2011; 31: 14243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzino L, Bove M, Pelosin E, Ogliastro C, Lagravinese G, Martino D. The cerebellum predicts the temporal consequences of observed motor acts. PLoS One 2015; 10: e0116607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L. Lateralization of the human mirror neuron system. J Neurosci 2006; 26: 2964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari R, Kirilina E, Caan M, Suttrup J, de Sanctis T, De Angelis L, et al. Does higher sampling rate (Multiband + SENSE) benefit the detection of task correlated BOLD for cognitive neuroscience applications at 3T? bioRxiv; 2019: 762831. [Google Scholar]
- Broersen R, Onuki Y, Abdelgabar AR, Owens CB, Picard S, Willems J, et al. Impaired spatio-temporal predictive motor timing associated with spinocerebellar ataxia type 6. PLoS One 2016; 11: e0162042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner IC, Skouen JS, Ersland L, Grüner R. Plasticity and response to action observation: a longitudinal fMRI study of potential mirror neurons in patients with subacute stroke. Neurorehabil Neural Repair 2014; 28: 874–84. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, et al. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron 2004; 42: 323–34. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage 2006; 33: 430–48. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 2010; 50: 1148–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catmur C, Gillmeister H, Bird G, Liepelt R, Brass M, Heyes C. Through the looking glass: counter-mirror activation following incompatible sensorimotor learning. Eur J Neurosci 2008; 28: 1208–15. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Fasanelli M, Andreatta O, Bonifati DM, Barchiesi G, Caruana F. Your actions in my cerebellum: Subclinical deficits in action observation in patients with unilateral chronic cerebellar stroke. Cerebellum 2012; 11: 264–71. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, et al. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol 2006; 495: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare G, Di Dio C, Marchi M, Rizzolatti G. Expressing our internal states and understanding those of others. Proc Natl Acad Sci 2015; 112: 10331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res 1992; 91: 176–80. [DOI] [PubMed] [Google Scholar]
- Du X, Wang J, Zhu H, Rinaldo L, Lamar K-M, Palmenberg AC, et al. Second cistron in CACNA1A gene encodes a transcription factor mediating cerebellar development and SCA6. Cell 2013; 154: 118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Thonnard J-L, Vandermeeren Y, Sébire G, Cosnard G, Olivier E. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain 2003; 126: 732–47. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 2006; 32: 570–82. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 2007; 36: 511–21. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005; 25: 1325–35. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Yu J. Effects of corticospinal lesions upon treadmill locomotion by cats. Exp brain Res 1981; 43: 101–3. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Geslerich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: From action organisation to intention understanding. Science (80-) 2005; 308: 662–7. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Eliasson AC, Redon-Zouitenn C, Mercuri E, Dubowitz L. Impaired grip-lift synergy in children with unilateral brain lesions. Brain 1999; 122: 1157–68. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. ‘Motor cognition’-What is it and is the cerebellum involved? Cerebellum 2007; 6: 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain 1996; 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, et al. A cortico-cerebellar loop for motor planning. Nature 2018; 563: 113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex 2009; 19: 1239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage 2007a; 35: 1674–84. [DOI] [PubMed] [Google Scholar]
- Gazzola V, van der Worp H, Mulder T, Wicker B, Rizzolatti G, Keysers C. Aplasics born without hands mirror the goal of hand actions with their feet. Curr Biol 2007b; 17: 1235–40. [DOI] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol 2004; 174: I–89. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, et al. Two different areas within the primary motor cortex of man. Nature 1996; 382: 805–7. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage 1999; 10: 63–83. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. 2. Spatial normalization to standard anatomical space. Neuroimage 2000; 11: 684–96. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: Observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage 2001; 14: 617–31. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 2001; 13: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, Joyce DW, Flanagan JR, Frith CD, Wolpert DM. Kinematic cues in perceptual weight judgement and their origins in box lifting. Psychol Res 2007; 71: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel B, Thier P, Haarmeier T. Visual motion perception deficits due to cerebellar lesions are paralleled by specific changes in cerebro-cortical activity. J Neurosci 2009; 29: 15126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsdörfer J, Hagl E, Nowak DA, Marquardt C. Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol 2003; 114: 915–29. [DOI] [PubMed] [Google Scholar]
- Hillis AE. Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain 2014; 137: 981–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Iacoboni M, Cross KA, Korb A, Lee J, Nori P, et al. Self-reported empathy and neural activity during action imitation and observation in schizophrenia. NeuroImage Clin 2014; 5: 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, et al. Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci U S A 2001; 98: 13995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science 1999; 286: 2526–8. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Abdollahi RO, Orban GA. Acting alters visual processing: Flexible recruitment of visual areas by one’s own actions. Cereb Cortex 2012; 22: 2930–42. [DOI] [PubMed] [Google Scholar]
- Jelsone-Swain L, Persad C, Burkard D, Welsh RC. Action processing and mirror neuron function in patients with amyotrophic lateral sclerosis: An fMRI study. PLoS One 2015; 10: e0119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 2003; 23: 8432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol 2009; 19: 666–71. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci 2010; 11: 417–28. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kohler E, Umiltà MA, Nanetti L, Fogassi L, Gallese V. Audiovisual mirror neurons and action recognition. Exp brain Res 2003; 153: 628–36. [DOI] [PubMed] [Google Scholar]
- Keysers C, Paracampo R, Gazzola V. What neuromodulation and lesion studies tell us about the function of the mirror neuron system and embodied cognition. Curr Opin Psychol 2018; 24: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umiltà MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science (80-) 2002; 297: 846–8. [DOI] [PubMed] [Google Scholar]
- Miall RC. Connecting mirror neurons and forward models. Neuroreport 2003; 14: 2135–7. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev 2012; 36: 341–9. [DOI] [PubMed] [Google Scholar]
- Nawrot M, Rizzo M. Motion perception deficits from midline cerebellar lesions in human. Vis Res 1995; 35: 723–31. [DOI] [PubMed] [Google Scholar]
- Orr ELR, Lacourse MG, Cohen MJ, Cramer SC. Cortical activation during executed, imagined, and observed foot movements. Neuroreport 2008; 19: 625–30. [DOI] [PubMed] [Google Scholar]
- Peirce JW. Generating Stimuli for Neuroscience Using PsychoPy. Front Neuroinform 2009; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata Bello J, Modroño C, Marcano F, González-Mora JL. The mirror neuron system and motor dexterity: what happens? Neuroscience 2014; 275: 285–95. [DOI] [PubMed] [Google Scholar]
- Pobric G, Hamilton AF. Action understanding requires the left inferior frontal cortex. Curr Biol 2006; 16: 524–9. [DOI] [PubMed] [Google Scholar]
- Rentiya Z, Khan N-S, Ergun E, Ying SH, Desmond JE. Distinct cerebellar regions related to motor and cognitive performance in SCA6 patients. Neuropsychologia 2017; 107: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cogn Brain Res 1996; 3: 131–41. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci 2010; 11: 264–74. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Filippi M. FMRI correlates of execution and observation of foot movements in left-handers. J Neurol Sci 2010; 288: 34–41. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci 2008; 28: 1569–88. [DOI] [PubMed] [Google Scholar]
- Sasaki AT, Kochiyama T, Sugiura M, Tanabe HC, Sadato N. Neural networks for action representation underlying automatic mimicry: a functional magnetic-resonance imaging and dynamic causal modeling study. Front Hum Neurosci 2012; 6: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saute JAM, Donis KC, Serrano-Munuera C, Genis D, Ramirez LT, Mazzetti P, et al. Ataxia rating scales—psychometric profiles, natural history and their application in clinical trials. Cerebellum 2012; 11: 488–504. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Spampinato D, Celnik PA. Laterality differences in cerebellar-motor cortex connectivity. Cereb Cortex 2015; 25: 1827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Verstynen TD, Ivry RB, Spencer RMC. Evidence of a novel somatopic map in the human neocerebellum during complex actions. J Neurophysiol 2010; 103: 3330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Prelunate, occipitotemporal, and parahippocampal projections to the basis pontis in rhesus monkey. J Comp Neurol 1993; 337: 94–112. [DOI] [PubMed] [Google Scholar]
- Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006; 66: 1717–20. [DOI] [PubMed] [Google Scholar]
- Sokolov AA, Gharabaghi A, Tatagiba MS, Pavlova M. Cerebellar engagement in an action observation network. Cereb Cortex 2010; 20: 486–91. [DOI] [PubMed] [Google Scholar]
- Sokolov AA, Miall RC, Ivry RB. The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 2017; 21: 313–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res 2000; 124: 141–72. [DOI] [PubMed] [Google Scholar]
- Thomas RM, De Sanctis T, Gazzola V, Keysers C. Where and how our brain represents the temporal structure of observed action. Neuroimage 2018; 183: 677–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Avenanti A. Neuroanatomical substrates of action perception and understanding: an anatomic likelihood estimation meta-analysis of lesion-symptom mapping studies in brain injured patients. Front Hum Neurosci 2014; 8: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valchev N, Gazzola V, Avenanti A, Keysers C. Primary somatosensory contribution to action observation brain activity-combining fMRI and cTBS. Soc Cogn Affect Neurosci 2016; 11: 1205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valchev N, Tidoni E, Hamilton AF de C, Gazzola V, Avenanti A. Primary somatosensory cortex necessary for the perception of weight from other people’s action: a continuous theta-burst TMS experiment. Neuroimage 2017; 152: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. Neuroimage 2014; 86: 554–72. [DOI] [PubMed] [Google Scholar]
- Weyer A, Abele M, Schmitz-Hübsch T, Schoch B, Frings M, Timmann D, et al. Reliability and validity of the scale for the assessment and rating of ataxia: A study in 64 ataxia patients. Mov Disord 2007; 22: 1633–7. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Doya K, Kawato M. A unifying computational framework for motor control and social interaction. Philos Trans R Soc L B Biol Sci 2003; 358: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci 2000; 3: 1212–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available online at https://openeuro.org.


