Abstract
Objective
This study aimed to evaluate the association between acute kidney injury (AKI) and bronchopulmonary dysplasia (BPD) in infants born <32 weeks of gestational age (GA).
Study Design
Present study is a secondary analysis of premature infants born at <32 weeks of GA in the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) retrospective cohort (n = 546). We stratified by gestational age and used logistic regression to determine association between AKI and moderate or severe BPD/mortality.
Results
Moderate or severe BPD occurred in 214 of 546 (39%) infants, while death occurred in 32 of 546 (6%); the composite of moderate or severe BPD/death occurred in 246 of 546 (45%). For infants born ≤29 weeks of gestation, the adjusted odds ratio (OR) of AKI and the primary outcome was 1.15 (95% confidence interval [CI] = 0.47–2.86; p = 0.76). Infants born between 29 and 32 weeks of gestation with AKI had four-fold higher odds of moderate or severe BPD/death that remained after controlling for multiple factors (adjusted OR = 4.21, 95% CI: 2.07–8.61; p < 0.001).
Conclusion
Neonates born between 29 and 32 weeks who develop AKI had a higher likelihood of moderate or severe BPD/death than those without AKI. Further studies are needed to validate our findings and evaluate mechanisms of multiorgan injury.
Keywords: acute renal failure, acute lung injury, bronchopulmonary dysplasia, chronic lung disease, organ crosstalk, prematurity, neonate
Bronchopulmonary dysplasia (BPD) is a chronic lung disease diagnosed in approximately 14,000 infants born in the United States each year.1 BPD is the most prevalent comorbidity of prematurity and is associated with high mortality and morbidity in the beginning years of life.2 In preterm infants, BPD is associated with high-medical costs and lifetime risk of impaired pulmonary function.3,4 The pathophysiology of BPD is complex, with contributions from pulmonary parenchymal damage, abnormally developed vasculature, infectious complications, and systemic inflammatory insults.4
One common systemic inflammatory insult in premature infants is acute kidney injury (AKI). AKI occurs in up to 40% of those born prematurely.5 While originally thought to be an isolated organ dysfunction, growing evidence suggests that AKI results in systemic disease involving other organs, such as the lung.6 The systemic effects of kidney injury appear to be more than just a marker of the severity of the underlying illness.7,8 In both critically ill adults and children, AKI is associated with higher morbidity and mortality for those with both kidney and lung injury, compared with those with isolated lung or kidney injury.9–11
There is a paucity of data evaluating the relationship between AKI and lung disease in premature infants. One prospective single-center study found that premature infants with AKI had a higher likelihood of BPD.12 We hypothesized that the presence of AKI during the first 28 days of life in premature infants would be independently associated with more severe degrees of BPD at 36 weeks of corrected gestational age (CGA). To determine if there is an association between AKI and BPD, we evaluated premature infants in the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) cohort born at less than 32 weeks of gestational age (GA).
Materials and Methods
Study Design and Participants
The AWAKEN study is a multicenter, multinational retrospective observational cohort of neonates admitted to the neonatal intensive care unit (NICU) at 24 institutions in four countries between January 1 and March 31, 2014. A complete description of the development of the AWAKEN database and the epidemiology of neonatal AKI have been published elsewhere.13 Briefly, the AWAKEN cohort included infants, who were admitted to participating institutions from January 1 to March 31, 2014, and who received intravenous fluids for at least the first 48 hours. Infants were excluded if they were admitted after 14 days of age, underwent surgical repair of congenital heart disease within 7 days of birth, had a lethal chromosomal anomaly, died within 48 hours of NICU admission, or had severe congenital kidney and urinary tract abnormalities. We limited our cohort to those born at <32 weeks of GA and excluded infants with abdominal wall defects and severe congenital heart defects to focus on the interplay between kidney injury and immature lung parenchyma. The Institutional Review Board (IRB) at the University of Alabama at Birmingham approved this collaborative study, and each center received approval from their respective IRBs for participation. The study design allowed for a waiver of informed consent or parental permission. This study is registered with ClinicalTrials.org, number NCT02443389.
Definitions
AKI during the first month of age, defined by modified Kidney Disease Improving Global Outcomes (KDIGO) definition, was our exposure of interest.14 Information on AKI status obtained as part of clinical care was included in this study, including serum creatinine data throughout hospitalization and urine output for the first week of age. AKI was defined by a rise in serum creatinine (SCr) of ≥0.3 mg/dL within 48 hours or a >150% increase from a previous lowest value, or a urinary output <1 mL/kg/h during a 24-hour period on postnatal days 2 to 7 (►Supplementary Table S1, available in the online version).15 The maximum AKI severity (stages 1–3) was determined based on KDIGO definitions using the highest stage based on either serum creatinine or urinary output criteria (►Supplementary Table S1, available in the available version).16
The primary outcome of interest was development of moderate or severe BPD ordeath. We used the standardized National Institute of Child Health and Human Development (NICHD) definition of BPD.17,18 An infant who required supplemental oxygen at 28 days of life was diagnosed with BPD with severity classification at 36 weeks of CGA (►Supplemental Table S2, available in the online version). BPD was considered mild if the infant required no respiratory support at 36 weeks of CGA, moderate if the fraction of inspired oxygen (FiO2) was <0.30 and severe if the infant required ≥0.30 FiO2 and/or positive pressure ventilation.17 We chose to use moderate and severe BPD as our primary outcome to focus on premature infants most at risk for long-term pulmonary disease.4,18 We utilized a composite outcome of moderate or severe BPD/mortality as our primary outcome given they are competing risks.12,19 Because the pathophysiology of BPD in premature infants differs depending on the degree of prematurity, we divided the cohort into two GA groups (≤ 29 and 29–32 weeks).1,20,21
Statistical Analysis
We used descriptive statistics to determine differences between infants with and without moderate and severe BPD/death. Categorical variables were analyzed by proportional differences with either χ2 or Fisher’s exact tests as appropriate. For normally distributed continuous variables, we reported means and standard deviations (SD) and compared groups using the Student’s t-test. For nonnormally distributed variables, we reported medians and interquartile range (IQR) and compared groups with the Wilcoxon’s rank-sum test. To examine the association between AKI, different AKI stages and defining criteria (serum creatinine vs. urine output) with our primary outcome, we performed chi-squared test and reported p-Values; in addition, we estimated crude odds ratios (ORs) and associated 95% confidence intervals (CIs) and p-values using a general estimating equation (GEE) unconditional logistic regression, GEE ordinal logistic regression, and a GEE multinomial logistic regression, respectively. The use of the GEE methodology allows the analyses to account for clustering by study center.
A GEE unconditional logistic regression was used to estimate ORs and 95% CIs for the association between any AKI and our outcome. For adjusted models, we first created a model adjusting only for GA. We then selected those variables that had a p-value < 0.20 for inclusion in the final adjusted model. For the final model, a backward selection process was then used to create the most parsimonious model in which all independent variables had a p-value < 0.10. The final adjusted model included the variables GA, ethnicity, birth weight, and 1- and 5-minute Apgar’s scores. We created models for the entire cohort and for the GA groups (<29 weeks and 29–32 weeks). In a post hoc analysis, the main independent variable was changed from any AKI to stage-2 or −3 AKI (severe AKI). Findings were reported as adjusted odds ratios (aORs) with 95% CI and p-values. For all analyses, two-sided p-value of <0.05 was considered statistically significant. All statistical analyses were performed at the University of Alabama at Birmingham using SAS version 9.4 (Statistical Analysis Software Institute Inc., Cary, NC).
Results
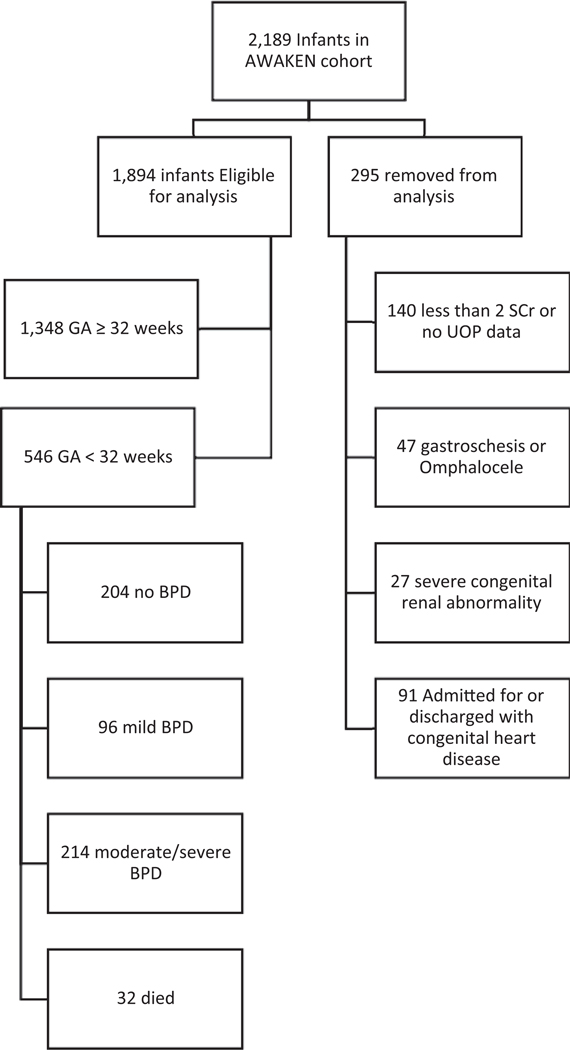
Of the 2,189 infants in the AWAKEN cohort, 295 met at least one exclusion criteria (►Fig. 1). Of the remaining 1,894 infants, 546 (29%) were <32 weeks of GA and made up the cohort for this analysis. More infants in the cohort were male (58%), white race (51%), and the majority identified as non-Hispanic ethnicity (74%). The mean GA of 28.0 weeks (SD = 2.6) and mean birthweight 1,150 g (SD = 426).
Fig. 1.
Breakdown of the eligible, enrolled and non-enrolled infants in the study cohort. AWAKEN, assessment of worldwide acute kidney injury epidemiology in neonates; BPD, bronchopulmonary dysplasia; GA, gestational age; SCr, SCr, serum creatinine; UOP, urine output.
Moderate or severe BPD occurred in 214 of 546 (39%) infants, while death occurred in 32 (6%); thus, the composite of moderate and severe BPD/death occurred in 246 of 546 (45%) infants. Infants with moderate or severe BPD/death had lower birth weight (876 vs. 1,374 g, p < 0.0001), and lower GA at birth (26.0 vs. 29.5 weeks, p < 0.0001; ►Table 1). They also had lower Apgar’s scores at 1 (median, 4 vs. 6, p < 0.0001) and 5 minutes (median, 7 vs. 8, p < 0.0001) of life. Eighty percent of infants with moderate and severe BPD/death were intubated as part of initial resuscitation, compared with 35% of those without moderate and severe BPD/death (p < 0.0001). There were no significant differences in sex, race, oligohydramnios, or maternal corticosteroid exposure between groups.
Table 1.
Univariate characteristics for prematurely born infants with and without moderate or severe bronchopulmonary dysplasia or death
| Infant characteristics | No BPD/mild BPD (n = 300) | Moderate or severe BPD/death (n = 246) | p-Value |
|---|---|---|---|
| Sex (male) | 167 (55.5) | 147 (60.0) | 0.40 |
| Ethnicity | |||
| Hispanic | 32 (14.3) | 27 (11.0) | 0.14 |
| Non-Hispanic | 212 (70.4) | 191 (78.0) | |
| Unknown | 46 (15.3) | 27 (11.0) | |
| Race | |||
| White | 158 (52.5) | 122 (50.0) | 0.43 |
| Black | 68 (22.6) | 67 (27.3) | |
| Other | 75 (25.0) | 56 (22.9) | |
| Mean gestational age (wk) | 29.5 ± 1.5 | 26.0 ± 2.2 | <0.0001 |
| Mean Birth Weight (g) | 1374.4 ± 383.5 | 875.9 ± 295.7 | <0.0001 |
| 1-min Apgar’s score) | 6 (IQR = 4–8) | 4 (IQR = 2–6) | <0.0001 |
| 5-min Apgar’s score) | 8 (IQR 7–9) | 7 (IQR 5–8) | <0.0001 |
| Mode of delivery | |||
| C-section (scheduled) | 18 (6.0) | 9 (3.7) | 0.05 |
| C-section (Unscheduled) | 175 (58.1) | 140 (57.1) | |
| Vaginal birth | 86 (28.6) | 88 (35.9) | |
| Unknown | 22 (7.3) | 8 (3.3) | |
| Maternal characteristics | |||
| Multiple gestations | 96 (31.9) | 64 (26.1) | 0.14 |
| Intrapartum infection | 35 (11.6) | 31 (12.7) | 0.71 |
| Diabetes | 37 (12.3) | 27 (11.0) | 0.65 |
| Hypertension | 35 (11.6) | 28 (11.4) | 0.94 |
| Amniotic fluid | 0.54 | ||
| Polyhydramnios | 5 (1.7) | 3 (1.2) | 0.28 |
| Oligohydramnios | 11 (3.7) | 16 (6.5) | |
| Normohydramnios | 285 (94.6) | 226 (92.2) | |
| Intrauterine growth restriction | 25 (8.3) | 20 (8.2) | 0.95 |
| Maternal steroids | 215 (71.4) | 180 (73.5) | 0.60 |
| Delivery characteristic | |||
| Neonatal intubation | 105 (34.9) | 195 (80.0) | <0.0001 |
| Meconium at delivery | 4 (1.3) | 10 (4.1) | 0.06 |
| Chest compressions | 9 (3.0) | 27 (11.0) | <0.001 |
| Reason for admission | |||
| Respiratory failure | 222 (73.8) | 201 (82.0) | 0.02 |
| Sepsis evaluation | 176 (58.5) | 155 (63.3) | 0.25 |
| Hypoglycemia | 28 (9.3) | 14 (5.7) | 0.12 |
| HIE | 5 (1.7) | 8 (3.3) | 0.22 |
| AKI | 53 (17.6) | 128 (52.2) | <0.0001 |
Abbreviations: AKI, acute kidney injury; BPD, bronchopulmonary dysplasia; HIE, hypoxic ischemic encephalopathy.
Note: Data in table are presented as the number with the percentage (n [%]) in parenthesis, or as the mean ± standard deviation (SD).
AKI occurred in 181 of 546 (33%) infants. Of those infants with AKI, 89 of 181 (49%) had stage-1 AKI and 92 of 181 (51%) had stage-2 or −3 AKI. The likelihood of AKI was five times higher for those with moderate or severe BPD or death compared with those with no or mild BPD (OR = 5.11, 95% CI: 2.78–9.42), and those with moderate or severe BPD or death were also more likely to have a higher stage of AKI (OR = 4.99, 95% CI: 2.59–9.59; ►Table 2).
Table 2.
Association between AKI and moderate/severe BPD or death
| No BPD/mild BPD (n = 300) | Moderate or severe BPD/death (n = 246) | |
|---|---|---|
| AKIa | ||
| No AKI | 248 (82.4) | 117 (47.8) |
| AKI | 53 (17.6) | 128 (52.2) |
| Odds ratio (95% CI) | Referent | 5.11 (2.78–9.42) |
| AKI stageb | ||
| Stage 1 AKI | 29 (9.6) | 60 (24.5) |
| Stage 2/3 AKI | 24 (8.0) | 68 (27.8) |
| Odds ratio (95% CI) | Referent | 4.99 (2.59–9.59) |
| AKI typec | ||
| UOP only | 8 (15.0) | 5 (3.9) |
| SCr only | 42 (79.2) | 118 (92.2) |
| Odds ratio (95% CI) | Referent | 4.50 (1.51–13.41) |
| SCr and UOP | 3 (5.7) | 5 (3.9) |
| Odds ratio (95% CI) | Referent | 2.67 (0.34–20.85) |
Abbreviations: AKI, acute kidney injury; BPD, bronchopulmonary dysplasia; CI, confidence interval; SCr, serum creatinine; UOP, urine output.
Note: Data in table are presented as the number with the percentage (n [%]).
Odds ratios estimated from general estimating equation (GEE) unconditional logistic regression.
Odds ratios estimated from GEE ordinal logistic regression.
Odds ratios estimated among those with AKI from GEE multinomial logistic regression with urine output as the referent group.
After adjusting for GA, birth weight, ethnicity, and 1- and 1-minute Apgar’s scores, moderate or severe BPD or death was associated with a two-fold increased likelihood of AKI (OR = 2.10, 95% CI: 1.15–3.83; ►Table 3). This association, though, was limited only to those between 29 and 32 weeks of GA (OR = 4.21, 95% CI: 2.07–8.61).For those <29 weeks of GA, there was no association seen (OR = 1.15, 95% CI: 0.47–2.86). There was no association with severe AKI overall and the composite outcome, (OR = 1.66, 95% CI: 0.64–4.34), among those <29 weeks (OR = 1.26, 95% CI: 0.44–3.62), or 29–32 weeks (OR = 2.33, 95% CI: 0.44–12.23).
Table 3.
Crude and adjusted odds ratiosa for moderate or severe bronchopulmonary dysplasia or death stratified by GA group
| Crude OR (95% CI) | GA-adjustedb OR (95% CI) | p-Value | Adjustedc OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Overall (n = 546) | |||||
| Any AKI | 5.12 (2.78–9.42) | 2.25 (1.26–4.01) | 0.01 | 2.10 (1.15–3.83) | 0.02 |
| Severe AKI | 4.43 (1.73–11.40) | 1.87 (0.75–4.64) | 0.18 | 1.66 (0.64–4.34) | 0.30 |
| <29 weeks (n = 267) | |||||
| Any AKI | 3.13 (1.52–6.41) | 1.35 (0.62–2.93) | 0.45 | 1.15 (0.47–2.86) | 0.76 |
| Severe AKI | 2.55 (1.15–5.66) | 1.36 (0.56–3.26) | 0.50 | 1.26 (0.44–3.62) | 0.67 |
| 29–32 weeks (n = 279) | |||||
| Any AKI | 4.17 (2.07–8.38) | 3.82 (1.84–7.96) | <0.001 | 4.21 (2.07–8.61) | <0.001 |
| Severe AKI | 2.64 (0.67–10.31) | 2.65 (0.59–11.97) | 0.20 | 2.33 (0.44–12.23) | 0.32 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; GA, gestational age; OR, odds ratio.
Estimated from a general estimating equation logistic regression.
Adjusted for gestational age.
Adjusted for gestational age, ethnicity birth weight, and 1-/5-minute Apgar’s score.
Discussion
The results of this study suggest that AKI is associated with development of BPD in prematurely born neonates. After controlling for known potential confounders, premature infants born between 29 and 32 weeks of GA who developed AKI and had an increased prevalence of moderate or severe BPD or death.
Infants born between 29 and 32 weeks of GA have lower rates of BPD than more prematurely born neonates (those born ≤29 weeks of GA) given their more developed lung parenchyma and pulmonary vasculature.1,18,21 However, it is in this GA group that we found the strongest relationship between AKI and BPD. There are several possible reasons for this finding. First, premature infants may have different pathophysiologic processes contributing to the development of BPD depending on GA.22 Growing evidence suggest that infants in this GA may be at a critical point of pulmonary angiogenesis.23 It may be, therefore, that AKI may potentiate lung injury more prominently in this group due to disruption of postnatal lung development and impaired angiogenesis leading to impaired alveolarization.24 Second, this finding could represent organ crosstalk and an interplay between AKI and BPD, two inflammatory multihit processes that lead to substantial transcription changes in both organ systems.25 Additionally, given the less-critically-ill status of these infants, fewer confounders may exist in the relationship between kidney and lung injury allowing a more clear assessment of this association.
We did not find a dose-dependent association between severity of AKI and the combined outcome. Neonates with AKI of all stages had similar likelihood of developing lung disease, regardless of the severity of their kidney injury. This suggests that either the currently used neonatal AKI diagnostic criteria do not fully capture the degree of injury, or that any degree of kidney injury is important and has clinical impact.26 Our findings further support the need to define AKI not only with functional markers (such as urine output and serum creatinine) but also with structural markers of kidney injury.27
Studies in other critically-ill patients with AKI report similar associations of worse lung outcomes in those with AKI.11,25,28,29 While the precise mechanisms underlying this relationship require further elucidation, this bidirectional interaction between kidney and lung appears to be mediated by cytokine activation, endovascular damage, and systemic inflammatory responses, leading to pulmonary injury and fibrosis.6 Existing animal-based research shows a systemic cascade of inflammatory responses disrupting lung homeo-stasis following AKI.8 AKI increases pulmonary neutrophil activity and increases the sensitivity of lungs to ongoing injury.6 This likely impacts premature infants who require proinflammatory mechanical ventilation to survive.19
Limitations
To our knowledge, this is the first study to examine the incidence and associations between AKI and BPD in premature infants using a contemporary definition of AKI including both serum creatinine and urinary output criteria and a standard definition of BPD. We acknowledge several limitations of this study. While utilizing AKI based on KDIGO definitions for infants is the current standard for this patient population, this may not be the optimal marker for detection of kidney dysfunction in prematurely born infants. Additionally, we included urine output criteria where available, which increased the diagnosis rate of AKI among this premature population but recognize that, there is little evidence for the urine output cutoffs in premature infants. Finally, as enrolled infants were from many NICUs with various standards of clinical care, differences in management including respiratory support may contribute to variable BPD rates between centers.3,20
Conclusion
In conclusion, we show that in premature infants born between 29 and 32 weeks of gestation, AKI is associated with moderate or severe BPD or death. These findings may have significant clinical care, cost, and quality of life implications given the long-term implications of BPD.21 Further work is needed to understand the bidirectional lung–kidney interaction in this fragile patient population through larger, prospective studies.
Supplementary Material
Acknowledgments
The authors would like to thank Krysta Smith (Department of Pediatrics, University of Alabama at Birmingham) for help with technical editing and proofreading of this manuscript.
Funding
Cincinnati Children’s Hospital Center for Acute Care Nephrology provided funding to create and maintain the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study Medidata Rave electronic database. The Pediatric and Infant Center for Acute Nephrology (PICAN) provided support for web meetings and for the Neonatal Kidney Collaborative (NKC) steering committee annual meeting at The University of Alabama at Birmingham (UAB), as well as support for two of the AWAKEN study investigators at UAB (D.A., R.G., and L.B.). PICAN is part of the Department of Pediatrics at UAB and is funded by Children’s of Alabama hospital, UAB Department of Pediatrics, UAB School of Medicine, and UAB Center for Clinical and Translational Sciences (National Institutes of Health grant UL1TR001417). Finally, the AWAKEN study at The University of New Mexico was supported by the Clinical and Translational Science Center at The University of New Mexico (National Institutes of Health grant UL1TR001449) and by The University of Iowa Institute for Clinical and Translational Science (grant U54TR001356). The AWAKEN study investigators at the Canberra Hospital at the Australian National University Medical School were supported by the Canberra Hospital Private Practice Fund, and investigators at University of Virginia Children’s Hospital were supported by a 100 Women Who Care Grant from the 100 Women Charitable Foundation. The funding sources for this study had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
All authors report no real or perceived conflicts of interest that could affect the study design, collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication. This study was supported by the NIH U01NS077953 (L.B., R.G., L.L., and D.A.) and T32DK007662 (MS). For full disclosure, we provide the additional list of authors’ other commitments and funding sources that are not directly related to this study. D.A. reports serving on the speaker board for Baxter and for the Acute Kidney Injury Foundation; he also reported receiving grant funding for studies not related to this work, grant R01 DK103608 from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, and grant R01 FD005092 from the National Institutes of Health/US Food and Drug Administration. S.G. reports receiving grant funding for studies not related to this work, grant R01 DK103608 from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. No other disclosures were reported.
References
- 1.Fanaroff AA, Stoll BJ, Wright LL, et al. ; NICHD Neonatal Research Network. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 2007;196(02):147.e1–147.e8 [DOI] [PubMed] [Google Scholar]
- 2.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129(06):1019–1026 [DOI] [PubMed] [Google Scholar]
- 3.Allen J, Zwerdling R, Ehrenkranz R, et al. ; American Thoracic Society. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med 2003; 168(03):356–396 [DOI] [PubMed] [Google Scholar]
- 4.Shima Y, Kumasaka S, Migita M. Perinatal risk factors for adverse long-term pulmonary outcome in premature infants: comparison of different definitions of bronchopulmonary dysplasia/chronic lung disease. Pediatr Int 2013;55(05):578–581 [DOI] [PubMed] [Google Scholar]
- 5.Jetton JG, Boohaker LJ, Sethi SK, et al. ; Neonatal Kidney Collaborative (NKC). Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 2017;1(03):184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd-O JM, Hristopoulos M, Scharfstein D, et al. Interactive effects of mechanical ventilation and kidney health on lung function in an in vivo mouse model. Am J Physiol Lung Cell Mol Physiol 2009;296(01):L3–L11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: the role of the kidney. Curr Opin Crit Care 2009;15(06):481–487 [DOI] [PubMed] [Google Scholar]
- 8.Basu RK, Wheeler DS. Kidney-lung cross-talk and acute kidney injury. Pediatr Nephrol 2013;28(12):2239–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland SM, Ji J, Sheikhi FH, et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 2013;8(10):1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996;275(19):1489–149 [PubMed] [Google Scholar]
- 11.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 2017;376(01):11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Askenazi D, Patil NR, Ambalavanan N, et al. Acute kidney injury is associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr Nephrol 2015;30(09):1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jetton JG, Guillet R, Askenazi DJ, et al. ; Neonatal Kidney Collaborative. Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front Pediatr 2016;4(10):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr 2012;24(02):191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selewski DT, Charlton JR, Jetton JG, et al. Neonatal acute kidney injury. Pediatrics 2015;136(02):e463–e473 [DOI] [PubMed] [Google Scholar]
- 16.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17(01):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163(07):1723–1729 [DOI] [PubMed] [Google Scholar]
- 18.Ehrenkranz RA, Walsh MC, Vohr BR, et al. ; National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(06):1353–1360 [DOI] [PubMed] [Google Scholar]
- 19.Balena-Borneman J, Ambalavanan N, Tiwari HK, Griffin RL, Halloran B, Askenazi D. Biomarkers associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr Res 2017;81 (03):519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen EA, Wright CJ. Bronchopulmonary Dysplasia: The Ongoing Search for One Definition to Rule Them All. J Pediatr 2018; 197:8–10 [DOI] [PubMed] [Google Scholar]
- 21.Baker CD, Abman SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology 2015;107(04):344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jobe AH. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am J Perinatol 2016;33(11):1076–1078 [DOI] [PubMed] [Google Scholar]
- 23.Thébaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 2007;175(10): 978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Paepe ME, Mao Q, Powell J, et al. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med 2006;173(02):204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 2008;19(03):547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zappitelli M, Ambalavanan N, Askenazi DJ, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res 2017;82(04): 569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endre ZH, Kellum JA, Di Somma S, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. ADQI Consensus on AKI Biomarkers and Cardiorenal Syndromes 2013:18230–44 [DOI] [PubMed] [Google Scholar]
- 28.Madershahian N, Liakopoulos OJ, Wippermann J, et al. The impact of intraaortic balloon counterpulsation on bypass graft flow in patients with peripheral ECMO. J Card Surg 2009;24(03): 265–268 [DOI] [PubMed] [Google Scholar]
- 29.McNicholas BA, Rezoagli E, Pham T, et al. ; ESICM Trials Group and the Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE (LUNG SAFE) Investigators. Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: a secondary analysis of a multicenter observational study. Crit Care Med 2019; 47(09):1216–1225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.