Abstract
Computerized targeted cognitive training (TCT) of auditory processing has been shown to improve verbal learning in several clinical trials of schizophrenia outpatients. Less is known, however, about the effectiveness of this promising intervention in more chronic, treatment-refractory patients who are treated in non-academic settings. This study aimed to determine whether TCT improves auditory processing, verbal learning, and clinical symptoms in SZ patients mandated to receive care at a locked residential rehabilitation center. Secondarily, potential factors that moderate TCT’s effectiveness including age, symptom severity, antipsychotic medication load, and duration of illness were examined. Schizophrenia patients were randomized to treatment as usual (TAU; n=22) or TAU augmented with TCT (TAU+TCT; n=24). Outcomes included a measure of auditory perception (Word-In-Noise test, WIN), verbal learning domain scores from the MATRICS Consensus Cognitive Battery (MCCB), and clinical symptoms (Scale for the Assessment of Positive Symptoms, SAPS; Scale for the Assessment of Negative Symptoms, SANS). TCT produced significant improvements in auditory perception (d=0.67) and verbal learning (d=0.65); exploratory analyses revealed a statistically significant reduction in auditory hallucinations (d =−0.58). TCT’s effects were only weakly, and mostly non-significantly, moderated by age, clinical symptoms, medication, and illness duration. These findings indicate that even highly symptomatic, functionally disabled patients with chronic illness benefit from this emerging treatment. Ongoing studies will examine the predictive utility of neurophysiological biomarkers and other characteristics assessed at baseline.
Keywords: Schizophrenia, Targeted Cognitive Training, Cognitive Remediation, Chronic Psychosis, Effectiveness
1. Introduction
Schizophrenia (SZ) is a disabling mental illness characterized by a wide range of impairments across multiple cognitive domains (Bilder et al., 2000; Butler et al., 1991; Gur et al., 2015; Kalkstein et al., 2010; Seidman et al., 2010). Deficits in higher-order cognitive processes can be explained, in part, by abnormalities in early auditory information processing, which have been consistently identified in chronic, recent onset, and unmedicated SZ patients, as well as in individuals at high clinical risk for developing psychosis (Frommann et al., 2008; Jahshan et al., 2012; Javitt and Sweet, 2015; Joshi et al., 2018; Perez et al., 2017; Rissling et al., 2012; Thomas et al., 2017).
Although currently approved medications do not meaningfully improve deficits in early auditory information processing, targeted cognitive training (TCT) is an emerging therapeutic intervention that has shown promise in remediating auditory perception and verbal learning in randomized controlled trials conducted with patients diagnosed with SZ (Fisher et al., 2016). Specifically, TCT is a computerized, neuroplasticity-based form of cognitive training that utilizes a suite of exercises that target specific auditory-based sensory, memory, and learning processes. Exercises are self-paced, designed to be engaging for the user, and adaptive in order to maintain difficulty levels at a user’s upper performance threshold by both incrementally adjusting exercise properties (e.g., exposure duration, stimulus similarity, span length) and providing trial-by-trial performance feedback (Vinogradov et al., 2012). Engaging SZ patients with incremental, recursive, feedback-driven learning targeting the auditory system has been shown to drive plasticity in cortical activation patterns related to both sensory representations as well as higher-level cognitive processes (Dale et al., 2016; Fisher et al., 2009; Loewy et al., 2016; Mahncke et al., 2006; Nahum et al., 2013; Perez et al., 2017; Tarasenko et al., 2016; Vinogradov et al., 2012).
Despite the initial successes which convincingly demonstrated the efficacy of TCT under controlled laboratory settings, it is unclear whether TCT could be similarly beneficial in chronically symptomatic, functionally disabled cohorts of SZ patients, who are perhaps most in need of treatment. Treatment studies conducted in academic settings often exclude such subgroups of SZ patients, particularly those who are currently, or were recently, in inpatient or other residential treatment centers (Bowie et al., 2012; Dale et al., 2016; Fisher et al., 2009). Indeed, meta-analyses of cognitive training interventions in SZ tend to be heavily represented by studies of patients with mild to moderate impairment (McGurk et al., 2007; Wykes et al., 2011). These strategies are not without merit: such exclusion criteria are critical for the early determination of whether an intervention is efficacious under optimized conditions. It has also been suggested that patients with significant functional disability, longer disease chronicity, higher levels of clinical symptoms, or those who are treatment-resistant to antipsychotic medications may not benefit from treatments such as TCT (e.g., Medalia and Richardson, 2005; Ramsay et al., 2018).
While several different approaches to cognitive remediation have been used in SZ patients across a wide range of symptoms severity (Lewandowski, 2016; Lindenmayer et al., 2017; Loewy et al., 2016; McGurk et al., 2007; Wykes et al., 2011), results are mixed regarding variables that moderate outcomes. Some reports suggest that better cognitive training outcomes are found in SZ patients with a higher level of baseline cognitive functioning and lower symptom severity, whereas others have shown that illness severity does not significantly moderate outcomes (Bell et al., 2003; Lewis et al., 2003).
In order to address this knowledge gap, we aimed to determine whether TCT improves outcomes in a cohort of stabilized but symptomatic, treatment-refractory patients who have been involuntarily mandated to receive care at a non-academic, locked residential treatment facility. Consistent with prior randomized controlled studies of schizophrenia outpatients (Fisher et al., 2009; Fisher et al., 2010), we hypothesized that TCT would drive improvements in auditory and verbal domains, particularly verbal learning. Additionally, we aimed to examine factors that could moderate TCT’s effectiveness, hypothesizing that older age, more severe clinical symptoms, greater medication load, and longer illness duration would negatively moderate the positive effects of TCT.
2. Methods
2.1. Participants and Design
Participants included 46 patients with psychosis recruited from a community-based residential treatment program following acute hospitalization and then at least 1-month of stabilization and acclimation to the center. The transitional care facility serves as a bridge between acute crisis and independent living. All participants enrolled in the study were under public guardianship/conservatorship by San Diego or Los Angeles Counties, or by a private party; in California, this requires individuals to be “gravely disabled” (i.e., unable to provide for basic needs) and/or is a danger to himself or herself or others. While in the program, patients undergo phased community reintegration contingent upon the ability to maintain safety, instrumental activities of daily living (e.g., basic hygiene), engage in treatment, etc. Patients often discharge to a lower level of care (e.g., Board and Care Home), usually after at least 6 months of care.
The study used a parallel design with stratified random assignment by sex, age, and ethnicity to either treatment as usual (TAU; n = 22) or TAU augmented with TCT (TAU+TCT; n = 24). This was accomplished by stratifying individuals into discrete levels of sex, age (over age 44 vs. under age 45), and race, and then using a random number generator to assign participants to conditions in a manner that ensured balanced sample size. The primary inclusion criterion was meeting formal diagnostic criteria for schizophrenia or schizoaffective disorder verified using an abbreviated Structured Clinical Interview for DSM-IV-TR (First et al., 2002). Exclusion criteria included inability to consent, limited English proficiency, previous significant head injury with greater than 30 minutes loss of consciousness, neurological illness, severe systemic illness, or current mania (due to concerns that patients with current mania might not actively engage in treatment or might disrupt others). Additionally, we excluded a small number of participants who, by rule of the facility, lost their privileges to leave their locked units due to symptom exacerbation and/or disruptive behavior. Initial eligibility was determined by patients’ clinical treatment teams who were blinded to treatment assignment, assessment results, and study activities. After potential participants indicated interest in the study, written informed consent was obtained with subsequent written approvals ultimately granted from public guardians/conservators before initiating any study activities. The Institutional Review Board of University of California, San Diego approved all experimental procedures (IRB#130874).
2.2. Intervention
Participants randomized to the TAU group received their usual care and study assessments only. TAU followed a biopsychosocial model, including medication management, individual and group therapy, and structured social activities. Participants randomized to TAU+TCT additionally completed 1 hour of training per day 3–5 days per week for up to 40 hours. TCT was administered in a dedicated classroom located in a building on the program grounds with groups of up to five participants at a time using individual laptop computers with headphones. Six exercises supplied by BrainHQ by Posit Science Corporation were administered (Posit Science, 2016): Sound Sweeps (targets auditory processing speed): Two successive frequency-modulated tone sweeps are presented and participants indicate whether the frequency increased or decreased within each tone; Fine Tuning (targets auditory perception and processing speed): Participants indicate which one of two confusable syllables were presented; Syllable Stacks (targets auditory memory): Users report the order of presented syllables in a serial memory span task; Memory Grid (targets auditory memory): Participants match identical cards representing syllables; To-Do List Training (targets auditory memory): Participants see a grid of everyday items (e.g., plant, carrots, shovel) and select the items in accordance with spoken instructions; Rhythm Recall (target auditory memory): Participants recreate auditory melodies.
2.3. Outcome Measures
Participants were assessed on measures of cognition, auditory perception, and clinical symptoms at baseline and at the end of treatment (approximately 12–13 weeks post randomization). Cognition was assessed using age and gender corrected T-scores from the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2008). The Verbal Learning subscale served as the primary dependent variable; secondary cognition measures included the Neurocognitive Composite (MCCB-NC), Speed of Processing, Attention and Vigilance, Working Memory, Visual Learning, and Reasoning and Problem Solving domain T-scores. Auditory perception was assessed using a modified version of the Words-in-Noise (WIN) test (Wilson and Watts, 2012). The WIN requires examinees to listen to, and accurately repeat 5 words embedded in background noise (conversations) at several different dB levels. Auditory perception was operationalized as total correct scores on the WIN (maximum score = 35). Symptoms were assessed using the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984a) and the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984b). Negative and positive symptom were operationalized as summed SANS and SAPS global rating scores, respectively. Chlorpromazine equivalent doses (CPZs) were calculated (Woods, 2003) using data on patients’ prescribed medication.
2.4. Analyses
Data were analyzed using linear mixed models (Hox, 2010). Consistent with the intent-to-treat approach, all data were analyzed regardless of dropout or study compliance. This approach avoids bias that might be introduced if the data are not missing completely at random. Preliminary analyses suggested approximately one to three statistical outliers per outcome (see Cohen et al., 2003) that were likely due to variability in some patients’ clinical states over time. To minimize the impact of outliers, robust linear mixed-effects models were fitted to the data using the ‘robustlmm’ package for R (Koller, 2016). We found that using this more robust estimator produced effects size estimates that were smaller in comparison to estimates based on data where outliers were simply removed. Degrees of freedom were approximated using the Kenward-Roger approach implemented in the ‘pbkrtest’ package (Halekoh and Højsgaard, 2014). Simple approximations of effect size reported as Cohen’s d (small = 0.2; medium = 0.5; large = 0.8) were produced using the ‘compute.es’ package (Del Re, 2010) with the estimated t statistic and observed degrees of freedom. This approach is expected to be slightly conservative in comparison to others used to approximate Cohen’s d within the context of linear mixed-effects models (cf. Kleiman, 2017). Effect sizes for moderator analyses were quantified using standardized regression coefficients (bSTD).
Outcomes were regressed onto contrast-coded treatment and time variables as well as their interaction. Only the interaction terms were examined for significance and effect size. Models also included random intercepts. The moderating effects of age, clinical symptoms, medication, and illness duration were assessed in separate models that included all three-way interactions, two-way interactions, and main effects. Analyses of moderation were restricted to a priori hypothesized outcomes in auditory/verbal domains. Only the three-way interactions between moderators, treatment, and time were examined for significance and effect size.
3. Results
Demographic characteristics, clinical symptoms, CPZs, days to follow-up assessment, and training hours completed are reported in Table 1. TAU+TCT participants who completed training acquired significantly fewer hours (M = 27.94) than the 40 targeted (t(15)= −4.73, p < 0.001). The TAU and TAU+TCT groups did not differ on any of the baseline demographic variables or outcome measures. A CONSORT flow diagram with enrollment, exclusion, and discontinuation totals is shown in Figure 1. A total of 8 participants randomized to TAU+TCT and 2 assigned to TAU did not complete the study; however, the completion rate did not significantly differ between groups (χ2(1) = 2.668, p =.10). Dropout in the TAU+TCT group was generally caused by the time required (1h/day) to comply with the training protocol; no participants dropped out because of difficulty using the training program or computer. Dropout was not significantly correlated with age, gender, and baseline MCCB, SAPS, SANS, or CPZ scores (all ps > .05).
Table 1.
Demographic and Clinical Characteristics at Baseline
| Treatment as Usual | Targeted Cognitive Training | p | |
|---|---|---|---|
| Sample Size | 22 | 24 | |
| Age | 35.73 (13.00) | 34.54 (12.13) | 0.75 |
| Gender: Male | 9 (41%) | 13 (54%) | 0.55 |
| Hispanic | 6 (27%) | 4 (17%) | 0.61 |
| Race | |||
| African American | 3 (14%) | 5 (21%) | 0.51 |
| Asian | 2 (9%) | 1 (4%) | |
| Caucasian | 12 (55%) | 13 (54%) | |
| More than one race | 5 (23%) | 3 (12%) | |
| Native American | 0 (0%) | 2 (8%) | |
| Education | 11.95 (2.17) | 11.71 (1.99) | 0.69 |
| Chlorpromazine Equivalents | 982.54 (758.10) | 1329.42 (972.78) | 0.82 |
| Illness Duration | 15.23 (12.78) | 16.12 (13.67) | 0.82 |
| SAPS | 5.36 (5.02) | 6.46 (4.26) | 0.62 |
| PSYRATS-AH | 7.32 (11.10) | 8.79 (11.64) | 0.66 |
| SANS | 13.09 (3.41) | 12.96 (4.19) | 0.22 |
| MCCB-NC Composite | 23.95 (13.71) | 23.12 (12.14) | 0.83 |
| Days to Follow-Up | 99.30 (24.26) | 89.44 (19.79) | 0.20 |
| Hours of Training | 27.94 (10.20) |
Note: Means and standard deviations are reported for continuous variables. Counts and percentages are reported for discrete variables. Groups were compared using regression for continuous variables and χ2 tests for categorical variables. Education is in years completed. SAPS = Scale for the Assessment of Positive Symptoms reported as total global rating scores; SANS = Scale for the Assessment of Negative Symptoms reported as total global rating scores; PSYRATS-AH = Psychotic Symptom Rating Scales auditory hallucination subscale reported as total scores; MCCB-NC = MATRICS Consensus Cognitive Battery neurocognitive composite reported as age and gender corrected T-scores.
Figure 1.
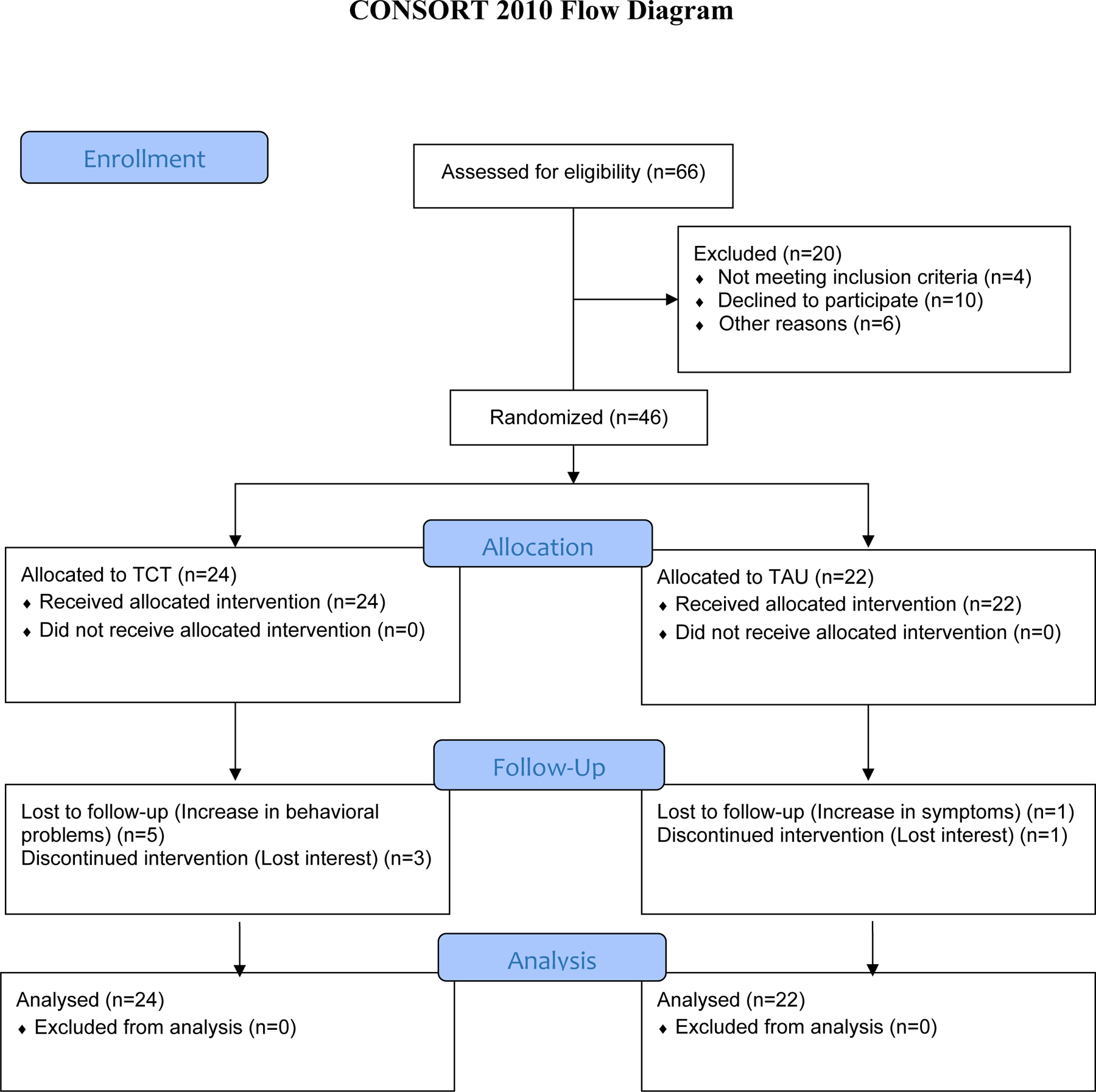
CONSORT flow diagram. Analyses included participants lost to follow-up. Participants with increased symptoms or behavioral problems, by rule of the facility, lost their privileges to leave their locked units and participate in the study.
Treatment-by-time interaction effects for cognition and auditory perception are reported in Table 2. TCT produced medium-sized, positive, and significant effects on MCCB Verbal Learning T-scores and WIN total scores. Figure 2 displays the results for MCCB Verbal Learning T-scores as line plots. No other cognitive outcomes demonstrated statistically significant effects. Treatment by time interaction effects for clinical symptoms and medication (SAPS, SANS, and CPZs) are also reported in Table 2. Although none of the interaction effects reached statistical significance, TCT yielded a moderate effect size on reducing global SAPS ratings. Exploratory analyses of individual SAPS items revealed statistically significant reductions in auditory hallucinations (b = −0.58, SE = 0.49, df = 36.77, t = −1.96, d = −0.58) and voices conversing (b = −1.33, SE = 0.59, df = 37.62, t = −2.25, d = −0.67).
Table 2.
Effects of Targeted Cognitive Training on Cognition, Auditory Perception, Clinical Symptoms, and Medication
| TAU | TCT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Time 1 M (SD) | Time 2 M (SD) | Time 1 M (SD) | Time 2 M (SD) | b | SE | df | t | d |
| Speed of Processing | 27.73 (12.88) | 29.75 (10.62) | 29.25 (12.83) | 31.00 (13.44) | −0.07 | 2.74 | 32.33 | −0.03 | −0.01 |
| Attention & Vigilance | 31.09 (14.47) | 32.65 (16.65) | 29.83 (11.13) | 33.81 (13.66) | −0.26 | 3.00 | 32.05 | −0.09 | −0.03 |
| Working Memory | 32.36 (14.90) | 36.05 (17.50) | 31.75 (13.98) | 35.31 (11.25) | −2.33 | 2.36 | 31.43 | −0.99 | −0.29 |
| Verbal Learning | 33.09 (6.09) | 33.75 (9.33) | 32.75 (6.19) | 37.62 (6.18) | 4.13 | 1.86 | 33.22 | 2.21* | 0.65 |
| Visual Learning | 31.27 (11.22) | 33.35 (11.53) | 29.88 (10.83) | 32.25 (12.12) | −1.70 | 3.09 | 33.10 | −0.55 | −0.16 |
| Reas. & Prob. Solving | 39.82 (9.66) | 41.60 (10.31) | 41.12 (7.99) | 44.00 (7.51) | 1.35 | 4.35 | 37.61 | 0.31 | 0.09 |
| MCCB-NC | 23.95 (13.71) | 26.80 (14.77) | 23.12 (12.14) | 28.56 (9.80) | 0.69 | 2.48 | 31.72 | 0.28 | 0.08 |
| Words-In-Noise | 25.91 (3.70) | 25.15 (3.17) | 24.71 (2.93) | 26.38 (2.13) | 2.16 | 0.95 | 33.87 | 2.28* | 0.67 |
| SAPS | 4.45 (5.14) | 3.79 (4.08) | 5.12 (4.00) | 3.25 (4.37) | −1.38 | 0.88 | 31.12 | −1.57 | −0.46 |
| SANS | 6.18 (3.97) | 7.26 (4.81) | 7.75 (4.50) | 7.31 (3.91) | −0.74 | 1.62 | 33.80 | −0.46 | −0.13 |
| CPZs | 982 (758) | 993 (851) | 1329 (972) | 1190 (1082) | −13.42 | 94.38 | 28.44 | −0.14 | −0.04 |
Note. TAU = Treatment as Usual; TCT = Targeted Cognitive Training; MCCB-NC = MATRICS Consensus Cognitive Battery Neurocognitive Composite; SAPS = Scale for the Assessment of Positive Symptoms; SANS = Scale for the Assessment of Negative Symptoms; CPZs = chlorpromazine equivalent doses.
p < .05. Cohen’s d based on parameter estimates from linear mixed-effects models.
Figure 2.

Line plots (with standard errors) showing mean Verbal Learning T-scores before and after treatment (Time 1 vs. Time 2) between TAU and TAU+TCT participants. Data from statistical outliers have been omitted in the results used to produce this figure.
Given that participants completed significantly fewer hours of TCT than were targeted for training, we examined whether the number of TCT hours completed moderated outcomes. The resulted indicated that neither change in MCCB Verbal Learning T-scores (p = 0.75) nor WIN total scores (p = 0.51) was moderated by hours completed.
Moderating effects of age, clinical symptoms (SAPS and SANS), medication (CPZs), and illness duration on outcomes in auditory and verbal domains are reported in Table 3. Only two of the effects were statistically significant: positive moderating effects of age (bSTD = 0.20) and CPZs (bSTD = 0.23) on Verbal Learning T-scores. That is, TCT was significantly more effective for older adults and also those who were prescribed higher antipsychotic loads. Otherwise, the majority of the moderating effects were small (i.e., absolute bSTD ≤ .12), suggesting that the effectiveness of TCT was not meaningfully affected in a negative manner by age, clinical symptoms, medication, and illness duration.
Table 3.
Moderating Effects of Age, Clinical Symptoms, Medication, and Illness Duration
| Outcome | Moderator | b | SE | df | t | bSTD |
|---|---|---|---|---|---|---|
| Verbal Learning | Age | 0.47 | 0.15 | 31.70 | 3.06* | 0.20 |
| Words-In-Noise | Age | 0.02 | 0.09 | 33.25 | 0.19 | 0.02 |
| Verbal Learning | SAPS | 0.27 | 0.41 | 30.84 | 0.65 | 0.04 |
| Words-In-Noise | SAPS | −0.20 | 0.21 | 31.21 | −0.95 | −0.07 |
| Verbal Learning | SANS | −0.09 | 0.36 | 32.16 | −0.26 | −0.02 |
| Words-In-Noise | SANS | −0.07 | 0.19 | 33.01 | −0.35 | −0.03 |
| Verbal Learning | CPZs | 0.50 | 0.15 | 32.48 | 3.24* | 0.23 |
| Words-In-Noise | CPZs | 0.06 | 0.09 | 34.09 | 0.71 | 0.06 |
| Verbal Learning | Illness Duration | −0.05 | 0.39 | 31.71 | −0.13 | −0.01 |
| Words-In-Noise | Illness Duration | −0.29 | 0.18 | 32.06 | −1.56 | −0.12 |
Note. SAPS = Scale for the Assessment of Positive Symptoms; SANS = Scale for the Assessment of Negative Symptoms; CPZs = chlorpromazine equivalent doses.
p < .05.
4. Discussion
The field of cognitive training is still in its relative infancy, described as existing somewhere between the “wild west” and the “golden age” (Vinogradov et al., 2013). Nevertheless, the literature has supported the efficacy of targeted cognitive training of auditory processing for improving cognition in patients with SZ when studied in academic research settings. Our study extends this literature by providing support for the effectiveness of TCT in chronic, treatment refractory patients mandated to locked residential care in the community; a subgroup of patients that—while relatively small—relies on a disproportionately large share of mental healthcare resources (Zhu et al., 2008). In this context, TCT significantly improved verbal learning and auditory perception scores, and also reduced the severity of auditory hallucinations. The results are particularly salient given that the TCT modules selected were specifically designed to improve auditory systems. TCT-associated improvements occurred in the absence of changes in antipsychotic load as measured by CPZ equivalent scores. The magnitudes of these effects were consistent with meta-analytic research showing improvements in cognition following other cognitive remediation approaches (McGurk et al., 2007; Wykes et al., 2011), as well as specific research examining auditory-based TCT (Fisher et al., 2009).
It is important to note the challenges of implementing a structured and time-consuming treatment within the context of a real-world residential care facility. Obstacles encountered during the course of this effectiveness trial included maintaining participants’ willingness and motivation to engage in daily treatment, participants losing their privileges to leave their locked units due to symptom exacerbation and/or disruptive behavior, as well as several challenges related to maintaining the adequate resources, including facility support, physical space and personnel necessary to implement and monitor the treatment. Also noteworthy is the observation that some patients expressed concern that participation in the study would detract from other psychosocial treatments they were receiving. Despite this concern, we have previously reported that participants randomized to TAU+TCT engaged in a greater number of psychosocial treatment activities compared to participants randomized to TAU (Thomas et al., in press).
Meta-analyses have suggested that demographic and clinical factors such as symptoms have only a minor impact on outcomes after a wide range of cognitive remediation interventions (McGurk et al., 2007; Wykes et al., 2011). However, recent studies suggest that cognitive training may be significantly more effective for less symptomatic patients with lower antipsychotic load (e.g., Lindenmayer et al., 2017; Vita et al., 2013). These findings raise the concern that certain forms of cognitive training may not be effective for severely- and persistently-ill patients who are often treated, at some point over the course of illness, in inpatient and other residential treatment centers.
Our results do not support the concern that chronically-ill, highly disabled patients do not benefit from TCT. Participants showed improvements in both auditory perception and verbal learning, and the effects were not negatively moderated by age, clinical symptoms, medication, and illness duration. Notably, the average MCCB-NC Composite score among patients enrolled in the present study was nearly three standard deviations below the normative sample mean, and approximately 5 to 10 T-score points lower than means obtained in more typical outpatient samples (e.g., August et al., 2012). Also, compared to samples collected in more typical, academic-based outpatient cognitive training studies, participants in this study were treated with approximately twice the average antipsychotic medication dose (e.g., Sartory et al., 2005). Such antipsychotic loads are similar to those used in state hospitals for treatment-resistant psychosis (Owen et al., 2003; Owen et al., 2002). That TCT was effective in those with high antipsychotic loads is encouraging since many antipsychotics are known to have significant anticholinergic properties, and higher anticholinergic burden has been previously reported to moderate the effectiveness of TCT (Minzenberg et al., 2004; Vinogradov et al., 2009).
Previous clinical trials of TCT and related interventions often exclude more symptomatic patients (Bowie et al., 2012; Dale et al., 2016; Fisher et al., 2009), a choice which strengthens methodological rigor, but also might imply an expectation that these patients cannot, or will not, participate, or that they cannot benefit. These expectations are rooted in literature that is evolving; for example, baseline cognition has been reported to be a strong predictor of functional outcomes (Kurtz et al., 2008). Our findings, however, indicate that patients with SZ can both participate and benefit from TCT even at high levels of disability, and that such participation may even promote robust improvements in functionally relevant domains of cognition. The present results of enhanced verbal learning is particularly salient given previous seminal reports which suggest that better verbal learning is associated with improved functional outcomes (Green, 1996; Green et al., 2000; Kurtz et al., 2008) and offers hope that more can be done to remediate these disabling cognitive impairments, even in patients with chronic and refractory illness.
Results of this study should be interpreted in light of certain caveats. First, the non-completion rate was modestly higher in patients randomized to TAU+TCT compared to TAU alone, and TAU+TCT participants completed, on average, only 70% of the training hours targeted. Thus, there are still feasibility and tolerability concerns to be addressed in the context of delivering TCT within locked residential settings. Most notably, TCT is demanding. TCT participants in our study had to maintain approval to leave their unit and come to the building where TCT was administered multiple times per week for at least 1 hour. The privilege to leave their building was based on appropriate behavior. Participants who lost their privilege were able to continue with TAU, but not TCT, which may therefore exaggerate differences in attrition rates between groups. Beyond these setbacks, some participants occasionally declined training on certain days due to lack of interest and/or motivation. Thus, while other studies have demonstrated efficacy in SZ patients using an active control condition, future studies with an active control or other treatment comparison group are needed to replicate and extend the present findings in more impaired populations. Despite lost subjects and fewer hours of training, we were nonetheless able to detect significant treatment effects in critical cognitive and clinical domains, which underscores the effectiveness of the training. Second, medications were not experimentally controlled. We cannot exclude the possibility that medication interactions with TCT that may have influenced the results. Future larger-scale investigations are needed to carefully parse the impact of medications on cognitive training outcomes. Lastly, although all participants were stabilized at the time of enrollment, accounting for the heterogeneity of the sample and the complexity of implementing TCT in a locked community-based residential facility should not be underestimated in future trials that aim to “scale up” this intervention for more widespread use. We adhered to a statistical analysis plan to conservatively minimize the influence of potential outliers on outcome variables (robust regression). When outliers were simply removed entirely, it is noteworthy that effect sizes for TCT were larger. Specifically, the Cohen’s d for verbal learning changed from 0.65 to 0.82 and the d for auditory perception changed from 0.67 to 0.77.
Rather than being an outlier, our study adds to those by McGurk and colleagues already showing that cognitive training therapies such as TCT can be implemented in a variety of settings, including psychosocial clubhouses (McGurk et al., 2010), supported employment programs (McGurk et al., 2015), and other community-based psychiatric rehabilitation programs (McGurk et al., 2017). However, it is also important to recognize that not all patients benefit. In the current study, approximately 31% of patients randomized to TCT showed essentially no response in verbal learning (i.e., using Cohen’s d equivalent of less than .20 as a cutoff). Non-response in SZ patients is common, not only in trials of TCT specifically, but also with cognitive training in general, even in trials which demonstrate medium-to-large effect size gains at the cohort-level (Biagianti et al., 2016). Although we aimed for participants to complete 40 hours of cognitive training, they completed, on average, only 75% of this total. Nonetheless, we found that number of training hours completed did not moderate effectiveness. This is in contrast to the literature, which suggests that hours of training does, specifically, moderate the impact of cognitive training on verbal learning outcomes (McGurk et al., 2007).
Developing strategies that improve TCT delivery in a way that further reduces the rate of non-response is of critical importance to the field for scaling up TCT as a rehabilitative strategy. Future studies are needed to identify biomarkers and other variables that can be used to predict and monitor response to cognitive training, and that can help inform treatment stratification. Given the specificity of our results—that is, the auditory based trainings specifically improved auditory domains of cognition—biomarkers and training modules might be paired to target specific perceptual and cognitive systems. Acute biomarker sensitivity to cognitive training (Perez et al., 2017) offers great promise for “bending the curve” on psychosis outcomes, even in symptomatic patients with chronic illness receiving care in real-world community settings (Light and Swerdlow, 2015).
Bottom-up models suggest that improvements in early auditory information processing—as was targeted by the TCT modules selected for this study—should result in better functional outcomes via improved cognition and reduced clinical symptoms (Javitt, 2009; Thomas et al., 2017). However, the pathway from improved information processing to improved functioning might require assistance. Evidence suggests that encouraging patients to use newly improved cognitive skills in complex and demanding situations can better prepare them to translate their cognitive gains into improved real-world functioning (Medalia and Choi, 2009; Medalia and Richardson, 2005). Moreover, cognitive training has been found to be more beneficial when implemented within a comprehensive rehabilitation program (McGurk et al., 2007). Additional studies are needed to determine not only whether TCT improves functional outcomes within this population, but also, if so, how best to encourage positive change.
Supplementary Material
Acknowledgements
The authors wish to thank George B. Handran and the Sidney R. Baer, Jr. Foundation for their generous support of this research. We also wish to thank all of the participants and non-author support staff that made this study possible, including the following key personnel: Sean Pianka, Sonia Rackelmann, and Alexandra L. Shiluk.
5 Author Disclosure
Funding Body Agreements and Policies
Research reported in this publication was supported by the Sidney R. Baer, Jr. Foundation, the Brain and Behavior Research Foundation, VISN-22 Mental Illness Research Education and Clinical Center (MIRECC), and National Institute of Mental Health of the National Institutes of Health (K23 MH102420).
Footnotes
Conflict of Interest
Dr. Light reports having been a consultant to Astellas, Boehringer-Ingelheim, Dart Neuroscience, Heptares, Lundbeck, Merck, NeuroSig, Neuroverse, and Takeda.
Dr. Attarha is a research scientist and a stock holder at Posit Science Corporation, the company that developed the computerized brain training program.
6 References
- Andreasen NC, 1984a. Modified Scale for the Assessment of Negative Symptoms (SANS). University of Iowa, Iowa City. [Google Scholar]
- Andreasen NC, 1984b. Scale for the Assessment of Positive Symptoms (SAPS). University of Iowa, Iowa City. [Google Scholar]
- August SM, Kiwanuka JN, McMahon RP, Gold JM, 2012. The MATRICS Consensus Cognitive Battery (MCCB): Clinical and cognitive correlates. Schizophrenia Research 134(1), 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M, Bryson G, Wexler BE, 2003. Cognitive remediation of working memory deficits: Durability of training effects in severely impaired and less severely impaired schizophrenia. Acta Psychiat Scand 108(2), 101–109. [DOI] [PubMed] [Google Scholar]
- Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S, 2016. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology 30(8), 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA, 2000. Neuropsychology of first-episode schizophrenia: Initial characterization and clinical correlates. Am J Psychiat 157(4), 549–559. [DOI] [PubMed] [Google Scholar]
- Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD, 2012. Combined Cognitive Remediation and Functional Skills Training for Schizophrenia: Effects on Cognition, Functional Competence, and Real-World Behavior. Am J Psychiat 169(7), 710–718. [DOI] [PubMed] [Google Scholar]
- Butler RW, Jenkins MA, Geyer MA, Braff DL, 1991. Wisconsin card sorting deficits and diminished sensorimotor gating in a discrete subgroup of schizophrenic patients, in: Tamminga CA, Schulz SC (Eds.), Advances in Neuropsychiatry & Psychopharmacology, Volume I: Schizophrenia. Raven Press, New York, pp. 163–168. [Google Scholar]
- Cohen J, Cohen J, West SG, Aiken LS, 2003. Applied multiple regression/correlation analysis for the behavioral sciences, 3rd ed. L. Erlbaum Associates, Mahwah, N.J. [Google Scholar]
- Dale CL, Brown EG, Fisher M, Herman AB, Dowling AF, Hinkley LB, Subramaniam K, Nagarajan SS, Vinogradov S, 2016. Auditory cortical plasticity drives training-induced cognitive changes in schizophrenia. Schizophrenia Bull 42(1), 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re AC, 2010. compute.es: Compute Effect Sizes. R package version 0.2 [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S, 2009. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiat 166(7), 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S, 2010. Neuroplasticity-based cognitive training in schizophrenia: An interim report on the effects 6 months later. Schizophrenia Bull 36(4), 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommann I, Brinkmeyer J, Ruhrmann S, Hack E, Brockhaus-Dumke A, Bechdolf A, Wolwer W, Klosterkotter J, Maier W, Wagner M, 2008. Auditory P300 in individuals clinically at risk for psychosis. Int J Psychophysiol 70(3), 192–205. [DOI] [PubMed] [Google Scholar]
- Green MF, 1996. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiat 153(3), 321–330. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J, 2000. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophrenia Bull 26(1), 119–136. [DOI] [PubMed] [Google Scholar]
- Gur RC, Braff DL, Calkins ME, Dobie DJ, Freedman R, Green MF, Greenwood TA, Lazzeroni LC, Light GA, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Gur RE, 2015. Neurocognitive performance in family-based and case-control studies of schizophrenia. Schizophrenia Research 163(1–3), 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halekoh U, Højsgaard S, 2014. A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models: The R package pbkrtest. J Stat Softw 59(9). [Google Scholar]
- Hox JJ, 2010. Multilevel analysis: Techniques and applications. Routledge/Taylor & Francis Group, New York. [Google Scholar]
- Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA, 2012. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychological Medicine 42(1), 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2009. When doors of perception close: Bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psycho 5, 249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Sweet RA, 2015. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nature Reviews Neuroscience 16(9), 535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi YB, Breitenstein B, Tarasenko M, Thomas ML, Chang WL, Sprock J, Sharp RF, Light GA, 2018. Mismatch negativity impairment is associated with deficits in identifying real-world environmental sounds in schizophrenia. Schizophrenia Research 191, 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC, 2010. Neurocognition in schizophrenia. Current Topics in Behavioral Neurosciences 4, 373–390. [DOI] [PubMed] [Google Scholar]
- Kleiman E, 2017. EMAtools: Data Management Tools for Real-Time Monitoring/Ecological Momentary Assessment Data, CRAN package repository. [Google Scholar]
- Koller M, 2016. robustlmm: An R package for robust estimation of linear mixed-effects models. J Stat Softw 75(6), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Wexler BE, Fujimoto M, Shagan DS, Seltzer JC, 2008. Symptoms versus neurocognition as predictors of change in life skills in schizophrenia after outpatient rehabilitation. Schizophrenia Research 102(1–3), 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, 2016. Cognitive Remediation for the Treatment of Cognitive Dysfunction in the Early Course of Psychosis. Harvard Rev Psychiat 24(2), 164–172. [DOI] [PubMed] [Google Scholar]
- Lewis L, Unkefer EP, O’Neal SK, Crith CJ, Fultz J, 2003. Cognitive rehabilitation with patients having persistent, severe psychiatric disabilities. Psychiatr Rehabil J 26(4), 325–331. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, 2015. Bending the curve on psychosis outcomes. Lancet Psychiat 2(5), 365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JP, Ozog VA, Khan A, Ljuri I, Fregenti S, McGurk SR, 2017. Predictors of response to cognitive remediation in service recipients with severe mental illness. Psychiatr Rehabil J 40(1), 61–69. [DOI] [PubMed] [Google Scholar]
- Loewy R, Fisher M, Schlosser DA, Biagianti B, Stuart B, Mathalon DH, Vinogradov S, 2016. Intensive auditory cognitive training improves verbal memory in adolescents and young adults at clinical high risk for psychosis. Schizophrenia Bull 42, S118–S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM, 2006. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res 157, 81–109. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, Watkins MA, Dalton CM, Deutsch H, 2017. The Feasibility of Implementing Cognitive Remediation for Work in Community Based Psychiatric Rehabilitation Programs. Psychiatr Rehabil J 40(1), 79–86. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, Xie HY, Welsh J, Kaiser S, Drake RE, Becker DR, Bailey E, Fraser G, Wolfe R, McHugo GJ, 2015. Cognitive Enhancement Treatment for People With Mental Illness Who Do Not Respond to Supported Employment: A Randomized Controlled Trial. Am J Psychiat 172(9), 852–861. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Schiano D, Mueser KT, Wolfe R, 2010. Implementation of the Thinking Skills for Work Program in a Psychosocial Clubhouse. Psychiatr Rehabil J 33(3), 190–199. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT, 2007. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiat 164(12), 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Choi J, 2009. Cognitive remediation in schizophrenia. Neuropsychol Rev 19(3), 353–364. [DOI] [PubMed] [Google Scholar]
- Medalia A, Richardson R, 2005. What predicts a good response to cognitive remediation interventions? Schizophrenia Bull 31(4), 942–953. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S, 2004. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiat 161(1), 116–124. [DOI] [PubMed] [Google Scholar]
- Nahum M, Lee H, Merzenich MM, 2013. Principles of neuroplasticity-based rehabilitation, in: Merzenich MM, Nahum M, Vleet TMV (Eds.), Changing Brains: Applying Brain Plasticity to Advance and Recover Human Ability. Elsevier, Oxford, UK, pp. 141–171. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 1: Test selection, reliability, and validity. Am J Psychiat 165(2), 203–213. [DOI] [PubMed] [Google Scholar]
- Owen RR, Fischer EP, Kirchner JE, Thrush CR, Williams DK, Cuffel BJ, Elliott CE, Booth BM, 2003. Clinical practice variations in prescribing antipsychotics for patients with schizophrenia. Am J Med Qual 18(4), 140–146. [DOI] [PubMed] [Google Scholar]
- Owen RR, Thrush CR, Hudson TJ, Mallory SR, Fischer EP, Clardy JA, Williams DK, 2002. Using an explicit guideline-based criterion and implicit review to assess antipsychotic dosing performance for schizophrenia. Int J Qual Health C 14(3), 199–206. [DOI] [PubMed] [Google Scholar]
- Perez VB, Tarasenko M, Miyakoshi M, Pianka ST, Makeig SD, Braff DL, Swerdlow NR, Light GA, 2017. Mismatch negativity is a sensitive and predictive biomarker of perceptual learning during auditory cognitive training in schizophrenia. Neuropsychopharmacol 42(11), 2206–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posit Science, 2016. Brain Training that works.
- Ramsay IS, Fryer S, Boos A, Roach BJ, Fisher M, Loewy R, Vinogradov S, Mathalon DH, 2018. Response to Targeted Cognitive Training Correlates with Change in Thalamic Volume in a Randomized Trial for Early Schizophrenia. Neuropsychopharmacol 43(3), 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling AJ, Braff DL, Swerdlow NR, Hellemann G, Rassovsky Y, Sprock J, Pela M, Light GA, 2012. Disentangling early sensory information processing deficits in schizophrenia. Clinical Neurophysiology 123(10), 1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartory G, Zorn C, Groetzinger G, Windgassen K, 2005. Computerized cognitive remediation improves verbal learning and processing speed in schizophrenia. Schizophrenia Research 75(2–3), 219–223. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RS, Heinssen R, Cornblatt BA, North American Prodrome Longitudinal Study, G., 2010. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry 67(6), 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko M, Perez VB, Pianka ST, Vinogradov S, Braff DL, Swerdlow NR, Light GA, 2016. Measuring the capacity for auditory system plasticity: An examination of performance gains during initial exposure to auditory-targeted cognitive training in schizophrenia. Schizophrenia Research 172(1–3), 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ML, Green MF, Hellemann G, et al. , 2017. Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA Psychiatry 74(1), 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ML, Treichler EBH, Bismark A, Shiluk AL, Tarasenko M, Zhang W, Joshi YB, Sprock J, Cardoso L, Tiernan K, Light GA, in press. Computerized cognitive training is associated with improved psychosocial treatment engagement in schizophrenia. Schizophrenia Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E, 2012. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacol 37(1), 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Nagarajan S, 2013. Cognitive training in schizophrenia: Golden age or wild west? Biol Psychiat 73(10), 935–937. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG, 2009. The Cognitive Cost of Anticholinergic Burden: Decreased Response to Cognitive Training in Schizophrenia. Am J Psychiat 166(9), 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A, Deste G, De Peri L, Barlati S, Poli R, Cesana BM, Sacchetti E, 2013. Predictors of cognitive and functional improvement and normalization after cognitive remediation in patients with schizophrenia. Schizophrenia Research 150(1), 51–57. [DOI] [PubMed] [Google Scholar]
- Wilson RH, Watts KL, 2012. The Words-in-Noise Test (WIN), list 3: A practice list. J Am Acad Audiol 23(2), 92–96. [DOI] [PubMed] [Google Scholar]
- Woods SW, 2003. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry 64(6), 663–667. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P, 2011. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. Am J Psychiat 168(5), 472–485. [DOI] [PubMed] [Google Scholar]
- Zhu BJ, Ascher-Svanum H, Faries DE, Peng XM, Salkever D, Slade EP, 2008. Costs of treating patients with schizophrenia who have illness-related crisis events. Bmc Psychiatry 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


