Abstract
Kidney transplantation at the time of the COVID-19 pandemic is challenging. Modifying the immunosuppression protocols is controversial and not evidence based. In this study, we aim to review the published literature of kidney transplant recipients who encountered COVID-19.
A literature review was performed using PubMed, ScienceDirect, and World Health Organization databases to identify relevant English-language articles published up to May 7, 2020.
There were 24 articles that reported 129 kidney transplant recipients who encountered COVID-19. The age mean was 54.2 years with 73.7% as males. The most commonly reported presentations in order were fever (82.3%), cough (58%), shortness of breath (33.2%), and fatigue (30.7%). Acute kidney injury was observed in 34.1% of patients. Kidney transplant patients encountered COVID-19 were maintained on tacrolimus (Tac, 92%), mycophenolate mofetil (MMF, 78.8%), and prednisone (Pred, 77%) and were manage by holding MMF in 79.1% of patients and holding Tac in 34.4% of patients. In all, 20% of patients needed Intensive Care Unit (ICU) admission and 24.6% of patients required mechanical ventilation. In all, 18.8% of patients had died compared to the reported general population COVID-19 mortality of 3.4%.
The clinical presentation of COVID-19 in kidney transplant recipients may be different from the general population with a higher rate of severe disease, complications including renal failure, and mortality.
MeSH Keywords: COVID-19, Kidney Transplantation, Organ Transplantation
Background
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV2) [1]. The disease was initially confirmed in China and then rapidly spread worldwide with more than 2 million infected individuals and over 200 000 deaths worldwide [2]. This disease is especially fatal in elderly patients (patients older than 70 years) with comorbidities [3]. Most published data regarding COVID-19 and organ transplant recipients is nonspecific and lacks quality evidence. Data about demographics, characteristics, and clinical presentations of COVID-19 in kidney transplant recipients is scarce [4]. In this study, we aimed to review the published literature regarding kidney transplant patients who encountered COVID-19.
Methods
Literature search
A systematic literature review was performed using PubMed and ScienceDirect databases to identify relevant English-language articles published through May 6, 2020. Search terms included COVID-19, coronavirus, severe acute respiratory syndrome coronavirus 2, 2019-nCoV, SARS-CoV-2, SARS-CoV, MERS-CoV and transplantation. All article types were included: case reports, case series, commentaries, and review articles. A search in the database of the COVID-19 global research on coronavirus disease section of the World Health Organization (WHO) website through May 6, 2020 was performed using the following criteria: transplantation without any additional limits or filters [5]. Additional articles were retrieved by screening the reference lists of the included studies. The search strategy was approved and reviewed by all authors.
Eligibility criteria and study selection
The authors independently reviewed the titles and abstracts for inclusion. Figure 1 displays the flow diagram for this systematic review, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 [6]. Databases were screened, filtered, and assessed for eligibility. Cases of COVID-19 in kidney transplant patients were included in this study. Articles with unrelated topics and/or with missed information were excluded.
Figure 1.
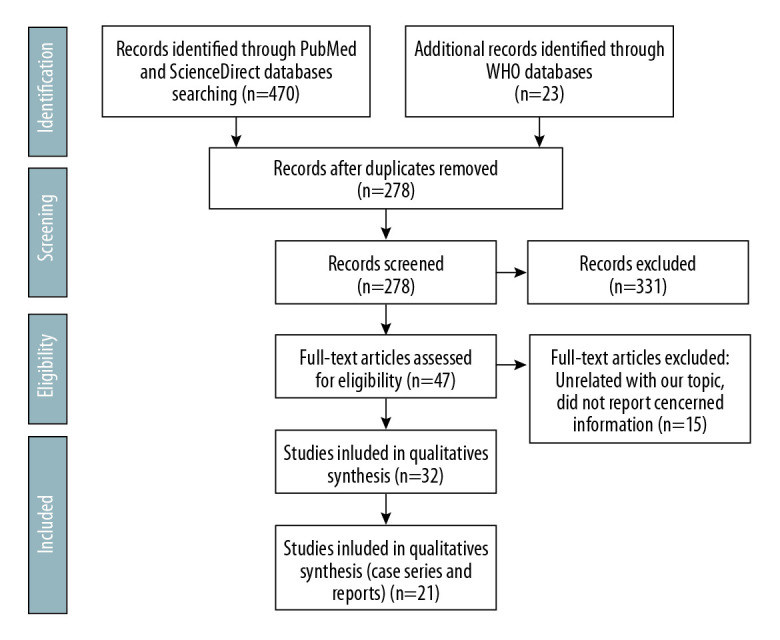
PRISMA flow chart for the present study.
Risk of bias
The National Institutes of Health Quality Assessment Tool for Case Series Studies was used to qualify the reviewed articles [7]. Table 1 shows the results of the 2 reviewers who independently rated the quality of the included studies.
Table 1.
Quality ratings of included studies according to NIH quality assessment tool for case series studies.
| Study | Q 1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Q 8 | Q 9 | Reviewer 1 | Reviewer 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Banerjee | Yes | No | CD | Yes | CD | Yes | No | Yes | Yes | Fair | Fair |
| Bartiromo | Yes | Yes | NA | NA | Yes | Yes | No | NA | Yes | Fair | Fair |
| Chen | Yes | Yes | CD | No | Yes | Yes | Yes | NA | Yes | Fair | Fair |
| Gandolfini | Yes | Yes | CD | Yes | Yes | Yes | Yes | NA | Yes | Fair | Fair |
| Guillen | Yes | Yes | NA | NA | No | Yes | No | NA | Yes | Fair | Fair |
| Huang | Yes | No | CD | Yes | Yes | No | Yes | NA | No | Poor | Poor |
| Ning | Yes | Yes | NA | NA | Yes | Yes | Yes | NA | Yes | Fair | Fair |
| Seminari | Yes | Yes | NA | NA | Yes | Yes | No | NA | Yes | Fair | Fair |
| Wang | Yes | No | NA | NA | Yes | Yes | No | NA | Yes | Fair | Fair |
| Zhang | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Yes | Fair | Fair |
| Zhu | Yes | Yes | NA | NA | Yes | Yes | No | NA | Yes | Fair | Fair |
| Arpali | Yes | No | CD | NA | CD | Yes | No | Yes | Yes | Fair | Fair |
| Billah | Yes | No | CD | NA | CD | Yes | No | Yes | Yes | Fair | Fair |
| Fernández-Ruiz | Yes | Yes | CD | NA | Yes | Yes | Yes | Yes | Yes | Fair | Fair |
| Fontana | Yes | No | CD | NA | CD | Yes | No | Yes | Yes | Fair | Fair |
| Hsu | Yes | No | CD | NA | Yes | Yes | No | Yes | Yes | Fair | Fair |
| Johnson | Yes | Yes | NA | NA | CD | Yes | No | Yes | Yes | Fair | Fair |
| Kates | Yes | No | CD | Yes | CD | Yes | Yes | Yes | Yes | Fair | Fair |
| Kim | Yes | Yes | NA | Yes | Yes | Yes | No | Yes | Yes | Fair | Fair |
| Nair | Yes | No | CD | Yes | Yes | Yes | Yes | Yes | Yes | Fair | Fair |
| Zhu | Yes | Yes | NA | Yes | CD | Yes | Yes | Yes | Yes | Fair | Fair |
NIH – National Institutes of Health; NR – not reported; CD – cannot determine; NA – not applicable. The NIH Quality Assessment Tool for Case Series Studies [7] poses nine questions: 1=Was the study question or objective clearly stated?, 2=Was the study population clearly and fully described, including a case definition?, 3=Were the cases consecutive?, 4=Were the subjects comparable?, 5=Was the intervention clearly described?, 6=Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants?, 7=Was the length of follow-up adequate?, 8=Were the statistical methods well-described?, 9=Were the results well-described?
Data extraction and synthesis
Data was independently extracted from reports by 2 reviewers. All reported patients’ demographic and clinical characteristics (country, age, sex, time from transplant, donor type, comorbidities, clinical presentation and maximum body temperature, initial complete blood count (CBC), C-reactive protein (CRP), baseline creatinine (Cr), blood urea nitrogen (BUN), renal involvement, baseline immunosuppressant medications, need for intensive care unit (ICU) and mechanical ventilation (MV), duration of illness and outcomes) were extracted, collected and analyzed. Due to the lack of sufficient data, a meta-analysis to assess the association of various patients’ findings with demographic data, disease and patient characteristics, or outcomes was not performed. The principal summary measures used were the median, mean, standard deviation, and incidence.
Results
Overview of the included studies
A total of 493 articles were retrieved using the search strategy. After duplication removal, 378 articles were screened; 331 articles were excluded due to unrelated content. The remaining 47 articles were assessed for eligibility through full-text screening. There were 15 articles excluded due to unrelated content or lack of relevant information. There were 32 articles included but only 21 articles reported kidney transplant recipients encountered COVID-19 (Figure 1). For quality assessment, we used the NIH Quality Assessment Tool for Case Series Studies [7]. Five case series and 16 case reports included 58 kidney transplant patients encountered COVID-19. Patients’ characteristics and demographics were included in Tables 2–4.
Table 2.
Characteristics of all available reported kidney transplant recipients infected with COVID-19.
| Study; Country | Number | Age; Sex | Years post-Tx; Donor | Comorbidities | Clinical presentation; Max Temp | Initial WBCC, NC, LC (×109/L) | Initial CRP (mg/L) | Baseline Cr (mg/dL)^ | Initial Cr (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| Banerjee; UK [12] | #1 | 48;M | 31; deceased | HTN | Cough, Fever, SOB; N/A | N/A | N/A | 3.96 | N/A |
| #2 | 67;F | 1; DBD | HTN, DM | Cough, Fever, SOB, Hypoxia; N/A | 6, N/A, 0.8 | 83 | 1.7 | 2.54; AKI | |
| #3 | 54;F | 0.25; deceased | DM, CMV infection | SOB, Hypoxia; N/A | 11.2, N/A, 0.5 | 329 | 1 | 2.71; AKI | |
| #4 | 65;M | 1.5; deceased | HTN | SOB, Chest pain; N/A | N/A | N/A | 2 | N/A | |
| #5 | 69;F | 0.083; deceased | HTN, DM | SOB, Fever, V/D; 39°C | 9.4, N/A, 0.3 | N/A | 1.9 | N/A | |
| #6 | 54;M | 7; N/A | HTN, HHA | Cough, Fever; 38.5°C | 10, N/A, 4 | N/A | 1.6 | 2.1; AKI | |
| #7 | 45;M | 2.5; N/A | HTN | Fever, Flu-like symptoms, Cough, SOB, Hypoxia; N/A | 5.5, N/A, 0.3 | 198 | 5.1 | 11; AKI | |
| Bartiromo; Italy [3] | #1 | 36; F | 25; deceased | SLS | Cough, Coryza, Fatigue; 36.3°C | N/A, High, Normal | 67 | 1.5 | 1.77 |
| Chen; China [24] | #1 | 49; M | 6; DBD | HTN | Hyporexia, Cough, Fever, SOB; 38.6°C | 3.4, 2.59, 0.4 | 74 | 1.24 | 1.89 |
| Gandolfini; Italy [25] | #1 | 75; M | 10; deceased | COPD, HTN, Obesity, CAD | Fever, SOB, Flu-like symptoms; 37.5°C | 6.5, N/A, 0.8 | 180 | 2.1 | 2.2 |
| #2 | 52; F | 0.66; DCD | HTN | Fever, SOB, D, Flu-like symptoms; 37.5°C | 2.5, N/A, 0.11 | 158 | 1.3 | 2.4; AKI | |
| Guillen; Spain [26] | #1 | 50; M | 4; deceased | HTN, PTLD, SP | Fever, V, Dehydration, Cough, Conjunctivitis, Hypoxia; 38.2°C | 10.5, N/A, 1.8 but developed lymphopenia | 5 | 1.3 | 1.6; AKI |
| Huang; China [27] | #1 | 58; M | 12; N/A | None | Cough, Fever, SOB, Hypoxia; 37.6°C | N/A but developed lymphopenia | N/A | N/A | N/A |
| Ning; China [28] | #1 | 29; M | 1.5; Living | HTN | Fever, Fatigue, Chills, Hyporexia, N/V, Chest tightness, nasal stuffiness, Dizziness, Hematuria; 37.7°C | 11.4, N/A, 1.5 | N/A | N/A | 1.1; AKI |
| Seminari; Italy [29] | #1 | 50; M | 4; N/A | HTN, DM | Cough, Fever; 37.5°C | 3.5, 1.8, 1.2 but developed lymphopenia | 18.6 | N/A | 1.7 |
| Wang; China [30] | #1 | 49; M | 2; N/A | HTN, DM | Fever, Respiratory symptoms; N/A | 7, 6, 0.6 | 22.7 | N/A | 1.4 |
| Zhang; China [31] | #1 | 38; M | 0.5; DCD | None | Cough, Fever; 38.9°C | 4.7, 2.6, 0.6 | 6 | N/A | 1.1 |
| #2 | 64; M | 4; DCD | Bladder cancer | Fever, Anuria, Cough,, SOB, Flu-like symptoms; 38.3°C | 17.6, 16, 0.5 | 337 | N/A | 4.6; AKI | |
| #3 | 37; F | 0.66; DCD | HTN | Cough, Fever; 39°C | 5.6, 3.9, 0.3 | 9.7 | N/A | 1.5 | |
| #4 | 47; M | 1.2; DCD | None | Cough, Fever, Flu-like symptoms; 39.8°C | 4, 2.3, 0.5 | 13.3 | N/A | 1.6 | |
| #5 | 38; M | 3; DCD | HTN, DM | Cough, Fever, Flu-like symptoms; 39.1°C | 6.4, 3.2, 0.9 | 33.7 | N/A | 1.5 | |
| Zhu; China [32] | #1 | 52; M | 12; Living | None | Fatigue, SOB, Chest tightness and pain, N, Hyporexia, Abd.P, Cough, Fever, Headache, Weight loss; 38.9°C | 9, 7, 1.13 but developed lymphopenia | 30 | 1.57 | 1.62 |
| Arpali; Turkey [37] | #1 | 28; F | 0.5; Living | LLS | Fever, Fatigue, Sore throat, Rhinorrhea; 38°C | 3.1, N/A, 0.3 | 5.7 | 0.92 | |
| Billa; USA [38] | #1 | 44; M | 7; deceased | None | SOB; N/A | 2.3 | 2.3; AKI | ||
| Fontana; Italy [39] | #1 | 61; M | 15; deceased | NMZL, PD, NB | Fever, Chills; 38°C | 5.4, 4.2, 1.2 but developed lymphopenia | 41 | 1.5 | 1.9 |
| Hsu; USA [40] | #1 | 39; M | 3; | DCM, DM, HTN, Obesity | Fever, Headache, Sore throat, Cough, SOB, Fatigue, Myalgia, Dizziness, Chills; 38.8°C | 2.5, N/A, 0.2 | 67 | 1 | 0.85 |
| Kates, USA [41] | #1 | 54; M | 20; deceased | HTN, DM, | Fever, Chills, Fatigue, Cough, SOB, N/V/D; 40°C | 6.2, N/A, 2 but developed lymphopenia | 1.9 | 3.4; AKI | |
| Johnson; USA [42] | #1 | 57; M | 0.66; deceased | None | Fever, Chills, Hyporexia, Abd. bloating, Back pain, Fatigue, Myalgia, SOB, Anorexia, D, Oliguria; 38.2°C | 1.4, 0.7, 0.3 | 2 | 3.2; | |
| Kim; Korea [43] | #1 | 36; M | 4; Living | None | Fever, Cough, Rhinorrhea, D, Oliguria, Chest discomfort; 38.5°C | 6.6, 5.4, 0.6 | 46 | 1.47 | 2 |
| #2 | 46; M | 9; deceased | DM | Cough; N/A | 4, 2, 1.3 | 27 | 2 | 1.85 | |
| Fernández-Ruiz; Spain [44] | #1 | 78; M | 8.3; N/A | HTN, Prostate CA | Fever, SOB | ||||
| #2 | 73; M | 1.8; N/A | HTN, DM | Fever, SOB, Cough, | |||||
| #3 | 80; M | 3.8; N/A | HTN, DM | SOB, Cough, Myalgia, Hyporexia | |||||
| #4 | 71; F | 6; N/A | HTN | Fever, SOB, Cough, Sore throat | |||||
| #5 | 71; M | 30; N/A | HTN, DM, CAD | Fever, Abd.P | |||||
| #6 | 76; M | 14.8; N/A | HTN, Obesity | Fever, Rhinorrhea | |||||
| #7 | 39; M | 16.8; N/A | HTN | Fever, Myalgia | |||||
| #8 | 65: M | 6.5; N/A | HTN, DM, OSA | Fever, SOB, Cough | |||||
| Nair; USA [45] | #1 | 51; M | 0.42; deceased | HTN, DM, CAD | Fever, Chills, Cough | 9.2, N/A, 1.1 | 0.88 | ||
| #2 | 37; M | 7; living | HTN, DM | Cough, Chills, Nasal congestion, Myalgia | 5, N/A, 2.4 | 180 | 1.93 | ||
| #3 | 63; F | 11.6; living | HTN | Fever, Chills, Cough, Myalgia, Headache; | 9, N/A, 1.2 | 34 | 1.2 | ||
| #4 | 30; F | 3.7; living | HTN, DM | Fever, Myalgia, Headache, V | 3.7, N/A, 1.2 | 1.5 | |||
| #5 | 56; M | 20; deceased | HTN, DM | Fever, Cough, Fatigue | 4, N/A, 0.3 | 306 | 4.8; AKI | ||
| #6 | 80; M | 13.8; living | HTN, DM, CAD | Fever, Chills, Fatigue, Myalgia, D | 5, N/A, 0.2 | 87 | 1.9 | ||
| #7 | 45; M | 3.4; deceased | HTN, DM | Fever, Cough, Myalgia, D | 5.2, N/A, 1.1 | 38 | 1.74 | ||
| #8 | 68; M | 11.6; N/A | HTN, DM | Fever, Cough, SOB | 6.7, N/A, 0.5 | 240 | 1.46; AKI | ||
| #9 | 75; F | 8.6; living | HTN, CA | Fever, Cough, SOB, Fatigue | 6.3, N/A, 0.4 | 50 | 1.3 | ||
| #10 | 57; F | 11.6; deceased | HTN, DM | Cough, SOB, Chills, Fatigue | 11, N/N, 1.4 | 230 | 1.6; AKI | ||
| Zhu; China [46] | #1 | 24; M | None | Fever; 38°C | N/A | 30 | 2.24 | ||
| #2 | 55; M | CAD | Cough, SOB, Fatigu | N/A, N/A, 0.3 | 80 | 3.48 | |||
| #3 | 29; M | None | Fever, Cough, SOB, Fatigue, D; 38.8°C | N/A, N/A, 0.47 | 118 | 2.84 | |||
| #4 | 30; M | HTN | Fever, Cough, SOB, Fatigue; 39°C | N/A, N/A, 0.6 | 42 | 2.36 | |||
| #5 | 50; M | HTN | Fever, Cough, SOB, Fatigue; 38.6°C | N/A, N/A, 0.4 | 40 | N | |||
| #6 | 65; F | None | Fever, Cough, SOB, Fatigue, D; 38°C | N/A, N/A, 0.7 | 40 | N | |||
| #7 | 52; M | HTN, CAD | Fever, Cough, SOB, Fatigue; 38.9°C | N/A, N/A, 1 | 54 | N | |||
| #8 | 49; M | None | Fever, Cough, SOB, Fatigue, D; 39.2°C | N/A | 49 | N | |||
| #9 | 59; M | HTN, COPD | Fever, Cough, SOB, Fatigue; 38.4°C | N/A, N/A, 0.4 | 100 | 5.2; AKI | |||
| #10 | 37; F | HTN | Fever, Cough, SOB, Fatigue; 40°C | N/A, N/A, 0.2 | 34 | 2.14 | |||
| Study; Country | Initial D-dimer, (μg/L) | Initial ALT/AST (U/L) | Initial LDH (U/L) | Baseline ISMs | Management | ICU & MV | Outcomes* | ||
| Banerjee; UK [12] | N/A | N/A | N/A | Aza, Pred | No change in ISMs | None | Recovered, N/A | ||
| 2032 | N/A | 1226 | Tac, MMF, Pred | Tac, MMF stopped, Abx | Both | Died after 12d | |||
| N/A | N/A | N/A | Tac, MMF, Pred | Tac, MMF stopped, Abx, oseltamivir | MV | Alive but suffers, 7d | |||
| N/A | N/A | N/A | Tac, MMF, Pred | MMF stopped | Both | Alive but suffers | |||
| N/A | N/A | N/A | Tac, MMF, Pred | MMF stopped, Abx, PC, furosemide, Blood transfusion | ICU | Alive but suffers, 7d | |||
| N/A | N/A | N/A | Tac, MMF | MMF stopped, PC | None | Alive but suffers, 11d | |||
| 1907 | N/A | 502 | Tac, Aza, Pred | Aza stopped, Tac reduced, Pred increased, HD | None | Recovered, 13d | |||
| Bartiromo; Italy [3] | N/A | N/A | N/A | Tac, Pred | Tac stopped, hydroxychloroquine, Abx, lopinavir/ritonavir (for 2 days), darunavir/cobicistat | None | Recovered, 14d | ||
| Chen; China [24] | N/A | 57/50 | N/A | Tac, MMF, Pred | ISMs stopped, Ribavirin, Abx, IVIG, IVMP | ICU | Recovered, 32d | ||
| Gandolfini; Italy [25] | N/A | 25/45 | 301 | Tac, MMF, Pred | Tac, MMF, stopped, Hydroxychloroquine, Abx, Lopinavir/ritonavir | None | Died, 5d | ||
| 832 | 26/62 | 718 | Tac, MMF, Pred | Tac, MMF, stopped, Abx, Hydroxychloroquine, Darunavir/cobicistat | None | Recovered, 8d | |||
| Guillen; Spain [26] | 8900 | N/A | N/A | Tac, Pred, Eve | Tac, Eve stopped, Abx, hydroxychloroquine, Lopinavir/Ritonavir, INF-β | Both | Alive but suffers, 12d | ||
| Huang; China [27] | N/A | N/A | N/A | MMF, Pred | MMF, Pred stopped, Abx, oseltamivir, Lopinavir/ritonavir, IVMP | MV | Died, 40d | ||
| Ning; China [28] | N/A | 20/23 but elevated later | N/A | MMF, Pred, CsA | No change in ISMs, Abx, lopinavir/ritonavir, IVIG | None | Recovered, 12d | ||
| Seminari; Italy [29] | N/A | 14/22 | 167 then 277 | Tac, MMF | No change in ISMs, Abx | None | Recovered, 13d | ||
| Wang; China [30] | N/A | N/A | N/A | MMF, Pred, CsA | No change in ISMs, INF-α, ribavirin, lopinavir/ritonavir IVMP | None | Recovered, 12d | ||
| Zhang; China [31] | 185 | 66/41 | 193 | Tac, MMF, Pred | MMF stopped, Tac reduced, oseltamivir | None | Recovered, 16d | ||
| 630 | 21/31 | 180 | MMF, Pred | ISMs stopped, Abx, oseltamivir | None | Alive but suffers, 7d | |||
| 1015 | 70/49 | 160 | Tac, MMF, Pred | Tac, MMF stopped, oseltamivir, IVIG | None | Recovered, 11d | |||
| 225 | 7/26 | 235 | Tac, MMF, Pred | ISMs stopped, oseltamivir | None | Recovered, 19d | |||
| 195 | 20/21 | 248 | Tac, MMF, Pred | No change in ISMs, oseltamivir | None | Recovered, 7d | |||
| Zhu; China [32] | N/A | 30/29 but elevated later | N/A | Tac, MMF, Pred | ISMs stopped, Abx, IVMP, IVIG, INF-α, GAD | None | Recovered, 18d | ||
| Arpali; Turkey [37] | Tac, Pred | No change in ISMs, oseltamivir | None | Recovered, 7d | |||||
| Billa; USA [38] | 1100 | 285 | Tac, MMF, Pred | Tac reduced, IVMP, | MV | Alive but suffers, 31d | |||
| Fontana; Italy [39] | N | N | Pred, CsA | CsA stopped, Pred increased, Abx, Hydroxycloroquine, Tocilizumab, IVIG, | None | Recovered, 22d | |||
| Hsu; USA [40] | 1124 | 54/44 | 361 | Tac, MMF, Pred | MMF stopped, hydroxychloroquine, remdesivir | ICU | Recovered, 15d | ||
| Kates, USA [41] | 34/48 | Tac, MMF, | MMF stopped, Tac reduced, Pred, Abx, chloroquine, hydroxychloroquine, | None | Recovered, 16d | ||||
| Johnson; USA [42] | Tac, MMF, | Tac, MMF reduced, Abx, hydroxychloroquine, | None | Recovered, 23d | |||||
| Kim; Korea [43] | 35/32 | Tac, MMF, Pred | Tac, MMF stopped, lopinavir/ritonavir, IVMP, hydroxychloroquine | None | Recovered, 23d | ||||
| 10/14 | Tac, MMF, Pred | MMF stopped, hydroxychloroquine, Abx, | None | Recovered, 17d | |||||
| Fernández-Ruiz; Spain [44] | Tac, Pred | Tac reduced, lopinavir/ritonavir | None | Died, 5d | |||||
| Tac, MMF, Pred | Tac reduced, MMF, Pred stopped, lopinavir/ritonavir, hydroxychloroquine, IVIG | None | Alive but suffers, 23d | ||||||
| Tac, MMF, Pred | Tac reduced, MMF stopped, lopinavir/ritonavir, hydroxychloroquine | None | Alive but suffers, 28d | ||||||
| Tac, MMF, Pred | Tac reduced, MMF, Pred stopped, lopinavir/ritonavir, hydroxychloroquine, IVIG, IVMP | None | Died, 16d | ||||||
| Tac | Tac reduced, hydroxychloroquine, IVIG, | None | Alive but suffers, 9d | ||||||
| MMF, Pred, Srl | MMF stopped, hydroxychloroquine, IVMP | None | Recovered, 13d | ||||||
| Tac, Pred, Eve | Tac, Eve stopped, hydroxychloroquine, IVMP, Tocilizumab, | None | Alive but suffers, 16d | ||||||
| Tac, MMF, Pred | Tac, MMF reduced, lopinavir/ritonavir, hydroxychloroquine | None | Alive but suffers, 17d | ||||||
| Nair; USA [45] | Tac, MMF, Pred, Eve | No change in ISMs | None | Recovered | |||||
| Tac, MMF, Pred | MMF stopped, hydroxychloroquine, azithromycin | None | Recovered | ||||||
| Tac, MMF | MMF stopped, hydroxychloroquine, azithromycin, Abx | None | Recovered | ||||||
| Tac, MMF, Pred | MMF stopped, hydroxychloroquine, azithromycin, Abx | None | Recovered | ||||||
| Tac, MMF, Pred | Tac, MMF stopped, hydroxychloroquine, azithromycin, Abx | Both | Died | ||||||
| Tac, MMF | Tac, MMF stopped, hydroxychloroquine, azithromycin, Abx, IVMP | Both | Recovered | ||||||
| Tac, MMF, Pred | MMF stopped, hydroxychloroquine, azithromycin, Abx, IVMP | None | Recovered | ||||||
| Tac, MMF, Pred | MMF stopped, hydroxychloroquine, azithromycin, Abx | ICU | Recovered | ||||||
| Pred, Srl | Srl stopped, hydroxychloroquine, azithromycin | Both | Died | ||||||
| Tac, MMF | MMF stopped, hydroxychloroquine, azithromycin, Abx, Pred | Both | Died | ||||||
| Zhu; China [46] | N | Tac, MMF, Pred | No change in ISMs, Avx | None | Recovered, 43d | ||||
| N | Tac, MMF, Pred | MMF stopped, Tac reduced, IVIG, Avx | MV | Recovered, 48d | |||||
| N | Tac, MMF, Pred | MMF stopped, IVMP, Avx | None | Recovered, 37d | |||||
| N | Tac, MMF, Pred | Tac, MMF stopped, IVIG, IVMP, Avx | None | Recovered, 37d | |||||
| 104; N/A | Tac, MMF, Pred | Tac, MMF stopped, IVIG, IVMP, Avx | None | Recovered, 34d | |||||
| N | Tac, MMF | Tac, MMF stopped, IVIG, IVMP, Avx | MV | Alive but suffers, 49d | |||||
| 94; N/N | Tac, MMF, Pred | Tac, MMF stopped, IVIG, IVMP, Avx | None | Recovered, 20d | |||||
| 97; N/A | Tac, MMF | Tac, MMF stopped, IVMP, Avx | None | Recovered, 34d | |||||
| 61; N/A | CsA, mizoribine | ISMs stopped, IVIG, IVMP, Avx | MV | Died, 6d | |||||
| 163; N/A | Tac, MMF, Pred | Tac, MMF stopped, IVIG, IVMP, Avx | None | Recovered, 31d | |||||
Recovered indicates recovery of clinical symptoms and signs not negative COVID-19 testing;
Initial Creatinine (Cr) indicates Cr serum level in mg/dL unit before encountering COVID-19.
ISMs – immunosuppressant medications; Max Temp – maximum temperature; DOI – duration of illness:; ICU – Intensive Care Unit; MV – mechanical ventilation; CRP – C-reactive protein; WBCC – white blood cell count; normal is 3.5–10 (×109/L); NC – neutrophil count, normal is 1.5–8 (×109/L); LC – lymphocyte count, normal is 1–3.5 (×109/L); AKI – acute kidney injury; Aza – azathioprine; MMF – mycophenolate mofetil; Tac – tacrolimus; Pred – prednisone; N/A – not available; DCD – donor after cardiac death; DBD – donor after brain death; Abx – antibiotics; Avx – antivirals; V/D – vomiting/diarrhea; PC – Paracetamol; SLS – Senior-Loken syndrome; LOS – loss of appetite; IVMP – intravenous methylprednisolone; IVIG – intravenous immunoglobin; D – Diarrhea; PTLD – post-transplant lymphoproliferative disease; SP – splenectomy; V – vomiting; Eve – everolimus:; INF-β – interferon beta; CsA – ciclosporin; N/V – nausea/vomiting; INF-α – interferon α; Abd.P – abdominal pain; GAD – glycyrrhizic acid diamine; CMV – cytomegalovirus; HHA – hereditary haemolytic anaemia; HD – hemodialysis; LDH – lactate dehydrogenase; ALT – alanine aminotransferase; AST – aspartate aminotransferase; LLS – lupus-like syndrome; NMZL – nodal marginal zone lymphoma; PD – Parkinson disease; NB – neurogenic bladder; DCM – dilated cardiomyopathy; CAD – coronary artery disease; OSA – obstructive sleep apnea; CA – cancer; Srl – Sirolimus.
Table 3.
Clinical Characteristics for the 58 Reported Kidney Transplant Patients Who Encountered COVID-19.
| Variable (n=58) | Value |
|---|---|
| Age (mean, range) | 52.69 (24–80) |
| Sex | |
| Male | 44/58 (75.9%) |
| Female | 14/58 (24.1%) |
| Kidney transplant years age (mean, range) | 7.68 (0.083–31) |
| Within 1 year | 9/58 (15.5%) |
| Beyond 1 year | 49/58 (84.5%) |
| Type of donor | |
| DCD | 6/58 (10.3%) |
| DBD | 2/58 (3.4%) |
| Deceased, unknown | 16/58 (27.5%) |
| Living | 9/58 (15.5%) |
| Not available | 25/58 (43.1%) |
| Comorbidities | |
| HTN | 40/58 (68.9%) |
| DM | 21/58 (36.2%) |
| CAD | 6/58 (10.3%) |
| COPD | 2/58 (3.4%) |
| Obesity | 3/58 (5.2%) |
| None | 11/58 (18.9%) |
| Clinical presentation | |
| Fever | 49/58 (84.5%) |
| Max. temp (average, SD) | 38.47 (±0.79) |
| Chills | 10/58 (17.2%) |
| Cough | 40/58 (70%) |
| SOB | 33/58 (56.9%) |
| Chest pain/discomfort/tightness | 4/58 (6.9%) |
| Flu-like symptoms | 6/58 (10.3%) |
| Fatigue | 20/58 (34.5%) |
| Myalgia | 9/58 (15.5%) |
| Vomiting | 4/58 (6.9%) |
| Diarrhea | 10/58 (17.2%) |
| Abdominal pain/bloating | 3/58 (5.2%) |
| Nausea | 3/58 (5.2%) |
| Hyporexia/anorexia | 6/58 (10.3%) |
| Headache | 4/58 (6.9%) |
| Dizziness | 2/58 (3.4%) |
| Sore throat | 3/58 (5.2%) |
| Rhinorrhea/nasal congestion/stuffiness | 5/58 (8.6%) |
| Oliguria | 2/58 (3.4%) |
| Laboratory | |
| White Cell Count | |
| Median (SD) per l | 6×109 (±3.4×109) |
| Leukopenia (<4×109/l) | 8/35 (22.8%) |
| Neutrophils count | |
| Median (SD) per l | 3.2×109 (±3.9×109) |
| Neutropenia (<1.5×109/l) | 1/13 (7.6%) |
| Lymphocyte count | |
| Median (SD) per l | 0.6*×109 (±0.72×109) |
| Lymphopenia (<1×109/l) | 34/43 (79%) |
| Initial C-reactive protein (CRP) | |
| Median (SD) | 49 (±92.4) |
| High CRP (>5 mg/dl) | 36/37 (97.2%) |
| Baseline serum creatinine (Cr) | |
| Median (SD) | 1.65 (±0.96) |
| High Cr (>1.2 mg/dL) | 17/20 (85%) |
| Initial serum Creatinine (Cr) | |
| Median (SD) | 1.9 (±1.7) |
| AKI | 13/46 (28.2%) |
| Initial D-dimer | |
| Median (SD) | 1015 (±2485) |
| High D-dimer (>500 μg/L) | 8/11 (72.7%) |
| Initial ALT | |
| Median (SD) | 34 (±39.8) |
| High ALT (>50 U/l) | 11/25 (44%) |
| Initial AST | |
| Median (SD) | 32 (±13.6) |
| High AST (>54 U/l) | 3/20 (15%) |
| Initial lactate dehydrogenase (LDH) | |
| Median (SD) | 266.5 (±311) |
| High LDH (>225 U/l) | 9/13 (69.2%) |
Table 4.
Management and Outcomes of the 58 Reported Kidney Transplant Patients Who Encountered COVID-19.
| Variable | Value |
|---|---|
| Baseline Immunosuppression | |
| Tacrolimus | 48/58 (82.7%) |
| Mycophenolate mofetil | 46/58 (79.3%) |
| Prednisone | 47/58 (81%) |
| Azathioprine | 2/58 (3.4%) |
| Everolimus | 3/58 (5.2%) |
| Ciclosporin | 4/58 (6.8%) |
| Sirolimus | 2/58 (3.4%) |
| Mizoribine | 1/58 (1.7%) |
| Management | |
| Immunosuppression | |
| Held tacrolimus | 23/48 (47.9%) |
| Reduced tacrolimus | 10/48 (20.8%) |
| No change tacrolimus | 15/48 (25.8%) |
| Held mycophenolate mofetil | 37/46 (80.4%) |
| Reduced mycophenolate mofetil | 2/46 (4.3%) |
| No change mycophenolate mofetil | 7/46 (15.2%) |
| Held prednisone | 7/47 (14.8%) |
| Increased prednisone | 2/47 (4.2%) |
| No change prednisone | 38/47 (80.8%) |
| Azathioprine | Held 1; No change 1 |
| Everolimus | Held 2; No change 1 |
| Ciclosporin | Held 2; No change 2 |
| Sirolimus | Held 1; No change 1 |
| Mizoribine | Held 1 |
| Other Tx | |
| Lopinavir/Ritonavir | 12/58 (20.6%) |
| Hydroxychloroquine | 25/58 (43.1%) |
| Azithromycin | 9/58 (15.5%) |
| Oseltamivir | 8/58 (13.8%) |
| Antibiotics | 24/58 (41.3%) |
| Intravenous methylprednisolone | 19/58 (32.7%) |
| Intravenous immunoglobulin | 15/58 (25.8%) |
| Unspecified antivirals | 10/58 (17.2%) |
| Interferon α, β | 3/58 (5.2%) |
| Tocilizumab | 2/58 (3.4%) |
| Remdesivir | 1/58 (1.7%) |
| ICU admission | 11/58 (19%) |
| MV requirement | 13/58 (22.4%) |
| Outcomes | |
| Clinically recovered/Discharged | 36/58 (62%) |
| Illness days duration (median, Range) | 17.5 (7–48) |
| Alive but suffers/In hospital | 13/58 (22.4%) |
| Illness days duration (median, range) | 14 (7–49) |
| Death | 9/58 (15.5%) |
| Illness days duration (median, range) | 9 (5–40) |
Patients demographics and characteristics
The 21 articles reported 58 kidney transplant patients who encountered COVID-19. There were 20 patients from China, 14 patients from the USA, 9 patients from Spain, 7 patients from the United Kingdom, 5 patients from Italy, 2 patients from Korea, and 1 patient from Turkey. There were 44 male patients (75.9%) and 14 female patients (14 out of 58; 24.1%). The mean age was 52.69 years (range, 24 to 80 years). Transplants were from unknown decreased persons in 16 cases (27.5%), living donors in 9 cases (15.5%), DCD (donor after cardiac death) in 6 cases (10.3%), DBD (donor after brain death) in 2 cases (3.4%), and the remaining 25 cases (43.1%) were from unknown sources. The mean post-transplant period was 7.68 years (range, 0.083 to 31 years). There were 9 patients (15.5%) who were within their first year after transplantation. The most common reported comorbidities were hypertension in 40 patients (68.9%), diabetes mellitus in 21 patients (36.2%), coronary artery/heart disease in 6 patients (10.3%), COPD in 2 patients (3.4%), and obesity in 3 patients (5.2%). In 11 patients (18.9%) there was no comorbidities reported. These variables are presented in the Table 3.
Clinical presentation
The most frequently reported clinical presentation was fever; it was reported in 49 patients (84.5%) with a mean maximum temperature of 38.47°C (±0.79°C). Other reported clinical symptoms were cough (70%), shortness of breath (SOB) (56.9%), flu-like symptoms including myalgia and fatigue (60%), gastrointestinal symptoms including vomiting, diarrhea, nausea, abdominal pain/bloating and hyporexia/anorexia (44.8%), chills (17.2%), and chest pain/tightness/discomfort (6.9%). Less frequently reported symptoms included headache, dizziness, sore throat, rhinorrhea, nasal congestion, and stuffiness, coryza, dehydration, conjunctivitis, hematuria, and oliguria. These variables are presented in the Table 3.
Laboratory results
The initial white blood cell count median was 6×109 (±3.4×109). The initial median lymphocyte cell count was 0.6×109 (±0.72×109). Initial leukopenia and lymphopenia were reported in 22.8% and 63%, respectively. However, lymphopenia was eventually developed in 79% of patients. Median of the initial CRP was 49 mg/L (±92.4 mg/L) and high CRP (>5 mg/dL) levels were noted in 97.2% of cases. Median of the baseline serum Cr was 1.65 mg/dL (±0.96 mg/dL) and 85% of patients had baseline serum Cr >1.2 mg/dL. Median initial (post infection) serum Cr was 1.9 mg/dL (±1.7 mg/dL) and acute kidney injury (AKI) was observed in 28.2% of patients. High D-dimer (>500 μg/L), ALT (>50 U/L), AST (>54 U/L), and LDH (>225 U/L) levels were observed in 72.7%, 44%, 15%, and 69.2%, respectively. These variables are presented in the Table 3.
Immunosuppression management
Patients were treated with different immunosuppressive regimens though the most frequently reported regimen included tacrolimus (Tac), mycophenolate mofetil (MMF), and prednisone (Pred) which was reported in 33 patients (56.9%).
Pred was prescribed in 81% of patients and discontinued in 14.8%, increased in 4.2% and not changed in 80.8%. MMF was prescribed in 79.3% of patients and discontinued in 72%, reduced in 4.3%, and not changed in 15.2%. Tac was prescribed in 82.7% of patients and discontinued in 47.9%, reduced in 20.8%, and not changed in 25.8%. Other baseline immunosuppression medications were azathioprine, everolimus, ciclosporin, sirolimus, and mizoribine. These medications were held in some patients and not changed in others; 13.8% of patients recovered with no change in immunosuppression medications. These variables are presented in the Table 4.
Patients who died had their immunosuppression medications held (Tac 50%, MMF 100%, Pred 28.5%, ciclosporin 100%, sirolimus 100%, mizoribine100%), reduced (Tac 33.3%) or continued unchanged (Tac 16.6%, Pred 71.4%). These variables are presented in the Table 5.
Table 5.
Characteristics and management of the COVID-19 kidney transplant patients with death as the outcome.
| Variable (n=9) | Value |
|---|---|
| Age in years (mean) | 66.2 |
| >65y | 55.5% |
| <65y | 44.5% |
| Sex, Male | 55.5% |
| Kidney transplant years age (Mean) | 9.7 |
| Comorbidities | |
| HTN | 88.8% |
| DM | 33.3% |
| COPD | 100% |
| Laboratories | |
| Lymphopenia (<1*109/l) | 66.6% |
| High CRP (>5 mg/dl) | 66.6% |
| Acute kidney injury | 44.4% |
| Baseline Immunosuppression regimen | |
| Tac, MMF, Pred | 44.4% |
| Management | |
| Immunosuppression | |
| Held tacrolimus | 3/6 (50%) |
| Reduced tacrolimus | 2/6 (33.3%) |
| No change tacrolimus | 1/6 (16.6%) |
| Held mycophenolate mofetil | 6/6 (100%) |
| Held prednisone | 2/7 (28.5%) |
| No change prednisone | 5/7 (71.4%) |
| Held ciclosporin | 1/1 (100%) |
| Held sirolimus | 1/1 (100%) |
| Held mizoribine | 1/1 (100%) |
| Tx targets COVID-19 | |
| Lopinavir/Ritonavir | 44.4% |
| Hydroxychloroquine | 55.5% |
| Oseltamivir | 11.1% |
| Antibiotics | 55.5% |
| Intravenous methylprednisolone | 33.3% |
| Intravenous immunoglobulin | 22.2% |
| ICU admission | 33.3% |
| MV requirement | 55.5% |
| Disease days duration (median, range) | 9 (5–40) |
Overall, patients had their immunosuppression medications held (Tac 47.9%, MMF 80.4%, Pred 14.8%, ciclosporin 50%, sirolimus 50%, mizoribine100%, azathioprine 50%), reduced (Tac 33.3%, MMF 4.3%) or continued unchanged (Tac 25.8%, MMF 15.2%, Pred 80.8%). Overall, 4.2% of patients were managed by increasing the dose of prednisone. These variables are presented in the Table 4.
COVID-19 directed management
Treatment targeted COVID-19 included antibiotics (41.3%), hydroxychloroquine (43.1%), intravenous methylprednisolone (32.7%), intravenous immunoglobulin (25.8%), lopinavir/ritonavir (20.6%), unspecified antivirals (17.2%), azithromycin (15.5%), oseltamivir (13.8%), interferon a,b (5.2%), tocilizumab (3.4%), and remdesivir (1.7%). These variables are presented in the Table 4.
COVID-10 targeting treatment used in patient who died included antibiotics (55.5%), hydroxychloroquine (55.5%), lopinavir/ritonavir (44.4%), intravenous methylprednisolone (33.3%), and intravenous immunoglobulin (22.2%).
Patients outcomes
Overall, 19% of patients needed intensive care unit (ICU) admission and 22.4% of patients required mechanical ventilation (MV). Overall, 62% of patients recovered and were discharged with median of the illness duration of 17.5 days (range, 7 to 48 days). At the time of report publication, 22.4% of patients were alive and hospitalized, with median of the illness duration of 14 days (range, 7 to 49 days). Overall, 15.5% of patients had died with median of the illness duration of 9 days (range, 5 to 40 days).
Characteristics of patients who died
The mean age was 66.2 years with 55.5% of patients older than 65 years; 55.5% of patients were male and the mean of the transplant age was 9.7 years. Hypertension, diabetes mellitus, and COPD were reported in 88.8%, 33.3%, and 100% of patients, respectively. In addition, 66.6% of patients had lymphopenia and 66.6% had high CRP serum levels. Acute kidney injury (AKI) was reported in 44.4% of patients (Table 5).
Pooled results
Three studies reported 36, 20, and 15 patients, respectively. These studies reported results without demographics and characteristics for each patient and thus were not included in our tabulated results. However, these 3 studies were used to draw pooled measures for all reported kidney transplant patients who encountered COVID-19, found in the literature. The total number of kidney transplant patients who encountered COVID-19 reported in the literature was 129 cases. The age mean was 54.2 years with 73.7% of the patients were males. The transplant age mean was 8.2 years with 65.4% of the transplants from a deceased source. Hypertension, diabetes mellitus, and coronary artery disease were reported in 82.6%, 40%, and 14% of cases, respectively. The most commonly reported presentations in order were fever (82.3%), cough (58%), SOB (33.2%), fatigue (30.7%), diarrhea (19.7%), myalgia (17.3%), sore throat (7.6%), and vomiting (6.9%) (Table 6).
Table 6.
Clinical characteristics of the 58 reported kidney transplant patients who encountered covid-19 compared with previous published studies.
| Variable | This study | Akalin et al. [47] | Alberici et al. [48] | CUKTP [49] | Pooled (mean) |
|---|---|---|---|---|---|
| Number of patients | 58 | 36 | 20 | 15 | 129 |
| Age (mean) | 52.69 | 59 | 51 | 54.2 | |
| Sex | |||||
| Male | 75.9% | 72% | 80% | 67% | 73.7% |
| Female | 24.1% | 28% | 20% | 33% | 26.2% |
| Kidney transplant years age (mean) | 7.68 | 13 | 4 | 8.2 | |
| Type of donor | |||||
| Deceased | 41.2% | 75% | 80% | 65.4% | |
| Comorbidities | |||||
| HTN | 68.9% | 94% | 85% | 82.6% | |
| DM | 36.2% | 69% | 15% | 40% | |
| CAD | 10.3% | 17% | 15% | 14% | |
| Clinical presentation | |||||
| Fever | 84.5% | 58% | 100% | 87% | 82.3% |
| Cough | 70% | 53% | 50% | 60% | 58% |
| SOB | 56.9% | 44% | 5% | 27% | 33.2% |
| Fatigue | 34.5% | 27% | 30.7% | ||
| Myalgia | 15.5% | 36% | 5% | 13% | 17.3% |
| Vomiting | 6.9% | 7% | 6.9% | ||
| Diarrhea | 17.2% | 22% | 20% | 19.7% | |
| Sore throat | 5.2% | 10% | 7.6% | ||
| Laboratory | |||||
| White cell count | |||||
| Mean per l (×109) | 6 | 5.3 | 5.4 | 4.8 | 5.37 |
| Leukopenia (<4×109/l) | 22.8% | 21% | 21.9% | ||
| Lymphocyte count | |||||
| Mean per l (×109) | 0.6 | 0.6 | 1.1 | 0.8 | 0.77 |
| Lymphopenia (<1×109/l) | 79% | 79% | 79% | ||
| Initial C-reactive protein (CRP) | |||||
| Mean | 49 | 7.9 | 49 | 104 | 52.4 |
| High CRP (>5 mg/dl) | 97.2% | 46% | 71.6% | ||
| Initial serum Creatinine (Cr) | |||||
| Mean | 1.9 | 1.8 | 1.85 | ||
| AKI | 28.2% | 40% | 34.1% | ||
| Initial D-dimer | |||||
| Mean | 1015 | 1020 | 279 | 771.3 | |
| High D-dimer (>500 μg/L) | 72.7% | 57% | 64.8% | ||
| Initial lactate dehydrogenase (LDH) | |||||
| Mean | 266.5 | 336 | 231 | 275 | 277 |
| High LDH (>225 U/l) | 69.2% | 36% | 52.6% | ||
The initial white blood cell count mean was 5.37×109. The initial mean lymphocyte cell count was 0.77×109. Leukopenia and lymphopenia were reported in 21.9% and 79% of patients, respectively. Mean initial CRP was 52.4 mg/L and high CRP (>5 mg/dL) levels were noted in 71.6% of cases. Mean initial (post infection) serum Cr was 1.85 mg/dL and AKI was observed in 34.1% of patients. High D-dimer (>500 μg/L) and lactate dehydrogenase (LDH, >225 U/L) levels were observed in 64.8% and 52.6%, respectively. Kidney transplant patients encountered COVID-19 were maintained on Tac (92%), MMF (78.8%), Pred (77%), and azathioprine (5.2%) and were manage by holding MMF in 79.1% of patients and holding Tac in 34.4% of patients. These patients received treatments targeted for COVID-19 which included hydroxychloroquine (77.6%), lopinavir/ritonavir (49.8%), antibiotics (48%), azithromycin (40.5%), and tocilizumab (11.8%). Overall, 20% of patients needed ICU admission, 24.6% of patients required MV, 42% of patients recovered and were discharged, 41.3% of patients were alive and hospitalized at the time of the study publication, and 18.8% of patients had died (Tables 6, 7).
Table 7.
Management and outcomes of the 58 reported kidney transplant patients who encountered COVID-19 compared with previous published studies.
| Variable | This study | Akalin et al. [47] | Alberici et al. [48] | CUKTP [49] | Pooled (mean) |
|---|---|---|---|---|---|
| Baseline Immunosuppression | |||||
| Tacrolimus | 82.7% | 97% | 95% | 93% | 91.9% |
| Mycophenolate mofetil | 79.3% | 86% | 70% | 80% | 78.8% |
| Prednisone | 81% | 94% | 65% | 67% | 77% |
| Azathioprine | 3.4% | 7% | 5.2% | ||
| Management | |||||
| Immunosuppression | |||||
| Held tacrolimus | 47.9% | 21% | 34.4% | ||
| Held mycophenolate mofetil | 80.4% | 86% | 71% | 79.1% | |
| Other Tx | |||||
| Lopinavir/Ritonavir | 20.6% | 79% | 49.8% | ||
| Hydroxychloroquine | 43.1% | 86% | 95% | 86.6% | 77.6% |
| Azithromycin | 15.5% | 46% | 60% | 40.5% | |
| Antibiotics | 41.3% | 55% | 48.1% | ||
| Tocilizumab | 3.4% | 7% | 30% | 7% | 11.8% |
| ICU admission | 19% | 20% | 20% | ||
| MV requirement | 22.4% | 39% | 10% | 27% | 24.6% |
| Outcomes | |||||
| Clinically recovered/Discharged | 62% | 36% | 15% | 53% | 42% |
| Alive but suffers/In hospital | 22.4% | 43% | 60% | 40% | 41.3% |
| Death | 15.5% | 28% | 25% | 7% | 18.8% |
Discussion
Fever in COVID-19 has been reported in 99% of patients [18,19]. Our study has found that 15% of the kidney transplant patients had no fever on presentation or during their hospitalization. On the other hand, cough, shortness of breath, myalgia, headache, sore throat, and gastrointestinal symptoms were more common than the typical COVID-19 presentation [18–21].
Additionally, this review found that there were several unreported symptoms that appeared in the COVID-19 positive kidney transplant patients, like chest tightness and pain, coryza, dehydration, conjunctivitis, dizziness, and weight loss [20]. Interestingly, the previously unreported chest tightness and pain symptoms in other cohorts had an incidence of 7% in this cohort.
COVID-19 causes of pneumonia can be severe enough to be lethal, especially in patients with advanced age or underlying medical comorbidities [22]. Those comorbidities include cardiovascular disease, diabetes mellitus, hypertension, chronic lung disease, cancer, chronic kidney disease, and obesity (body mass index ≥30 kg/m2) [3,22]. Kidney transplant patients infected with COVID-19 are a fragile and high-risk group due to immunosuppressant medications (ISMs), kidney disease, and common comorbidities. In this review, 81% of patients had comorbidities, with diabetes mellitus and hypertension as the most common reported comorbidities. This fact exposes those patients to a more severe COVID-19 infection, not to mention the additional unclear risk of the immunosuppression. Kidney transplant recipients and candidates are as a rule in a high-risk group due to the high incidence and prevalence of hypertension, diabetes, obesity, and advanced age in this group.
It was previously reported that a progressive decline in lymphocyte count was observed in non-survivors compared to more stable levels in survivors [18]. In the present study, 66.6% of patients who died had lymphopenia. Given the fact that ISMs can induce lymphopenia, many kidney transplant patients can have a baseline lymphopenia that might further deteriorate and worsen the prognosis [8].
AKI, proteinuria, and hematuria have all been reported in COVID-19 patients. AKI has been reported to have occurred in 25% to 29% of critically ill COVID-19 patients in Wuhan, China [23,24]. The incidence of AKI among patients who are less severely ill is unknown. In our cohort, AKI was reported in 28.2% of patients. However, the pooled AKI prevalence was 34.1% (see Table 6). This may indicate that kidney transplant patients infected with COVID-19 are more likely to have AKI than other cohorts. AKI has been associated with worse outcomes [25] and subsequently, kidney transplant patients infected with COVID-19 may have worse outcomes and mortality.
Several studies and a report from the Chinese Center for Disease Control and Prevention have classified COVID-19 severity [23,26] as mild, severe, and critical disease that were reported in 81%, 14%, and 5% of patients. However, in our cohort, mild disease was only reported in 43% of patients while severe disease that presents with SOB or hypoxia was reported in 57% of patients.
The WHO reported that recovery time appears to be around 2 weeks for mild infections and 3 to 6 weeks for severe disease [27]. In this report, it appears that 62% of patients recovered clinically from the COVID-19 with a median duration of illness of 17.5 days (range, 7 to 48 days). Rates of ICU admission were reported to range between 5% to 12% while the rate of MV was 2.3% in the general population [28,29]. In our cohort, 19% of patients were admitted to the ICU and 22.4% of patients required MV.
The most recently reported mortality rate of COVID-19 was 3.77% [30]. The mortality rate of kidney transplant patients infected with COVID-19 in this cohort was 15.5% (9 out of 58 patients) and the pooled mean was 18.8%. Despite the small number of patients included in the present study, this high mortality rate indicates that COVID-19 in kidney transplant patients may portend an ominous outcome. We found that 11.1% of the 1-year transplant age patients had died while 16.3% mortality was found for patients with transplant age more than 1 year.
It has been reported that 81% of COVID-19 patients present with mild disease and can be managed at home, while severe and critical COVID-19 disease should be managed with prompt hospitalization while ensuring appropriate infection control and supportive care [23,31,32]. The hospitalized patients should be managed with empiric treatment for bacterial pneumonia in selected patients, prevention of venous thromboembolism, and avoiding nebulized medications. The WHO and CDC recommend against systemic glucocorticoids in COVID-19 patients unless there are other indications [31,32]. To date, all suggested medications are under investigation with no proven efficacy against COVID-19. These medications include remdesivir, hydroxychloroquine/chloroquine, azithromycin and hydroxychloroquine, convalescent plasma, tocilizumab, favipiravir, interferon beta, and lopinavir/ritonavir. A registry of international clinical trial can be found at https://www.clinicaltrials.gov/ct2/results?cond=covid19&term=&cntry=&state=&city=&dist. Most of our cohort had been hospitalized and could not be managed at home due to the severity of the disease. They had been managed with antibiotics (41.3%), hydroxychloroquine (43.1%), intravenous methylprednisolone (32.7%), intravenous immunoglobulin (25.8%), lopinavir/ritonavir (20.6%), unspecified antivirals (17.2%), azithromycin (15.5%), oseltamivir (13.8%), interferon a,b (5.2%), tocilizumab (3.4%), and remdesivir (1.7%).
The effect of immunosuppression on the progression of COVID-19 is not clear yet. There are 2 aspects that cannot be ignored when dealing with immunosuppression and COVID-19. Firstly, it was proven that COVID-19 patients have a high prevalence of lymphopenia [33] but it is not clear yet whether lymphopenia is a risk factor for COVID-19 or a result of it. Secondly, it is thought that the severity of COVID-19 may be the result of a hyperinflammatory response (cytokine storm). Thus, many have questioned the role of immunomodulation in the treatment of severe cases [34,35].
Interestingly, D’Antiga from Italy recently published a study that concluded that immunocompromised patients do not have an increased risk of developing severe pulmonary disease compared to the general population [36].
Conclusions
Kidney transplant patients may present with atypical clinical picture of the COVID-19. Fever may be absent while other atypical symptoms may prevail. Therefore, a high index of suspicion for COVID-19 and perhaps even surveillance in this population may help in early diagnosis and prevention of further transmission. The role of immunosuppression therapy should be assessed in every case individually.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.World Health Organization (WHO) Novel coronavirus (2019 nCoV) 2020. available from: https://www.who.int/health-topics/coronavirus#tab=tab_1.
- 2.World Health Organization (WHO) Coronavirus disease 2019 (COVID-19) Situation Report – 88. 2020. available from https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study [published correction appears in Lancet, 2020; 395(10229): 1038. [published correction appears in Lancet, 2020; 395(10229): 1038] Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartiromo M, Borchi B, Botta A, et al. Threatening drug-drug interaction in a kidney transplant patient with coronavirus disease 2019 (COVID-19) Transpl Infect Dis. 2020 doi: 10.1111/tid.13286. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) COVID-19 global literature on coronavirus disease. 2020. available from https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Heart and Blood Institute. Study quality assessment tools. 2020. available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 8.Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–82. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Yin Q, Shi H, et al. A familial cluster, including a kidney transplant recipient, of Coronavirus Disease 2019 (COVID-19) in Wuhan, China. Am J Transplant. 2020 doi: 10.1111/ajt.15903. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020 doi: 10.1111/ajt.15891. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020 doi: 10.1111/ajt.15874. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Lin H, Wu Y, et al. COVID-19 in posttransplant patients-report of 2 cases. Am J Transplant. 2020 doi: 10.1111/ajt.15896. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ning L, Liu L, Li W, et al. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: Case report. Am J Transplant. 2020 doi: 10.1111/ajt.15897. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seminari E, Colaneri M, Sambo M, et al. SARS Cov-2 infection in a renal-transplanted patient: A case report. Am J Transplant. 2020 doi: 10.1111/ajt.15902. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Li X, Cao G, et al. COVID-19 in a kidney transplant patient. Eur Urol. 2020;77(6):769–70. doi: 10.1016/j.eururo.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77(6):742–47. doi: 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020 doi: 10.1111/ajt.15869. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–69. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19), (2020) 2020. available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 21.Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020 Apr;8(4): e26] Lancet Respir Med. 2020;8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published correction appears in BMJ, 2020;3 68: m1295] BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kujawski S, Wong K, Collins J, et al. COVID-19 Investigation Team. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020 doi: 10.1038/s41591-020-0877-5. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Director-General’s opening remarks at the media briefing on COVID-19. Feb 24, 2020. available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---24-february-2020.
- 28.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA. 2020 doi: 10.1001/jama.2020.4031. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–72. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prevention, Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed 2019 novel coronavirus (2019-nCoV) infection. 2020 [Google Scholar]
- 32.World Health Organization. Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts. 2020 [Google Scholar]
- 33.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv. 2020 2002.2006.20020974. [Google Scholar]
- 34.Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395:1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area [published correction appears in JAMA. 2003 Jul 16;290(3): 334] JAMA. 2003;289(21):2801–9. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 36.D’Antiga L. Coronaviruses and immunosuppressed patients: The facts during the third epidemic. Liver Transpl. 2020;26(6):832–34. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 37.Arpali E, Akyollu B, Yelken B, et al. Case report: A kidney transplant patient with mild COVID-19. Transpl Infect Dis. 2020:e13296. doi: 10.1111/tid.13296.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billah M, Santeusanio A, Delaney V, Cravedi P, Farouk SS. A catabolic state in a kidney transplant recipient with COVID-19. Transpl Int. 2020 doi: 10.1111/tri.13635. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fontan Fontana F, Alfano G, Mori G, et al. COVID-19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine. Am J Transplant. 2020 doi: 10.1111/ajt.15935. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu JJ, Gaynor P, Kamath M, et al. COVID-19 in a high-risk dual heart and kidney transplant recipient. Am J Transplant. 2020 doi: 10.1111/ajt.15936. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kates OS, Fisher CE, Stankiewicz-Karita HC, et al. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transplant. 2020 doi: 10.1111/ajt.15944. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson KM, Belfer JJ, Peterson GR, et al. Managing COVID-19 in renal transplant recipients: A review of recent literature and case supporting corticosteroid-sparing immunosuppression. Pharmacotherapy. 2020 doi: 10.1002/phar.2410. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y, Kwon O, Paek JH, et al. Two distinct cases with COVID-19 in kidney transplant recipients. Am J Transplant. 2020 doi: 10.1111/ajt.15947. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am J Transplant. 2020 doi: 10.1111/ajt.15929. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020 doi: 10.1111/ajt.15891. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: A summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77(6):748–54. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020 doi: 10.1056/NEJMc2011117. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–88. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150–56. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]


