Abstract
Background
Quercetin was reported to be crucial for a broad range of activities, including attenuating inflammation, platelet aggregation, capillary permeability, and lipid peroxidation. However, the effect of quercetin in hypertension during pregnancy, was not fully understood.
Methods
The model of hypertension in pregnancy was established in rats by reduced uterine perfusion pressure (RUPP). Quercetin was administrated by gavage. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using the CODA 6 BP system. Plasma concentrations of Endothelin-1 (ET-1), soluble fms-like tyrosine kinase-1 (sFlt-1), and vascular endothelial growth factor (VEGF) were detected using enzyme-linked immunosorbent assay kits. The mRNA and protein levels of ET-1 and endothelin-1 type A receptor (ETAR) were determined by RT-PCR and Western blotting. The ETAR antagonist BQ-123 was performed by osmotic minipumps.
Results
In RUPP induced rats, quercetin treatment decreased SBP and DBP, fetal resorptions percentage, plasma ET-1 and sFlt-1 concentrations, ET-1 and ETAR levels, but increased fetal body weight and VEGF expression. BQ-123 administration attenuated SBP and DBP, suppressed fatal resorptions percentage, and increased fetal body weight of RUPP rats.
Conclusion
Quercetin attenuates RUPP induced hypertension in pregnant rats through the regulation of ET-1 and ETAR.
Keywords: Quercetin, Reduced uterine perfusion pressure, Hypertension, Pregnant rats, Pharmacology
Introduction
Hypertension in pregnancy is the disease that developed in mother gestation of 20 weeks or longer and is one of the leading causes of morbidity and mortality for mother and baby around the world [1, 2]. Hypertension in pregnancy is characterized by sudden hypertension development with over 140/90 blood pressure, proteinuria, elevated liver enzymes, hemolytic anemia, and low platelet count [3, 4]. The disorder during hypertension in pregnancy begins with a normal pregnant process and will finally cause severe maternal and fetal health problems [5, 6]. Moreover, when comparing with normal pregnant women, women with history of hypertension in pregnancy have a higher risk to develop higher blood pressure, long term endothelial dysfunction, and low-density lipoprotein cholesterol [7]. Hypertension in pregnancy commonly leads to complications in the brain, heart, and kidneys [7]. Early diagnosis is crucial for the therapy of hypertension in pregnancy. However, the mechanism that regulated the pathogenesis of hypertension in pregnancy in not totally understood, which limits the options for potential treatment [8].
Quercetin could be widely found in vegetables, fruits, and soybeans [9]. Various studies reported the effect of quercetin in anti-hypertension both in human and animal models. Daily intake of 150–730 mg quercetin for 4 to 10 weeks showed the anti-hypertensive effect in human. For stage 1 hypertensive patients, a high dose (730 mg daily) of quercetin intake significantly suppressed systolic blood pressures (SBP) and diastolic blood pressures (DBP), while high dose quercetin showed no effect for prehypertension patients [10]. In patients with metabolic syndrome, Daily intake of 150 mg quercetin for 5 weeks suppressed SBP [11]. In a hypertensive rat model, 10− 5 M quercetin treatment increased acetylcholine-induced vascular relaxation [12], demonstrating that quercetin suppressed blood pressure by attenuating the blood vessel elastance. However, the effect of quercetin on hypertension in pregnancy remains unclear and the mechanism is still waiting for exploration.
In this study, the reduced uterine perfusion pressure (RUPP) rat model [13] was used to explore the effect of quercetin in hypertension during pregnancy. In RUPP rats, quercetin treatment decreased blood pressure, and fetal resorptions percentage, and increased fetal body weight. Mechanically, quercetin treatment inhibited the Endothelin-1 (ET-1) / endothelin-1 type A receptor (ETAR) pathway. These results supply evidence that quercetin may be used for the treatment of hypertension in pregnancy in the clinic.
Methods
PUPP rat model
Sprague-Dawley female rats (210–240 g) and male rats (260–300 g) procured from HFK Bioscience (Beijing, China), aged 10 weeks, were monitored in sterile cages under laboratory conditions (12 h day/night cycle; 22–23 °C; humidity, 55–60%) at a ratio of 2:1 with the free access of standard diet and water. On the second day, the female rats were inspected for the presence of a vaginal plug. The day that the vaginal plug was found was counted as day 0 of pregnancy.
The pregnant rats were randomly divided into different groups. RUPP group: The RUPP rat model was established as previously described [13]. Briefly, on gestational day (GD) 14, rats undergoing RUPP procedure were anesthetized with 2% isoflurane (Sigma, St. Louis, MO, USA), and after a midline incision, a 0.203 mm internal diameter silver clip was placed around subrental abdominal aorta. On branches of both ovarian artery, 0.1 mm internal diameter clips were placed above the uterine arteries. Control group: on gestational day 14, the pregnant rats were performed by Sham surgery that have the same abdominal incision and suturing, but don’t have the clip placement. RUPP+Quercetin groups: From GD 14 to GD 21, RUPP rats were performed intragastric administration of different doses of freshly prepared quercetin (Sigma, St. Louis, MO, USA; 10 mg/kg/d, 20 mg/kg/d, 50 mg/kg/d) that was dissolved in 1 mL of 0.9% saline solution. The doses of quercetin used in this study are based on previous reports [14, 15]. RUPP+BQ-123 group: From GD 14 to GD 21, RUPP rats were performed 100 nmol/kg/d BQ-123 (Sigma, St. Louis, MO, USA) treatment through osmotic minipumps.
On day 21 of gestation, the pregnant rats were anaesthetized using isoflurane, fetuses and corresponding placentas were obtained and weighted. Placenta tissues were quick-frozen in liquid nitrogen, then stored at − 80 °C until use. There are no rats were in the process of giving birth on GD21, and the fetuses extracted from the mother. There are 6 rats in each group. All experiments were approved by the Animal Ethics Committee of Qingdao Municipal Hospital (#QDSLYY2018271).
Blood pressure measure
From GD 13 to GD19, the CODA 6 BP system was used to test blood pressure. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were tested every 2 days (GD13, GD15, GD17, and GD19). In each session, SBP and DBP were measured for 3 times. On GD21, the rats were anaesthetized, and blood pressure on GD21 was not taken into analysis. The average SBP and DBP were recorded.
ELISA
Blood of rats on GD21 from different groups was collected and spun at 2500 rpm for 12 min at 4 °C, and plasma was stored at − 80 °C for analysis. The plasma concentrations of ET-1, sFlt-1 and VEGF were detected using commercial ELISA kits according to the manufacturer’s protocols. The sensitivity of the ELISA kits for ET-1, sFlt-1 and VEGF were 0.207 pg/mL, 15.2 pg/mL, 25 pg/mL, respectively. All EILSA kits are purchased from R&D Biosystems (Minneapolis, MN, USA).
RT-PCR
RNA from placenta tissue on GD21 was extracted by Trizol kit (Thermo Fisher, Waltham, MA, USA). RT-qPCR was carried out using SYBR TaqTM kit (Qiagen, Valencia, CA, USA) in 7300 Real-Time PCR machine (Thermo Fisher, Waltham, MA, USA). The primers used in the study are: preproET-1, Forward: 5′- CTA GGT CTA AGC GAT CCT TGA A-3′ Reverse: 5′- CTT GAT GCT GTT GCT GAT GG-3′; ETAR, Forward: 5′-TCA AGC AGC GTC GAG AGG TG-3′, Reverse: 5′-TGT GGC TGC TCC GTT CTG TG-3′; GAPDH, Forward: 5′-AAC TGA GGG CTC TGC TCG CT-3′, Reverse: 5′-GTG ACA CAC CGC AAG GCT T-3′. Ct (cycle threshold) value was collected. The relative expression levels of genes were determined by the 2−△△Ct method.
Western blotting
On GD21, placental tissue proteins were first extracted, followed by measuring the concentration of the proteins using the BCA kit (Pierce™ BCA, Thermo Fisher Scientific). Proteins were loaded onto a 10% polyvinylidene fluoride membrane (Merck Millipore, Burlington, MA, USA). Then, the samples were blocked using 5% milk for 1 h, followed by incubated using primary antibodies for ET-1 (1:1000, ab2786, Abcam, Shanghai, China) and ETAR (1:500, ab85163, Abcam, Shanghai, China) overnight at 4 °C. Horseradish peroxidase-conjugated secondary antibodies were used to incubate temperature for 1 h at room temperature. Finally, chemiluminescent reagents (Merck Millipore, Burlington, MA, USA) were used to detect the signals. Image J was used for quantifying the signals.
Statistical analysis
SPSS 19.0 was used to analyze all the data. Data were shown as mean ± standard deviation (SD). Kruskal-Wallis test or one-way ANOVA followed with a Tukey post hoc test or two-way ANOVA followed with a Bonferroni post hoc test were used for determining the statistical significance.
Results
Quercetin decreased blood pressure during the middle to late gestation period
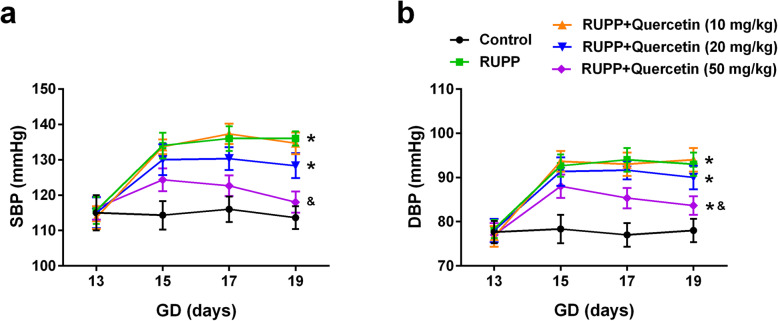
The animal model of hypertension in pregnant rats was first established using RUPP. After the surgery on day 14 of gestation, RUPP rats had significantly increased SBP from day 15 to day 19 when compared with control rats. Quercetin treatment by intragastric administration dramatically suppressed SBP of RUPP rats in a dose-dependent manner, as 50 mg/kg quercetin is most effective and decreased SBP from 124.3 ± 3.2 mmHg to 118.0 ± 3.0 mmHg (Fig. 1a). Similarly, Quercetin treatment significantly suppressed diastolic blood pressure (DBP) of RUPP rats from day 15 to day 19 (Fig. 1b). These results demonstrated that quercetin treatment decreased blood pressure during the middle to the late gestation period of RUPP rats.
Fig. 1.
Quercetin decreased systolic (a) and diastolic (b) blood pressure during the middle to late gestation period. n = 6 for each group. Data were shown as mean ± SD. *P < 0.0083 compared with the Control group; &P < 0.0083 compared with the RUPP group. Two-way repeated-measures ANOVA with a Bonferroni pos hoc test
Quercetin treatment protects fetal body of RUPP rats
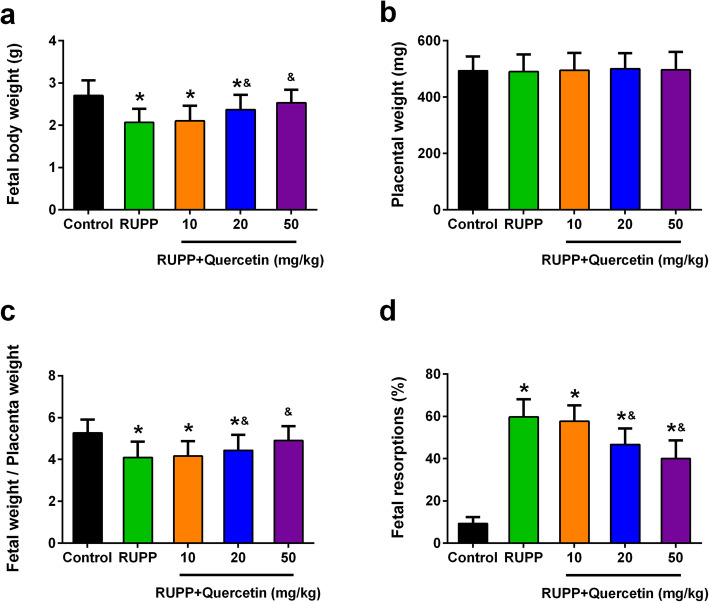
The effect of quercetin on protecting the fetal body of RUPP rats was further explored. As shown in Fig. 2a, the fetal body weight of RUPP rats (2.07 ± 0.32 g) significantly decreased when compared with the control rats (2.70 ± 0.36 g), but quercetin treatment, especially with high dose dramatically enhanced fetal body weight (2.53 ± 0.31 g). However, quercetin treatment has no effect on placental weight (Fig. 2b). The fetal weight/placenta weight ratio of RUPP rats significantly enhanced after quercetin treatment (Fig. 2c). Moreover, the percentage of fetal resorptions in RUPP rats (59.7 ± 8.4%) was increased as compared with the control group (9.3 ± 3.1%), while high dose quercetin treatment dramatically suppressed the percentage of fetal resorptions in RUPP rats (40.0 ± 8.7%) (Fig. 2d). These results demonstrated that quercetin treatment protects the fetal body of RUPP rats.
Fig. 2.
Effects of quercetin administration on features of fetal rats and placenta in each group. Fetal body weight (a), placental weight (b), fetal/placental weight ratio (c) and fetal resorptions (d) were determined in pregnant rats at day 21 of gestation. n = 55, 24, 30, 36 and 41 in control, RUPP, RUPP+Quercetin (10 mg/kg), RUPP+Quercetin (20 mg/kg) and RUPP+Quercetin (50 mg/kg) group respectively. Data were shown as mean ± SD. *P < 0.05 compared with the Control group; &P < 0.05 compared with the RUPP group. One-way ANOVA with a Tukey’s post hoc test (a-c), Kruskal-Wallis test (d)
Quercetin treatment attenuates ET-1 and sFLT1, and increased VEGF in plasma of RUPP rats
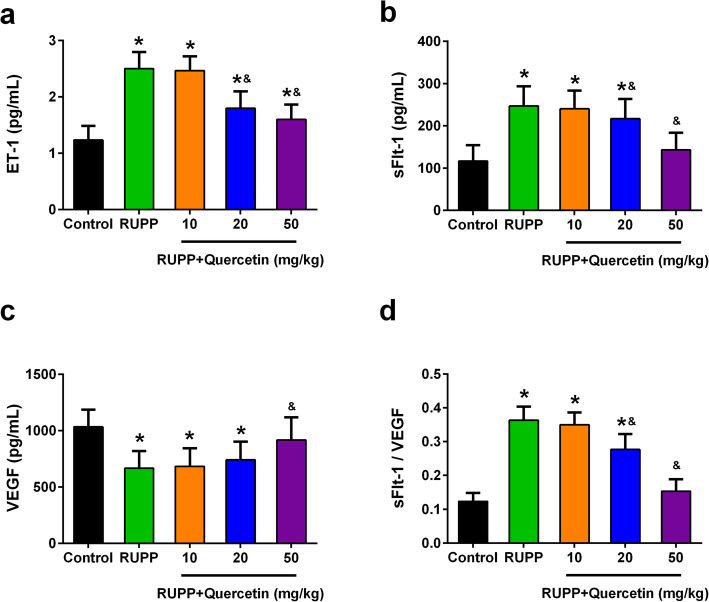
Endothelin-1 (ET-1) was reported to be crucial for preeclampsia pathogenesis and arterial pressure regulation [16–21]. The plasma ET-1 level of RUPP rats (2.51 ± 0.30 pg/mL) was significantly higher when compared with the control rats (1.23 ± 0.25 pg/mL). Quercetin treatment dramatically suppressed the plasma ET-1 level of RUPP rats (1.62 ± 0.27 pg/mL) (Fig. 3a). Circulating fms-like tyrosine kinase-1 (sFlt-1) as well as the VEGF are generally believed to be associated with hypertension in pregnant [22, 23]. The increased plasma level of soluble fms-like tyrosine kinase-1 (sFLT1) in RUPP rats was also inhibited after quercetin treatment (247 ± 47 pg/mL vs. 143 ± 40 pg/mL) (Fig. 3b). As expected, the decreased plasma level of VEGF in RUPP rats was enhanced after quercetin treatment (668 ± 152 pg/mL vs. 916 ± 202 pg/mL) (Fig. 3c). Consistently, the ratio of sFLT1/VEGF in plasma of RUPP rats significantly decreased after quercetin treatment (Fig. 3d). These results demonstrated that quercetin treatment attenuated ET-1 and sFLT1, but increased VEGE in plasma of RUPP rats.
Fig. 3.
Effects of quercetin on plasma levels of ET-1, sFlt-1 and VEGF of each group at day 21 of gestation. Plasma levels of ET-1 (a), sFlt-1 (b) and VEGF (c) were measured by using ELISA kits. d Quercetin administration reduced the sFlt-1/VEGF ratio. n = 6 for each group. Data were shown as mean ± SD. *P < 0.05 compared with the Control group; &P < 0.05 compared with the RUPP group. One-way ANOVA with a Tukey’s post hoc test
Quercetin inhibited ET-1, ETAR expression of RUPP rats
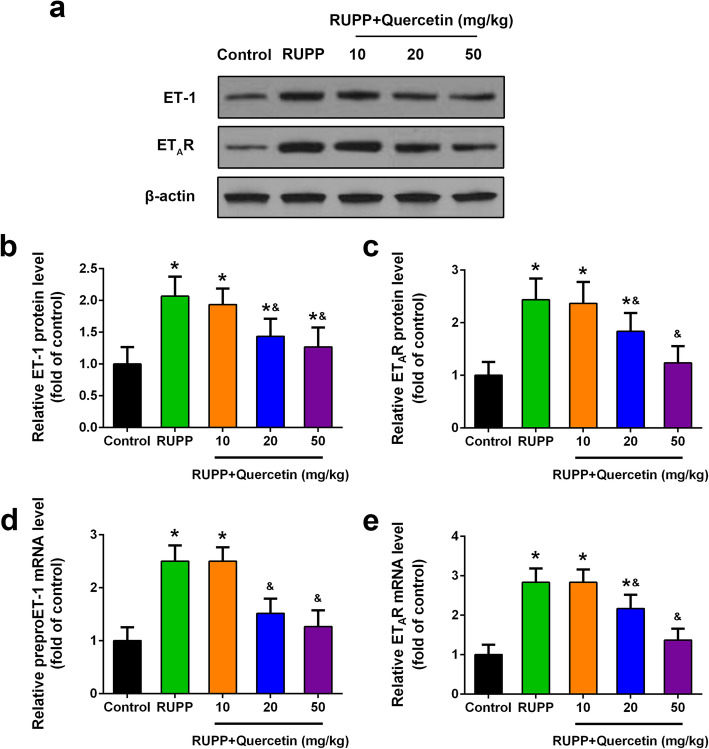
ETAR is one of the endothelin receptors (ETR) which were widely believed to be associated with hypertension in pregnancy [24]. The effect of quercetin treatment on ET-1 and ETAR expression levels in the placenta tissue of RUPP rats was further detected. On day 21 of gestation, protein expression levels of ET-1 and ETAR in the placenta tissue of RUPP rats significantly increased as compared with control rats, while dramatically decreased after quercetin treatment (Fig. 4a to c). Consistently, the mRNA expression levels of ET-1 and ETAR significantly increased in the placenta tissue of RUPP rats, while dramatically decreased after quercetin treatment (Fig. 4d and e). These results demonstrated that quercetin inhibited ET-1 and ETAR expression of RUPP rats.
Fig. 4.
Quercetin inhibited ET-1, ETAR expression in placenta tissue of RUPP rats at day 21 of gestation. Western blotting was used to analyze ET-1 and ETAR expression (a) and relative protein levels were quantified (b, c). Relative mRNA levels of preproET-1 (d) and ETAR (e) were detected by qRT-PCR. GAPDH was used as the internal control. n = 6 for each group. Data were shown as mean ± SD. *P < 0.05 compared with the Control group; &P < 0.05 compared with the RUPP group. One-way ANOVA with a Tukey’s post hoc test
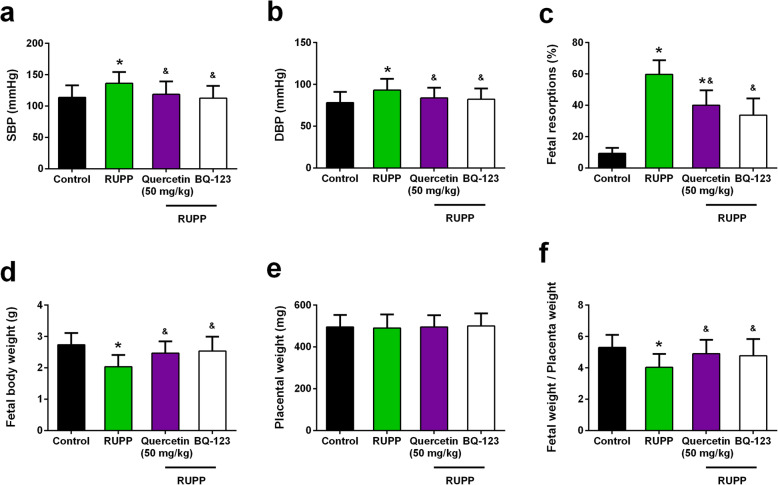
Effects of BQ-123 on blood pressure and outcomes in RUPP rats
To further confirm the effect of quercetin on RUPP rats was achieved by inhibiting ETAR, the effects of ETAR antagonist BQ-123 [25] on blood pressure and outcomes of RUPP rats were determined. Previous study indicated that quercetin has no significant effect on litter size and fetal death of control groups [26]. The procedure and dosage of BQ-123 followed the previous study [25]. BQ-123 administration suppressed systolic (112.3 ± 4.3 mmHg) (Fig. 5a) and diastolic (82.0 ± 3.1 mmHg) (Fig. 5b) blood pressure of RUPP rats. BQ-123 administration suppressed fetal resorptions percentage of RUPP rats (33.7 ± 10.7%) (Fig. 5c), increased fetal body weight (2.53 ± 0.46 g) (Fig. 5d), and showed no influence on placental weight (Fig. 5e). As expected, BQ-123 administration enhanced the ratio of fetal/placental weight (Fig. 5f). The effect of BQ-123 on RUPP rats is similar to the effect of quercetin on RUPP rats.
Fig. 5.
Comparison of the effect of quercetin with the activity of the ETAR antagonist BQ-123 on blood pressure and outcomes in RUPP rats. BQ-123 abolishes the increase in systolic (a) and diastolic (b) blood pressure. Values were measured at day 19 of gestation. Fetal resorptions (c), fetal body weight (d), placental weight (e) and fetal/placental weight ratio (f) and were determined in pregnant rats at day 21 of gestation. n = 6 for each group in (a-c), n = 53, 26, 29 and 45 pups in control, RUPP, RUPP+Quercetin (50 mg/kg) and RUPP+BQ-123 group respectively in (d-f). Data were shown as mean ± SD. *P < 0.05 compared with the Control group; &P < 0.05 compared with the RUPP group. One-way ANOVA with a Tukey’s post hoc test (a, b, d-f), Kruskal-Wallis test (c)
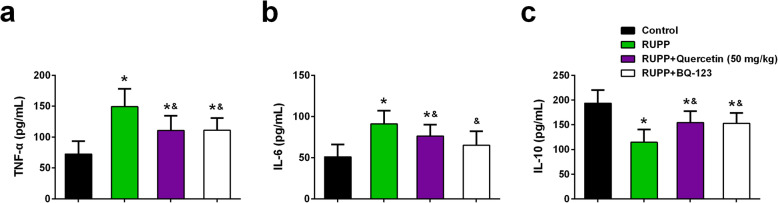
Effects of quercetin and BQ-123 on inflammatory cytokines
Quercetin was reported to be crucial for attenuating inflammation. Accordingly, the plasma levels of TNF-α, IL-6, and IL-10 in different groups were determined. The inflammatory cytokine (TNF-α and IL-6) levels of RUPP rats significantly increased when compared with the control rats. Quercetin treatment and the administration of ETAR antagonist BQ-123 dramatically suppressed the plasma levels of TNF-α and IL-6 in RUPP rats (Fig. 6a, b).
Fig. 6.
Effects of quercetin and BQ-123 on plasma levels of TNF-α, IL-6 and IL-10 of each group at day 21 of gestation. Plasma levels of TNF-α (a), IL-6 (b) and IL-10 (c) were measured by using ELISA kits. n = 6 for each group. Data were shown as mean ± SD. *P < 0.05 compared with the Control group; &P < 0.05 compared with the RUPP group. One-way ANOVA with a Tukey’s post hoc test
Oppositely, the plasma level of anti-inflammatory cytokine IL-10 in RUPP rats significantly decreased when compared with the control rats. Quercetin treatment and the administration of ETAR antagonist BQ-123 significantly enhanced the plasma level of IL-10 in RUPP rats (Fig. 6c).
Discussion
Hypertension in pregnancy influences many women, while the treatment for hypertension in pregnancy is limited. Quercetin was reported to be effective to suppress both SBP and DBP in human patients with high blood pressures and in animal models. Accordingly, the effect of quercetin in established hypertension in the pregnant rat model was explored. In RUPP-induced high blood pressure in pregnant rats, different doses of quercetin treatment (10, 20, 50 mg/kg) decreased SBP and DBP, increase fetal body weight, decreased fetal resorptions percentage. Mechanically, quercetin treatment inhibited ET-1/ ETAR pathway, and the ETAR antagonist suppressed SBP and DBP, and increased fetal body weight, which is similar to the effect of cetin treatment, indicating that quercetin attenuates RUPP induced hypertension in pregnant rats through regulation of ET-1/ ETAR pathway.
RUPP was widely used to induce hypertension in pregnant rats. In this study, the pregnant rats were first confirmed. On gestational day 14, the pregnant rats were performed by RUPP as described in methods. Successful hypertension in the pregnant rat model was confirmed by enhanced SBP and DBP of RUPP rats when compared with control rats.
Quercetin was reported to inhibit blood pressure [27]. Patients with type 2 diabetes showed dramatically decreased systolic blood pressure after 10 weeks intake of 500 mg/day quercetin [28]. From one summary of meta-analysis in human data, quercetin intake over 500 mg/day for 8 weeks will benefit patients that have high blood pressure, as evidenced by the decreased SBP and DBP [27]. In animal models of high blood pressure, quercetin also showed the effect to suppress blood pressure. In deoxycorticosterone acetate induced hypertensive model in rats, chronic oral administration of quercetin suppressed blood pressure and improved endothelial dysfunction and aortic dilatation [29]. In spontaneously hypertensive rats, quercetin showed the effect of anti-hypertensive action, enhanced the sensitivity of the baroreflex, suppressed the oxidative stress in serum, indicating that quercetin attenuated blood pressure via reducing oxidative stress [30, 31]. Some other studies reported that quercetin has the ability to destroy renin-angiotensin system through decreasing the expression level and activity of angiotensin converting enzyme [32]. Therefore, the effect of quercetin in anti-hypertensive actions was achieved by regulating vascular compliance, renin-angiotensin system, and the total blood volume. Consistently, quercetin treatment significantly suppressed SBP and DBP of RUPP-induced hypertension rats. More importantly, quercetin treatment improved the outcome of pregnant RUPP rats, as evidenced by increased fetal body weight and decreased fetal resorptions percentage.
ET-1 is expressed by smooth muscle cells and vascular endothelial cells. ET-1 is crucial in regulating vascular functions such as modifying blood flow, altering vessel diameter, and controlling basal arterial tone [33, 34]. ET-1 is effective in blood vessel constriction, and the overexpression of ET-1 always results in high blood pressure. During pregnancy, the effect of ET-1 is enhanced, which will lead to hypertension in pregnancy, even potential heart disease [35, 36]. ET-1 concentration in plasma was reported to be increased in preeclamptic patients that have high blood pressure and be associated with sFlt-1 [37, 38]. Moreover, in animal models of hypertension in pregnancy, the expression levels of ET-1 in kidney and placental increased as compared with normal pregnant rats [39, 40]. All these studies demonstrated that ET-1 may regulate the progression of hypertension in pregnancy. In the present study, ET-1 expression increased in RUPP rats, while quercetin treatment suppressed RUPP expression levels. More importantly, ETAR antagonist BQ-123 abolishes SBP and DBP of RUPP rats, which is similar to the effect of quercetin in RUPP rats, demonstrated that the effect of quercetin treatment in RUPP rats is achieved through ET-1/ ETAR pathway.
Study strength and limitations
It was worth noting that there are several limitations for the current study. First, the sample size varied significantly in control, RUPP, RUPP+Quercetin (10 mg/kg), RUPP+Quercetin (20 mg/kg) and RUPP+Quercetin groups, which may lead to false positive results. Second, the conclusion of the involvement of ET-1/ETAR pathway in such protective actions of quercetin could be strengthened by employing pharmacological method to active the ET-1/ETAR pathway in quercetin-treated RUPP rats, to check whether the activation of the ET-1/ETAR pathway will abolish the protective effect of quercetin on RUPP induced hypertensive rats. Last, although we did not study the side effects of quercetin on pregnancy, embryo, fetus, and placenta, the safety of quercetin could be supported by previous studies. It was reported that quercetin given throughout organogenesis represented no teratogenic threat in the rats [41]. In addition, quercetin treatment did not cause any obvious effects on maternal weight gain, placenta weight, fetal weight and length [26].
Conclusion
Quercetin attenuates RUPP induced hypertension in pregnant rats through regulation of ET-1 and ETAR. Quercetin may be a potential choice for hypertension in pregnancy in the clinic. The effect of quercetin in the clinic should be determined in the future.
Supplementary information
Acknowledgements
None.
Abbreviations
- RUPP
Reduced uterine perfusion pressure
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- ET-1
Endothelin-1
- sFlt-1
Soluble fms-like tyrosine kinase-1
- VEGF
Vascular endothelial growth factor
- ELISA
Enzyme-linked immunosorbent assay
- ETAR
Endothelin-1 type A receptor
- GD
Gestational day
Authors’ contributions
Xia Sun, Shuping Zhang and Haitao Song conducted the experiments, analyzed the data. Shuping Zhang and Haitao Song wrote the manuscript, supervised the study. The author (s) read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree the publication of this paper.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuping Zhang, Email: zhangshuping95@126.com.
Haitao Song, Email: Songhaitao0377@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12944-020-01357-w.
References
- 1.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jameil N, Aziz Khan F, Fareed Khan M, Tabassum H. A brief overview of preeclampsia. J Clin Med Res. 2014;6:1–7. doi: 10.5897/JCMR11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25:124–132. doi: 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Ruan Y, Shen L, Gong Q. Protective effects of PPAR-gamma against pregnancy-induced hypertension by differential ETR expression in rat models. J Cell Biochem. 2018;119:3118–3128. doi: 10.1002/jcb.26454. [DOI] [PubMed] [Google Scholar]
- 5.Salmani D, Purushothaman S, Somashekara SC, Gnanagurudasan E, Sumangaladevi K, Harikishan R, Venkateshwarareddy M. Study of structural changes in placenta in pregnancy-induced hypertension. J Nat Sci Biol Med. 2014;5:352–355. doi: 10.4103/0976-9668.136182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo JY, Fu D, Wu YQ, Gao Y. Inhibition of the JAK2/STAT3/SOSC1 signaling pathway improves secretion function of vascular endothelial cells in a rat model of pregnancy-induced hypertension. Cell Physiol Biochem. 2016;40:527–537. doi: 10.1159/000452566. [DOI] [PubMed] [Google Scholar]
- 7.Ren Y, Wang H, Qin H, Yang J, Wang Y, Jiang S, Pan Y. Vascular endothelial growth factor expression in peripheral blood of patients with pregnancy induced hypertension syndrome and its clinical significance. Pak J Med Sci. 2014;30:634–637. doi: 10.12669/pjms.303.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jido TA, Yakasai IA. Preeclampsia: a review of the evidence. Ann Afr Med. 2013;12:75–85. doi: 10.4103/1596-3519.112395. [DOI] [PubMed] [Google Scholar]
- 9.Marunaka Y, Marunaka R, Sun H, Yamamoto T, Kanamura N, Inui T, Taruno A. Actions of Quercetin, a polyphenol, on blood pressure. Molecules. 2017;22:209. [DOI] [PMC free article] [PubMed]
- 10.Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137:2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- 11.Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102:1065–1074. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 12.Choi S, Ryu KH, Park SH, Jun JY, Shin BC, Chung JH, Yeum CH. Direct vascular actions of quercetin in aorta from renal hypertensive rats. Kidney Res Clin Pract. 2016;35:15–21. doi: 10.1016/j.krcp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 14.Suganya N, Dornadula S, Chatterjee S, Mohanram RK. Quercetin improves endothelial function in diabetic rats through inhibition of endoplasmic reticulum stress-mediated oxidative stress. Eur J Pharmacol. 2018;819:80–88. doi: 10.1016/j.ejphar.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Toumi ML, Merzoug S, Tahraoui A. Effects of quercetin on predator stress-related hematological and behavioral alterations in pregnant rats and their offspring. J Biosci. 2016;41:237–249. doi: 10.1007/s12038-016-9613-1. [DOI] [PubMed] [Google Scholar]
- 16.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24:964–969. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.HYP.38.3.718. [DOI] [PubMed] [Google Scholar]
- 18.LaMarca BD, Alexander BT, Gilbert JS, Ryan MJ, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med. 2008;5(Suppl A):S133–S138. doi: 10.1016/j.genm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 20.Clark BA, Halvorson L, Sachs B, Epstein FH. Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment. Am J Obstet Gynecol. 1992;166:962–968. doi: 10.1016/0002-9378(92)91372-H. [DOI] [PubMed] [Google Scholar]
- 21.Wilkins FC, Jr, Alberola A, Mizelle HL, Opgenorth TJ, Granger JP. Systemic hemodynamics and renal function during long-term pathophysiological increases in circulating endothelin. Am J Phys. 1995;268:R375–R381. doi: 10.1152/ajpregu.1995.268.2.R375. [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, Feng Y, Yu J, Yuan H, Yin Y, Ding J, Zhao J, Xu Y, Xu J, Che H. Fasudil attenuates soluble fms-like tyrosine kinase-1 (sFlt-1)-induced hypertension in pregnant mice through RhoA/ROCK pathway. Oncotarget. 2017;8:104104–104112. doi: 10.18632/oncotarget.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tandon V, Hiwale S, Amle D, Nagaria T, Patra PK. Assessment of serum vascular endothelial growth factor levels in pregnancy-induced hypertension patients. J Pregnancy. 2017;2017:3179670. doi: 10.1155/2017/3179670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Xiao D, Hu Y, Wang Z, Paradis A, Mata-Greenwood E, Zhang L. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension. 2013;62:599–607. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim KA, Eleyan M, Abd El-Rahman HA, Khwanes SA, Mohamed RA. Quercetin attenuates the oxidative injury-mediated Upregulation of apoptotic gene expression and catecholaminergic neurotransmitters of the fetal Rats’ brain following prenatal exposure to fenitrothion insecticide. Neurotox Res. 2020;37:871–882. doi: 10.1007/s12640-020-00172-6. [DOI] [PubMed] [Google Scholar]
- 27.Serban MC, Sahebkar A, Zanchetti A, Mikhailidis DP, Howard G, Antal D, Andrica F, Ahmed A, Aronow WS, Muntner P, et al. Effects of quercetin on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e002713. [DOI] [PMC free article] [PubMed]
- 28.Zahedi M, Ghiasvand R, Feizi A, Asgari G, Darvish L. Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes: a double-blind randomized controlled clinical trial. Int J Prev Med. 2013;4:777–785. [PMC free article] [PubMed] [Google Scholar]
- 29.Galisteo M, Garcia-Saura MF, Jimenez R, Villar IC, Wangensteen R, Zarzuelo A, Vargas F, Duarte J. Effects of quercetin treatment on vascular function in deoxycorticosterone acetate-salt hypertensive rats. Comparative study with verapamil. Planta Med. 2004;70:334–341. doi: 10.1055/s-2004-818945. [DOI] [PubMed] [Google Scholar]
- 30.Merendino N, Molinari R, Costantini L, Mazzucato A, Pucci A, Bonafaccia F, Esti M, Ceccantoni B, Papeschi C, Bonafaccia G. A new “functional” pasta containing tartary buckwheat sprouts as an ingredient improves the oxidative status and normalizes some blood pressure parameters in spontaneously hypertensive rats. Food Funct. 2014;5:1017–1026. doi: 10.1039/C3FO60683J. [DOI] [PubMed] [Google Scholar]
- 31.Monteiro MM, Franca-Silva MS, Alves NF, Porpino SK, Braga VA. Quercetin improves baroreflex sensitivity in spontaneously hypertensive rats. Molecules. 2012;17:12997–13008. doi: 10.3390/molecules171112997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parichatikanond W, Pinthong D, Mangmool S. Blockade of the renin-angiotensin system with delphinidin, cyanin, and quercetin. Planta Med. 2012;78:1626–1632. doi: 10.1055/s-0032-1315198. [DOI] [PubMed] [Google Scholar]
- 33.Cao L, Cao YX, Xu CB, Edvinsson L. Altered endothelin receptor expression and affinity in spontaneously hypertensive rat cerebral and coronary arteries. PLoS One. 2013;8:e73761. doi: 10.1371/journal.pone.0073761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao L, Zhang Y, Cao YX, Edvinsson L, Xu CB. Cigarette smoke upregulates rat coronary artery endothelin receptors in vivo. PLoS One. 2012;7:e33008. doi: 10.1371/journal.pone.0033008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gillis EE, Sasser JM, Sullivan JC. Endothelin, sex, and pregnancy: unique considerations for blood pressure control in females. Am J Physiol Regul Integr Comp Physiol. 2016;310:R691–R696. doi: 10.1152/ajpregu.00427.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol. 2012;84:147–162. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am J Obstet Gynecol. 1991;165:724–727. doi: 10.1016/0002-9378(91)90317-K. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal PK, Chandel N, Jain V, Jha V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2012;26:236–241. doi: 10.1038/jhh.2011.29. [DOI] [PubMed] [Google Scholar]
- 39.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55:394–398. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willhite CC. Teratogenic potential of quercetin in the rat. Food Chem Toxicol. 1982;20:75–79. doi: 10.1016/S0278-6915(82)80012-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.