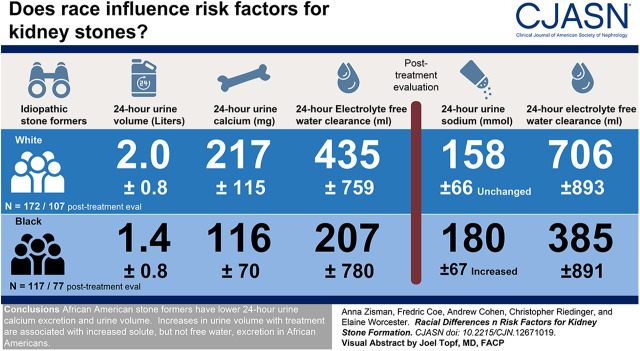
Visual Abstract
Keywords: kidney stones, ethnicity, Calcium Oxalate, Uric Acid, African Americans, Kidney Calculi, risk factors, calcium phosphate
Abstract
Background and objectives
Incidence of kidney stone disease is rising. It is not known whether mechanisms of stone formation differ across racial groups. Our objective was to identify differing lithogenic risk factors across racial groups in idiopathic nephrolithiasis.
Design, setting, participants, & measurements
We conducted a retrospective cohort study evaluating metabolic risk factors in black and age-matched white idiopathic stone formers at our tertiary referral center. We compared serum and urine metabolic risk factors pre- and post-treatment across racial groups using analysis of covariance. Generalized linear modeling was used to build regression models for risk of stone formation in both groups.
Results
Among 117 black and 172 white stone formers, urine volume was lower in black stone formers (1.4±0.8 versus 2.0±0.8 L/d, P<0.001). Urine calcium was lower in black stone formers (116±70 versus 217±115 mg/d, P<0.001). Supersaturations for calcium oxalate were similar among the groups, whereas calcium phosphate supersaturation was higher in white stone formers, and uric acid supersaturation was higher in black stone formers. Electrolyte free water clearance was significantly lower in black stone formers (207±780 versus 435±759 ml/d, P=0.02). In the subgroup of 77 black patients and 107 white patients with post-treatment evaluations, urine volume rose significantly and similarly in both groups. Urine sodium was unchanged in whites but increased in blacks by 40 mmol/d (95% confidence interval, 32 to 48 mmol/d). Electrolyte free water clearance remained lower in black stone formers (385±891 versus 706±893 ml/d, P=0.02). Post-treatment supersaturations were similar across the groups except for calcium phosphate, which improved with treatment in whites.
Conclusions
Black stone formers have lower 24-hour urine calcium excretion and urine volume. Increases in urine volume with treatment were associated with increased solute, but not free water, excretion in black stone formers.
Introduction
Kidney stones have long plagued mankind, with the first writings about the disease going back to Mesopotamian times. In modern times, with one in 11 individuals in the United States affected during his or her lifetime, the disease is highly prevalent and increasingly so (1). According to the National Health and Nutrition Examination Survey, a 70% increase in disease prevalence has been noted in the general population (from 5.2% in the 1988–1994 dataset to 8.4% in the 2007–2010 dataset) over the 15 years (1). Notably, the fastest-growing stone demographic is blacks, with the prevalence rising from 1.7% in the early group to a population-adjusted prevalence of 4.5% in the 2007–2010 cohort, reflecting a relative increase of >150% (1). In addition to the acute morbidity of stone events, kidney stone formation is associated with a multitude of comorbidities, including hypertension (2,3), cardiovascular disease (4–6), and CKD (7–10), as well as a lower quality of life than in individuals without kidney stones (11). Nephrolithiasis is also highly costly to the health care system, accounting for >$10 billion in spending yearly, not accounting for productivity or time lost from work (12).
The relative roles of environment (lifestyle, diet, and local climate) and genetics in stone pathogenesis have not been elucidated. Several studies have noted similar prevalence of stone disease between those of African ancestry and whites in a given geographic region (13,14), whereas others have invoked the increasing rates of obesity in the black population as the etiology of the more quickly rising prevalence of stone disease in the black population (15). Whether because of the perceived lack of disease burden in blacks (16,17), because of an assumption of similar pathogenesis of disease, or because of societal factors (18), this population of stone formers is poorly characterized, as evidenced by <40 articles on the subject of racial differences in nephrolithiasis that were noted in a comprehensive review (19). Most of the existing data approach the question of racial differences in stone formation from an epidemiologic perspective, and only five of these articles evaluated 24-hour lithogenic risk factors to understand underlying physiology (20–24). The largest study comparing these parameters involved only 44 black patients and concluded that few significant differences in the metabolic risk factors exist (24). Critically, no data are currently available on the racial differences in the response of the metabolic parameters with targeted treatment of individual risk factors.
Given the limited data currently available, we sought to characterize a population of self-identified black idiopathic stone formers to elucidate the physiologic mechanisms of stone formation. As a large tertiary referral center for stone disease that is located in a highly diverse area of Chicago, we are uniquely positioned to identify potential metabolic racial differences in the predilection for stone formation and in the response to the standard stone prevention treatment.
Materials and Methods
All patients presenting to the University of Chicago Kidney Stone Prevention Program complete two or three 24‐hour urine collections, with corresponding fasting blood sample (“pretreatment”), prior to the initial evaluation by a physician where treatment recommendations are made. The laboratory data and accompanying clinical and demographic information are entered into the clinical database. Racial data are collected from the initial intake questionnaire completed by every new patient. All subsequent urine and serum testing results, as ordered by the treating physician, are also entered into the database and classified as “post-treatment.” For this study, the average of the first two to three collections is reported as the pretreatment value, and only the first post-treatment sample was included in the post-treatment analysis. At their initial evaluation, virtually all patients are counseled on a low-sodium diet (<2300 mg/d) and educated regarding the critical importance of increasing fluid intake to at least 3 L/d. Additional individualized recommendations and medication prescriptions targeting specific metabolic abnormalities noted on the metabolic evaluation are provided to each patient. All patients in our program are offered the opportunity to consent to a University of Chicago–approved institutional review board protocol (11943A), which allows the use of their deidentified clinical and demographic data for our research studies.
Patient Selection
We retrospectively identified all consenting black patients who entered our program from July 1, 2008 (when we initially began collecting race data) to June 30, 2017 who had completed the initial evaluation, including complete urine and serum studies. Patients with any systemic diseases potentially contributing to stone risk, such as sarcoidosis, primary hyperparathyroidism, metastatic malignancy, inflammatory bowel disease, hereditary renal tubular acidosis, and cystinuria, were excluded. All consenting white stone formers in our program who completed the initial evaluation in the same calendar year as a corresponding age-matched (±3 years) black patient made up the initial comparison group. Patients who had previously or were currently receiving treatment for stone prevention were excluded. A subset of patients from each racial group who completed at least one post-treatment evaluation was selected and divided into groups by race for comparison of post-treatment outcomes. Stone type was collected where available and classified as calcium oxalate, calcium phosphate, or uric acid if >50% composition was of a given type. Concurrent use of thiazides was ascertained with each collection.
Laboratory Methods
All laboratory measurements were completed in the University of Chicago Kidney Stone Laboratory. All urine samples are evaluated for volume, pH, creatinine, sodium, potassium, chloride, calcium, magnesium, ammonium, phosphate, oxalate, citrate, uric acid, sulfate, and urea nitrogen by methods we have previously published (25). Blood samples are measured for calcium, phosphate, uric acid, creatinine, magnesium, sodium, potassium, chloride, and total CO2 content. Serum calcium was measured by atomic absorption on a PerkinElmer AAnalyst 100 spectrometer. Urine pH was measured by a glass electrode on a Beckman f390 pH meter. Urine sulfate was measured by assaying turbidity (OD630, turbidity-corrected optical density at wavelength of 630 nm) after barium precipitation. The remainder of the analyses was completed on the Beckman DxC 600.
Calculations
Supersaturation was calculated with respect to calcium oxalate, calcium phosphate, and uric acid using established open-source software (EQUIL 2) (26). Electrolyte free water clearance was calculated conventionally: Electrolyte free water clearance = V × (1 − (U/P)Na+k), where U and P are urine and plasma concentrations of Na + K, and V is urine flow rate in liters per day.
We also expressed electrolyte free water clearance per unit creatinine clearance: Electrolyte free water clearance per unit creatinine clearance = V × (1 − (U/P)Na+k)/V × (U/P)Cr = (1 − (U/P)Na+k)/(U/P)Cr.
Statistical Analyses
Mean baseline and post-treatment serum concentrations and urine excretions, as well as the changes between them, were compared between black and white subjects using analysis of covariance models. All values were adjusted for sex. Serum creatinine and all urine values except supersaturation were adjusted for weight. All urine values except pH, supersaturations, and creatinine clearance were adjusted for urine creatinine. All post-treatment values were adjusted for their pretreatment values. Significances were calculated by Bonferroni corrected post hoc hypothesis testing within analysis of covariance models (version 13; Systat, Chicago, IL). We used generalized linear models to evaluate the relative contributions of the urine variables to supersaturation. To graphically visualize associations for a given variable of interest, we used the coefficients from our generalized linear models to create regressions for calcium oxalate supersaturation and calcium phosphate supersaturation as functions of urine volume, pH, calcium, oxalate, and citrate excretions. Differences in proportions of patients completing a follow-up collection by race as well as in proportions using thiazides before and after treatment were analyzed using the Pearson chi-squared test.
Results
This study included 117 black patients and 172 white patients (Table 1). Stone analyses were available for 159 patients (55%), with similar proportions across racial groups (53% black and 56% white). The majority of stones were calcium based, with calcium oxalate representing 73% and 69% of stones in black and white patients, respectively. Calcium phosphate stones accounted for 15% and 18% of stones in black and white patients, respectively. Uric acid stones were found in 13% and 12% of black and white patients, respectively. Thiazide use was noted in 12.8% and 26.5% of black patients at baseline and during therapy, respectively. In whites, the baseline proportion was similar at 11.6%, but it was significantly higher at 50.6% after treatment (chi square =33.490, P<0.001).
Table 1.
Baseline demographic data and pretreatment laboratory values (SD) with adjusted differences by race (95% confidence interval)
| Black | White | Adjusted Difference Black versus Whitea | P Value | |
|---|---|---|---|---|
| N | 117 | 172 | ||
| Sex, men/women | 39/78 | 84/88 | ||
| Age, yr | 51 (14) | 47 (15) | ||
| Weight, kg | 87 (22) | 79 (23) | ||
| Stone analysis (% of available stone analyses) | 62 | 96 | ||
| Calcium oxalate | 45 (73) | 66 (69) | ||
| Calcium phosphate | 9 (15) | 17 (18) | ||
| Uric acid | 8 (13) | 12 (12) | ||
| Serum values | ||||
| Sodium, mmol/L | 140.4 (2.0) | 139.5 (1.5) | 0.9 (0.5 to 1.3) | <0.001 |
| Potassium, mmol/L | 4.2 (0.5) | 4.3 (0.3) | −0.07 (−0.15 to 0.01) | 0.07 |
| Bicarbonate, mmol/L | 26.4 (2.2) | 26.6 (0.2) | −0.1 (−0.6 to 0.5) | 0.77 |
| Calcium, mg/dl | 9.5 (0.5) | 9.6 (0.3) | −0.1 (−0.18 to −0.03) | 0.005 |
| Phosphate, mg/dl | 3.3 (0.6) | 3.5 (0.04) | −0.2 (−0.3 to −0.1) | <0.001 |
| Creatinine, mg/dl | 1.1 (0.3) | 0.9 (0.3) | 0.2 (0.1 to 0.3) | 0.001 |
| 24-h urine values | ||||
| Volume, L/d | 1.4 (0.8) | 2.0 (0.8) | −0.6 (−0.8 to −0.4) | <0.001 |
| pH | 5.9 (0.5) | 6.0 (0.5) | −0.1 (−0.2 to 0.0) | 0.15 |
| Creatinine, mg/d | 1432 (500) | 1395 (439) | 42 (−24 to 109) | 0.23 |
| Sodium, mmol/d | 135 (55) | 160 (62) | −30 (−41 to −19) | <0.001 |
| Potassium, mmol/d | 42 (18) | 58 (21) | −16 (−20 to −12) | <0.001 |
| Calcium, mg/d | 116 (70) | 217 (115) | −110 (−131 to −88) | <0.001 |
| Magnesium, mg/d | 76 (39) | 104 (41) | −29 (−37 to −21) | <0.001 |
| Sulfate, meq/d | 34 (14) | 44 (19) | −12 (−15 to −9) | <0.001 |
| Ammonium, meq/d | 28 (13) | 32 (13) | −1 (−4 to 2)b | 0.54 |
| Phosphate, mg/d | 645 (239) | 939 (321) | −314 (−360 to −268) | <0.001 |
| Oxalate, mg/d | 37 (14) | 39 (16) | −2.5 (−6 to 1) | 0.15 |
| Citrate, mg/d | 446 (282) | 556 (310) | −133 (−200 to −66) | <0.001 |
| Uric acid, mg/d | 503 (177) | 612 (214) | −135 (−163 to −106) | <0.001 |
| Urea nitrogen, g/d | 8.4 (3.1) | 10.5 (4.2) | −2.0 (−3.2 to −0.9) | 0.001 |
| Sodium + potassium, meq/d | 177 (65) | 218 (75) | −46 (−59 to −33) | <0.001 |
| Clearance H2O, ml/d | 207 (780) | 435 (759) | −222 (−400 to −42) | 0.02 |
| Creatinine clearance, ml/min | 96 (33) | 106 (32) | −12 (−19 to −5) | 0.001 |
| Supersaturation calcium oxalate | 7.6 (4.8) | 7.1 (3.3) | 0.6 (−0.3 to 1.6) | 0.20 |
| Supersaturation calcium phosphate | 0.8 (0.7) | 1.2 (0.9) | −0.4 (−0.6 to −0.2) | <0.001 |
| Supersaturation uric acid | 1.2 (0.9) | 0.9 (0.8) | 0.3 (0.1 to 0.5) | 0.003 |
All values are adjusted for sex. Serum creatinine and all urine values except supersaturation are adjusted for weight. All urine values are adjusted for 24-hour urine creatinine (except pH, supersaturation, and creatinine clearance).
Adjusted for urine sulfate. All P values are Bonferroni adjusted.
Pretreatment
Baseline laboratory characteristics are provided in Table 1. Serum sodium, calcium, and phosphate were lower in blacks than whites. Serum creatinine was higher in black patients.
The 24-hour urine creatinine and creatinine clearance were lower in black patients; 24-hour urine sodium, potassium, calcium, magnesium, sulfate, phosphate, uric acid, and urea nitrogen, adjusted for 24-hour urine creatinine, were all lower in blacks than whites. Urine volume, adjusted for urine creatinine, was significantly lower in black patients relative to their white counterparts. Urine pH and 24-hour oxalate excretion, adjusted for urine creatinine, were not different across racial groups. Supersaturations for calcium oxalate were similar across racial groups. Supersaturation for calcium phosphate was significantly higher in whites, whereas blacks had higher uric acid supersaturations. At baseline, black patients had significantly lower electrolyte free water clearance compared with controls.
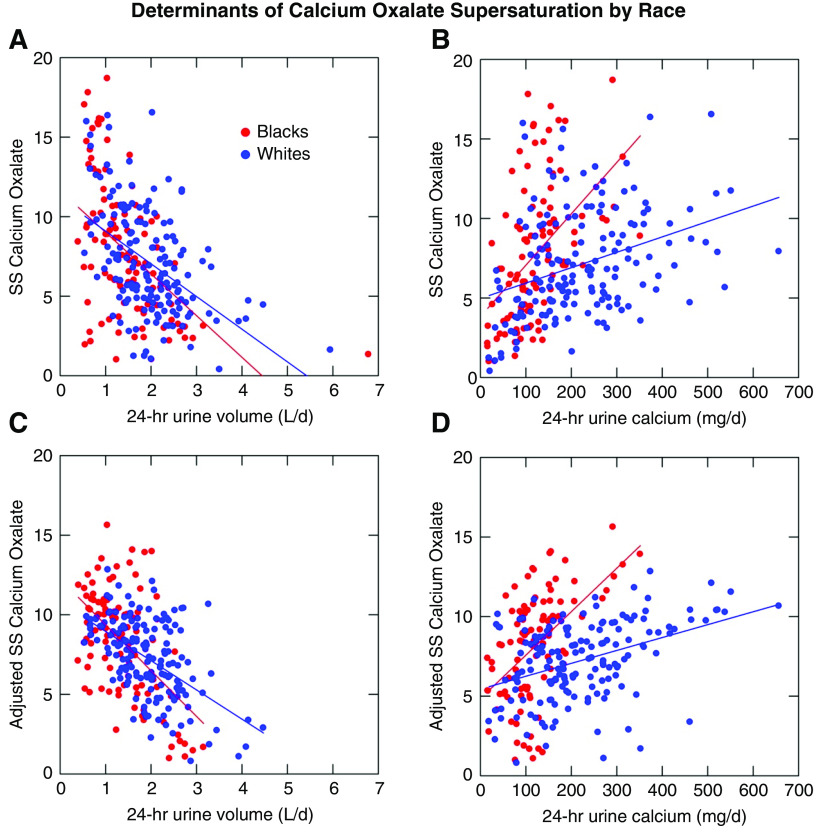
Although black and white patients have similar supersaturations for calcium oxalate, they differ in how they arrive at a given supersaturation. In unadjusted models, the relationship between the supersaturation for calcium oxalate and urine volume is not significantly different between black and white stone formers (Figure 1A). The relationship between calcium oxalate supersaturation and unadjusted 24-hour urine calcium is significantly different (Figure 1B), with any incremental rise in urine calcium having more of an effect in black patients than in white patients. When fully adjusted for sex, age, urine creatinine, urine oxalate, urine pH, and interaction terms (Figure 1C and D), the model demonstrates significant slope differences for calcium oxalate supersaturation versus 24-hour volume, oxalate, and calcium (P<0.001, R2=0.68). The coefficients and statistical values used for the adjustment models can be found in Table 2.
Figure 1.
Determinants of calcium oxalate supersaturation vary by race. Upper panels demonstrate unadjusted relationships between 24-hour urine volume and urine calcium and calcium oxalate supersaturation (SS) by race. Lower panels demonstrate fully adjusted models (sex, age, urine creatinine, urine pH, urine oxalate, and interaction terms). R2 for the model is 0.68.
Table 2.
Associations of 24-hour urine parameters with calcium oxalate and calcium phosphate supersaturation by race
| Calcium Oxalate Supersaturation | Calcium Phosphate Supersaturation | |||||
|---|---|---|---|---|---|---|
| Calcium, 10 mg/d | Oxalate,a,b 10 mg/d | Volume, L/d | Calcium,a 10 mg/d | pH, U/d | Volume,a L/d | |
| Black | 0.35 (0.31 to 0.39) | 1.35 (1.27 to 1.43) | −4.34 (−4.40 to −4.28) | 0.06 (0.05 to 0.07) | 0.60 (0.58 to 0.62) | −0.61 (−0.65 to −0.57) |
| White | 0.17 (0.14 to 0.21) | 0.31 (0.29 to 0.34) | −3.15 (−3.29 to −3.01) | 0.03 (0.02 to 0.04) | 1.30 (1.22 to 1.38) | −0.45 (−0.51 to −0.39) |
| Difference in slope estimate between whites and blacks | −0.18 (−0.23 to 0.13) | −1.04 (−1.09 to 0.99) | 1.19 (1.09 to 1.29) | −0.03 (−0.04 to −0.02) | 0.70 (0.64 to 0.76) | 0.16 (0.13 to 0.19) |
| P value for difference in slope between whites and blacks | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Values represent slope estimates for each clinical variable by racial group as seen in Figures 1 and 2, as well as an estimate of differences between groups. Where significant, any two- and three-way interactions are noted.
Interaction between sex and race present: P value for interaction <0.001.
Three-way interaction between sex, oxalate, and race present: P value for interaction <0.001.
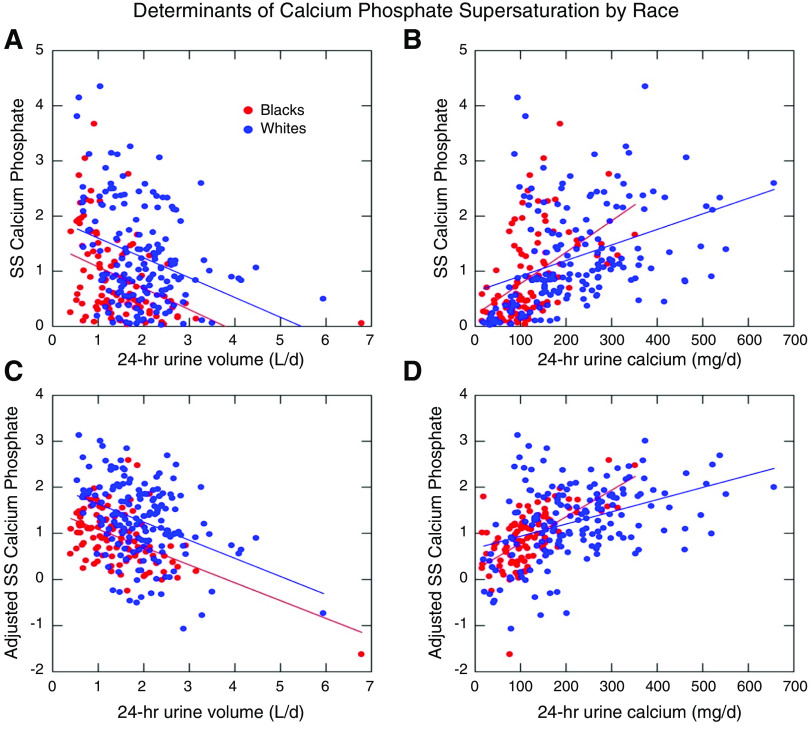
A similar unadjusted analysis for calcium phosphate supersaturation demonstrates a parallel relationship between urine volume and supersaturation for calcium phosphate between black and white patients, with the difference of intercept accounting for the lower urine volume in black patients (Figure 2A). Urine pH was largely similar between the groups. As with the calcium oxalate supersaturation, calcium phosphate supersaturation was more highly dependent on 24-hour urine calcium in black patients than in white patients (Figure 2B). The fully adjusted model including sex, age, urine creatinine, urine citrate, urine pH, and interaction terms (Figure 2C and D, Table 2) confirmed these relationships (P<0.001, R2=0.84). As urine pH is the primary determinant of the uric acid supersaturation and was not different between black and white patients, no significant racial differences in risk parameters were noted (data not shown).
Figure 2.
Determinants of calcium phosphate supersaturation vary by race. Upper panels demonstrate unadjusted relationships between 24-hour urine volume and urine calcium and calcium phosphate SS by race. Lower panels demonstrate fully adjusted models (sex, age, urine creatinine, urine citrate, urine pH, and interaction terms). R2 for the model is 0.84.
Post-Treatment
Seventy-seven (66%) black patients and 107 (62%) white patients completed follow-up testing. Similar proportions of men and women completed a follow-up analysis in the two groups (72% and 63% of black men and women, respectively; 66% and 58% of white men and women, respectively; P=0.73). Notably, there were no significant differences in either the demographic variables or the baseline metabolic parameters between those who did and did not complete a follow-up evaluation.
Table 3 demonstrates the post-treatment values in both groups and the differences between pre- and post-treatment values across the two groups. After treatment, serum sodium, calcium, and phosphate were no longer different between groups. Although serum sodium decreased significantly in both black and white patients, there were no recorded instances of hyponatremia in white patients, whereas three of 28 (11%) male black patients developed at least one episode of hyponatremia (serum Na <135 mmol/L). The lowest Na level attained on follow-up was 131 mmol/L. Serum potassium was lower in white patients, associated with a significant decrease in serum values compared with black patients. Serum bicarbonate was higher in white patients, also associated with a significant Δ in pre- to post-treatment values.
Table 3.
Pre- and post-treatment laboratory values and changes with treatment
| Black | White | Adjusted Difference in Δ between Whites and Blacksa (95% Confidence Interval) | P Value (Difference in Δ between Whites and Blacks) | |||||
|---|---|---|---|---|---|---|---|---|
| Pre (SD) | Post (SD) | Δ (95% Confidence Interval)a | Pre (SD) | Post (SD) | Δ (95% Confidence Interval)a | |||
| N | 77 | 107 | ||||||
| Age, yr | 52 (12) | 49 (14) | ||||||
| Weight, kg | 87 (20) | 80 (24) | ||||||
| Men/women | 28/49 | 56/51 | ||||||
| Serum values | ||||||||
| Sodium, mmol/L | 140.7 (1.8) | 140.1 (1.8) | −0.5 (−0.7 to 0.4) | 139.7 (1.8) | 139.5 (2.0) | −0.3 (−0.4 to −0.2) | 0.2 (−0.3 to 0.7) | 0.35 |
| Potassium, mmol/L | 4.2 (0.3) | 4.2 (0.4) | 0.02 (−0.02 to 0.06) | 4.3 (0.3) | 4.1 (0.4) | −0.2 (−0.3 to −0.1) | −0.2 (−0.3 to −0.1) | 0.002 |
| Bicarbonate, mmol/L | 26.7 (2.1) | 26.5 (2.6) | −0.2 (−0.4 to 0.0) | 26.6 (2.0) | 27.6 (2.5) | 1.1 (0.9 to 1.3) | 1.3 (0.7 to 1.8) | <0.001 |
| Calcium, mg/dl | 9.5 (0.3) | 9.6 (0.3) | 0.05 (0.02 to 0.08) | 9.6 (0.3) | 9.7 0.4) | 0.1 (0.07 to 0.03) | 0.06 (−0.1 to 0.03) | 0.18 |
| Phosphate, mg/dl | 3.2 (0.4) | 3.3 (0.5) | 0.07 (0.02 to 0.11) | 3.5 (0.4) | 3.4 (0.6) | −0.06 (−0.15 to 0.03) | −0.1 (−0.3 to 0.01) | 0.06 |
| Creatinine, mg/dl | 1.1 (0.3) | 1.2 (0.4) | 0.08 (0.04 to 1.12) | 1.0 (0.3) | 1.0 (0.4) | −0.02 (−0.05 to 0.01) | −0.1 (−0.2 to 0.0) | 0.05 |
| 24-h urine values | ||||||||
| Volume, L/d | 1.4 (0.7) | 1.9 (0.8) | 0.4 (0.3 to 0.5) | 1.9 (0.6) | 2.4 (0.8) | 0.5 (0.4 to 0.6) | 0.00 (−0.25 to 0.27) | 0.95 |
| pH | 5.9 (0.4) | 6.2 (0.4) | 0.3 (0.2 to 0.4) | 6.0 (0.4) | 6.1 (0.4) | 0.2 (0.1 to 0.3) | −0.1 (−0.2 to −0.01) | 0.04 |
| Creatinine, mg/d | 1500 (281) | 1602 (272) | 143 (112 to 174) | 1412 (279) | 1425 (269) | −17 (−43 to 8) | −160 (−242 to −77) | <0.001 |
| Sodium, mmol/d | 138 (48) | 180 (67) | 40 (32 to 48) | 163 (48) | 158 (66) | −4 (−11 to 3) | −45 (−65 to −24) | <0.001 |
| Potassium, mmol/d | 42 (18) | 55 (24) | 9 (7 to 11) | 59 (18) | 64 (23) | 8 (6 to 10) | −1 (−8 to 6) | 0.89 |
| Calcium, mg/d | 115 (94) | 158 (79) | 6 (−3 to 15) | 233 (101) | 169 (76) | −38 (−46 to −30) | −44 (−68 to −20) | <0.001 |
| Magnesium, mg/d | 75 (37) | 97 (32) | 10 (6 to 14) | 105 (37) | 98 (31) | 0 (−3 to 3) | −9 (−19 to 0.3) | 0.08 |
| Sulfate, meq/d | 33 (13) | 39 (12) | 4 (3 to 6) | 46 (13) | 47 (11) | 2 (1 to 3) | −2 (−6 to 1) | 0.21 |
| Ammonium, meq/db | 29 (12) | 31 (15) | 3 (2 to 4) | 32 (13) | 35 (14) | 3 (2 to 4) | 0 (−4 to 4) | 0.91 |
| Phosphate, mg/d | 638 (202) | 795 (228) | 104 (76 to 132) | 962 (207) | 902 (278) | −22 (−46 to 2) | −125 (−199 to 52) | 0.001 |
| Oxalate, mg/d | 37 (15) | 42 (14) | 5 (4 to 7) | 39 (15) | 41 (13) | 1 (−1 to 3) | −5 (−10 to 0) | 0.04 |
| Citrate, mg/d | 417 (307) | 516 (316) | 74 (45 to 103) | 553 (310) | 514 (309) | −21 (−45 to 3) | −95 (−170 to 19) | 0.01 |
| Uric acid, mg/d | 489 (131) | 581 (132) | 83 (61 to 99) | 634 (134) | 641 (134) | 14 (11 to 17) | −70 (−117 to −23) | 0.004 |
| Urea nitrogen, g/d | 8.6 (8.5) | 10.4 (4.8) | 1.4 (0.9 to 1.9) | 10.6 (3.4) | 10.9 (1.9) | 0.3 (0.2 to 0.4) | −1.2 (−2.6 to 0.2) | 0.10 |
| Sodium + potassium, meq/d | 180 (57) | 233 (68) | 51 (43 to 59) | 222 (61) | 224 (67) | 3 (−4 to 10) | −46 (−69 to −24) | <0.001 |
| Clearance H2O, ml/d | 163 (470) | 385 (891) | 92 (−9 to 192) | 426 (445) | 706 (893) | 410 (321 to 499) | 318 (49 to 588) | 0.02 |
| Creatinine clearance, ml/min | 98 (32) | 108 (23) | 5 (3 to 7) | 106 (31) | 103 (23) | −1 (−3 to 1) | −6 (−13 to 0.4) | 0.06 |
| Supersaturation calcium oxalate | 8.0 (3.7) | 5.8 (3.5) | −1.9 (−2.4 to −1.5) | 7.2 (3.8) | 5.6 (3.5) | −1.7 (−2 to −1.4) | 0.2 (−1 to 1.4) | 0.73 |
| Supersaturation calcium phosphate | 0.8 (0.7) | 0.9 (0.6) | 0 (−0.1 to 0.1) | 1.2 (0.8) | 0.8 (0.6) | −0.3 (−0.4 to −0.2) | 0.3 (−0.5 to −0.05) | 0.02 |
| Supersaturation uric acid | 1.3 (0.7) | 0.8 (0.6) | −0.4 (−0.5 to −0.3) | 1.0 (0.8) | 0.7 (0.6) | −0.4 (−0.5 to −0.3) | 0.1 (−0.1 to 0.3) | 0.48 |
All values are adjusted for individual pretreatment values and sex. All urine values are adjusted for 24-hour urine creatinine and weight (except pH, supersaturations, and creatinine clearance).
Adjusted for urine sulfate. All P values are Bonferroni adjusted.
Urine volumes increased similarly across the two racial groups (Table 3), and the differences in total urine volume were no longer significant. Strikingly, electrolyte free water clearance increased markedly more in white patients compared with black patients, contributing to a persistent disparity in free water clearance from the pretreatment state. Notably, urine sodium excretion, which remained stable in white patients, rose markedly in black patients, despite a similar increase in total urine volume. In other words, black patients appear to functionally raise their urine volume only by raising urine solute excretion, whereas white patients separate volume increase from solute increase. Urine calcium was similar post-treatment in the two groups, with a significant decrement in values in the white population. Supersaturations for calcium oxalate improved similarly for black and white patients. Calcium phosphate supersaturation did not change significantly with therapy for black patients, whereas there was a marked improvement in the white population. This resulted in similar final values for calcium phosphate supersaturation. For uric acid, the improvements in supersaturations were similar for both groups, with the final values not statistically different.
Discussion
In our cohort of black idiopathic stone formers, the largest reported to date, we have identified key differences in how supersaturation is achieved compared with in white stone formers as well as in the responses to standard treatment. Supersaturation has been shown to be associated with stone formation (27), and decrements in supersaturation are associated with reduced frequency of stone recurrence (28–30). Although the laws of chemistry dictate that urine must be supersaturated relative to the specific salt in question in order for precipitation and stone formation to occur, individual risk factors determine how this is achieved. Given that all patients in our cohort are stone formers, it is not surprising that the supersaturations for the various stones types were largely similar.
The lower urine volume and initial serum sodium in black patients may reflect higher levels of vasopressin, an evolutionary benefit given the African climate and need for water conservation. Bankir et al. (31) have shown lower 24-hour urine volumes in black men and women fed similar diets and allowed ad lib fluid intake in a clinical research center setting, and several studies have noted higher serum vasopressin levels in those of African ancestry compared with whites (32–34). Although perhaps not problematic alone, the baseline lower urine volumes in the setting of any abnormalities of calcium, oxalate, or pH may lead to a higher risk for stone formation compared with in whites with a similar level of perturbations, whether from increased supplementary calcium use, dietary oxalate intake, or a high-protein diet commonly undertaken by patients for purported health benefit.
Despite the well described lower urine calcium levels in black individuals (35,36), their vastly lower urine volumes allow black patients to attain a comparable level of risk at the lower calcium levels. Any incremental increase in urinary calcium level will lead to a significantly higher increase in risk for stone formation in a black patient compared with a white patient. Epidemiologic data by Curhan et al. (37) show risk inflection points for calcium at 200 and 250 mg/24 h for women and men, respectively, but these may not be consistent for black patients who have consistently lower urine volumes, as demonstrated in our work. Lower thresholds for treatment of urine calcium values than whites may be beneficial for black patients, who were not well represented in the cohorts from the aforementioned study.
Furthermore, although it would seem reasonable to advise black patients to increase fluid intake to ameliorate risk, our data indicate that a blanket approach to fluid and sodium intake goals for the stone former as currently advocated for in guidelines may not be appropriate. Although white patients were able to increase free water excretion when challenged with increased free water intake, black patients in our cohort were unable to do so, potentially resulting in hyponatremia in three black men. Although the average increase of less than half a liter of urine volume was similar in both racial groups (and overall less than optimal in a stone former), the black population was only able to do so by increasing solute intake. The obligatory increase in sodium intake, despite standard counseling regarding salt restriction that is provided to all patients in our practice, suggests underlying physiologic drive to preserve and maintain normal serum sodium levels. Indeed, studies in normal black volunteers demonstrate a decreased ability to excrete a free water load compared with whites (38). In our population, even though a significantly higher proportion of white patients was treated with thiazides, which impair free water excretion, the free water clearance was significantly higher in whites. The higher proportion of white patients receiving thiazides for treatment may also explain the lower post-treatment potassium and higher bicarbonates noted in the group.
The potential harm to patients is not only physiologic with hyponatremia and ongoing risks of stone formation, but repeated failure to improve their 24-hour urine results with regard to volume and sodium can lead to frustration for patients and may compromise the patient-physician relationship, particularly if the patients are labeled as noncompliant. In the black stone former, it may be more effective to treat other targets, such as calcium, even if the values are not overtly in the abnormal range. Further studies will be required to test this approach to stone prevention in black patients. Our data underscore the critical need to include black patients in clinical trials of stone patients.
Significant racial differences in the mechanisms of stone formation include lower 24-hour urine volume and urine calcium in black idiopathic stone formers, with a limited ability to increase free water clearance in the face of a markedly increased free water load. Furthermore, any incremental change even within the normal range in calcium, oxalate, or any other risk variable may be more impactful in a black patient given the lower urine volume. Individualized approaches to stone prevention must account for racial background to optimize outcomes and patient safety.
Disclosures
All authors have nothing to disclose.
Funding
F. Coe, E. Worcester, and A. Zisman are supported by National Institute of Diabetes and Digestive and Kidney Diseases grant P01 DK056788.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Scales C Jr., Smith A, Hanley J, Saigal C; Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cupisti A, D’Alessandro C, Samoni S, Meola M, Egidi M: Nephrolithiasis and hypertension: Possible links and clinical implications. J Nephrol 27: 477–482, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Kittanamongkolchai W, Mara K, Mehta R, Vaughan L, Denic A, Knoedler J, Enders F, Lieske J, Rule A: Risk of hypertension among first-time symptomatic kidney stone formers. Clin J Am Soc Nephrol 12: 476–482, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferraro P, Taylor E, Eisner B, Gambaro G, Rimm E, Mukamal K, Curhan G: History of kidney stones and the risk of coronary heart disease. JAMA 310: 408–415, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Li S, Zeng Z, Wang J, Xie L, Li T, He Y, Qin X, Zhao J: Kidney stones and cardiovascular risk: A meta-analysis of cohort studies. Am J Kidney Dis 64: 402–410, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Rule A, Roger V, Melton L 3rd, Bergstralh E, Li X, Peyser P, Krambeck A, Lieske J: Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 21: 1641–1644, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rule A, Krambeck A, Lieske J: Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol 6: 2069–2075, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhondup T, Kittanamongkolchai W, Vaughan L, Mehta R, Chhina J, Enders F, Hickson L, Lieske J, Rule A: Risk of ESRD and mortality in kidney and bladder stone formers. Am J Kidney Dis 72: 790–797, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoag J, Halpern J, Goldfarb D, Eisner B: Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol 192: 1440–1445, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Alexander R, Hemmelgarn B, Wiebe N, Bello A, Morgan C, Samuel S, Klarenbach S, Curhan G, Tonelli M; Alberta Kidney Disease Network : Kidney stones and kidney function loss: A cohort study. BMJ 345: e5287, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bensalah K, Tuncel A, Gupta A, Raman J, Pearle M, Lotan Y: Determinants of quality of life for patients with kidney stones. J Urol 179: 2238–2243, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Litwin M, Saigal C: Table 14-47: Economic Impact of Urologic Disease. Urologic Diseases in America, National Institutes of Health Publication 12-7865 Washington, DC, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, US Department of Health and Human Services, 2012 [Google Scholar]
- 13.Mason J, Miles B, Belville W: Urolithiasis and race: Another viewpoint. J Urol 134: 501–502, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Tasian G, Ross M, Song L, Sas D, Keren R, Denburg M, Chu D, Copelovitch L, Saigal C, Furth S: Annual incidence of nephrolithiasis among children and adults in South Carolina from 1997 to 2012. Clin J Am Soc Nephrol 11: 488–496, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akoudad S, Szklo M, McAdams M, Fulop T, Anderson C, Coresh J, Köttgen A: Correlates of kidney stone disease differ by race in a multi-ethnic middle-aged population: The ARIC study. Prev Med 51: 416–420, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarmina I, Spirnak J, Resnick M: Urinary lithiasis in the black population: An epidemiological study and review of the literature. J Urol 138: 14–17, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Soucie J, Thun M, Coates R, McClellan W, Austin H: Demographic and geographic variability of kidney stones in the United States. Kidney Int 46: 893–899, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Ghiraldi E, Reddy M, Li T, Lawler A, Friedlander J: Factors associated with compliance in submitting 24-hour urine collections in an underserved community. J Endourol 31: S64–S68, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers A: Race, ethnicity and urolithiasis: A critical review. Urolithiasis 41: 99–103, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Modlin M: The aetiology of renal stone: A new concept arising from studies on a stone-free population. Ann R Coll Surg Engl 40: 155–178, 1967. [PMC free article] [PubMed] [Google Scholar]
- 21.Whalley N, Martins M, Van Dyk R, Meyers A: Lithogenic risk factors in normal black volunteers, and black and white recurrent stone formers. BJU Int 84: 243–248, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Whalley N, Moraes M, Shar T, Pretorius S, Meyers A: Lithogenic risk factors in the urine of black and white subjects. Br J Urol 82: 785–790, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Abdel Goad E, Bereczky Z: Metabolic risk factors in patients with renal stones in KwaZulu Natal: An inter-racial study (Asian and Whites). BJU Int 93: 120–123, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Maloney M, Springhart W, Ekeruo W, Young M, Enemchukwu C, Preminger G: Ethnic background has minimal impact on the etiology of nephrolithiasis. J Urol 173: 2001–2004, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Worcester E, Bergsland K, Gillen D, Coe F: Evidence for increased renal tubule and parathyroid gland sensitivity to serum calcium in human idiopathic hypercalciuria. Am J Physiol Renal Physiol 305: F853–F860, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werness P, Brown C, Smith L, Finlayson B: EQUIL2: A BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Prochaska M, Taylor E, Ferraro P, Curhan G: Relative supersaturation of 24-hour urine and likelihood of kidney stones. J Urol 199: 1262–1266, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferraro P, Ticinesi A, Meschi T, Rodgers A, Di Maio F, Fulignati P, Borghi L, Gambaro G: Short-term changes in urinary relative supersaturation predict recurrence of kidney stones: A tool to guide preventive measures in urolithiasis. J Urol 200: 1082–1087, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A: Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol 155: 839–843, 1996. [PubMed] [Google Scholar]
- 30.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A: Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 346: 77–84, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Bankir L, Perucca J, Weinberger M: Ethnic differences in urine concentration: Possible relationship to blood pressure. Clin J Am Soc Nephrol 2: 304–312, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Bakris G, Bursztyn M, Gavras I, Bresnahan M, Gavras H: Role of vasopressin in essential hypertension: Racial differences. J Hypertens 15: 545–550, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Crofton J, Dustan H, Share L, Brooks D: Vasopressin secretion in normotensive black and white men and women on normal and low sodium diets. J Endocrinol 108: 191–199, 1986. [DOI] [PubMed] [Google Scholar]
- 34.Bursztyn M, Bresnahan M, Gavras I, Gavras H: Pressor hormones in elderly hypertensive persons. Racial differences. Hypertension 15[Suppl]: I88–I92, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Taylor E, Curhan G: Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18: 654–659, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Aloia J, Shieh A, Mikhail M, Islam S: Urinary calcium excretion in postmenopausal African American women. Clin Nephrol 84: 130–137, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curhan G, Willett W, Speizer F, Stampfer M: Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int 59: 2290–2298, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Weder A, Gleiberman L, Sachdeva A: Whites excrete a water load more rapidly than blacks. Hypertension 53: 715–718, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]