Abstract
Assessment of GFR is central to clinical practice, research, and public health. Current Kidney Disease Improving Global Outcomes guidelines recommend measurement of serum creatinine to estimate GFR as the initial step in GFR evaluation. Serum creatinine is influenced by creatinine metabolism as well as GFR; hence, all equations to estimate GFR from serum creatinine include surrogates for muscle mass, such as age, sex, race, height, or weight. The guideline-recommended equation in adults (the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine equation) includes a term for race (specified as black versus nonblack), which improves the accuracy of GFR estimation by accounting for differences in non-GFR determinants of serum creatinine by race in the study populations used to develop the equation. In that study, blacks had a 16% higher average measured GFR compared with nonblacks with the same age, sex, and serum creatinine. The reasons for this difference are only partly understood, and the use of race in GFR estimation has limitations. Some have proposed eliminating the race coefficient, but this would induce a systematic underestimation of measured GFR in blacks, with potential unintended consequences at the individual and population levels. We propose a more cautious approach that maintains and improves accuracy of GFR estimates and avoids disadvantaging any racial group. We suggest full disclosure of use of race in GFR estimation, accommodation of those who decline to identify their race, and shared decision making between health care providers and patients. We also suggest mindful use of cystatin C as a confirmatory test as well as clearance measurements. It would be preferable to avoid specification of race in GFR estimation if there was a superior, evidence-based substitute. The goal of future research should be to develop more accurate methods for GFR estimation that do not require use of race or other demographic characteristics.
Keywords: human, Cystatin C, creatinine, glomerular filtration rate, African Americans, Public Health, Decision Making, Shared, Body Weights and Measures, Renal Insufficiency, Chronic, Health Personnel, Demography, Kidney Function Tests
Introduction
Assessment of GFR is central to clinical practice, research, and public health (1). In clinical practice, GFR is used to interpret the symptoms, signs, and laboratory abnormalities that might signify kidney disease; to adjust drug doses; and to detect, assess risk, and manage acute kidney diseases and disorders (AKD) and CKD. In clinical research, GFR is used as an exposure, outcome, or characteristic in stratification or adjustment. In public health, GFR is used to estimate the burden of kidney disease. For these reasons, improving or worsening the accuracy of GFR assessment has implications at the individual and population levels.
AKD and CKD are worldwide public health problems, with variation in incidence, prevalence, and outcomes by race, which may reflect region, ethnicity, and ancestry and which may be the result of biologic, socioeconomic, and behavioral risk factors as well as access to and quality of health care (2–7). In particular, in the United States, blacks are at higher risk for progression of CKD, including kidney failure (7–9). Of particular importance are the large number of modifiable factors that contribute to this risk as well as the discovery of genetic susceptibility due to inheritance of high-risk APOL1 mutations (10). It is now widely accepted that race is a social rather than a biologic construct, and research has sought to understand the reasons for this disparity by exploring factors that ameliorate the association of race with kidney disease (Supplemental Box) (11–22). Importantly, some interventions directed at the underlying causes have been tested, such as in the African-American Study of Kidney Disease (AASK) (23).
Criteria for the definitions of AKD and CKD are independent of race as are methods to assess measured GFR (mGFR) and mGFR itself, although data are limited (1,24). However, eGFR from serum creatinine (eGFRcr), the initial test in GFR assessment, requires specification of race in adults, and eGFRcr is recommended by current guidelines in combination with assessment of albuminuria and cause of disease to guide prevention, detection, evaluation, and treatment of CKD (25,26). A recent article by Eneanya et al. (27) suggests that using race to guide clinical care is justified only if (1) the use confers substantial benefit; (2) the benefit cannot be achieved through other feasible approaches; (3) patients who reject race categorization are accommodated fairly; and (4) the use of race is transparent. They recommend reconsidering the use of race in eGFRcr because in their opinion, it fails to meet these criteria (27). In this feature, we will review the strengths and limitations of the use of race in GFR estimation, and we propose a way forward to overcome some of the limitations while maintaining accuracy of GFR estimation and fulfill the criteria suggested by Eneanya et al. (27).
Current Recommendations and Limitations
Current guidelines from Kidney Disease Improving Global Outcomes (KDIGO) (Box 1) recommend eGFRcr as the initial step in GFR evaluation (25). Confirmatory tests include measurement of serum cystatin C for use in GFR estimation (eGFR from the combination of serum creatinine and cystatin C [eGFRcr-cys] or eGFR from serum cystatin [eGFRcys] if eGFRcr is likely to be inaccurate) or a clearance measurement (using an exogenous filtration marker [mGFR] or urinary creatinine clearance if mGFR is not available). Serum creatinine is influenced by creatinine metabolism as well as GFR; hence, eGFRcr includes surrogates for muscle mass, such as age, sex, race, height, or weight. The guideline-recommended eGFRcr for adults, the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, includes a term for race (specified as black versus nonblack), which improves the accuracy by accounting for differences in non-GFR determinants of serum creatinine that differ by race (28). The recommendations were made despite an appreciation of some limitations: race can be incorrectly ascertained when inferred from physical or other attributes; variation and sometimes, inconsistency exist in how race is assigned in clinical settings and throughout the world; races other than black and country are not specified; and the entire biologic basis for the observed racial difference in non-GFR determinants of serum creatinine may not be captured by race per se. GFR estimation and clinical decisions on the basis of GFR estimation should take into account these limitations.
Box 1.
Recommendation for GFR evaluation by Kidney Disease Improving Global Outcomes 2012 clinical practice guideline for the evaluation and management of CKD
|
Information is from the 2012 Kidney Disease Improving Global Outcomes CKD guideline (25). eGFRcr, eGFR from serum creatinine; eGFRcys, eGFR from serum cystatin C; eGFRcr-cys, eGFR from the combination of serum creatinine and cystatin C; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
GFR Estimating Equations
GFR estimating equations enable assessment of GFR from serum concentrations of endogenous filtration markers without clearance measurements (1). Endogenous filtration markers include metabolites (approximately <1000 daltons; including creatinine and more recently recognized filtration markers pseudouridine, acetylthreonine, myoinositol, phenylacetylglutamine, and tryptophan) and low molecular weight proteins (approximately 1000–20,000 daltons; including cystatin C, β-2 microglobulin, and β-trace protein).
In principle, the serum concentration of an endogenous filtration marker is inversely related to the GFR and directly related to other physiologic processes affecting the marker, termed “non-GFR determinants,” which include generation, renal tubular handling (reabsorption and secretion), and extrarenal elimination of the marker. The non-GFR determinants are difficult to measure, but they may be associated with demographic variables, such as age, sex, and race, or clinical variables, such as height and weight, which can be included as surrogates of these determinants in GFR estimating equations. To facilitate clinical practice, research, and public health, a single equation for each filtration marker or a combination of markers is selected for routine eGFR reporting.
Most GFR estimating equations are predictive models generally developed using linear regression to relate the observed mGFRs to the observed serum concentrations of the filtration marker (both on the logarithmic scale) and to observed demographic and clinical variables as surrogates for the unmeasured non-GFR determinants. By design, an estimating equation provides a more accurate estimate of mGFR than the serum concentration of the filtration marker alone. Error in a single eGFR arises from systematic or random variation in non-GFR determinants that is not accounted for by surrogates and from measurement error in serum assays and mGFR. Systematic error and random variation in mGFR methods have been underemphasized as a source of error in eGFR (1,24). eGFRcys has similar accuracy as eGFRcr, but because the non-GFR determinants of serum creatinine and cystatin C differ from each other, using the combination of both markers (eGFRcr-cys) reduces systematic or random variation and is more accurate than using either marker alone (1). Other methodologic approaches have been reported, such as the Full Age Spectrum equations, which use assumed age-adjusted values for normal GFR, and sex- and race-adjusted normal values for serum creatinine and cystatin C (29,30). In children, eGFRcr requires specification of age, sex, and height but not race, likely reflecting differences between children and adults in the relationships among these variables with muscle mass (25).
Race Coefficients in GFR Estimating Equations
The 2009 CKD-EPI creatinine equation includes the variables age, sex, and race (specified as black versus nonblack) in addition to serum creatinine (Table 1) (28,31–37). The coefficient of 1.16 reflects that mGFR was 16% higher in blacks than nonblacks with similar age, sex, and creatinine in the dataset used to develop the equation. The CKD-EPI development dataset included 2601 blacks (31.5% of the total population), of whom the largest proportion was from the AASK, in which race was self-reported and all study participants were residents of the United States (Supplemental Table) (23,28). Of note, the black coefficient was 1.21 in the 1999 Modification of Diet in Renal Disease (MDRD) study equation; others have reported smaller differences (38). Reported differences in the magnitude of the black coefficients may relate to variation among blacks in the non-GFR determinants of serum creatinine (Table 1) (39). In one study, the black coefficient was too large for European blacks (40). Furthermore, the black coefficient did not predict well in Africa; several studies showed more accurate eGFRcr using the CKD-EPI or MDRD study equations without versus with the black coefficient, indicating heterogeneity among people of African descent in the non-GFR determinants of serum creatinine (41–45). In general, a black coefficient was smaller or was not required at all in eGFRcys or GFR estimating equations using serum β-2 microglobulin or β-trace protein (Table 1). The black coefficient for eGFRcr-cys was intermediate between the coefficients in eGFRcr and eGFRcys as were the coefficients for age and sex (Table 1).
Table 1.
Age, sex, and race coefficients in Modification of Diet in Renal Disease study and CKD-EPI GFR estimating equations developed in CKD populations and diverse populationsa
| Filtration Marker (eGFR)a | CKD Population | Diverse Population | ||||||
|---|---|---|---|---|---|---|---|---|
| Year (Reference) | Age (per 1 yr) | Women | Black Race | Year (Reference) | Age (per 1 yr) | Women | Black Race | |
| Creatinine (eGFRcr) | 1999 (31,32,37) | Age−0.203 | 0.74 | 1.21 | 2009 (28) | 0.993Age | 0.75b | 1.16 |
| Cystatin C (eGFRcys) | 2008 (33,34) | Age−0.13 | 0.91 | 1.06 | 2012 (35) | 0.996Age | 0.93b | NA |
| Creatinine-cystatin C (eGFRcr-cys) | 2008 (33,34) | Age−0.20 | 0.82 | 1.11 | 2012 (35) | 0.995Age | 0.83b | 1.08 |
| β-2 microglobulin (eGFRβ2M) | 2015 (36) | 0.998Age | 0.90 | NA | In progress | |||
| β-trace protein (eGFRβTP) | 2015 (36) | NA | NA | NA | In progress | |||
| β-2 microglobulin–β-trace protein (eGFRβ2M-βTP) | 2015 (36) | NA | NA | NA | In progress | |||
NA, not applicable.
All equations are CKD-EPI equations except the 1999 creatinine equation, which is the Modification of Diet in Renal Disease study equation. Equations developed in diverse populations (populations with and without CKD and with multiple geographic regions, races, and ethnicities) are preferred for clinical use. Equations are expressed on the raw scale. Coefficients for age, sex, and race are multiplication factors.
For serum creatinine >0.7 mg/dl in women and >0.9 mg/dl in men and for serum cystatin C >0.8 mg/L.
Coefficients for races other than black for eGFRcr are not agreed on. Using a four-level race variable in the 2009 CKD-EPI creatinine equation development dataset, coefficients for Asians (n=100; 1.2%) and a combined category for American Indians and Hispanics (n=353; 4.3%) were smaller (1.05 and 1.01, respectively) than for blacks (1.15) compared with whites and others, and use of the four-level variable compared with the two-level variable did not improve performance in the validation datasets (45). Other studies demonstrated that calibration factors improved the accuracy of the CKD-EPI creatinine or MDRD study equations in some Asian countries, which has often been interpreted as evidence in favor of use of a race coefficient for Asians (46). However, the calibration factors did not seem to be generalizable across countries, which may also reflect population differences in the non-GFR determinants. In general, the CKD-EPI cystatin C equation seemed more accurate than the CKD-EPI creatinine equation in Asian countries, and it did not require a calibration factor (46). It is important to point out that differences in the performance of an eGFRcr equation among studies may reflect differences among studies in methods for mGFR or serum creatinine determination as well as differences in the non-GFR determinants of serum creatinine (47). An ideal study design to determine the explanation for the apparent need for race coefficients in a GFR estimating equation would be a large multicenter study with a diverse development population composed of multiple racial groups from around the world; with a wide range of biologic (including muscle mass), socioeconomic, and behavioral risk factors; with measurement procedures for GFR and endogenous filtration markers traceable to standardized methods; and with consistent ascertainment of race, ethnicity, and ancestry (including use of genetic ancestral markers) as well as access to and quality of health care.
Possible Causes for Race Differences in GFR Estimating Equations
In principle, race could affect the relationship of GFR to serum concentrations of endogenous filtration markers if race is associated with errors in measurement methods for mGFR or serum concentrations of endogenous filtration markers or with variation in the non-GFR determinants of the markers. To our knowledge, there are no reported associations of race with errors in clearance measurements or laboratory measurement procedures for the endogenous filtration markers, although one study showed a nonsignificant larger difference in within-person variability for creatinine but not cystatin C in blacks than nonblacks (48). For the remainder of the discussion, we will focus on associations of race with variation in the non-GFR determinants of the markers (Table 2).
Table 2.
Non-GFR determinants of serum concentrations of endogenous filtration markers and clinical conditions associated with their variation
| Endogenous Filtration Markers | ||||
|---|---|---|---|---|
| Creatinine | Cystatin C | β-2 Microglobulin | β-Trace Protein | |
| Non-GFR determinants | ||||
| Generation | Muscle mass | All nucleated cells | All nucleated cells | CNS, testis, ovary |
| Tubular handling | Receptor-mediated tubular secretion (trimethoprim, cimetidine, fenofibrate, ritonavir, dolutegravir), level of GFR, cause of CKD (PKD versus others), antihypertensive agents (diuretics and CCB) | Receptor-mediated uptake and degradation in proximal tubular cells | Receptor-mediated uptake and degradation in proximal tubular cells | Receptor-mediated uptake and degradation in proximal tubular cells |
| Extrarenal elimination | GI (bacterial creatininase) | Multiple sites | Not known | Liver |
| Variation in non-GFR determinantsa | ||||
| Associated clinical conditions | Muscle mass | Fat mass, inflammation (higher CRP, lower serum albumin), smoking, thyroid and corticosteroid hormone | Lymph-proliferative and plasma cell disorders, smoking, inflammation (higher CRP, lower serum albumin), proteinuria | Proteinuria, weight |
There are two main methods for use in clinical studies to ascertain the association of race and other factors with the non-GFR determinants of endogenous filtration markers. For metabolites, such as creatinine, which are filtered and excreted in the urine, generation can be ascertained from urinary excretion in the steady state, tubular handling can be ascertained by comparison of urinary clearance with measured GFR, and extrarenal elimination can be ascertained by comparing plasma with urinary clearance. Then, variation in these processes can be assessed among racial groups (however determined) with and without adjustment for other explanatory factors. For low molecular weight proteins, such as cystatin C, which are filtered, reabsorbed, and metabolized with only small quantities excreted in the urine, inferences about variation in non-GFR determinants among racial groups are generally assessed by differences in the measured GFR filtration marker associations with and without adjustment for other factors. However, this latter method does not provide information about which physiologic process is affected. These methods could be supplemented by laboratory studies of the relevant mechanisms. CNS, central nervous system; PKD, polycystic kidney disease; CCB, calcium-channel blocker; GI, gastrointestinal; CRP, C-reactive protein.
Adjusted for age, sex, and race.
It is well known that body size and composition may differ among racial groups. Creatinine generation is affected by diet, particularly meat intake, and muscle mass, which varies by age, sex, and race; on average, creatinine excretion rates are higher in men than women, younger than older people, and people with self-reported black race than nonblack race (Table 2) (49–58). Self-reported race correlates strongly but imperfectly with genomically determined ancestry (59). Two studies reported positive and graded associations between the percentage of African ancestry (determined through genomic markers) and serum creatinine level (60). These differences are consistent with the magnitude and direction of the coefficients in the 2009 CKD-EPI creatinine equation. However, the aforementioned studies did not control for many possible confounding variables, and the causes of the observed racial differences in muscle mass and creatinine excretion are not well understood. One study showed that creatinine secretion in the AASK participants was lower than expected, but it did not include a comparison group of nonblacks (61). Enzymatic and transport processes involved in generation, tubular secretion, or extrarenal elimination of creatinine may reflect genetic variation. A meta-analysis of genetic determinants of eGFRcr did not find evidence for heterogeneity by ancestry at the vast majority of loci (62). Factors affecting the non-GFR determinants of novel metabolite filtration markers are less well known than for creatinine. As mentioned above, it is important to acknowledge that nonbiologic factors may be partly responsible for the higher serum and urine creatinine values observed in blacks.
After accounting for mGFR, cystatin C, β-2 microglobulin, and β-trace protein have weaker associations than creatinine with age, sex, and black race, findings that are generally interpreted, albeit without direct evidence, as reflecting smaller effects of muscle mass on generation of the markers (63–65). Associations with other clinical factors vary, including adiposity, smoking, inflammation, alterations in thyroid or corticosteroid hormones, proteinuria, and weight (Table 2) (63–68).
In principle, the use of multiple markers with non-GFR determinants that are not correlated with each other can reduce the contribution of non-GFR determinants of each marker and reduce error in GFR estimation. We have hypothesized that the use of a panel of filtration markers (panel eGFR) may avoid the need for these variables while providing more personalized estimates at the individual level and greater accuracy at the population level. Our results so far estimating GFR from a panel of novel metabolites provide proof of the concept that age, sex, and race are not necessary for GFR estimation and can be as accurate as provided by eGFRcr-cys (69,70). Studies evaluating combinations of metabolites and low molecular weight proteins in diverse populations are needed to develop a more accurate confirmatory test than eGFRcr-cys that would be convenient, acceptable, not costly, and widely available (71).
Consequences of Eliminating Race from GFR Estimates Using Creatinine
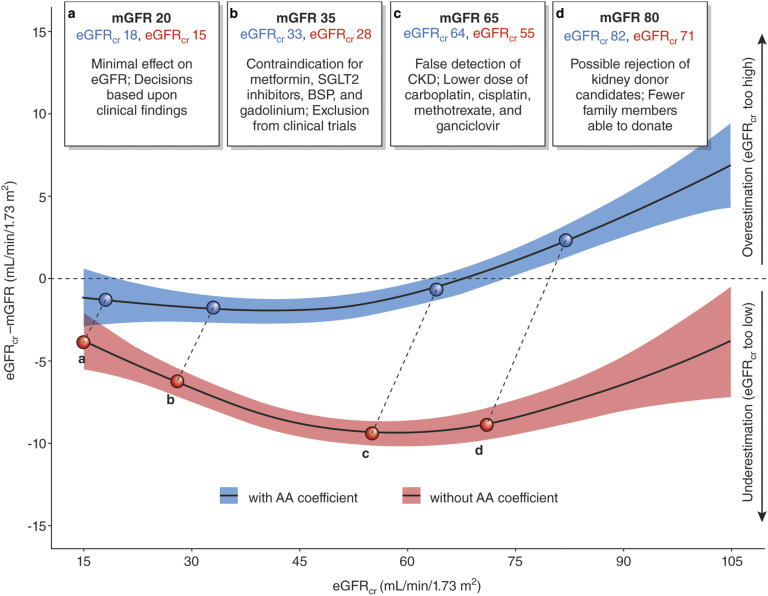
The benefits of nonrace-based inferences for care should be judged alongside the risk and harms of doing this. Eneanya et al. (27) suggest elimination of the black coefficient by substituting objective data on body size and composition, such as height or weight. Elimination of the black coefficient in the CKD-EPI creatinine equation would introduce a systematic error in eGFRcr in blacks, an underestimation of mGFR (Figure 1). Development of a new equation without a race variable would worsen the performance in estimating mGFR (root mean square error of 0.244 versus 0.236) more in blacks (0.258 versus 0.243) than in nonblacks (0.238 versus 0.232) (Table 3) (72). Inclusion of height and weight in addition to race does not meaningfully reduce the effect of race on eGFRcr (coefficient 1.15 versus 1.16) or improve performance (root mean square error of 0.235). Even when height and weight are included, omission of race worsens equation performance (root mean square error of 0.243) more in blacks than in nonblacks (0.255 and 0.238, respectively). To date, our experiences with the CKD-EPI equation and those of others collecting and analyzing data have not yielded other routinely available clinical data that could improve the accuracy of eGFRcr.
Figure 1.
Clinical decisions can be affected by accuracy of GFR assessment among blacks. Data from 2601 black participants from the Chronic Kidney Disease Epidemiology Collaboration development and internal validation sample were used in this plot (28). We computed eGFR from serum creatinine (eGFRcr) values with (blue) and without (red) the application of the black coefficient and removed values below and above the 2.5 and 97.5 percentiles of these distributions, leaving 2463 participants for the analysis. The model plotted represents generalized additive models for eGFRcr (milliliters per minute per 1.73 m2) on the difference between eGFRcr and measured GFR (mGFR) (milliliters per minute per 1.73 m2). We truncated the horizontal axis to eGFRcr values from 15 to 105 ml/min per 1.73 m2, excluding 190 participants from the plot. The colored areas along the line represent 95% confidence intervals of the estimate. Upper boxes represent hypothetical situations of eGFRcr values for the same mGFR, with numbers in blue representing the eGFRcr with the black coefficient and the numbers in red representing the eGFRcr without the black coefficient. AA, black; BSP, bisphosphonate; SGLT2, sodium-glucose transport protein 2. Modified from ref. 72, with permission.
Table 3.
Can use of height and weight eliminate the need for race in GFR estimation in the CKD-EPI creatinine equation?
| Variables Used in Equations for eGFR | All, n=8254 | Black, n=2601, Root Mean Square Errorb | Nonblack, n=5653, Root Mean Square Errorb | |
|---|---|---|---|---|
| Black Race Coefficient (95% CI)a | Root Mean Square Errorb | |||
| Serum creatinine, age, sex, race | 1.16 (1.14 to 1.17) | 0.236 (0.229 to 0.242) | 0.243 (0.232 to 0.254) | 0.232 (0.225 to 0.241) |
| Serum creatinine, age, sex | NA | 0.244 (0.238 to 0.251) | 0.258 (0.248 to 0.268) | 0.238 (0.230 to 0.247) |
| Serum creatinine, age, sex, race, height, weight | 1.15 (1.14 to 1.17) | 0.235 (0.229 to 0.242) | 0.242 (0.232 to 0.253) | 0.232 (0.225 to 0.241) |
| Serum creatinine, age, sex, height, weight | NA | 0.243 (0.237 to 0.250) | 0.255 (0.245 to 0.265) | 0.238 (0.230 to 0.246) |
Data are from the pooled CKD-EPI development and internal validation datasets. Mean (SD) measured GFR =68 (40) ml/min per 1.73 m2 (28). Other GFR estimating equations using serum creatinine were not considered because they are not more accurate than the CKD-EPI creatinine equations in external validation datasets. 95% CI, 95% confidence interval; NA, not applicable. Modified from ref. 72, with permission.
Coefficient for equation expressed on the multiplicative scale of GFR.
Root mean square error for the regression of measured GFR on eGFR computed on the logarithmic scale. A lower root mean square error indicates higher accuracy of the eGFR.
It is important to differentiate the consequences of the loss of accuracy of eGFRcr from eliminating the black coefficient from the CKD-EPI creatinine equation at the individual versus population level. For individuals, imprecision in eGFRcr is the main limitation in its accuracy; the clinical implications of further loss of accuracy would depend on the level of GFR, the clinical decisions that need to be made, and the availability of confirmatory tests (Figure 1) (72). For example, if the level of GFR is low, where elimination of the black coefficient does not lead to a large change in eGFR, or if the clinical decision does not require highly accurate GFR assessment, then ignoring the black coefficient may be of little consequence. However, if the level of GFR is moderate to high, where elimination of the black coefficient may lead to a large change in eGFR, or if the clinical decision requires accurate GFR assessment and race is uncertain, then confirmatory, guideline-recommended testing would be appropriate.
For populations, bias is the main limitation in the accuracy of eGFRcr. In the absence of routinely performed confirmatory tests, a systematic underestimation of mGFR in blacks throughout the GFR range is likely to have wide-ranging implications (Figure 1) (72). Eneanya et al. (27) call attention to the possibility that blacks may be disadvantaged from late referral for kidney transplantation because of systematically higher eGFRcr than nonblacks with the same serum creatinine, age, and sex. However, systematic underestimation of mGFR by eliminating the black coefficient could lead to unintended consequences. Although earlier referral for transplantation might be helpful in overcoming the disparity in kidney transplantation, inappropriate early transplantation or dialysis initiation could be harmful. Other consequences might be overdiagnosis of CKD, underestimation of the relationship of risk of adverse outcomes to reduced GFR, inadequate use or dosing of drugs excreted by glomerular filtration (including metformin), and limited access to tests (including imaging procedures) and treatments that require a higher level of GFR (Figure 1), including living donor kidney donation, another important disparity affecting blacks (72,73).
Clinical research and public health might also be compromised by failure to recognize this systematic bias and failure to focus attention and resources where they are most needed. For example, recognition of the causes and consequences of increased incidence of kidney failure and faster rate of disease progression in blacks in population studies requires accurate assessment of and adjustment for baseline eGFRcr (74,75). Appropriately, identification of the mechanisms underlying these associations and developing therapies to target these differences are the focus of much current research.
Moving Forward
We agree with Eneanya et al. (27) that specification of race for GFR estimation in adults is sometimes difficult and inaccurate and that it ideally should be replaced by physiologic measures that reflect variation in non-GFR determinants of serum creatinine. However, in the absence of suitable surrogates for these physiologic variables, we are concerned that the loss of accuracy from eliminating specification of race in eGFRcr could disadvantage blacks both at the individual and population levels. Therefore, we propose a more cautious approach. In our view, the goals should be to maintain and improve accuracy of GFR estimates and to avoid disadvantaging any racial group (Box 2). In general, we suggest full disclosure of the use of race in GFR estimation, accommodation of those who decline, and shared decision making between health care providers and patients regarding GFR estimation. We also suggest mindful use of endogenous filtration markers in addition to creatinine, such as cystatin C, when important clinical decisions must be made. Furthermore, we encourage more research to understand the underlying causes of race differences, including muscle mass, diet, and tubular secretion, in the relationship of mGFR to serum creatinine and other filtration markers. In the future, confirmatory tests in addition to eGFRcys and eGFRcr-cys could include a panel of filtration markers (panel eGFR), either without or with creatinine, that is not affected by race. In principle, use of panel eGFR as an initial test might avoid the need for specification of race altogether. Much more work is required for development and implementation of panel eGFRs for routine clinical care (71). Measuring filtration markers in addition to creatinine would increase the cost of GFR estimation, but the benefit may be worth the cost if it improves accuracy, improves generalizability across populations, and avoids specification of race.
Box 2.
Suggestions for GFR estimation in adults and clinical decision making now and in the future
| Goals: Maintain and improve accuracy of GFR estimates and avoid disadvantaging any racial group |
| General Suggestions |
|
| Specific Suggestions: Now |
|
| Specific Suggestions: Future |
|
Until better methods are developed, continuing to assess eGFRcr using the CKD-EPI creatinine equation with the black coefficient in blacks and without the coefficient in nonblacks (including mixed race combinations) or if race is not specified seems reasonable. In some Asian countries, other equations that have been proved to be more accurate than the CKD-EPI creatinine equation can be used. If greater accuracy is needed, eGFRcys in which specification of race is not required and eGFRcr-cys in which the bias from ignoring the black race coefficient is smaller (8%) also seem reasonable. Other confirmatory tests for critical clinical decision making (e.g., medication use and performance of diagnostic tests) include measured creatinine clearance and mGFR, which do not require specification of race. We believe that these strategies are consistent with the current KDIGO guidelines and the criteria proposed by Eneanya et al. (27) for using race to guide clinical care. In addition, we remind clinicians that the appropriate detection, evaluation, and management of kidney disease in all races require evaluation of cause of disease in addition to both GFR and albuminuria (25).
In conclusion, kidney disease is a worldwide public health problem, with racial disparities in incidence, prevalence, and outcomes. Criteria for the definitions of AKD and CKD are independent of race, but eGFRcr, the initial test in GFR assessment, requires specification of race for optimal accuracy, which has limitations. Specification of race improves the accuracy of eGFRcr, in part because of purported race associations with muscle mass and creatinine generation, an important non-GFR determinant of serum creatinine. Serum cystatin C is less affected by muscle mass than serum creatinine; thus, eGFRcys and eGFRcr-cys, confirmatory tests in GFR assessment, are less dependent on specification of race. In the future, using a panel of multiple filtration markers with or without creatinine may enable more accurate GFR estimation without specification of race. We believe that the limitations of use of race in GFR evaluation can be lessened by greater transparency in the use of race in eGFRcr, by mindful use of filtration markers in addition to creatinine, and by future research to address the gaps in mechanistic understanding that we have described.
Disclosures
Dr. Coresh and Dr. Levey have participated in Kidney Disease Improving Global Outcomes Guideline Work Groups. Dr. Inker reports funding from Retrophin; Omeros; Dialysis Clinics, Inc.; and Reata Pharmaceuticals for research and contracts given to Tufts Medical Center outside of this work. Dr. Levey reports contracts from National Institutes of Health and National Kidney Foundation to Tufts Medical Center and a clinical trial contract with AstraZeneca. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors are grateful to Juhi Chaudhari at Tufts Medical Center (Boston, MA) for assistance with manuscript preparation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Perspective, “Precision in GFR Reporting: Let’s Stop Playing the Race Card,” on pages 1201–1202.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12791019/-/DCSupplemental.
Supplemental Box. Race, ethnicity, and ancestry.
Supplemental Table. Descriptive characteristics of Chronic Kidney Disease Epidemiology Collaboration participants by race.
References
- 1.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA: Strengths and limitations of estimated and measured GFR. Nat Rev Nephrol 15: 784, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators : Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1789–1858, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 Causes of Death Collaborators : Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1736–1788, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Thomas B, Matsushita K, Abate KH, Al-Aly Z, Ärnlöv J, Asayama K, Atkins R, Badawi A, Ballew SH, Banerjee A, Barregård L, Barrett-Connor E, Basu S, Bello AK, Bensenor I, Bergstrom J, Bikbov B, Blosser C, Brenner H, Carrero JJ, Chadban S, Cirillo M, Cortinovis M, Courville K, Dandona L, Dandona R, Estep K, Fernandes J, Fischer F, Fox C, Gansevoort RT, Gona PN, Gutierrez OM, Hamidi S, Hanson SW, Himmelfarb J, Jassal SK, Jee SH, Jha V, Jimenez-Corona A, Jonas JB, Kengne AP, Khader Y, Khang YH, Kim YJ, Klein B, Klein R, Kokubo Y, Kolte D, Lee K, Levey AS, Li Y, Lotufo P, El Razek HMA, Mendoza W, Metoki H, Mok Y, Muraki I, Muntner PM, Noda H, Ohkubo T, Ortiz A, Perico N, Polkinghorne K, Al-Radaddi R, Remuzzi G, Roth G, Rothenbacher D, Satoh M, Saum KU, Sawhney M, Schöttker B, Shankar A, Shlipak M, Silva DAS, Toyoshima H, Ukwaja K, Umesawa M, Vollset SE, Warnock DG, Werdecker A, Yamagishi K, Yano Y, Yonemoto N, Zaki MES, Naghavi M, Forouzanfar MH, Murray CJL, Coresh J, Vos T; Global Burden of Disease 2013 GFR Collaborators; CKD Prognosis Consortium; Global Burden of Disease Genitourinary Expert Group : Global Cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 28: 2167–2179, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, Balkrishnan R, Bragg-Gresham J, Cao J, Chen JL, Cope E, Dharmarajan S, Dietrich X, Eckard A, Eggers PW, Gaber C, Gillen D, Gipson D, Gu H, Hailpern SM, Hall YN, Han Y, He K, Hebert H, Helmuth M, Herman W, Heung M, Hutton D, Jacobsen SJ, Ji N, Jin Y, Kalantar-Zadeh K, Kapke A, Katz R, Kovesdy CP, Kurtz V, Lavalee D, Li Y, Lu Y, McCullough K, Molnar MZ, Montez-Rath M, Morgenstern H, Mu Q, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Pearson J, Pisoni R, Plattner B, Port FK, Potukuchi P, Rao P, Ratkowiak K, Ravel V, Ray D, Rhee CM, Schaubel DE, Selewski DT, Shaw S, Shi J, Shieu M, Sim JJ, Song P, Soohoo M, Steffick D, Streja E, Tamura MK, Tentori F, Tilea A, Tong L, Turf M, Wang D, Wang M, Woodside K, Wyncott A, Xin X, Zang W, Zepel L, Zhang S, Zho H, Hirth RA, Shahinian V: United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2016 [Google Scholar]
- 8.Powe NR: To have and have not: Health and health care disparities in chronic kidney disease. Kidney Int 64: 763–772, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Powe NR: CKD progression: Teasing out contributions of elements in the human exposome. Am J Kidney Dis 73: 581–582, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Pollak MR, Genovese G, Friedman DJ: APOL1 and kidney disease. Curr Opin Nephrol Hypertens 21: 179–182, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine : Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, Washington, DC, The National Academies Press, 2003 [PubMed] [Google Scholar]
- 12.Office of Management and Budget : Revisions to the standards for the classification of federal data on race and ethnicity, 1997. Available at: https://obamawhitehouse.archives.gov/omb/fedreg_1997standards. Accessed March 3, 2019
- 13.Anonymous : Genes, drugs and race. Nat Genet 29: 239–240, 2001. [DOI] [PubMed] [Google Scholar]
- 14.American Anthropology Association : AAA statement on race. Available at: https://www.americananthro.org/ConnectWithAAA/Content.aspx?ItemNumber=2583. Accessed March 3, 2019
- 15.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, Mountain JL, Pérez-Stable EJ, Sheppard D, Risch N: The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med 348: 1170–1175, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Sankar P, Cho MK: Genetics. Toward a new vocabulary of human genetic variation. Science 298: 1337–1338, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Race, Ethnicity, and Genetics Working Group : The use of racial, ethnic, and ancestral categories in human genetics research. Am J Hum Genet 77: 519–532, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravlee CC: How race becomes biology: Embodiment of social inequality. Am J Phys Anthropol 139: 47–57, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Foster MW: Looking for race in all the wrong places: Analyzing the lack of productivity in the ongoing debate about race and genetics. Hum Genet 126: 355–362, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Witzig R: The medicalization of race: Scientific legitimization of a flawed social construct. Ann Intern Med 125: 675–679, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Lorusso L: The justification of race in biological explanation. J Med Ethics 37: 535–539, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Shriver MD, Kittles RA: Genetic ancestry and the search for personalized genetic histories. Nat Rev Genet 5: 611–618, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Soveri I, Berg UB, Björk J, Elinder CG, Grubb A, Mejare I, Sterner G, Bäck SE; SBU GFR Review Group : Measuring GFR: A systematic review. Am J Kidney Dis 64: 411–424, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving global outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 26.Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 27.Eneanya ND, Yang W, Reese PP: Reconsidering the consequences of using race to estimate kidney function. JAMA 322: 113–114, 2019. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, De Souza V, Selistre L, Mariat C, Martens F, Delanaye P: An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 31: 798–806, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pottel H, Delanaye P, Schaeffner E, Dubourg L, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, De Souza V, Selistre L, Goffin K, Pauwels S, Mariat C, Flamant M, Ebert N: Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 32: 497–507, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, Waheed S, Coresh J: Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis 58: 682–684, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inker LA, Tighiouart H, Coresh J, Foster MC, Anderson AH, Beck GJ, Contreras G, Greene T, Karger AB, Kusek JW, Lash J, Lewis J, Schelling JR, Navaneethan SD, Sondheimer J, Shafi T, Levey AS: GFR estimation using β-trace protein and β2-microglobulin in CKD. Am J Kidney Dis 67: 40–48, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459–466, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Delanaye P, Mariat C, Maillard N, Krzesinski J-M, Cavalier E: Are the creatinine-based equations accurate to estimate glomerular filtration rate in African American populations? Clin J Am Soc Nephrol 6: 906–912, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Flamant M, Vidal-Petiot E, Metzger M, Haymann JP, Letavernier E, Delatour V, Karras A, Thervet E, Boffa JJ, Houillier P, Stengel B, Vrtovsnik F, Froissart M; NephroTest Study Group : Performance of GFR estimating equations in African Europeans: Basis for a lower race-ethnicity factor than in African Americans. Am J Kidney Dis 62: 182–184, 2013. [DOI] [PubMed] [Google Scholar]
- 41.van Deventer HE, George JA, Paiker JE, Becker PJ, Katz IJ: Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clin Chem 54: 1197–1202, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Yayo E, Ayé M, Yao C, Gnionsahé A, Attoungbré ML, Cavalier E, Pottel H, Monnet D, Delanaye P: Measured (and estimated) glomerular filtration rate: Reference values in West Africa. Nephrol Dial Transplant 33: 1176–1180, 2018. [DOI] [PubMed] [Google Scholar]
- 43.Moodley N, Hariparshad S, Peer F, Gounden V: Evaluation of the CKD-EPI creatinine based glomerular filtration rate estimating equation in Black African and Indian adults in KwaZulu-Natal, South Africa. Clin Biochem 59: 43–49, 2018. [DOI] [PubMed] [Google Scholar]
- 44.Bukabau JB, Yayo E, Gnionsahé A, Monnet D, Pottel H, Cavalier E, Nkodila A, Makulo JRR, Mokoli VM, Lepira FB, Nseka NM, Krzesinski J-M, Sumaili EK, Delanaye P: Performance of creatinine- or cystatin C-based equations to estimate glomerular filtration rate in sub-Saharan African populations. Kidney Int 95: 1181–1189, 2019. [DOI] [PubMed] [Google Scholar]
- 45.Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, Nelson RG, Van Deventer M, Wang HY, Zuo L, Zhang YL, Levey AS: Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int 79: 555–562, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teo BW, Zhang L, Guh J-Y, Tang SCW, Jha V, Kang D-H, Tanchanco R, Hooi LS, Praditpornsilpa K, Kong X, Zuo L, Chan GC, Lee EJC: Glomerular filtration rates in Asians. Adv Chronic Kidney Dis 25: 41–48, 2018. [DOI] [PubMed] [Google Scholar]
- 47.Rule AD, Teo BW: GFR estimation in Japan and China: What accounts for the difference? Am J Kidney Dis 53: 932–935, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waikar SS, Rebholz CM, Zheng Z, Hurwitz S, Hsu CY, Feldman HI, Xie D, Liu KD, Mifflin TE, Eckfeldt JH, Kimmel PL, Vasan RS, Bonventre JV, Inker LA, Coresh J; Chronic Kidney Disease Biomarkers Consortium Investigators : Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis 72: 538–546, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB: Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J Appl Physiol (1985) 83: 229–239, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Barondess DA, Nelson DA, Schlaen SE: Whole body bone, fat, and lean mass in black and white men. J Bone Miner Res 12: 967–971, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Goldwasser P, Aboul-Magd A, Maru M: Race and creatinine excretion in chronic renal insufficiency. Am J Kidney Dis 30: 16–22, 1997. [DOI] [PubMed] [Google Scholar]
- 52.James GD, Sealey JE, Alderman M, Ljungman S, Mueller FB, Pecker MS, Laragh JH: A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens 1: 124–131, 1988. [DOI] [PubMed] [Google Scholar]
- 53.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL: Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ Health Perspect 113: 192–200, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohn SH, Abesamis C, Zanzi I, Aloia JF, Yasumura S, Ellis KJ: Body elemental composition: Comparison between black and white adults. Am J Physiol 232: E419–E422, 1977. [DOI] [PubMed] [Google Scholar]
- 55.Harsha DW, Frerichs RR, Berenson GS: Densitometry and anthropometry of black and white children. Hum Biol 50: 261–280, 1978. [PubMed] [Google Scholar]
- 56.Worrall JG, Phongsathorn V, Hooper RJ, Paice EW: Racial variation in serum creatine kinase unrelated to lean body mass. Br J Rheumatol 29: 371–373, 1990. [DOI] [PubMed] [Google Scholar]
- 57.Aloia JF, Vaswani A, Feuerman M, Mikhail M, Ma R: Differences in skeletal and muscle mass with aging in black and white women. Am J Physiol Endocrinol Metab 278: E1153–E1157, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Ix JH, Wassel CL, Stevens LA, Beck GJ, Froissart M, Navis G, Rodby R, Torres VE, Zhang YL, Greene T, Levey AS: Equations to estimate creatinine excretion rate: The CKD epidemiology collaboration. Clin J Am Soc Nephrol 6: 184–191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banda Y, Kvale MN, Hoffmann TJ, Hesselson SE, Ranatunga D, Tang H, Sabatti C, Croen LA, Dispensa BP, Henderson M, Iribarren C, Jorgenson E, Kushi LH, Ludwig D, Olberg D, Quesenberry CP Jr., Rowell S, Sadler M, Sakoda LC, Sciortino S, Shen L, Smethurst D, Somkin CP, Van Den Eeden SK, Walter L, Whitmer RA, Kwok PY, Schaefer C, Risch N: Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) Cohort. Genetics 200: 1285–1295, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Udler MS, Nadkarni GN, Belbin G, Lotay V, Wyatt C, Gottesman O, Bottinger EP, Kenny EE, Peter I: Effect of genetic african ancestry on eGFR and kidney disease. J Am Soc Nephrol 26: 1682–1692, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coresh J, Toto RD, Kirk KA, Whelton PK, Massry S, Jones C, Agodoa L, Van Lente F: Creatinine clearance as a measure of GFR in screenees for the african-American study of kidney disease and hypertension pilot study. Am J Kidney Dis 32: 32–42, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M, Tin A, Wang L, Chu AY, Hoppmann A, Kirsten H, Giri A, Chai JF, Sveinbjornsson G, Tayo BO, Nutile T, Fuchsberger C, Marten J, Cocca M, Ghasemi S, Xu Y, Horn K, Noce D, van der Most PJ, Sedaghat S, Yu Z, Akiyama M, Afaq S, Ahluwalia TS, Almgren P, Amin N, Ärnlöv J, Bakker SJL, Bansal N, Baptista D, Bergmann S, Biggs ML, Biino G, Boehnke M, Boerwinkle E, Boissel M, Bottinger EP, Boutin TS, Brenner H, Brumat M, Burkhardt R, Butterworth AS, Campana E, Campbell A, Campbell H, Canouil M, Carroll RJ, Catamo E, Chambers JC, Chee ML, Chee ML, Chen X, Cheng CY, Cheng Y, Christensen K, Cifkova R, Ciullo M, Concas MP, Cook JP, Coresh J, Corre T, Sala CF, Cusi D, Danesh J, Daw EW, de Borst MH, De Grandi A, de Mutsert R, de Vries APJ, Degenhardt F, Delgado G, Demirkan A, Di Angelantonio E, Dittrich K, Divers J, Dorajoo R, Eckardt KU, Ehret G, Elliott P, Endlich K, Evans MK, Felix JF, Foo VHX, Franco OH, Franke A, Freedman BI, Freitag-Wolf S, Friedlander Y, Froguel P, Gansevoort RT, Gao H, Gasparini P, Gaziano JM, Giedraitis V, Gieger C, Girotto G, Giulianini F, Gögele M, Gordon SD, Gudbjartsson DF, Gudnason V, Haller T, Hamet P, Harris TB, Hartman CA, Hayward C, Hellwege JN, Heng CK, Hicks AA, Hofer E, Huang W, Hutri-Kähönen N, Hwang SJ, Ikram MA, Indridason OS, Ingelsson E, Ising M, Jaddoe VWV, Jakobsdottir J, Jonas JB, Joshi PK, Josyula NS, Jung B, Kähönen M, Kamatani Y, Kammerer CM, Kanai M, Kastarinen M, Kerr SM, Khor CC, Kiess W, Kleber ME, Koenig W, Kooner JS, Körner A, Kovacs P, Kraja AT, Krajcoviechova A, Kramer H, Krämer BK, Kronenberg F, Kubo M, Kühnel B, Kuokkanen M, Kuusisto J, La Bianca M, Laakso M, Lange LA, Langefeld CD, Lee JJ, Lehne B, Lehtimäki T, Lieb W, Lim SC, Lind L, Lindgren CM, Liu J, Liu J, Loeffler M, Loos RJF, Lucae S, Lukas MA, Lyytikäinen LP, Mägi R, Magnusson PKE, Mahajan A, Martin NG, Martins J, März W, Mascalzoni D, Matsuda K, Meisinger C, Meitinger T, Melander O, Metspalu A, Mikaelsdottir EK, Milaneschi Y, Miliku K, Mishra PP, Mohlke KL, Mononen N, Montgomery GW, Mook-Kanamori DO, Mychaleckyj JC, Nadkarni GN, Nalls MA, Nauck M, Nikus K, Ning B, Nolte IM, Noordam R, O’Connell J, O’Donoghue ML, Olafsson I, Oldehinkel AJ, Orho-Melander M, Ouwehand WH, Padmanabhan S, Palmer ND, Palsson R, Penninx BWJH, Perls T, Perola M, Pirastu M, Pirastu N, Pistis G, Podgornaia AI, Polasek O, Ponte B, Porteous DJ, Poulain T, Pramstaller PP, Preuss MH, Prins BP, Province MA, Rabelink TJ, Raffield LM, Raitakari OT, Reilly DF, Rettig R, Rheinberger M, Rice KM, Ridker PM, Rivadeneira F, Rizzi F, Roberts DJ, Robino A, Rossing P, Rudan I, Rueedi R, Ruggiero D, Ryan KA, Saba Y, Sabanayagam C, Salomaa V, Salvi E, Saum KU, Schmidt H, Schmidt R, Schöttker B, Schulz CA, Schupf N, Shaffer CM, Shi Y, Smith AV, Smith BH, Soranzo N, Spracklen CN, Strauch K, Stringham HM, Stumvoll M, Svensson PO, Szymczak S, Tai ES, Tajuddin SM, Tan NYQ, Taylor KD, Teren A, Tham YC, Thiery J, Thio CHL, Thomsen H, Thorleifsson G, Toniolo D, Tönjes A, Tremblay J, Tzoulaki I, Uitterlinden AG, Vaccargiu S, van Dam RM, van der Harst P, van Duijn CM, Velez Edward DR, Verweij N, Vogelezang S, Völker U, Vollenweider P, Waeber G, Waldenberger M, Wallentin L, Wang YX, Wang C, Waterworth DM, Bin Wei W, White H, Whitfield JB, Wild SH, Wilson JF, Wojczynski MK, Wong C, Wong TY, Xu L, Yang Q, Yasuda M, Yerges-Armstrong LM, Zhang W, Zonderman AB, Rotter JI, Bochud M, Psaty BM, Vitart V, Wilson JG, Dehghan A, Parsa A, Chasman DI, Ho K, Morris AP, Devuyst O, Akilesh S, Pendergrass SA, Sim X, Böger CA, Okada Y, Edwards TL, Snieder H, Stefansson K, Hung AM, Heid IM, Scholz M, Teumer A, Köttgen A, Pattaro C; Lifelines Cohort Study; V. A. Million Veteran Program : A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 51: 957–972, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Foster MC, Tighiouart H, Anderson AH, Beck GJ, Contreras G, Coresh J, Eckfeldt JH, Feldman HI, Greene T, Hamm LL, He J, Horwitz E, Lewis J, Ricardo AC, Shou H, Townsend RR, Weir MR, Inker LA, Levey AS; CRIC (Chronic Renal Insufficiency Cohort) Study Investigators : Non-GFR determinants of low-molecular-weight serum protein filtration markers in CKD. Am J Kidney Dis 68: 892–900, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foster MC, Levey AS, Inker LA, Shafi T, Fan L, Gudnason V, Katz R, Mitchell GF, Okparavero A, Palsson R, Post WS, Shlipak MG: Non-GFR determinants of low-molecular-weight serum protein filtration markers in the elderly: AGES-kidney and MESA-kidney. Am J Kidney Dis 70: 406–414, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS: Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75: 652–660, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A: Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem 38: 1–8, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004. [DOI] [PubMed] [Google Scholar]
- 68.White CA, Ghazan-Shahi S, Adams MA: β-Trace protein: A marker of GFR and other biological pathways. Am J Kidney Dis 65: 131–146, 2015. [DOI] [PubMed] [Google Scholar]
- 69.Coresh J, Inker LA, Sang Y, Chen J, Shafi T, Post WS, Shlipak MG, Ford L, Goodman K, Perichon R, Greene T, Levey AS: Metabolomic profiling to improve glomerular filtration rate estimation: A proof-of-concept study. Nephrol Dial Transplant 34: 825–833, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freed TA, Coresh J, Inker LA, Toal DR, Perichon R, Chen J, Goodman KD, Zhang Q, Conner JK, Hauser DM, Vroom KET, Oyaski ML, Wulff JE, Eiríksdóttir G, Gudnason V, Torres VE, Ford LA, Levey AS: Validation of a metabolite panel for a more accurate estimation of glomerular filtration rate using quantitative LC-MS/MS. Clin Chem 65: 406–418, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inker LA, Levey AS, Coresh J: Estimated glomerular filtration rate from a panel of filtration markers-hope for increased accuracy beyond measured glomerular filtration rate? Adv Chronic Kidney Dis 25: 67–75, 2018. [DOI] [PubMed] [Google Scholar]
- 72.Levey AS, Tighiouart H, Titan SM, Inker LA: Estimating GFR with and without race. JAMA Intern Med 180: 2020, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Purnell TS, Luo X, Cooper LA, Massie AB, Kucirka LM, Henderson ML, Gordon EJ, Crews DC, Boulware LE, Segev DL: Association of race and ethnicity with live donor kidney transplantation in the United States from 1995 to 2014. JAMA 319: 49–61, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derose SF, Rutkowski MP, Crooks PW, Shi JM, Wang JQ, Kalantar-Zadeh K, Kovesdy CP, Levin NW, Jacobsen SJ: Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis 62: 236–244, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, Appel LJ, Tin A, Coresh J: Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol 27: 2842–2850, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.