Medications are an indispensable cornerstone of modern health care. Clinicians prescribe drugs in the treatment of innumerable medical conditions, whereas patients and the general population self-treat various maladies with over-the-counter medications and natural and herbal products. For the most part, these drugs are well tolerated, but every drug is a double-edged sword with risk of adverse drug reactions (ADRs) that clearly can complicate therapy (1). Although any organ system can be affected by “drug toxicity,” the kidney, skin, liver, bone marrow, heart, and brain are frequently affected. Thus, understanding the causes and risk factors underlying drug toxicity is of great interest to the medical community because health care providers wish to uphold the “primum non nocere” (first, do no harm) approach by preventing and/or reducing adverse drug effects.
To develop strategies to prevent or reduce the likelihood of drug toxicity, it is critical to understand how a drug is handled by the body. This is the essence of pharmacology. Clinical pharmacokinetics can be simply classified as absorption, distribution, metabolism/transport, and excretion of the drug of interest (2). Pharmacokinetic properties are important determinants of the amount of drug reaching its site of action and help determine, in part, the drug’s effects on the body (clinical pharmacodynamics) after receptor binding, postreceptor effects, and other interactions (3,4). Although there are a number of factors (e.g., genetics, diseases, drug-drug interactions) that affect clinical pharmacokinetics and pharmacodynamics (Figure 1), and a number of high-risk groups that can develop disturbances in them, we will focus on CKD.
Figure 1.
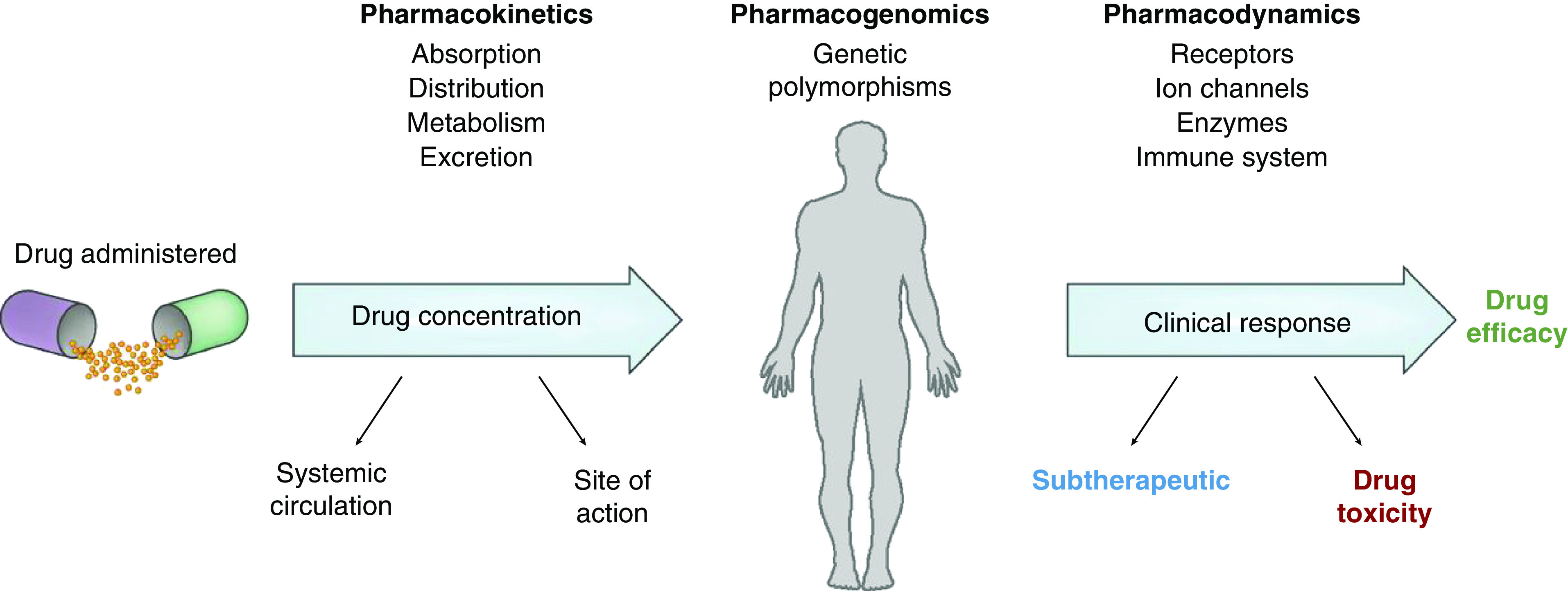
Factors that affect clinical pharmacology in humans. Reprinted from reference 2, with permission.
Identifying groups such as those with CKD that are high risk for drug toxicity due to altered clinical pharmacokinetics and pharmacodynamics can potentially limit toxicity. All pharmacokinetic parameters may be affected, most notably metabolism, transport, and excretion (3,4). Although most health care providers know that patients with a reduced GFR have impaired clearance of drugs excreted primarily by the kidneys, the effect of CKD on nonkidney drug clearance is underappreciated (3,4). For example, drug metabolism by the cytochrome P450 system and/or transport of drugs into enterocytes, hepatobiliary cells, or other cells of metabolism by various transporters (p-glycoprotein, organic anion/cation transporters, multidrug-resistant proteins, etc.) may be altered in CKD (3,4). In contrast to the well described pharmacokinetics in patients with CKD that lead to alterations in drug action, alterations in pharmacodynamic parameters have rarely been considered and are somewhat understudied (5). Pharmacodynamic parameters observed in patients with normal kidney function might change in the setting of CKD (5). For example, the maximum effect normally elicited by a given drug concentration at the target receptor may be diminished or enhanced depending on receptor sensitivity in patients with CKD (5). As a result of these variable effects in both pharmacokinetics and pharmacodynamics, underdosing with lower therapeutic efficacy or overdosing with resulting toxicity may occur in patients with CKD.
In this issue of CJASN, Laville et al. (6) undertook a study to examine medication prescribing in patients with CKD focusing on ADRs. Using the CKD-REIN cohort, which included 3033 patients with CKD (eGFR <60 ml/min per 1.73 m2 as calculated by the Chronic Kidney Disease Epidemiology Collaboration equation) from nephrology outpatient facilities in France with follow-up for 2 years, the authors estimated the incidence, preventability, and management of both overall and serious ADRs. An ADR was defined broadly, whereas serious ADRs were defined as those leading to death, hospitalization, disability/permanent damage, or another important medical event. Two pharmacists reviewed and classified ADRs, and a committee of four expert pharmacologists evaluated all ADRs deemed as serious. Drug prescriptions were continuously recorded from 3 months before inclusion until the end of follow-up. Medication adherence was measured using the Girerd score (six questions used to determine adherence to chronic medications). ADRs were identified from hospitalization reports, medical records, and participant interviews. The Begaud imputability method (cause-and-effect relationship based on highest intrinsic score) was used to identify drugs most responsible for ADRs. The Naranjo ten-question algorithm was utilized to confirm the causal relationship of each ADR by determining that an ADR was due to the drug (definite, probable, or possible) rather than another factor. The authors assessed for preventability using the Olivier ADR preventability scale (focuses on the drug, the patient, and the prescription), which categorizes ADRs as preventable, potentially preventable, not preventable, or not assessable. Immediate therapeutic management of ADRs (drug discontinuation, dose adjustment, or no change) was assessed by experts in pharmacology.
Over the 2-year period, study participants had a median age of 69 years, 45% had an eGFR <30 ml/min per 1.73 m2, and they were prescribed a median of 8 (range, 5–10) drugs. During the study period, a total of 751 ADRs occurred in 536 participants; of these, 150 serious ADRs developed in 125 participants, translating to rates of 14.4 and 2.7 per 100 person-years, respectively. Of the 150 serious ADRs, 145 and 16 were associated with hospitalization and death, respectively. Kidney/urinary disorders, primarily AKI and an increased serum creatinine, were the most frequent ADRs, followed by gastrointestinal and musculoskeletal disorders. Kidney disorders and hemorrhage/bleeding made up two thirds of the serious ADRs. Notably, 11 of 16 deaths were due to hemorrhage alone, which was associated with 40% of the serious ADRs in patients with eGFR <30 ml/min per 1.73 m2. Drugs with the most ADRs included the renin-angiotensin system (RAS) inhibitors (15%), antithrombotic agents (14%), and diuretics (10%), whereas antithrombotic agents (predominantly vitamin K antagonists) were associated with the most serious ADRs (34%).
In examining the factors associated with ADRs, adjusted hazard ratios (HRs) for all and serious ADRs were significantly higher in patients with eGFR <30 versus ≥30 ml/min per 1.73 m2 (all ADRs HR, 1.56: 95% confidence interval [95% CI], 1.3 to 1.87; serious ADRs HR, 1.8: 95% CI, 1.3 to 2.6), in those prescribed >10 versus <5 medications (all ADRs HR, 1.71: 95% CI, 1.21 to 2.41; serious ADRs HR, 2.4: 95% CI 1.1 to 5.2), and those with poor drug adherence (all ADRs HR, 1.36: 95% CI, 1.12 to 1.64; serious ADRs HR, 1.59: 95% CI, 1.05 to 2.42). In assessing the preventability of ADRs, 32% of the serious ADRs were considered either preventable (13%) or potentially preventable (19%). Participant self-medication was associated with 25% of the preventable ADRs. After the identification of an ADR, the responsible drug was discontinued in 71%, the dose was adjusted in 14%, and no change was made in 11%.
The authors conclude that ADRs, including serious ADRs, are relatively common in patients with moderate to advanced CKD. They note that certain pharmacological classes, particularly antithrombotic agents, are more commonly associated with serious ADRs and that they may be preventable. Cautious drug prescription in patients with CKD, particularly in those with stage 4 or greater CKD, as well as increased involvement of clinical pharmacists and enhanced patient education, are recommended to minimize the risk of ADRs.
This publication brings to light important information regarding the potential for drug-related toxicity in patients with CKD. The authors are to be congratulated on their in-depth assessment of a large number of patients with CKD, providing granular information about ADRs, including the drugs most frequently associated with their development, the factors enhancing risk, and the preventability of ADRs. Although other studies provide information on ADRs in patients with CKD, the process used to capture and verify data in this study provides additional insight. In particular, the inclusion of multiple sources to capture events and the review of data by experts in pharmacology further cements the information as reliable. However, as the authors note, there are study limitations such as potential underestimation of ADRs in outpatients, not using decision algorithms in causality assessment, and potential lack of generalizability to patients cared for by non-nephrologists.
One can also quibble about some of the ADRs reported in the study. RAS inhibitors and diuretics were associated with 26% of the ADRs noted, and these drugs were listed as commonly causing AKI (65 total and 33 serious ADRs) and increased serum creatinine (30 total and 0 serious). An increase in serum creatinine, including the 50% increase used to define AKI, may not be an ADR at all. Several studies have shown that “hypercreatininemia” is not necessarily harmful and is associated with improved outcomes (7,8). Also, a large number (32%) of ADRs associated with use of the five drug classes most frequently implicated were classified as “other.” Information related to specific ADRs may facilitate better risk–benefit assessment in patients with CKD, but further evaluation is necessary.
In summary, this study contributes to our understanding of the potential toxicity of drugs when used in patients with underlying CKD. It highlights the legitimate risk of agents acting in the kidney (RAS inhibitors and diuretics) and antithrombotic agents. Prescribers must be vigilant when using these drugs in patients with impaired kidney function, in whom the safe use of medications should focus on selection and dosing with the intent of maximizing efficacy and safety, minimizing systemic toxicity, including kidney injury, and optimizing risk–benefit. Specific strategies that may improve safety in patients with CKD include medication reconciliation and deprescribing (9). Importantly, greater patient and clinician education regarding the risks of medications in patients with kidney disease will enhance awareness and serve to improve medication safety in this high-risk population.
Disclosures
T. Nolin reports receiving personal fees from MediBeacon and CytoSorbents and other from McGraw-Hill Education, outside the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Adverse Drug Reactions in Patients with CKD,” on pages 1090–1102.
References
- 1.Tyson RJ, Park CC, Powell JR, Patterson JH, Weiner D, Watkins PB, Gonzalez D: Precision dosing priority criteria: Drug, disease, and patient population variables. Front Pharmacol 11: 420, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nolin TD, Perazella MA: Introduction to nephropharmacology for the clinician: A new CJASN series. Clin J Am Soc Nephrol 13: 1083–1084, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolin TD: A synopsis of clinical pharmacokinetic alterations in advanced CKD. Semin Dial 28: 325–329, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts DM, Sevastos J, Carland JE, Stocker SL, Lea-Henry TN: Clinical pharmacokinetics in kidney disease: Application to rational design of dosing regimens. Clin J Am Soc Nephrol 13: 1254–1263, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller F, Hann A: Clinical pharmacodynamics: Principles of drug response and alterations in kidney disease. Clin J Am Soc Nephrol 13: 1413–1420, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laville S, Gras-Champel V, Moragny J, Metzger M, Jacquelinet C, Combe C, Fouque D, Laville M, Frimat L, Robinson BM, Stengel B, Massy ZA, Liabeuf S: Adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol 15: 1090–1102, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, Felker GM, Hernandez AF, O’Connor CM, Sabbisetti VS, Bonventre JV, Wilson FP, Coca SG, Testani JM: Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation 137: 2016–2028, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH: Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: Importance of sustained decongestion. J Am Coll Cardiol 62: 516–524, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker CF, Miklich MA, Patel RS, Fink JC: Medication safety principles and practice in CKD. Clin J Am Soc Nephrol 13: 1738–1746, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


