Visual Abstract
Keywords: ANCA, kidney biopsy, Antibodies, Antineutrophil Cytoplasmic, Confidence Intervals, Observer Variation, glomerulonephritis, Renal Insufficiency, Cohort Studies, Prognosis, Biopsy
Abstract
Background and objectives
The histopathologic classification for ANCA-associated GN distinguishes four classes on the basis of patterns of injury. In the original validation study, these classes were ordered by severity of kidney function loss as follows: focal, crescentic, mixed, and sclerotic. Subsequent validation studies disagreed on outcomes in the crescentic and mixed classes. This study, driven by the original investigators, provides several analyses in order to determine the current position of the histopathologic classification of ANCA-associated GN.
Design, setting, participants, & measurements
A validation study was performed with newly collected data from 145 patients from ten centers worldwide, including an analysis of interobserver agreement on the histopathologic evaluation of the kidney biopsies. This study also included a meta-analysis on previous validation studies and a validation of the recently proposed ANCA kidney risk score.
Results
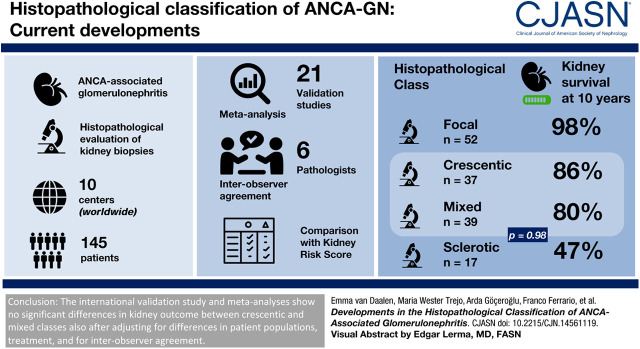
The validation study showed that kidney failure at 10-year follow-up was significantly different between the histopathologic classes (P<0.001). Kidney failure at 10-year follow-up was 14% in the crescentic class versus 20% in the mixed class (P=0.98). In the meta-analysis, no significant difference in kidney failure was also observed when crescentic class was compared with mixed class (relative risk, 1.15; 95% confidence interval, 0.94 to 1.41). When we applied the ANCA kidney risk score to our cohort, kidney survival at 3 years was 100%, 96%, and 77% in the low-, medium-, and high-risk groups, respectively (P<0.001). These survival percentages are higher compared with the percentages in the original study.
Conclusions
The crescentic and mixed classes seem to have a similar prognosis, also after adjusting for differences in patient populations, treatment, and interobserver agreement. However, at this stage, we are not inclined to merge the crescentic and mixed classes because the reported confidence intervals do not exclude important differences in prognosis and because an important histopathologic distinction would be lost.
Introduction
The ANCA-associated vasculitides represent a spectrum of diseases characterized by inflammation of small- to medium-sized blood vessels in the absence or paucity of immune deposits (1). The numbers of acute and chronic lesions in the kidney biopsy may vary considerably from patient to patient, as well as the patients’ outcome (2,3). In the past, several studies found associations between histopathologic parameters in diagnostic kidney biopsies and kidney outcomes. The most consistent findings were associations between the percentage of normal glomeruli and a favorable kidney outcome and between the percentage of sclerotic glomeruli and a worse kidney outcome (2,4–6). Moreover, the presence of active lesions, such as cellular crescents, seemed to be associated with higher probability of kidney function recovery with immunosuppressive therapy (2,4). These results were incorporated into a proposal for a histopathologic classification of ANCA-associated GN by an international working group in 2010 (7). The devised algorithm distinguished four classes: sclerotic, focal, crescentic, and mixed. The original study showed that this classification predicted kidney outcomes at 1- and 5-year follow-up visits in 100 patients.
Since 2010, 21 studies from Asia (8–15), North America (16–18), Australia (19), and Europe (14,20–27) have validated the histopathologic classification in adults, and four studies validated the classification in pediatric patients (14,28–30). The results from some of these studies were recently incorporated into a meta-analysis showing that all validation studies reported the best kidney outcomes in the focal class and the worst in the sclerotic class (13). In some studies, kidney outcomes were similar in the crescentic and mixed classes, whereas in others, they were significantly better in the mixed class than in the crescentic class (13). It has been suggested that these conflicting results regarding crescentic and mixed classes could be attributed to differences in patient populations, differences in treatment, and moderate interobserver agreement with regard to the kidney biopsy evaluation (31). It would seem appropriate to adjust the histopathologic classification for ANCA-associated GN only after the source of the discrepancies in results from various studies has been clarified. We therefore undertook an international validation study in newly collected data driven by the original investigators. Additionally, we analyzed the findings from this validation study in a meta-analysis that included results from previously performed validation studies.
In the meantime, Brix et al. (32) proposed a new predictive tool in the form of a kidney risk score on the basis of a cohort of German patients. This score includes kidney function at baseline, percentage of normal glomeruli, and percentage of interstitial fibrosis and tubular atrophy (IFTA). Similar to the histopathologic classification of ANCA-associated GN, the kidney risk score aims to predict kidney outcome, in particular to identify those patients with a high risk to develop kidney failure, but it aims to do so by also including a clinical parameter. We here present a validation of the recently proposed ANCA kidney risk score on the basis of an international cohort.
Materials and Methods
Study Cohort
Patients were enrolled from ten centers worldwide with histopathologically proven ANCA-associated GN and a follow-up for at least 3 years (including patients who developed kidney failure or died within the first 3 years). The follow-up visits took place at the participating centers and were not conducted according to any protocol. In 88% of the patients, follow-up exceeded 3 years. These follow-up data were also reported on. Exclusion criteria were age under 18 years, overlap syndrome, and participation in a previous validation study. This study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
Diagnostic Kidney Biopsies
Biopsy slides were assembled at Leiden University Medical Center. The original study proposed that a minimum of ten glomeruli was required to define the class (7). However, a recent study showed that the prognostic capability of the classification was also valid for biopsies that contained three to nine glomeruli (25). This study included biopsies with a minimum of five glomeruli. The biopsies were scanned with the Ultra-Fast Scanner at a magnification of ×40. The scanned slides were placed on a secured website, where the biopsies were scored by a group of six pathologists (I.M.B., F.F., K.J., L.-H.N., Y.O., and S.W.) blinded to clinical data. The scoring form on the website (Supplemental Material) was a slightly modified version of the original scoring tool for ANCA-associated GN (33). One marked level was used by all pathologists to classify the biopsies. Each case was scored by two pathologists; when pathologists disagreed on the histopathologic class, a third pathologist (I.M.B. or J.A.B.) made the final decision on the case. For analytic purposes, tubulointerstitial scores from two pathologists were averaged.
Clinical Data
We retrieved data on patient demographics, type of diagnosis (granulomatosis with polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis, or kidney-limited vasculitis), serum and urine laboratory values, and details on induction and maintenance therapy. Kidney function was expressed as eGFR, calculated with the Chronic Kidney Disease Epidemiology Collaboration equation, adjusted for race/ethnicity (34–36). The eGFR was calculated at the time of biopsy (eGFR0) and at 1- and 5-year follow-up visits (eGFR1 and eGFR5, respectively). In addition, the change (Δ) in eGFR over time was calculated, which was defined as the difference between the eGFR at that time point and eGFR at baseline. In the case of developing kidney failure, eGFR was considered zero for analytic purposes. Kidney failure was defined as a need for KRT (dialysis for at least 3 months or transplantation) or as eGFR below 15 ml/min per 1.73 m2 persisting for at least 3 months (37). Kidney survival was expressed as the time between diagnosis and kidney failure. In case of a single missing value in outcome, the patient remained included in the study but was omitted from any specific analysis for which this missing value was required.
Literature Search
The review protocol will be described in this section and was not published elsewhere. A trained librarian performed a literature search on previously published validation studies for the meta-analysis, updated until November 2019. Web of Science and Google Scholar were searched for articles that referred to the original study. Additionally, PubMed and Embase were searched on combinations of the following words: “biopsy,” “histopathological,” “classification,” “ANCA,” and “validation.” The selection of studies and data extraction were performed by one of the authors. Only studies that associated histopathologic class with kidney outcome in patients with ANCA-associated GN were selected (Supplemental Figure 1). The proportion of adult patients who had developed kidney failure at the end of follow-up was extracted from 21 validation studies, including this study and the original study by Berden et al. (7). Five studies reported adjusted hazard ratios (HRs), and these were analyzed in a separate meta-analysis. Four studies investigated the prognostic value of the histopathologic classification in pediatric patients (aged <18 years). Differences between the crescentic and mixed classes were analyzed.
ANCA Kidney Risk Score
For each patient, the ANCA kidney risk score was calculated, which includes eGFR0 (>15 or ≤15 ml/min per 1.73 m2), percentage of normal glomeruli (>25%, 10%–25%, or <10%), and IFTA (≤25% or >25% of cortical area). Following the risk score, each parameter was assigned points, resulting in a low, intermediate, or high risk for kidney failure (Supplemental Table 1) (32).
Statistical Analyses
Continuous variables are expressed as the mean ± SD. Groups were compared with the t test or one-way ANOVA. Categorical variables are expressed as numbers (percentages). Differences were assessed with the Fisher exact test or the chi-squared test. Kidney survival was analyzed with the Kaplan–Meier method and log-rank test. Cox regression analysis was used to calculate HRs with 95% confidence intervals (95% CIs) for outcomes in the crescentic and mixed classes. Interobserver agreement was investigated by calculating the κ or the intraclass correlation coefficient (ICC). Values of κ or ICC were interpreted as follows: >0.75, excellent agreement; 0.40–0.75, fair to good agreement; and <0.40, poor agreement (38,39). All analyses in the validation study were performed with SPSS version 23 (IBM Corp., Armonk, NY). The meta-analysis used random effects models and was performed in ReviewManager, version 5.3. The meta-analysis calculated relative risks (RRs) for the development of kidney failure. The variation in kidney outcome across studies, due to heterogeneity beyond chance, was estimated by I2. Values of I2 were interpreted as follows: 25%, low heterogeneity; 50%, moderate heterogeneity; and 75%, high heterogeneity (40). In all analyses, P values <0.05 were considered significant.
Results
Patient Characteristics
We assembled histopathologic and clinical data for 157 patients. Twelve cases were excluded due to missing clinical data (n=7) or an insufficient number of glomeruli (n=5). Patients were diagnosed between 1991 and 2011. The characteristics of the 145 included patients are summarized in Supplemental Table 2.
Histopathologic Classes and Kidney Function
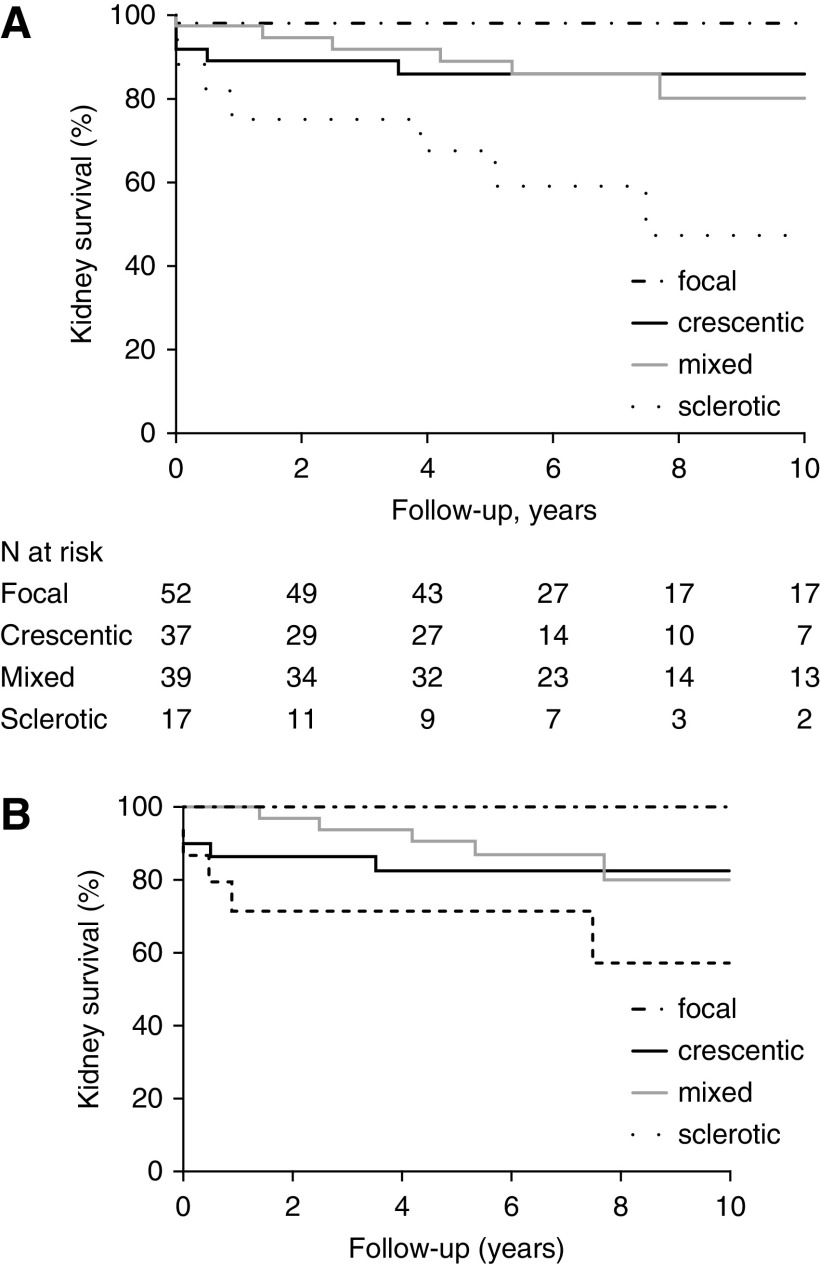
The evaluation indicated that 52 (36%) biopsies were focal, 37 (25%) were crescentic, 39 (27%) were mixed, and 17 (12%) were sclerotic (Table 1). The mean number of glomeruli per biopsy was 19 (range, 5–58). eGFRs at baseline and during follow-up were highest in the focal class (Tables 1 and 2). The lowest eGFR was observed in the sclerotic class. ΔeGFR at 1-year follow-up was highest in the crescentic class. ΔeGFR1 was significantly higher in the crescentic class than in the mixed class (21±19 versus 10±15, respectively; P=0.01), which is driven by the lower eGFR at baseline in the crescentic class. No difference in ΔeGFR5 was observed between the crescentic and mixed classes. During a mean follow-up duration of 8.0±5.4 years, 24 (17%) patients developed kidney failure. Kidney survival at the 10-year follow-up was significantly different between the four histopathologic classes but not between the crescentic and mixed classes (86% in crescentic class versus 80% in mixed class; P=0.98) (Figure 1A). When biopsies with five to ten glomeruli were excluded, kidney survival remained significantly different between the classes (Figure 1B). Forty-eight (33%) patients experienced at least one kidney relapse (Table 2). The number of patients who had a kidney relapse was similar between the crescentic and mixed classes (HR, 0.9 with crescentic as reference group; 95% CI, 0.4 to 2.0) (Table 3). In the total cohort, 45 (31%) patients died. When the combined outcome of death and/or kidney failure within 10 years was analyzed, no difference between crescentic and mixed classes was observed (47% versus 36%, respectively; P=0.57) (Supplemental Figure 2). Additionally, we performed an analysis comparing patients with proteinase-3 ANCA and patients with myeloperoxidase ANCA, showing no significant difference in kidney failure during 10-year follow-up (86% in proteinase-3 ANCA versus 84% in myeloperoxidase ANCA; P=0.61).
Table 1.
Baseline characteristics of the validation cohort according to histopathologic class
| Baseline Characteristics | Focal, n=52 | Crescentic, n=37 | Mixed, n=39 | Sclerotic, n=17 |
|---|---|---|---|---|
| Age at biopsy, yr, mean ± SD | 60±13 | 62±11 | 61±14 | 66±12 |
| Men (%) | 33 (64) | 24 (65) | 18 (46) | 8 (47) |
| Diagnosis (%)a | ||||
| Granulomatosis with polyangiitis | 33 (63) | 14 (40) | 8 (21) | 8 (50) |
| Microscopic polyangiitis | 17 (33) | 19 (54) | 27 (73) | 8 (50) |
| Eosinophilic granulomatosis with polyangiitis | 1 (2) | 0 (0.0) | 1 (3) | 0 (0) |
| Kidney limited vasculitis | 1 (2) | 2 (6) | 1 (3) | 0 (0) |
| ANCA specificity (%)b | ||||
| PR3 | 23 (48) | 13 (37) | 7 (20) | 7 (44) |
| MPO | 19 (40) | 21 (60) | 25 (69) | 8 (50) |
| Negative | 3 (6) | 0 (0) | 3 (8) | 0 (0) |
| Double positive | 3 (6) | 1 (3) | 1 (3) | 1 (6) |
| Diagnostic delay, mo, mean ± SD | 4.7±12.9 | 1.4±2.7 | 2.4±3.2 | 3.6±11.5 |
| eGFR0, ml/min per 1.73 m2, mean ± SD | 50±29 | 18±16 | 27±19 | 19±12 |
| Proteinuria class at biopsy (%)c | ||||
| Normal | 4 (9) | 1 (3) | 2 (5) | 0 (0) |
| Moderately increased | 18 (39) | 4 (13) | 3 (8) | 3 (19) |
| Severely increased | 24 (52) | 27 (84) | 33 (87) | 13 (81) |
PR3, proteinase-3; MPO, myeloperoxidase.
The diagnosis was specified in 140 patients.
ELISA test results were available in 135 patients.
In accordance with the Kidney Disease Improving Global Outcomes clinical guidelines, normal level of proteinuria was defined as protein excretion of <0.15 g/d or as a negative protein dipstick test, moderately increased proteinuria was defined as a protein excretion rate of 0.15–0.50 g/d or as trace on protein dipstick test, and severely increased proteinuria was defined as total protein excretion >0.50 g/d or as + or more on protein dipstick. The proteinuria class could be determined in 132 patients.
Table 2.
Kidney outcome of the validation cohort according to histopathologic class
| Kidney Outcome Parameters | Focal, n=52 | Crescentic, n=37 | Mixed, n=39 | Sclerotic, n=17 | P Value |
|---|---|---|---|---|---|
| eGFR1, ml/min per 1.73 m2, mean ± SDa | 61±24 | 37±21 | 38±21 | 20±16 | <0.001 |
| ΔeGFR1, ml/min per 1.73 m2, mean ± SDa,b | 11±21 | 21±19 | 10±15 | 0±8 | 0.003 |
| eGFR5, ml/min per 1.73 m2, mean ± SDc | 60±21 | 35±20 | 37±24 | 19±20 | <0.001 |
| ΔeGFR5, ml/min per 1.73 m2, mean ± SDb,c | 8±24 | 19±23 | 10±20 | −1±14 | 0.04 |
| Kidney relapse (%) | 20 (40) | 14 (40) | 11 (29) | 3 (19) | 0.33 |
| Kidney failure (%) | 1 (2) | 9 (24) | 6 (15) | 8 (47) | <0.001 |
| Death (%) | 15 (29) | 15 (41) | 9 (23) | 6 (35) | 0.40 |
(Δ)eGFR1 was available in 132 patients.
ΔeGFR at a time point was defined as the difference between the eGFR at that time point and eGFR at baseline.
(Δ)eGFR5 was available in 113 patients.
Figure 1.
Significantly different kidney survival between the histopathological classes in the validation cohort. (A) At 10-year follow-up, kidney survival was different between the four classes (log rank; P<0.001) but not between crescentic and mixed class (log rank; P=0.98). (B) When biopsies with five to ten glomeruli were excluded, kidney survival remained different between the four classes (log rank; P=0.003).
Table 3.
Subanalyses for outcomes in the crescentic and mixed classes
| Kidney Outcome Parameters | N (%) Events | Time to Event, yr, Mean ± SD | Unadjusted Hazard Ratio (95% Confidence Interval) |
|---|---|---|---|
| Kidney relapse | |||
| Crescentic | 14 (40) | 3.8±2.0 | Reference group |
| Mixed | 11 (29) | 3.4±3.0 | 0.9 (0.4 to 2.0) |
| Kidney failure | |||
| Crescentic | 9 (24) | 6.3±4.6 | Reference group |
| Mixed | 6 (15) | 7.9±5.6 | 0.5 (0.2 to 1.8) |
| Kidney failure within 10 yr | |||
| Crescentic | 5 (14) | 5.6±3.4 | Reference group |
| Mixed | 6 (15) | 6.5±3.2 | 1.0 (0.3 to 3.2) |
| Death | |||
| Crescentic | 15 (41) | 6.6±5.5 | Reference group |
| Mixed | 9 (23) | 4.6±4.5 | 0.6 (0.2 to 1.6) |
Calculated by using Cox regression analysis.
Treatment
The majority of patients received corticosteroids and cyclophosphamide to induce remission (74% of patients) (Supplemental Table 3). Patients treated with corticosteroids and cyclophosphamide had a similar 10-year kidney survival as patients treated with other regimens (excluding patients who only received corticosteroids for induction; 89% versus 94%, respectively; P=0.60). Maintenance therapy consisted most frequently of corticosteroids combined with azathioprine and/or mycophenolate mofetil (62% of patients). The 10-year kidney survival rates were similar between patients who received no or minimal maintenance therapy (only corticosteroids) and those who received corticosteroids combined with another immunosuppressive drug (81% versus 89%, respectively; P=0.37). Patients in the crescentic class received plasma exchange more frequently than patients in the other classes (24% versus 10%, respectively; P=0.04) (Supplemental Table 3). Within the crescentic class, kidney survival at 10 years was similar between patients who received and patients who did not receive plasma exchange (78% versus 89%, respectively; P=0.34).
Interobserver Agreement
Agreement on the histopathologic class among the two pathologists was observed in 99 (68%) cases (Supplemental Figure 3), corresponding to a κ of 0.56, which indicated fair to good agreement. During the re-evaluations of the cases that lacked agreement, we distinguished three possible causes of disagreement: technical (e.g., differences between histologic stains), interpretative (e.g., different interpretations of the definitions), and errors (e.g., miscalculations and incomplete scorings). The ICC between two pathologists was 0.57 for interstitial infiltrate, 0.46 for IFTA, and 0.36 for tubulitis, demonstrating poor to fair agreement.
Meta-Analyses
In the meta-analyses, the risk of kidney failure was similar in the crescentic and mixed classes (RR, 1.15 with mixed as reference group; 95% CI, 0.94 to 1.41) (Figure 2A), and also when adjusted HRs were pooled (RR, 1.08; 95% CI, 0.53 to 2.22) (Figure 2B). Pediatric patients in crescentic and mixed classes had similar risks of kidney failure (RR, 0.67 with mixed as reference group; 95% CI, 0.25 to 1.80) (Figure 2C). Heterogeneity was low in all three meta-analyses.
Figure 2.
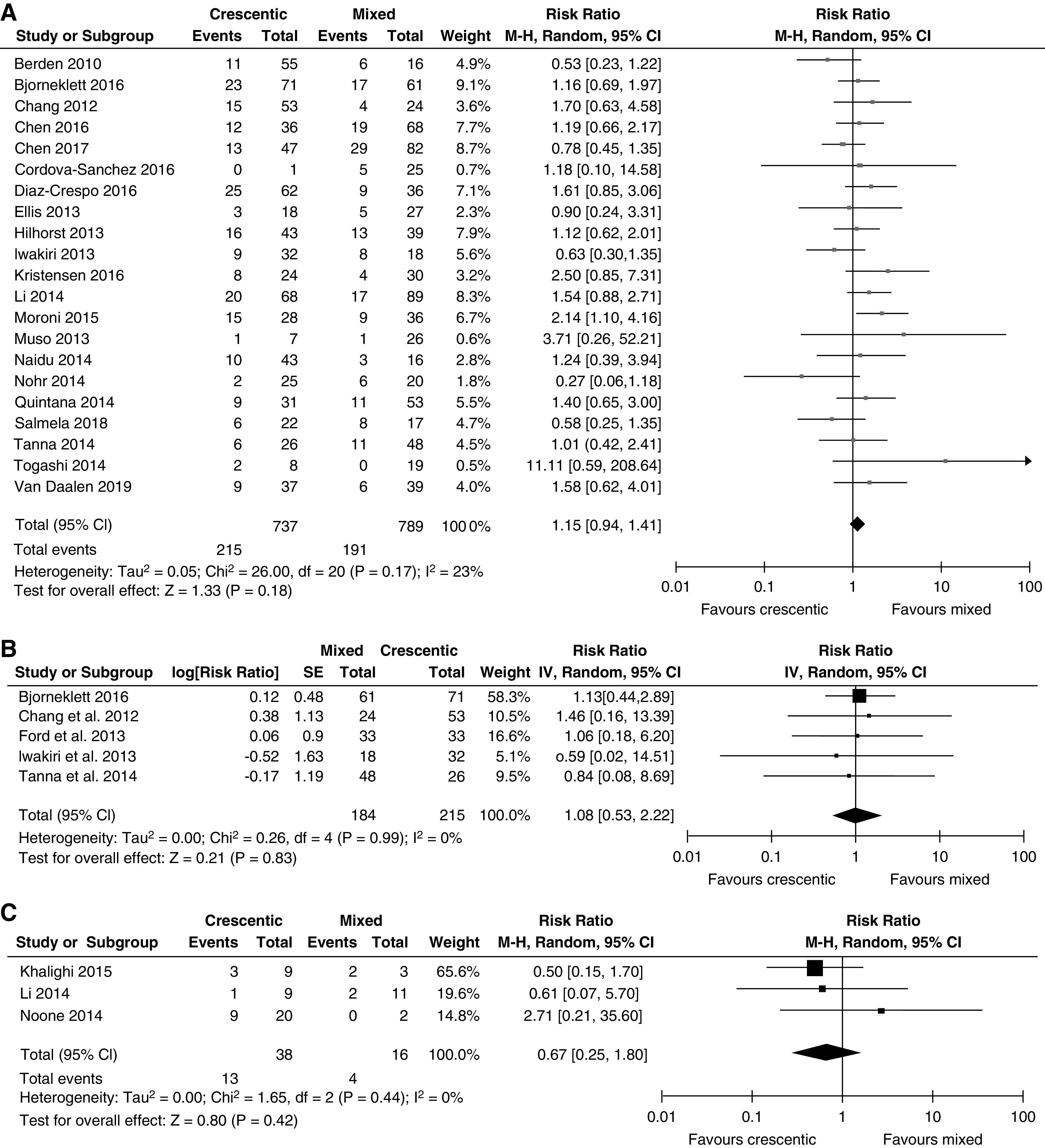
Forest plots of risk ratios showing no significantly differences between crescentic and mixed classes in the meta-analyses. (A) Using kidney failure-event rates in adult patients. (B) Using adjusted hazard ratios in adult patients. (C) Using kidney failure-event rates in pediatric patients. 95% CI, 95% confidence interval; IV, inverse variance; M-H, Mantel-Haenszel; SE, SEM.
ANCA Kidney Risk Score
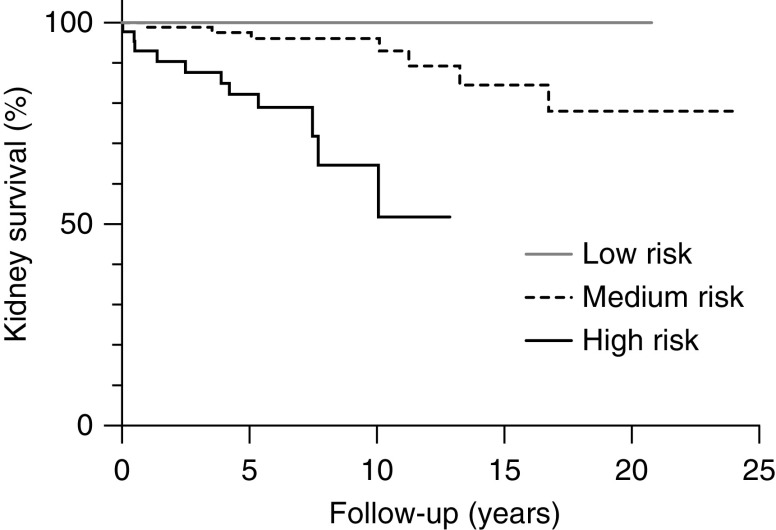
Patients in the low-risk group did not develop kidney failure during follow-up (mean duration in this group was 10.6±5.8 years). Nine patients (10%) in the medium-risk group and 15 patients (31%) in the high-risk group developed kidney failure (mean durations of follow-up were 8.8±5.9 and 6.3±3.5 years, respectively). Kidney survival was significantly different between the three risk groups (Figure 3) (P<0.001). One of the five patients with the maximum risk score of 11 developed kidney failure. In the study by Brix et al. (32), kidney survival was calculated at 3 years of follow-up. The comparison between our cohort and the cohort by Brix et al. (32) at 3 years of follow-up is depicted in Table 4.
Figure 3.
Significantly different kidney survival in the validation cohort according to kidney risk score. Kidney survival was significantly different between the kidney risk groups (log rank; P<0.001).
Table 4.
Kidney survival at 3 years in our cohort and the cohort of Brix et al. (32)
| Risk Group (Points) | This Cohort | The Training Cohort by Brix et al. (32) | ||
|---|---|---|---|---|
| N of Patients | Kidney Survival at 3 yr, % | N of Patients | Kidney Survival at 3 yr, % | |
| Low (0) | 6 | 100 | 30 | 100 |
| Medium (2–7) | 91 | 96 | 64 | 84 |
| High (8–11) | 48 | 77 | 21 | 32 |
Discussion
In this multicenter, international validation study on ANCA-associated GN, a favorable outcome in the focal class and a poor outcome in the sclerotic class were shown, consistent with our own original study (7) and previous validation studies (13). We found no significant difference in kidney survival between the crescentic and mixed classes (HR, 1.0 with crescentic as reference group; 95% CI, 0.3 to 3.2), which might be attributed to the relatively small number of patients in these subanalyses. The change in eGFR over time was significantly different between the crescentic and mixed classes, which is attributed to the lower eGFR at baseline in the crescentic class. We then performed a meta-analysis including 25 studies, which also showed no significant difference in the occurrence of kidney failure between the crescentic and mixed classes. These results are in line with the results of a slightly smaller meta-analysis by Chen et al. (13).
In order to clarify sources of the discrepant results in previous validation studies on the histopathologic classification of ANCA-associated GN, we evaluated differences in patient populations, differences in treatment, and interobserver agreement (31). Differences in patient populations do not seem to be an important factor on the basis of the results of our meta-analysis because we found no trends that suggested differences in outcomes between countries or continents, and moreover, similar results were obtained when only white patients were included (data not shown). As for differences in treatment, a subanalysis revealed that kidney survival was not affected by whether patients received plasma exchange, a finding that is in line with the outcomes of the the trial on plasma exchange and glucocorticoid dosing in the treatment of ANCA-associated vasculitis (PEXIVAS) (41). Firm conclusions on whether the effect of therapy on outcomes should be considered in addition to the histopathologic classification cannot, therefore, be drawn at the moment, but this should be subject to investigation in the near future. We carefully investigated whether interobserver variation could have influenced the results, but this seems unlikely given that the κ in our study was higher than in previous studies on interobserver variation between nephropathologists (19,33). Therefore, we conclude that discrepant results between validation studies have not been caused by differences in patient populations, differences in treatment, and interobserver agreement.
Because prognostic differences between crescentic and mixed classes have not been statistically significant in multiple studies, including our own international validation study, the questions arise of whether and how the classification should be adjusted. What needs to be addressed is whether prognostic value is the sole discriminator on which a classification scheme should be based. For ANCA-associated GN, this was the initial criterion on which the classification was based by us in 2010. Because it has been demonstrated sufficiently that crescentic and mixed classes have similar prognostic outcomes, we would be inclined to change the classification scheme into only three classes: focal, sclerotic, and crescentic/mixed. However, by doing so, the important histopathologic distinction between crescentic and mixed class would be lost. The crescentic class is characterized by a majority of glomeruli with cellular crescents. The mixed class is a more heterogenous class lacking a dominant histopathologic pattern. A clinical difference is that the crescentic class usually presents with lower eGFR values, which improve over time toward eGFR levels similar to those in the mixed class. Therefore, before merging the crescentic and mixed classes in a definitive new classification scheme, we are currently performing an analysis that evaluates histopathologic patterns of kidney biopsies with ANCA-associated GN in more detail, with a focus on the distinction between cellular, fibrocellular, and fibrous crescents. Moreover, this analysis will evaluate the necessity to add tubulointerstitial parameters to the histopathologic classification.
In the meantime, an ANCA kidney risk score was proposed in order to predict kidney survival (32). In our cohort, kidney survival rates at 3 years were 100%, 96%, and 77% in the low-, medium-, and high-risk groups, respectively. These percentages are much higher compared with the kidney survival in the study by Brix et al. (32). Moreover, only one patient from five with the maximum risk score of 11 developed kidney failure in our cohort versus all patients with a maximum score in the German cohort. It is possible that the relatively poor kidney survival in the study by Brix et al. (32) might result from the German practice of early dialysis initiation, which questions the applicability of the kidney risk score to other populations (42).
The strength of our study is its international patient cohort; only one previous validation study included patients from more than two countries (21). Moreover, this validation study was the first to include a large group of pathologists for scoring kidney biopsies. The international character of the study also led to some limitations. For instance, the variety of therapeutic regimens hindered, to some extent, the performance of statistical analyses on the effects of therapy. Another limitation of the study was its retrospective design; this design precluded the collection of complete clinical data. However, only <7% of data were missing on kidney function, diagnosis, serology, and therapy.
In conclusion, we here present results of an international validation study and meta-analyses of the histopathologic classification for ANCA-associated GN, showing no significant differences in kidney outcome between crescentic and mixed classes. We have ruled out differences in patient populations, differences in treatment, and interobserver agreement as sources for this finding. Before definitively merging the crescentic and mixed classes into a new classification scheme for ANCA-associated GN, we await results from a study taking into account histopathology in more detail. In addition, we here present results from a validation study for the recently proposed ANCA kidney risk score, showing major differences with the original study by Brix et al. (32). Future research will determine how the histopathologic classification for ANCA-associated GN and the kidney risk score will be of merit in the daily clinical practice of patients with ANCA-associated vasculitis.
Disclosures
W. Bos reports receiving grants from Zilveren Kruis Insurance, outside the submitted work. A. Kronbichler reports receiving personal fees from Miltenyi Biotech, Novartis, Terumo BCT, and Vifor Pharma, outside the submitted work. All remaining authors have nothing to disclose.
Funding
R. Kain reports receiving European Commission grant European Commission Health programme FP7 during the conduct of the study. E.E. van Daalen received Nierstichting Kolff Student Researcher grant 16OKK53.
Supplementary Material
Acknowledgments
We thank J. Schoones for his help in performing the literary search for the meta-analysis.
Part of this study was presented at the American Society of Nephrology Kidney Week 2016 in Chicago, Illinois (JASN Abstract Suppl. 27: abstract FR-PO681, 2016); at the 18th International Vasculitis and ANCA Workshop 2017 in Tokyo, Japan (Rheumatology [Oxford] 56[Suppl 3]: WS9_1, 2017); at the American Society of Nephrology Kidney Week 2017 in New Orleans, Louisiana (JASN Abstract Suppl. 28: abstract FR-PO720, 2017); and at the 19th International Vasculitis and ANCA Workshop 2019 in Philadelphia, Pennsylvania (Rheumatology [Oxford] 58[Suppl 2]: abstract 118, 2019).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Keeping Up with the Times: Prognostic Tools in ANCA-Associated Glomerulonephritis,” on pages 1078–1080.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.14561119/-/DCSupplemental.
Supplemental Figure 1. Flowchart illustrating how validation studies were selected for the meta-analyses.
Supplemental Figure 2. Combined outcome of kidney failure/death in the crescentic and mixed classes over time.
Supplemental Figure 3. Interobserver agreement on histopathologic class.
Supplemental Material. Scoring questionnaire.
Supplemental Table 1. The kidney risk score proposed by Brix et al. (32).
Supplemental Table 2. Characteristics of the validation cohort.
Supplemental Table 3. Treatment according to histopathologic class.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 65: 1–11, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Hauer HA, Bajema IM, Van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC; European Vasculitis Study Group (EUVAS) : Determinants of outcome in ANCA-associated glomerulonephritis: A prospective clinico-histopathological analysis of 96 patients. Kidney Int 62: 1732–1742, 2002. [DOI] [PubMed] [Google Scholar]
- 3.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, Noël LH, Ferrario F, Waldherr R, Hagen EC, Bruijn JA, Bajema IM: Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 17: 2264–2274, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bajema IM, Hagen EC, Hermans J, Noël LH, Waldherr R, Ferrario F, Van Der Woude FJ, Bruijn JA: Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int 56: 1751–1758, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Aasarød K, Bostad L, Hammerstrøm J, Jørstad S, Iversen BM: Renal histopathology and clinical course in 94 patients with Wegener’s granulomatosis. Nephrol Dial Transplant 16: 953–960, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Haroun MK, Stone JH, Nair R, Racusen L, Hellmann DB, Eustace JA: Correlation of percentage of normal glomeruli with renal outcome in Wegener’s granulomatosis. Am J Nephrol 22: 497–503, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Chang DY, Wu LH, Liu G, Chen M, Kallenberg CG, Zhao MH: Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis: A study of 121 patients in a single center. Nephrol Dial Transplant 27: 2343–2349, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Iwakiri T, Fujimoto S, Kitagawa K, Furuichi K, Yamahana J, Matsuura Y, Yamashita A, Uezono S, Shimao Y, Hisanaga S, Tokura T, Wada T, Kitamura K, Asada Y: Validation of a newly proposed histopathological classification in Japanese patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. BMC Nephrol 14: 125, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muso E, Endo T, Itabashi M, Kakita H, Iwasaki Y, Tateishi Y, Komiya T, Ihara T, Yumura W, Sugiyama T, Joh K, Suzuki K: Evaluation of the newly proposed simplified histological classification in Japanese cohorts of myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in comparison with other Asian and European cohorts. Clin Exp Nephrol 17: 659–662, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Togashi M, Komatsuda A, Nara M, Omokawa A, Okuyama S, Sawada K, Wakui H: Validation of the 2010 histopathological classification of ANCA-associated glomerulonephritis in a Japanese single-center cohort. Mod Rheumatol 24: 300–303, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Naidu GS, Sharma A, Nada R, Kohli HS, Jha V, Gupta KL, Sakhuja V, Rathi M: Histopathological classification of pauci-immune glomerulonephritis and its impact on outcome. Rheumatol Int 34: 1721–1727, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Chen YX, Xu J, Pan XX, Shen PY, Li X, Ren H, Chen XN, Ni LY, Zhang W, Chen N: Histopathological classification and renal outcome in patients with antineutrophil cytoplasmic antibodies-associated renal vasculitis: A study of 186 patients and metaanalysis. J Rheumatol 44: 304–313, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Tanna A, Guarino L, Tam FW, Rodriquez-Cubillo B, Levy JB, Cairns TD, Griffith M, Tarzi RM, Caplin B, Salama AD, Cook T, Pusey CD: Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: Evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant 30: 1185–1192, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Bao H, Liu Z, Liu X, Gao E, Zeng C, Zhang H, Liu Z, Hu W: Risk factors for renal survival in Chinese patients with myeloperoxidase-ANCA-associated GN. Clin J Am Soc Nephrol 12: 417–425, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis CL, Manno RL, Havill JP, Racusen LC, Geetha D: Validation of the new classification of pauci-immune glomerulonephritis in a United States cohort and its correlation with renal outcome. BMC Nephrol 14: 210, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nohr E, Girard L, James M, Benediktsson H: Validation of a histopathologic classification scheme for antineutrophil cytoplasmic antibody-associated glomerulonephritis. Hum Pathol 45: 1423–1429, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Córdova-Sánchez BM, Mejía-Vilet JM, Morales-Buenrostro LE, Loyola-Rodríguez G, Uribe-Uribe NO, Correa-Rotter R: Clinical presentation and outcome prediction of clinical, serological, and histopathological classification schemes in ANCA-associated vasculitis with renal involvement. Clin Rheumatol 35: 1805–1816, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Ford SL, Polkinghorne KR, Longano A, Dowling J, Dayan S, Kerr PG, Holdsworth SR, Kitching AR, Summers SA: Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidney Dis 63: 227–235, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Hilhorst M, Wilde B, van Breda Vriesman P, van Paassen P, Cohen Tervaert JW; Limburg Renal Registry : Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol 24: 1371–1375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quintana LF, Peréz NS, De Sousa E, Rodas LM, Griffiths MH, Solé M, Jayne D: ANCA serotype and histopathological classification for the prediction of renal outcome in ANCA-associated glomerulonephritis. Nephrol Dial Transplant 29: 1764–1769, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Moroni G, Binda V, Leoni A, Raffiotta F, Quaglini S, Banfi G, Messa P: Predictors of renal survival in ANCA-associated vasculitis. Validation of a histopatological classification schema and review of the literature. Clin Exp Rheumatol 33[Suppl 89]: S-56-S-63, 2015. [PubMed] [Google Scholar]
- 23.Diaz-Crespo F, Villacorta J, Acevedo M, Cavero T, Guerrero C, García Díaz E, Orradre JL, Martinez MA, Praga M, Fernandez-Juarez G: The predictive value of kidney biopsy in renal vasculitis: A multicenter cohort study. Hum Pathol 52: 119–127, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen T, Gregersen JW, Krag SR, Ivarsen P: The relation between histopathological classification and renal outcome, ANCA subtype and treatment regimens in ANCA-associated vasculitis. Clin Exp Rheumatol 34[Suppl 97]: S105–S110, 2016. [PubMed] [Google Scholar]
- 25.Bjørneklett R, Sriskandarajah S, Bostad L: Prognostic value of histologic classification of ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol 11: 2159–2167, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreiana I, Stancu S, Avram A, Taran L, Mircescu G: ANCA positive crescentic glomerulonephritis outcome in a central east European cohort: A retrospective study. BMC Nephrol 16: 90, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmela A, Törnroth T, Poussa T, Ekstrand A: Prognostic factors for survival and relapse in ANCA-associated vasculitis with renal involvement: A clinical long-term follow-up study. Int J Nephrol 2018: 6369814, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noone DG, Twilt M, Hayes WN, Thorner PS, Benseler S, Laxer RM, Parekh RS, Hebert D: The new histopathologic classification of ANCA-associated GN and its association with renal outcomes in childhood. Clin J Am Soc Nephrol 9: 1684–1691, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalighi MA, Wang S, Henriksen KJ, Bock M, Keswani M, Chang A, Meehan SM: Pauci-immune glomerulonephritis in children: A clinicopathologic study of 21 patients. Pediatr Nephrol 30: 953–959, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Sacri AS, Chambaraud T, Ranchin B, Florkin B, Sée H, Decramer S, Flodrops H, Ulinski T, Allain-Launay E, Boyer O, Dunand O, Fischbach M, Hachulla E, Pietrement C, Le Pogamp P, Stephan JL, Belot A, Nivet H, Nobili F, Guillevin L, Quartier P, Deschênes G, Salomon R, Essig M, Harambat J: Clinical characteristics and outcomes of childhood-onset ANCA-associated vasculitis: A French nationwide study. Nephrol Dial Transplant 30[Suppl 1]: i104–i112, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Rahmattulla C, Bruijn JA, Bajema IM: Histopathological classification of antineutrophil cytoplasmic antibody-associated glomerulonephritis: An update. Curr Opin Nephrol Hypertens 23: 224–231, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Brix SR, Noriega M, Tennstedt P, Vettorazzi E, Busch M, Nitschke M, Jabs WJ, Özcan F, Wendt R, Hausberg M, Sellin L, Panzer U, Huber TB, Waldherr R, Hopfer H, Stahl RAK, Wiech T: Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int 94: 1177–1188, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Bajema IM, Hagen EC, Hansen BE, Hermans J, Noël LH, Waldherr R, Ferrario F, van der Woude FJ, Bruijn JA: The renal histopathology in systemic vasculitis: An international survey study of inter- and intra-observer agreement. Nephrol Dial Transplant 11: 1989–1995, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS; Chronic Kidney Disease Prognosis Consortium : Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 307: 1941–1951, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teo BW, Xu H, Wang D, Li J, Sinha AK, Shuter B, Sethi S, Lee EJ: GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis 58: 56–63, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 38.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics 33: 159–174, 1977. [PubMed] [Google Scholar]
- 39.Fleiss JL, Cohen J: Equivalence of weighted kappa and intraclass correlation coefficient as measures of reliability. Educ Psychol Meas 33: 613–619, 1973 [Google Scholar]
- 40.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, Hawley CM, Khalidi N, Floßmann O, Wald R, Girard LP, Levin A, Gregorini G, Harper L, Clark WF, Pagnoux C, Specks U, Smyth L, Tesar V, Ito-Ihara T, de Zoysa JR, Szczeklik W, Flores-Suárez LF, Carette S, Guillevin L, Pusey CD, Casian AL, Brezina B, Mazzetti A, McAlear CA, Broadhurst E, Reidlinger D, Mehta S, Ives N, Jayne DRW; PEXIVAS Investigators : Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med 382: 622–631, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson BM, Akizawa T, Jager KJ, Kerr PG, Saran R, Pisoni RL: Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: Differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 388: 294–306, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.