Visual Abstract
Keywords: kidney transplantation, COVID-19, SARS-CoV2, coronavirus, Outpatients, Statistics, Nonparametric, creatinine, severe acute respiratory syndrome coronavirus 2, Cohort Studies, hospitalization, Dyspnea
Abstract
Background and objectives
Outcomes of kidney transplant recipients diagnosed with coronavirus disease 2019 as outpatients have not been described.
Design, setting, participants, & measurements
We obtained clinical data for 41 consecutive outpatient kidney transplant recipients with known or suspected coronavirus disease 2019. Chi-squared and Wilcoxon rank sum tests were used to compare characteristics of patients who required hospitalization versus those who did not.
Results
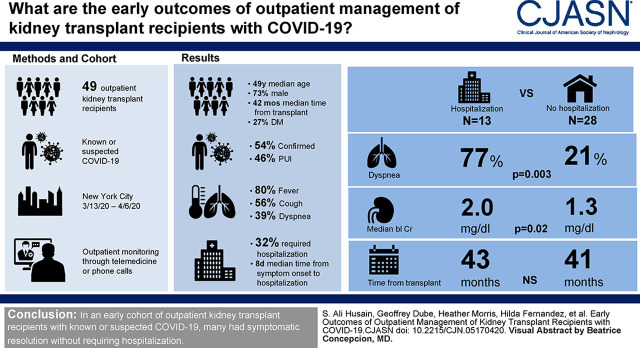
Of 41 patients, 22 (54%) had confirmed coronavirus disease 2019, and 19 (46%) were suspected cases. Patients most commonly reported fever (80%), cough (56%), and dyspnea (39%). At the end of follow-up, 13 patients (32%) required hospitalization a median of 8 days (range, 1–16) after symptom onset, and 23 (56%) had outpatient symptom resolution a median of 12 days (4–23) after onset. Patients who required hospitalization were more likely to have reported dyspnea (77% versus 21%, P=0.003) and had higher baseline creatinine (median, 2.0 versus 1.3 mg/dl, P=0.02), but there were no other differences between groups.
Conclusions
In an early cohort of outpatient kidney transplant recipients with known or suspected coronavirus disease 2019, many had symptomatic resolution without requiring hospitalization.
Introduction
The global pandemic of coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2, continues to spread, with cases now reported in almost every country in the world (1). As the burden of disease grows, there is increased emphasis on understanding disease presentation and management in high-risk subgroups. One such group is kidney transplant recipients, who many have suspected to have an increased risk of severe disease due to their underlying immunosuppression and a high prevalence of other comorbidities associated with the development of severe disease, such as hypertension, diabetes, cardiovascular disease, and CKD (2). On the other hand, it has also been suggested that some of the medications routinely prescribed for transplant immunosuppression may have a protective effect in patients with COVID-19—namely prednisone and mycophenolate (3).
We and others have previously described the presentation and outcomes for kidney transplant recipients who required inpatient hospitalization for COVID-19, noting similar presenting symptoms and early outcomes as have been reported for patients in the general population (4–6). However, the outpatient management of and outcomes for kidney transplant recipients diagnosed with COVID-19 are of particular interest because it is unclear whether immunosuppressed patients can be safely managed as outpatients or how their disease evolves. Here, we describe our early experience with outpatient kidney transplant recipients with established or suspected COVID-19 at Columbia University Irving Medical Center.
Materials and Methods
We identified 41 consecutive kidney-only transplant recipients followed at our center who were first tested and confirmed to have COVID-19 or who were suspected to have COVID-19 (person under investigation [PUI]) in the outpatient setting with symptom onset between March 13 and April 6, 2020. PUIs were defined as patients with presumed COVID-19 on the basis of symptoms but who did not have confirmatory testing performed given the limitations of access to testing during the early phase of the pandemic in New York City. Patients who were initially suspected to have COVID-19 but subsequently tested negative were excluded from this analysis, despite the fact that current testing may carry a false negative rate exceeding 40% (7). Patients were monitored by the clinical team through telemedicine visits and/or phone calls that were conducted every 6, 24, or 48 hours on the basis of the patient’s symptom severity.
Patient demographics, clinical data, symptoms, clinical course, and outcomes were obtained from a review of the medical record and patient interviews. Medical comorbidities and medication history were obtained from the medical record. Three patients did not have recent creatinine values available to assess baseline creatinine and were excluded for the reporting of this variable. The primary outcome was need for hospitalization. Among patients who did not require hospitalization, time to outpatient symptom resolution was assessed. Outcomes were recorded with end of follow-up on April 22, 2020. Chi-squared and Wilcoxon rank sum tests were used to compare characteristics of PUIs versus those with confirmed COVID-19 and to compare patients who eventually required hospital admission versus those who did not require admission. No subgroup or sensitivity analyses were performed. This study was approved by the Columbia University Medical Center Institutional Review Board. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Results
Of the 41 outpatients included in this analysis, 22 (54%) had testing that confirmed the diagnosis of COVID-19, and 19 (46%) were PUIs (Table 1). Overall, patients were predominantly men (73%), with a median age of 49 years (interquartile range [IQR], 41–63) and median time since transplant of 42 months (IQR, 18–66). Almost all (90%) had hypertension, and 27% had diabetes; 22% were taking either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker at the time of diagnosis. About half of the patients had an identifiable sick contact. There were no differences in any of these characteristics between PUIs and patients with confirmed COVID-19. Maintenance immunosuppression was also similar between both groups, with 76% of patients taking a calcineurin inhibitor and 76% taking either mycophenolate mofetil or mycophenolate sodium (Table 1). A minority of patients had immunosuppression regimens that included belatacept (22%), prednisone (39%), or azathioprine (12%).
Table 1.
Characteristics of outpatient kidney transplant recipients with known coronavirus disease 2019 versus suspected coronavirus disease 2019
| Characteristic | n (%) or Median (IQR) | ||
|---|---|---|---|
| All | Suspected COVID-19 (PUI) | Known COVID-19 | |
| n | 41 | 19 (46) | 22 (54) |
| Men | 30 (73) | 13 (68) | 17 (77) |
| Age, yr | 49 (41–63) | 49 (38–62) | 52 (42–64) |
| Time since transplant, mo | 42 (18–66) | 39 (18–61) | 43 (17–98) |
| Deceased donor kidney recipient | 23 (56) | 12 (63) | 11 (50) |
| Hypertension | 37 (90) | 17 (89) | 20 (91) |
| Diabetes mellitus | 11 (27) | 5 (26) | 6 (27) |
| Body mass index, kg/m2 | 26.6 (25.7–32.0) | 27.5 (24.8–32.5) | 26.5 (25.8–27.9) |
| Obese, body mass index ≥30 kg/m2 | 12 (29) | 7 (37) | 5 (22) |
| Angiotensin-converting enzyme inhibitor use | 3 (7) | 2 (11) | 1 (5) |
| Angiotensin receptor blocker use | 6 (15) | 1 (5) | 5 (22) |
| Maintenance immunosuppression | |||
| Calcineurin inhibitor | 31 (76) | 16 (84) | 15 (68) |
| Mycophenolate mofetil/mycophenolate sodium | 31 (76) | 14 (74) | 17 (77) |
| Belatacept | 9 (22) | 3 (16) | 6 (27) |
| Prednisone | 16 (39) | 7 (37) | 9 (41) |
| Azathioprine | 5 (12) | 4 (21) | 1 (5) |
| Known sick contact | 21 (51) | 13 (68) | 8 (36) |
| Symptoms | |||
| Fever | 33 (80) | 11 (58) | 22 (100) |
| Cough | 23 (56) | 14 (74) | 9 (41) |
| Dyspnea (exertional or rest) | 16 (39) | 6 (32) | 10 (45) |
| Gastrointestinal | 11 (27) | 6 (32) | 5 (23) |
| Myalgia/arthralgia | 11 (27) | 7 (37) | 4 (18) |
| Fatigue/malaise | 10 (24) | 8 (36) | 2 (11) |
| Headache | 3 (7) | 1 (5) | 2 (9) |
| Chills | 2 (5) | 0 (0) | 2 (9) |
| Symptoms improved as outpatient | 23 (56) | 15 (79) | 8 (36) |
| Admitted to hospital | 13 (32) | 1 (5) | 12 (55) |
IQR, interquartile range; COVID-19, coronavirus disease 2019; PUI, person under investigation.
The most common presenting symptom was fever, reported by 80% of patients overall, including 100% of confirmed cases (Table 1). Cough was reported by 56% of patients, with 39% reporting dyspnea, 27% reporting gastrointestinal symptoms, 27% reporting myalgia or arthralgia, and 24% reporting fatigue or malaise. Among 13 (32%) patients who eventually required hospitalization, all but one had positive COVID-19 testing, and the median time from symptom onset to hospital admission was 8 days but as long as 16 days in one patient (IQR, 6–9; range, 1–16) (Table 1). Among those whose symptoms resolved as outpatients (n=23, 56%), median time from symptom onset to resolution of symptoms was 12 days, but it was as long as 23 days (IQR, 8–15; range, 4–23). The remaining five patients (12%) have not had resolution of their presenting symptoms but have not required hospital admission to date (median follow-up, 19 days; range, 16–21).
A total of 26 patients (63%) had a reduction in their immunosuppression regimen (including 42% of PUIs and 82% of patients with confirmed cases; P=0.008), whereas the remaining 15 (37%) had no change (Table 2). There were no differences in initial presenting symptoms between patients who did or did not have a reduction in immunosuppression. Patients who eventually required hospital admission were more likely to have reported dyspnea than those who did not require admission (77% versus 21%, P=0.003) and had higher baseline creatinine (median, 2.0 mg/dl; IQR, 1.6–2.8 versus median, 1.3 mg/dl; IQR, 1.1–1.9; P=0.02). Otherwise, there were no differences in immunosuppression management strategy, comorbidities, or demographics between patients who were admitted to the hospital and those who were not. None of the patients who had their immunosuppression reduced have experienced acute rejection to date during this limited follow-up period.
Table 2.
Characteristics of patients admitted to the hospital versus those who remained outpatient
| Characteristic | n (%) or Median (IQR) | |
|---|---|---|
| Outpatient Only | Hospitalized | |
| n | 28 (68) | 13 (32) |
| Men | 19 (68) | 11 (85) |
| Age, yr | 48 (40–63) | 56 (43–64) |
| Time since transplant, mo | 41 (18–61) | 43 (15–100) |
| Deceased donor kidney recipient | 16 (57) | 7 (54) |
| Hypertension | 25 (89) | 12 (92) |
| Diabetes mellitus | 7 (25) | 4 (31) |
| Body mass index, kg/m2 | 26.7 (25.0–31.51) | 26.6 (26.0–34.0) |
| Angiotensin-converting enzyme inhibitor use | 2 (7) | 1 (8) |
| Angiotensin receptor blocker use | 3 (11) | 3 (23) |
| Baseline serum creatinine, mg/dla | 1.3 (1.1–1.9) | 2.0 (1.6–2.8) |
| Maintenance immunosuppression | ||
| Calcineurin inhibitor | 21 (75) | 10 (77) |
| Mycophenolate mofetil/mycophenolate sodium | 23 (82) | 8 (62) |
| Prednisone | 10 (36) | 6 (46) |
| Azathioprine | 4 (14) | 1 (8) |
| Belatacept | 7 (25) | 2 (15) |
| Known sick contact | 17 (61) | 4 (31) |
| Symptoms | ||
| Fever | 21 (75) | 12 (92) |
| Cough | 18 (64) | 5 (38) |
| Dyspnea (exertional or rest) | 6 (21) | 10 (77) |
| Gastrointestinal | 6 (21) | 5 (38) |
| Myalgia/arthralgias | 10 (36) | 1 (8) |
| Fatigue/malaise | 6 (21) | 4 (31) |
| Seen in clinic for evaluation | 4 (14) | 4 (31) |
| Any reduction in immunosuppression | 15 (54) | 11 (84) |
| Days to symptom improvement, n=23 | 12 (IQR, 8–15; range, 4–23) | — |
| Symptom onset to admission, d, n=13 | — | 8 (IQR, 6–9; range, 1–16) |
IQR, interquartile range.
n=38 due to three patients with missing data.
Discussion
As the COVID-19 pandemic continues to progress, we are likely to see an increasing number of kidney transplant recipients who will be exposed to and subsequently develop a COVID-19 infection. A better understanding of the disease course is needed to identify which patients can be managed safely at home and which are at a higher risk of severe disease or need closer monitoring. Herein, we describe our experience with 41 outpatient kidney transplant recipients known or suspected to have COVID-19.
By the end of follow-up, about one third of our outpatients required hospital admission, which occurred a median of 8 days after the onset of symptoms but as late as 16 days. This wide interval underscores the need for increased vigilance approximately 1 week following the onset of symptoms and the need for continued close outpatient follow-up for the early detection of clinical deterioration during the second week. This pattern is consistent with what has been described as the biphasic nature of the clinical course, the sometimes slow and protracted recovery in some individuals, and the median duration between symptom onset and hospitalization in the general population in the United States (median, 7 days; IQR, 3–9 on the basis of data from the Centers for Disease Control and Prevention) (8,9). Further, even among patients whose symptoms resolved without requiring admission, median time to resolution of symptoms was 12 days, with a maximum of 23 days. Clinicians caring for these patients must therefore be aware that a prolonged duration of symptoms does not necessarily portend a poor outcome. Additional data are needed to determine when in the clinical course immunosuppression should be returned to baseline and whether that decision can be safely made using clinical symptoms alone.
Notably, patients who reported dyspnea were more likely to eventually require hospitalization than those who did not report dyspnea, perhaps because of the eventual need for supplemental oxygen that would require hospitalization. As a result, patients with dyspnea at the outset should be monitored closely for the likely eventual need for hospitalization. However, the ideal strategy for monitoring has not been established. As hospitals reach overflow capacity, dyspnea and hypoxia have become the main decision point for admission decisions, so there is a need to establish pathways for close outpatient follow-up. Access to ambulatory clinics during the pandemic is often adversely affected by reductions in outpatient clinic hours and the transition to telemedicine to enhance social distancing policies and the redeployment of staff to assist with the surge of inpatients with COVID-19. This is likely further compounded by the inability of symptomatic patients to come to an in-person clinic visit and the difficulties in arranging such visits. In light of these circumstances, we implemented a telemedicine approach that included self-monitored temperature and (when available) pulse oximetry combined with frequent phone check-ins. However, additional experience is needed to determine whether this is sufficient and whether it will remain sustainable as the volume of patients with COVID-19 increases. Given the limits of telemedicine and need for laboratory follow-up in some patients, we have also partnered with our institution’s general internists who have developed a “Fever Clinic” that provides scheduled appointments for patients with mild/moderate symptoms to be assessed in person and have laboratory studies performed. The Fever Clinic avoids use of the emergency department, and earlier intervention may improve outcomes. Outpatients being monitored with telehealth who develop worsening dyspnea and/or hypoxia are referred to either the Fever Clinic or the emergency department for further evaluation, depending on the severity of their symptoms.
The majority of our patients were men, 29% were obese, and almost all had hypertension. However, despite all of these characteristics’ association with more severe disease in general population cohorts, there were no differences between patients who did and did not require hospital admission in our study, a finding that needs additional study in larger cohorts (9,10). Further, we observed that maintenance immunosuppression regimens were similar among patients who were hospitalized and those who did not require hospitalization. Timing and the degree of immunosuppression reduction were not uniform because patients first reported symptoms at varying time points during their illness and degree of immunosuppression was at each clinician’s discretion on the basis of illness severity and risk of immunosuppression reduction. A substantial proportion of patients did not undergo a reduction in immunosuppression, including half of patients who were not admitted to the hospital. The effect of this decision on symptom course and time to improvement is difficult to ascertain given selection bias and a lack of follow-up viral testing, and it underscores the need for further studies to determine the optimal immunosuppression management strategy in kidney transplant recipients with COVID-19. For now, it seems that more information is needed in order to identify which ambulatory patients are at a higher risk of progressing to severe disease. Management of patients who are COVID-19 positive on belatacept presents an additional challenge. We have administered maintenance belatacept to a patient who was COVID-19 positive in an examination room outside of our transplant center to avoid potential exposure to patients and staff, with the infusion given at the end of the day to allow time for the room to be cleaned before being used by other patients. Finally, half of the patients in our cohort had a known sick contact before developing symptoms. This finding underscores the need for kidney transplant recipients to practice social distancing at home when members of their household are ill, especially as they or their household contacts return to work.
Patients with confirmed COVID-19 cases were more likely than PUIs to require hospital admission. It is possible that this finding is a product of the limited testing initially available in New York City, resulting in patients with more severe symptoms being more likely to undergo testing. However, it is also possible that hospitalization was less frequent among PUIs because some did not truly have COVID-19. A better understanding of the natural history of this illness in kidney transplant recipients and the optimal outpatient management will require broader testing to accurately identify those with COVID-19. Subsequent antibody testing may also help us retrospectively confirm COVID-19 in PUIs who did not undergo testing while symptomatic.
Limitations of this study include the 12% of patients with ongoing illness. It is possible that as these cases progress, overall hospitalization rates may rise. Additionally, given the limited availability of COVID-19 testing in New York City, we are unable to determine what proportion of PUIs truly had COVID-19. Given that hospital admissions predominantly occurred among patients who were COVID-19 positive, it is possible either that these represented less severe disease manifestations that did not trigger a testing pathway or that many of these patients were experiencing an alternative illness. Further, because we were only able to identify and include patients who contacted us to report symptoms and seek treatment advice, it is possible that additional mild cases of COVID-19 among our transplant recipients were not included in our study. Additionally, because we lack data on PUIs who tested negative, we are unable to compare these patients with those in our cohort in order to identify characteristics associated with decreased likelihood of positive testing or the clinical course of PUIs who test negative. Such data are needed because it is possible that some PUIs did not have COVID-19 and that others had false negative test results. Overall hospitalization rates for the general population cannot be reliably determined for similar reasons (9). Additionally, given that the vast majority of patients were managed remotely while remaining at home, we lack imaging or laboratory data, such as inflammatory biomarkers, that may have helped identify patients at risk for clinical deterioration (6).
In conclusion, among 41 outpatient kidney transplant recipients at Columbia University Medical Center with known or suspected COVID-19, about one third required hospitalization by the end of follow-up, and there were no differences in demographics or medical comorbidities between those who were or were not admitted to the hospital. These patients therefore require close monitoring for clinical deterioration until symptom resolution. Further research is needed to determine the optimal management of COVID-19, particularly the risks and benefits of reduced immunosuppression, in this vulnerable population.
Disclosures
Dr. Mohan is a Scientific Advisory Board Member for Angion Pharmaceuticals and a Deputy Editor of Kidney International Reports outside the submitted work. All remaining authors have nothing to disclose.
Funding
Dr. Husain is supported by National Center for Advancing Translational Sciences grant KL2 TR001874. Dr. Mohan is supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK114893, R01-MD014161, and U01-DK116066.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “COVID-19 in Patients with Kidney Disease,” on pages 1087–1089.
References
- 1.WHO: Coronavirus Disease 2019 (COVID-19) Situation Report–85, 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200414-sitrep-85-covid-19.pdf?sfvrsn=7b8629bb_2. Accessed April 14, 2020
- 2.Ahmadpoor P, Rostaing L: Why the immune system fails to mount an adaptive immune response to a COVID-19 infection [published online ahead of print April 1, 2020]. Transpl Int doi:10.1111/tri.13611 [DOI] [PubMed] [Google Scholar]
- 3.Cheng KW, Cheng SC, Chen WY, Lin MH, Chuang SJ, Cheng IH, Sun CY, Chou CY: Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res 115: 9–16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Zambetti N, Moscato M, Venturini M, Affatato S, Gaggiotti M, Bossini N, Scolari F: A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia [published online ahead of print April 9, 2020]. Kidney Int doi:10.1016/j.kint.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M: COVID-19 infection in kidney transplant recipients [published online ahead of print April 9, 2020]. Kidney Int doi:10.1016/j.kint.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Columbia University Kidney Transplant Program: Early description of coronavirus 2019 disease in kidney transplant recipients in New York [published online ahead of print April 28, 2020]. J Am Soc Nephrol doi:10.1681/ASN.2020030375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L: Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases [published online ahead of print February 26, 2020]. Radiology doi:10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati F, Le Hingrat Q, Enouf V, Houhou-Fidouh N, Valette M, Mailles A, Lucet JC, Mentre F, Duval X, Descamps D, Malvy D, Timsit JF, Lina B, van-der-Werf S, Yazdanpanah Y: Clinical and virological data of the first cases of COVID-19 in Europe: A case series [published online ahead of print March 27, 2020]. Lancet Infect Dis doi:10.1016/S1473-3099(20)30200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC: Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, 2020. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm. Accessed April 14, 2020 [DOI] [PMC free article] [PubMed]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054–1062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]