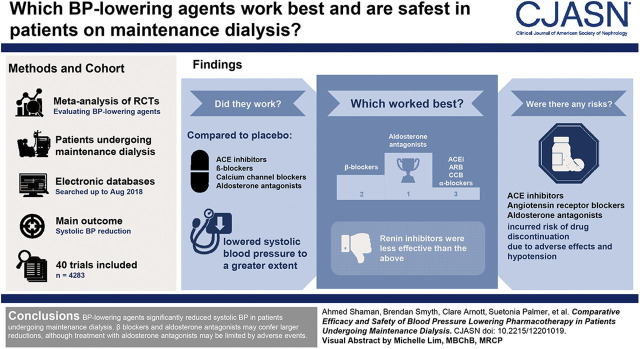
Visual Abstract
Keywords: hypertension, systolic blood pressure, chronic kidney disease, renal dialysis, Antihypertensive agents, Adverse events, Angiotensin-Converting Enzyme Inhibitors, Angiotensin Receptor Antagonists, Renin, Calcium Channel Blockers, blood pressure, Mineralocorticoid Receptor Antagonists, Peptidyl-Dipeptidase A, Cardiovascular Diseases, hypotension, risk factors
Abstract
Background and objectives
Elevated BP is an important risk factor for cardiovascular disease, with a prevalence of over 80% in patients undergoing maintenance dialysis. We assessed the comparative BP-lowering efficacy and the safety of BP-lowering drugs in patients undergoing maintenance dialysis.
Design, settings, participants, & measurements
We performed a frequentist random effects network meta-analysis of randomized, controlled trials evaluating BP-lowering agents in adult patients undergoing maintenance dialysis. Electronic databases (CENTRAL, MEDLINE, and Embase) were systematically searched (up to August 2018) for relevant trials. The main outcome was systolic BP reduction.
Results
Forty trials (4283 participants) met our inclusion criteria. Angiotensin-converting enzyme inhibitors, β-blockers, calcium-channel blockers, and aldosterone antagonists lowered systolic BP to a greater extent than placebo, with effect sizes ranging from −10.8 mm Hg (95% confidence interval, −14.8 to −6.7 mm Hg) for the aldosterone antagonists to −4.3 mm Hg (95% confidence interval, −7.2 to −1.5 mm Hg) for angiotensin-converting enzyme inhibitors. Aldosterone antagonists and β-blockers were superior to angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium-channel blockers, and renin inhibitors at lowering systolic BP. Compared with angiotensin-converting enzyme inhibitors, aldosterone antagonists and β-blockers lowered systolic BP by 6.4 mm Hg (95% confidence interval, −11.4 to −1.4 mm Hg) and 4.4 mm Hg (95% confidence interval, −7.4 to −1.3 mm Hg), respectively. Systolic BP reduction was not different with angiotensin receptor blockers, α-blockers, and calcium-channel blockers compared with angiotensin-converting enzyme inhibitors. Renin inhibitors were less effective. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists incurred risks of drug discontinuation due to adverse events and hypotension.
Conclusions
BP-lowering agents significantly reduced systolic BP in patients undergoing maintenance dialysis. β-Blockers and aldosterone antagonists may confer larger reductions, although treatment with aldosterone antagonists may be limited by adverse events.
Introduction
Cardiovascular diseases account for approximately half of all deaths in people with kidney failure receiving maintenance dialysis (1). Elevated BP is an important risk factor for cardiovascular disease, and its prevalence rises to 86% among patients undergoing maintenance dialysis (2). Although two earlier meta-analyses of randomized, controlled trials showed cardiovascular and survival benefit with BP-lowering pharmacotherapy (3,4), their comparative efficacy and safety are not well known because these patients have typically been excluded from large trials. Extrapolation of findings observed within patients not requiring maintenance dialysis is challenging as the benefits and tolerability of BP-lowering medications may be different, as has been observed for other cardiovascular medications (5).
A lack of head-to-head trials may limit the capacity of conventional pairwise meta-analyses to evaluate the comparative efficacy and safety of different classes of BP-lowering agents. Network meta-analysis uses direct and indirect evidence to simultaneously compare available treatments (6). In this review, we examine the comparative efficacy and safety of BP-lowering medications to reduce BP in patients with kidney failure requiring dialysis using network meta-analysis.
Materials and Methods
We conducted a systematic review according to the PRISMA guidelines (7). The study was preregistered with PROSPERO (CRD42016035487).
Data Sources and Searches
We searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from database inception up to August 2018 using medical subject headings and text words relevant to BP-lowering agents, kidney failure, and clinical trials designs (Supplemental Table 1) without date or language restriction. We hand searched reference lists from retrieved records and clinical trials registries to identify any additional studies.
The titles and abstracts of retrieved records were screened independently by two investigators (C.A. and A.M.S.) according to the review eligibility criteria on the basis of a standardized approach. Any disagreement on the selection of studies was resolved in discussion with additional reviewers (M.J. and V.P.).
We included randomized, controlled trials that recruited adults (≥18 years old) requiring dialysis and assessed the efficacy of any class of BP-lowering therapy: angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), α-blockers, β-blockers, calcium-channel blockers, diuretics, centrally acting vasodilators, aldosterone antagonists, or direct renin inhibitors alone or in combination compared with control (placebo or standard of care) or another class of BP-lowering agents. Trials aiming to achieve specific BP targets using a range of therapies were excluded.
Data Extraction
Two authors (C.A. and A.M.S.) extracted and verified data after entry into a spreadsheet. Collected data included baseline characteristics (age, sex, comorbidities [diabetes, hypertension, baseline BP, and history of cardiovascular disease], type of dialysis, and drug use); trial data (trial design, sample size, follow-up duration, country, and year of publication); and outcomes data.
Quality Assessment
We assessed risk of bias in included trials using the Cochrane tool (8). Two authors (A.M.S. and B.S.) classified risk of bias for each study domain as low, unclear, or high. Any disagreement in risk of bias assessment was adjudicated by additional reviewers (M.J. and V.P.). Grading of Recommendations, Assessment, Development and Evaluation (GRADE) domains for network meta-analysis proposed by Salanti et al. (9) were used to assess confidence in each treatment comparison estimates.
Outcomes
We collected data on BP and the method of measurement, heart rate, and adverse events. The main outcome was mean difference in systolic BP measurements between assigned treatment groups. Other outcomes included diastolic BP, heart rate, discontinuation due to adverse events, hypotension, hyperkalemia, and serum potassium concentration.
Data Synthesis and Analyses
We used random effects pairwise (10) and network meta-analyses within a frequentist environment to obtain summary estimates of study outcomes. Network meta-analysis combines evidence about treatments from direct head-to-head trials and indirectly from studies that used a common comparator for both treatments. For example, on the basis of direct comparisons of interventions A versus B and B versus C, one can investigate the effects of intervention A versus C using indirect comparisons. The direct and indirect comparisons are then pooled to form a network effect (9). We assumed that eligible participants can be randomized to any of the network interventions. We checked the transitivity assumption by investigating the distribution of potential effect modifiers (age, baseline systolic BP, follow-up duration, sample size, population, and study methodological quality) (11). We assessed agreement between direct and indirect estimates in every closed loop of evidence using loop-specific and node-splitting approaches and for the entire network using design-by-treatment interaction model (global inconsistency test) (12,13).
For continuous outcomes, the mean differences and corresponding 95% confidence intervals (95% CIs) were calculated using end of trial mean values, their corresponding SD, and treatment arm size. For crossover trials, we calculated the SEMs from paired t statistics using a method described elsewhere (14). For dichotomous outcomes, relative risks (RRs) and corresponding 95% CIs were calculated using total number of patients randomized in each group as the denominator. Evidence of statistical heterogeneity in estimates between studies beyond the level of chance was estimated using the I2 statistic in the pairwise meta-analysis (15). We investigated possible sources of heterogeneity by comparing summary results obtained from subsets of studies, which compared the efficacy of BP-lowering drugs compared with control, grouped by class of BP-lowering drugs, age, proportion of men, baseline systolic BP, dialysis modality, type of BP measurements, median study size, and follow-up duration. We assumed the same heterogeneity variance (τ2) for all comparisons in the network meta-analysis (16) and compared its value with outcome- and treatment-specific empirical distribution of variances to assess the magnitude of heterogeneity in the entire network (17,18).
Publication bias was assessed using comparison-adjusted funnel plots for all active drugs against control (19). Statistical analyses were performed with Stata, version 15.1 (Stata, College Station, TX) using published methods (20–22).
Results
Search Results and Characteristics of Included Studies
The electronic literature search retrieved 6406 records, of which 40 randomized trials met our inclusion criteria (Supplemental Figure 1). Supplemental Table 2 summarizes the characteristics of included studies, which were reported between 1986 and 2016. Among the 40 included trials, 32 included patients receiving hemodialysis, four enrolled patients receiving peritoneal dialysis, and four included both. Twenty-three trials were placebo controlled, six trials compared an intervention with standard of care, and 11 trials were head-to-head trials, of which two were three-arm trials. Thirty-seven trials had a parallel design, two were crossover trials, and one was a two-by-two factorial study.
The trials included a total of 4283 participants (sample size range, 8–469 participants), with a median proportion of men of 61%. Study follow-up duration ranged from 2 weeks to 3.5 years (median 6 months). The median age of trial participants was 57 years (range, 41–71 years). The mean baseline systolic BP was 148 mm Hg (range, 123–189 mm Hg). In 26 trials, mean baseline systolic BP was ≥140 mm Hg.
Included trials reported BP measurements in several ways: 19 (59%) studies reported predialysis BP measurement, five (16%) reported clinic BP, four (13%) reported home BP, three (9%) reported ambulatory BP measurement, and one (3%) trial measured postdialysis BP.
Risks of Bias and Confidence Rating
Overall, 14 trials (35%) were considered at low risk of bias; 10 (25%) were at unclear risk of bias, and 16 trials (40%) were at high risk of bias (Supplemental Figure 2, Supplemental Table 3). Of the included studies, 22 were double blinded, 15 were open label, and blinding was not specified in three trials.
For the systolic BP outcome, trials with some concerns regarding quality contributed 55% of direct evidence to the network of BP-lowering drugs. Trials with no concern regarding quality contributed 14% of direct evidence, whereas trials with major concerns contributed 31% of the direct evidence in the network (Supplemental Figure 3).
The GRADE confidence rating for effects of treatments on systolic BP is presented in Table 1. Confidence in network estimates varied; one comparison (β-blockers versus placebo) provided high confidence, six provided moderate confidence, 12 provided low confidence, and nine provided very low confidence.
Table 1.
Summary of confidence in effect estimates of BP-lowering agents in lowering systolic BP
| Comparison | Type of Evidence | Confidence Rating | Reasons for Downgrading |
|---|---|---|---|
| ACE inhibitors versus placebo | Mixed | Moderate | Study limitationsa |
| ARBs versus placebo | Mixed | Very low | Study limitationsa; imprecisionb; inconsistencyc |
| β-Blockers versus placebo | Mixed | High | — |
| Calcium-channel blockers versus placebo | Mixed | Moderate | Study limitationsa |
| Aldosterone antagonists versus placebo | Mixed | Low | Study limitationsa; heterogeneityd |
| α-Blockers versus placebo | Indirect | Very low | Study limitationsa; imprecisionb; inconsistencye |
| Renin inhibitors versus placebo | Indirect | Very low | Study limitationsa; imprecisionb; inconsistencye |
| ACE inhibitors versus ARBs | Mixed | Low | Study limitationsa; imprecisionb |
| ACE inhibitors versus β-blockers | Mixed | Moderate | Study limitationsa |
| ACE inhibitors versus calcium-channel blockers | Mixed | Moderate | Study limitationsa |
| ACE inhibitors versus α-blockers | Indirect | Very low | Study limitationsa; imprecisionb; inconsistencye |
| ACE inhibitors versus aldosterone antagonists | Indirect | Low | Study limitationsa; inconsistencye |
| ACE inhibitors versus renin inhibitors | Indirect | Low | Study limitationsa; inconsistencye |
| ARBs versus calcium-channel blockers | Mixed | Low | Study limitationsa; imprecisionb |
| ARBs versus renin inhibitors | Mixed | Low | Study limitationsa; inconsistencyc |
| ARBs versus α-blockers | Indirect | Very low | Study limitationsa; imprecisionb; inconsistencye |
| ARBs versus β-blockers | Indirect | Very low | Study limitationsa; imprecisionb; inconsistencye |
| ARBs versus aldosterone antagonists | Indirect | Low | Study limitationsa; inconsistencye |
| α-Blockers versus β-blockers | Mixed | Low | Study limitationsa; imprecisionb |
| α-Blockers versus calcium-channel blockers | Indirect | Very low | Study limitationsa; imprecisionb; inconsistencye |
| α-Blockers versus aldosterone antagonists | Indirect | Very low | Study limitationsa; imprecisionb; inconsistencye |
| α-Blockers versus renin inhibitors | Indirect | Low | Study limitationsa; inconsistencye |
| β-Blockers versus calcium-channel blockers | Mixed | Moderate | Study limitationsa |
| β-Blockers versus aldosterone antagonists | Indirect | Very low | Study limitationsa; imprecisionb; inconsistencye |
| β-Blockers versus renin inhibitors | Indirect | Low | Study limitationsa; inconsistencye |
| Calcium-channel blockers versus renin inhibitors | Mixed | Moderate | Study limitationsa |
| Calcium-channel blockers versus aldosterone antagonists | Indirect | Low | Study limitationsa; inconsistencye |
| Aldosterone antagonists versus renin inhibitors | Indirect | Low | Study limitationsa; inconsistencye |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
The majority of evidence was at high or unclear risk of bias.
Confidence intervals for network estimates include values that would favor either treatment.
Confidence intervals for direct and indirect evidence provided different interpretation.
Substantial heterogeneity present between the included trials.
Only indirect evidence existed, and inconsistency could not be assessed.
There was no evidence of disagreement between direct and indirect evidence for all outcomes, except for heart rate outcome (ACE inhibitors versus placebo; P=0.05). The “design-by-treatment” interaction models found no evidence for global inconsistency for all outcomes except for serum potassium concentration outcome (P<0.001). Low heterogeneity existed in the network for all outcomes except for diastolic BP, heart rate (high), and hypotension (low to moderate) (Supplemental Tables 4 and 5).
Effects of BP-Lowering Pharmacotherapy
Systolic BP.
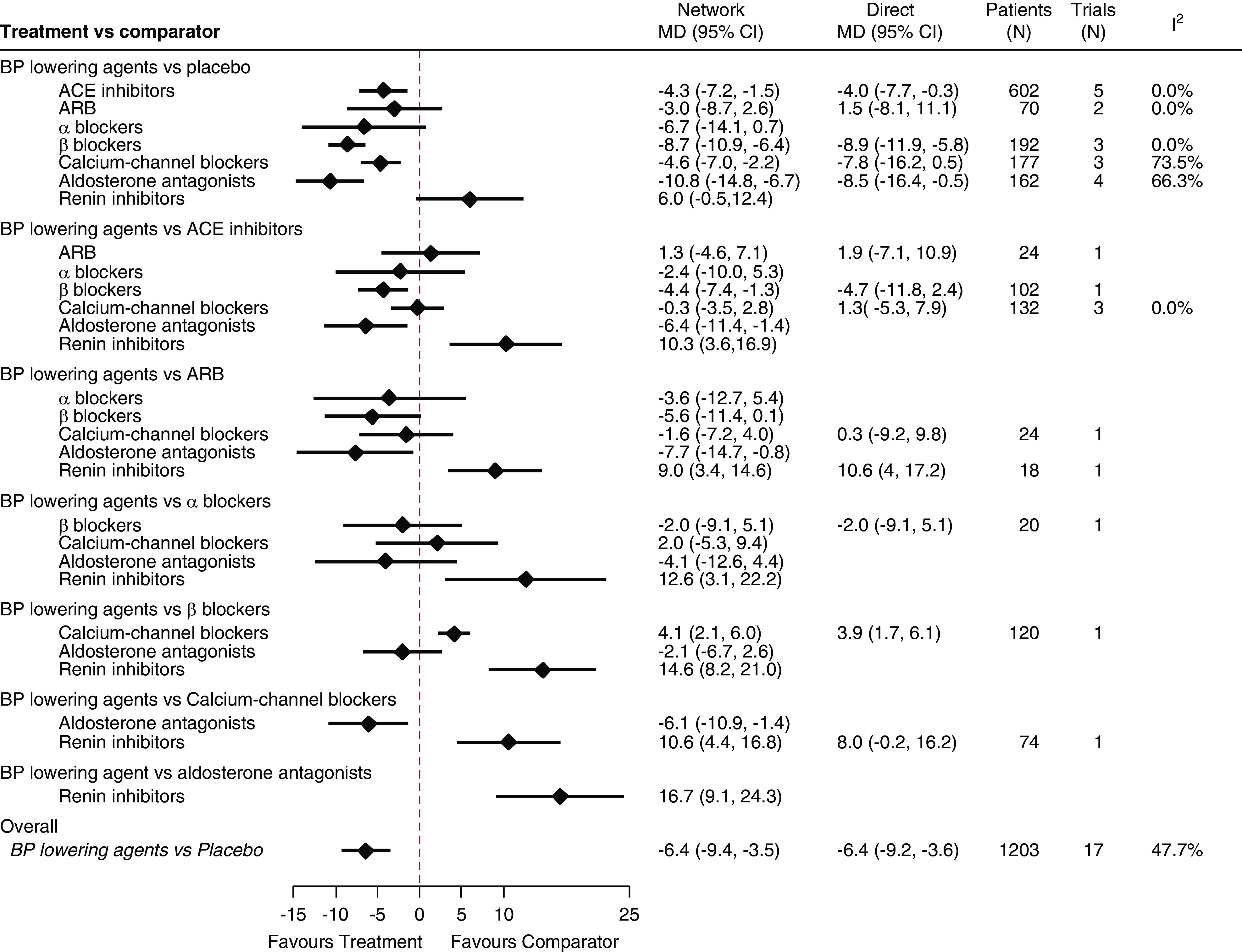
Twenty-five randomized trials involving 1681 participants formed the network for the systolic BP outcome (Figure 1). Four treatments (ACE inhibitors, β-blockers, calcium-channel blockers, and aldosterone antagonists) lowered systolic BP to a greater extent than placebo, with effect sizes ranging from −10.8 mm Hg (95% CI, −14.8 to −6.7 mm Hg) for aldosterone antagonists to −4.3 mm Hg (95% CI, −7.2 to −1.5 mm Hg) for ACE inhibitors (Figure 2). No separate effect was clearly identified for ARBs (−3.0 mm Hg; 95% CI, −8.7 to 2.6 mm Hg), α-blockers (−6.7 mm Hg; 95% CI, −14.1 to 0.7 mm Hg), or renin inhibitors (6.0 mm Hg; 95% CI, −0.5 to 12.4 mm Hg).
Figure 1.
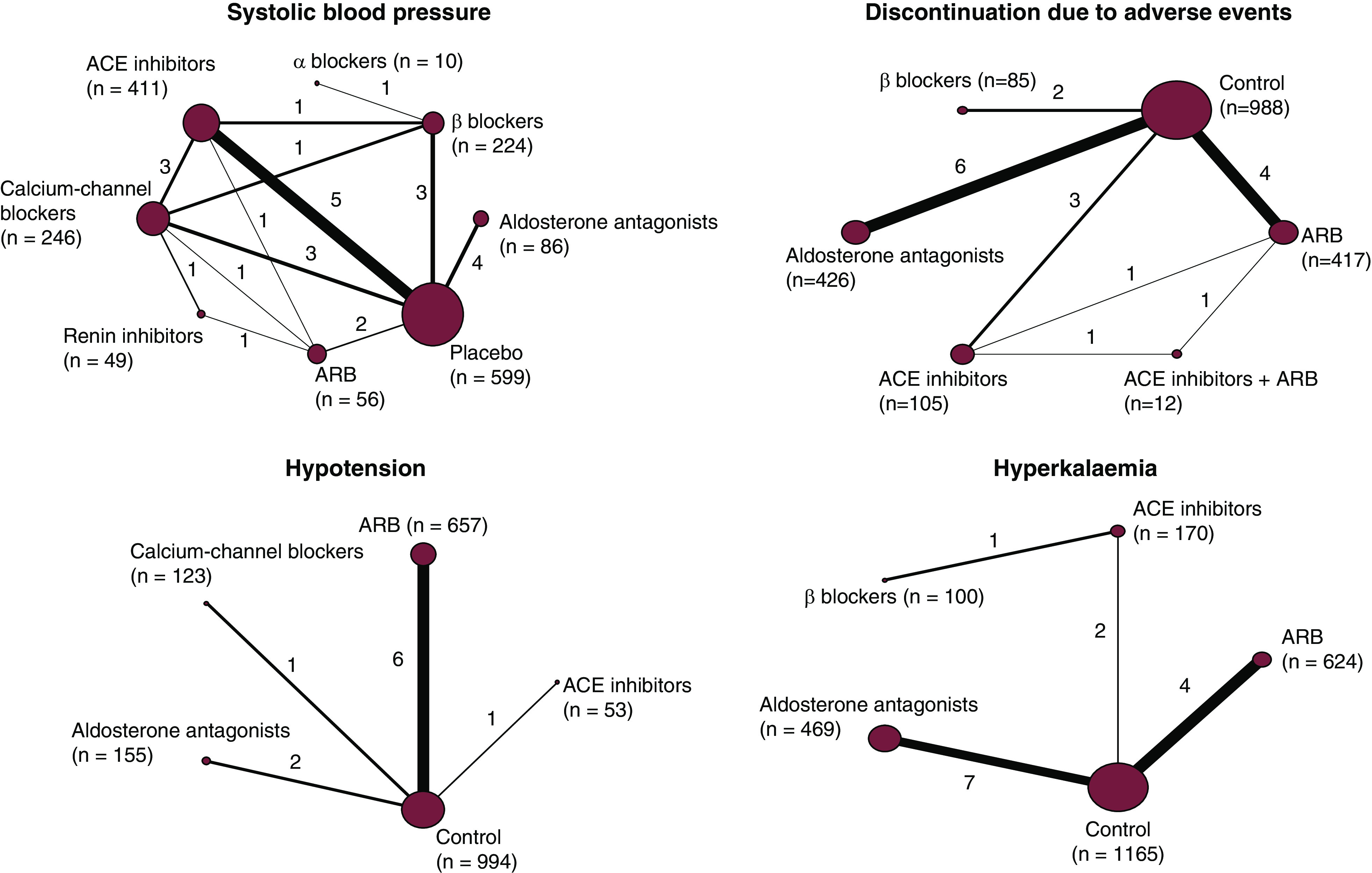
Diagram showing the networks of treatment comparison of BP-lowering agents for systolic BP and safety outcomes in the identified trials. The size of the node corresponds to the number of trials. The thickness of the line connecting two treatments corresponds to the number of patients. Numbers next to each line represent the number of trials that compared the connected treatments. Numbers in brackets correspond to the number of patients. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Figure 2.
BP-lowering agents lowered systolic BP (millimeters of mercury) to a greater extent than placebo, and aldosterone antagonists and β-blockers may confer larger reductions. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; 95% CI, 95% confidence interval; MD, mean difference.
Aldosterone antagonists and β-blockers lowered systolic BP to a greater extent than ACE inhibitors, ARBs, calcium-channel blockers, and renin inhibitors (Figure 2). For example, compared with ACE inhibitors, aldosterone antagonists and β-blockers lowered systolic BP by 6.4 mm Hg (95% CI, −11.4 to −1.4 mm Hg) and 4.4 mm Hg (95% CI, −7.4 to −1.3 mm Hg), respectively. Systolic BP reduction was not detectably different with ARBs, α-blockers, and calcium-channel blockers compared with ACE inhibitors. Renin inhibitors were less effective at lowering BP compared with all other classes.
The comparison-adjusted funnel plot of placebo-controlled trials of BP-lowering drugs’ effect on systolic BP in patients receiving dialysis suggested no evidence of a small study effect in the network (Supplemental Figure 4), although few trials were available for each drug class.
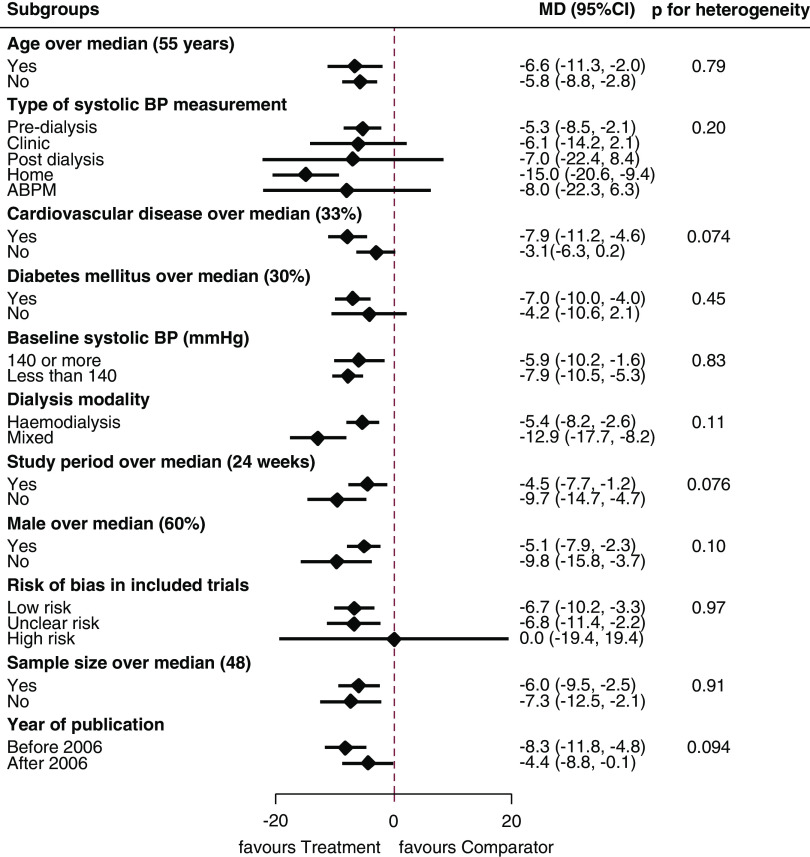
We did not observe significant differences in pooled estimates for systolic BP across any of the subgroups assessed (P value for heterogeneity for all subgroups >0.05) (Figure 3).
Figure 3.
Subgroup analyses for the effects of BP-lowering agents on systolic BP outcome compared with placebo showed no significant differences in pooled estimates across the assessed groups. ABPM, ambulatory BP monitoring; 95% CI, 95% confidence interval; MD, mean difference (millimeters of mercury).
Diastolic BP and Heart Rate.
A total of 22 trials (1553 patients) contributed to diastolic BP outcome (Supplemental Figure 11). β-Blockers showed an effect on diastolic BP compared with placebo (−4.4 mm Hg; −7.1 to −1.7 mm Hg). There was no detectable difference between other classes against placebo or each other (Supplemental Figure 5).
The network for the heart rate outcome is shown in Supplemental Figure 11. β-Blockers showed a reduction of heart rate by 21 beats per minute (95% CI, −26 to −16 beats per minute), a more marked effect than any other class (Supplemental Figure 6).
Discontinuation Due to Adverse Events.
Sixteen trials (1996 patients) contributed to the network for the risk of discontinuation due to adverse events (Figure 1). This risk was significantly increased with aldosterone antagonists (RR, 3.35; 95% CI, 1.32 to 8.49), ACE inhibitors (RR, 1.77; 95% CI, 1.09 to 2.87), and ARBs (RR, 1.57; 95% CI, 0.96 to 2.57) compared with control (Figure 4, Supplemental Figure 7), whereas there was no detectably increased risk with β-blockers (RR, 0.81; 95% CI, 0.27 to 2.39). Limited data were available for other classes.
Figure 4.
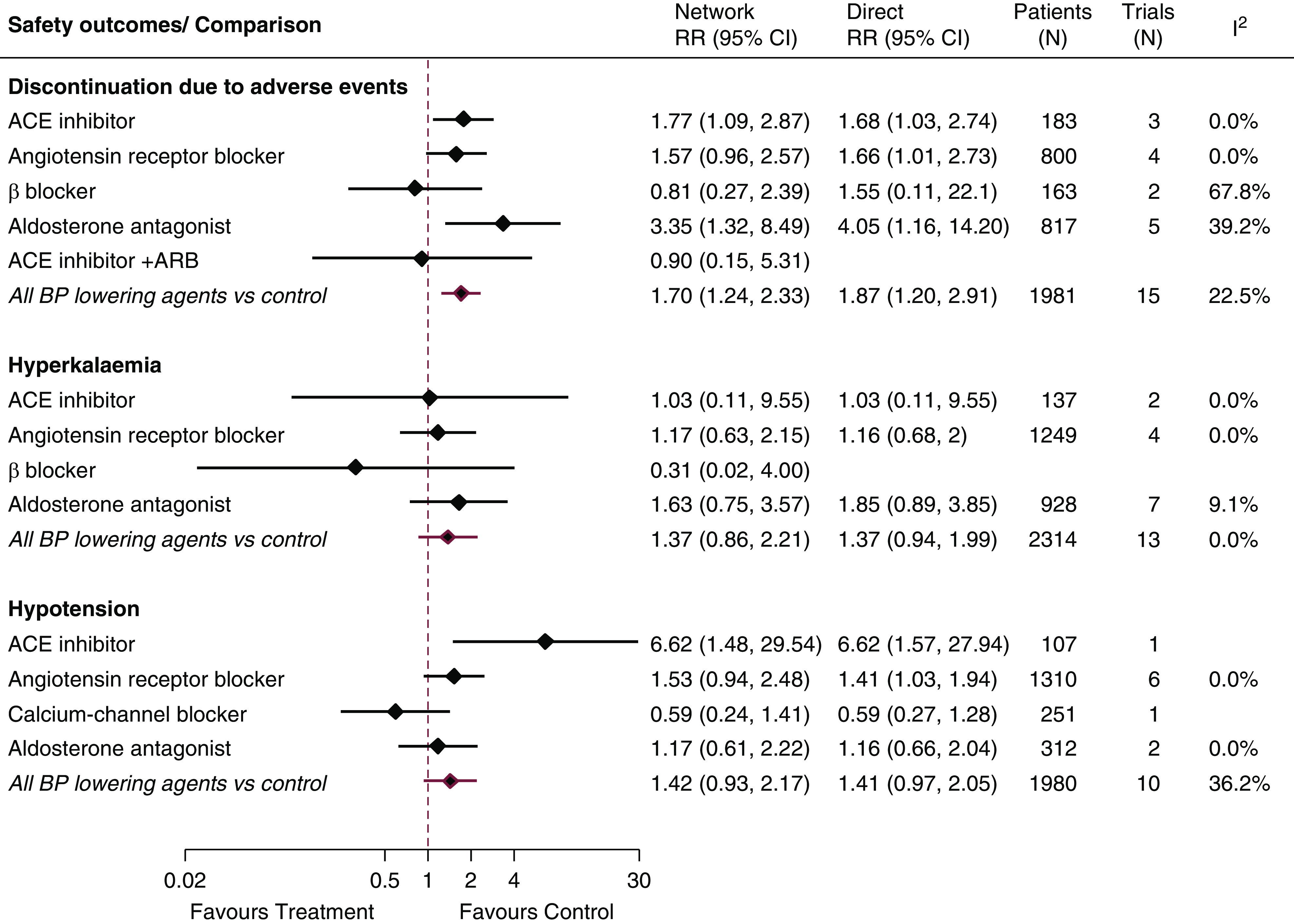
A number of BP-lowering agents were associated with adverse events compared with control, including treatment discontinuation and hypotension. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; 95% CI, 95% confidence interval; RR, relative risk.
Hypotension.
Ten trials (1980 patients) contributed to the network for hypotension (Figure 1). Compared with control, ACE inhibitors (RR, 6.62; 95% CI, 1.48 to 29.54) and ARBs (RR, 1.53; 95% CI, 0.94 to 2.48) increased the risk of hypotension (Figure 4). Calcium-channel blockers (RR, 0.09; 95% CI, 0.02 to 0.50) and aldosterone antagonists (RR, 0.18; 95% CI, 0.03 to 0.90) were superior to ACE inhibitors in this regard (Supplemental Figure 8). Limited data were available for other classes.
Hyperkalemia and Serum Potassium Concentration.
Fourteen trials (2514 patients) contributed to the network for hyperkalemia (Figure 1). No clear differences between medication classes for hyperkalemia risk were observed (Figure 4, Supplemental Figure 9).
In a network of 15 trials (959 patients) (Supplemental Figure 11), aldosterone antagonists increased serum potassium concentrations by 0.32 meq/L (95% CI, 0.15 to 0.49 meq/L) compared with control, by 0.40 meq/L (95% CI, 0.08 to 0.71 meq/L) compared with ACE inhibitors, and by 0.54 meq/L (95% CI, 0.20 to 0.88 meq/L) compared with ARBs (Supplemental Figure 10).
Sensitivity Analyses
Because of heterogeneity in some loops of evidence involving calcium-channel blockers for systolic BP outcome (Supplemental Table 4), we removed the study by London et al. (23) (baseline mean systolic BP was 189 mm Hg). Although loop-specific heterogeneity (τ2) in all loops dropped to zero, the treatment estimates did not change. In addition, for the serum potassium concentration outcome, we split the control node to placebo and standard of care separately. The global inconsistency test showed a nonsignificant P value of 0.91 with no changes in treatment estimates. In further sensitivity analysis, we excluded trials with unclear or high risk of bias from the systolic BP network, and overall results remained largely unchanged, although some comparisons did not reach statistical significance (Supplemental Figure 12).
Discussion
This meta-analysis provides evidence that should guide the use of BP-lowering agents in patients undergoing maintenance dialysis. The pooled analysis showed an overall significant effect of BP-lowering agents in lowering systolic BP and suggests that aldosterone antagonists and β-blockers may produce greater reductions in systolic BP. The data also suggest that ACE inhibitors and calcium-channel blockers have important BP-lowering effects. The effects of α-blockers and ARBs were less precise. These data suggest that β-blockers and perhaps aldosterone antagonists may be considered as BP-lowering agents of choice where they are tolerated for people with kidney failure requiring maintenance dialysis.
There seem to be differences in the BP-lowering efficacy of different drug classes. Specifically, aldosterone antagonists and β-blockers appear superior to other classes of BP-lowering drugs at lowering systolic BP, whereas the effects of ACE inhibitors and ARBs appear less potent. There is a potential pathophysiologic rationale for reduced efficacy of agents targeting the renin-angiotensin system because renin is produced by the kidney, and levels may be lower in people with kidney failure (24,25). Conversely, both increased aldosterone levels (so called “relative hyperaldosteronism”), irrespective of volume status, and increased sympathetic drive may be important mechanisms underpinning the increased BP observed in people with kidney failure, providing a potential rationale for superior efficacy of aldosterone antagonists and β-blockers in people receiving dialysis (26–28). It is noted, however, that our findings on the effects of aldosterone antagonists conflict with those reported in two recent trials (29,30), which showed no effect on systolic BP with spironolactone compared with placebo. It is possible that smaller size and suboptimal quality of earlier studies may have contributed to an overestimation of the treatment effect. Caution is thus warranted when interpreting these findings. The two ongoing clinical trials, ALCHEMIST (NCT01848639) and ACHIEVE (NCT03020303), should help better define the effectiveness and safety of spironolactone in patients undergoing maintenance dialysis.
Our study supports the use of β-blockers to lower BP in patients undergoing maintenance dialysis. However, β-blockers may be underused in clinical practice (31), and because included trials in our analysis are relatively small, future research is needed to evaluate the use of β-blockers as first-line BP-lowering agents in this patient population. In addition, water-soluble β-blockers are dialyzable, and they need to be supplemented after dialysis. This is important because observational evidence suggested possible harm with dialyzable compared with nondialyzable β-blockers (32).
Volume control is important for BP management. Achieving dry weight or increased dialysis frequency and/or time have been shown to lower BP in patients undergoing hemodialysis (33–37). However, because few data were available, it is not clear how volume control in the included trials could potentially affect or modify BP-lowering effects of BP-lowering drugs.
It is important to consider the potential risks of BP-lowering agents in this population. We found the risk of drug discontinuation due to adverse events was increased, especially for ACE inhibitors, ARBs, and aldosterone antagonists. Hypotension risk was increased with ACE inhibitors and ARBs, whereas aldosterone antagonists increased serum potassium concentrations but not hyperkalemia. β-Blockers may also increase hyperkalemia and hypotension risk, but data were limited to evaluate these outcomes in our study (38,39). These insights into the nature of adverse outcomes may be particularly important in patients undergoing maintenance dialysis, in whom intradialytic hypotension, hyperkalemia, and the use of low dialysate potassium have all been associated with worse outcomes (40,41). On a related note, the use of potassium binders is a potentially practice-changing strategy, which may facilitate the use of BP-lowering agents that might increase the risk of hyperkalemia as observed with patiromer in the phase 2 AMBER trial (42).
The superiority of β-blockers and aldosterone antagonists in systolic BP lowering may translate to better cardiovascular morbidity and mortality risk reduction. HDPAL study (43) showed that lisinopril was associated with 2.29 times higher cardiovascular events compared with atenolol in 200 predominately black patients undergoing maintenance hemodialysis. However, both lisinopril and atenolol improved left ventricular mass index, and the BP-lowering effect was not significantly different. In our analysis, β-blockers were superior at lowering systolic BP (by 4.4 mm Hg) compared with ACE inhibitors (Figure 2), consistent with these findings. Other meta-analyses reported cardiovascular and mortality benefits with β-blockers (39) and aldosterone antagonists (44), but no clear effects on major cardiovascular events and mortality with ACE inhibitors and ARBs were observed in patients receiving maintenance dialysis (45).
This study has several strengths. It aggregates a comprehensive, methodologically rigorous overview of the available data, incorporating direct and indirect treatment effects of BP-lowering drugs against placebo or against each other. It includes a substantial number of studies and a much larger number of participants than any previous study of BP lowering in patients undergoing maintenance dialysis. It also has some important limitations. As with any systematic review, it is dependent on the quality of the included studies, which varied substantially as did the size and design of the included trials. Most of the data in the network for the outcome of systolic BP were derived from trials with unclear or high risk of bias, although overall findings remained largely unchanged after excluding studies with unclear or high risk of bias. There were relatively few head-to-head studies, leading to limited network connectivity and power to detect statistical inconsistencies and publication bias. Limited data may have also affected the stability of the point estimates, including those reported for aldosterone antagonists (46). The majority of trials were reporting predialysis BP measurements, and thus, subgroup analysis may be underpowered to detect important differences between different BP measurements methods. This also limited our ability to draw conclusions regarding BP variability, a predictor of adverse outcomes in patients with kidney failure (47,48). Adverse events were also inconsistently captured and reported, and thus, they should be interpreted with caution. Because included trials used different agents from different classes, we could not assess the effect of the doses on transitivity assumption. However, most of the included agents had doses that are within the therapeutic ranges for BP control (49). Lastly, this analysis focused on BP effects and did not evaluate the effects of BP-lowering agents on patient-reported outcomes.
In conclusion, our study provides important evidence regarding the comparative efficacy and safety of different classes of BP-lowering agents in managing BP in patients undergoing maintenance dialysis. It suggests, within the limitations of the available evidence, that β-blockers and aldosterone antagonists may have greatest systolic BP-lowering effect, although aldosterone antagonists have a higher risk of adverse effects. Therefore, β-blockers should be evaluated in further research as the first class of agents considered for the treatment of hypertension in this patient population.
Disclosures
Dr. Gallagher reports receiving honoraria from Shire and Amgen for speaking at scientific meetings. Dr. Jardine is responsible for research projects that have received unrestricted funding from Gambro, Baxter, CSL, Amgen, Eli Lilly, and Merck; has served on advisory boards sponsored by Akebia, Baxter, and Boehringer Ingelheim; and has spoken at scientific meetings sponsored by Janssen, Amgen, and Roche, with any consultancy, honoraria, or travel support paid to her institution. Dr. Jun reports receiving grant support from National Health and Medical Research Council of Australia project grant 1148060 and unrestricted grant support from VentureWise (a wholly owned commercial subsidiary of NPSMedicineWise) to conduct a commissioned project funded by AstraZeneca. Dr. Perkovic has participated in advisory boards for both Relypsa and AstraZeneca and has received honoraria and consultation fees from Tricida, Novartis, Amgen, Janssen, GlaxoSmithKline, Astellas, Boeringer Ingelheim, Baxter, Mitsubishi Tanabe, Retrophin, Merck, Abbvie, Novo Nordisk, AstraZeneca, Gilead, Durect, Servier, Eli Lilly, Relypsa, Pharmalink, Bayer, Bristol-Myers Squibb, and Tufts, with payments paid to his institution. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
Dr. Perkovic and Dr. Shaman designed the study; Dr. Gallagher, Dr. Jardine, Dr. Jun, and Dr. Mihailidou revised the protocol; Dr. Shaman carried out the literature search; Dr. Arnott and Dr. Shaman independently screened trials for inclusion and extracted and verified data; Dr. Shaman and Dr. Smyth independently assessed the quality of included trials; Dr. Shaman analyzed the data and prepared figures and tables with support from Dr. Palmer; Dr. Shaman drafted the paper; Dr. Jun, Dr. Palmer, and Dr. Perkovic revised the paper; and Dr. Arnott, Dr. Gallagher, Dr. Jardine, Dr. Jun, Dr. Mihailidou, Dr. Palmer, Dr. Perkovic, Dr. Shaman, and Dr. Smyth approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Drug Selection for Treating Hypertension in Dialysis Patients: More to Consider than BP-Lowering Potency,” on pages 1084–1086.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12201019/-/DCSupplemental.
Supplemental Figure 1. Electronic searches in MEDLINE, Embase, and the Cochrane Central Register of Randomised Trials for trials on BP-lowering drugs in adults with kidney failure requiring dialysis.
Supplemental Figure 2. Domain-specific risk of bias assessment summary of included studies.
Supplemental Figure 3. Study limitations weighted by contribution of direct estimates to the network of BP-lowering drugs for systolic BP outcome.
Supplemental Figure 4. Comparison-adjusted funnel plot of placebo-controlled trials (n=17) of BP-lowering drugs effect on systolic BP in patients on dialysis.
Supplemental Figure 5. Network estimates of BP-lowering drugs effects on diastolic BP.
Supplemental Figure 6. Network estimates of BP-lowering drugs effects on heart rate.
Supplemental Figure 7. Network estimates of BP-lowering drugs effects on discontinuation due to adverse events.
Supplemental Figure 8. Network estimates of BP-lowering drugs effects on risk of hypotension.
Supplemental Figure 9. Network estimates of BP-lowering drugs effects on the risk of hyperkalemia.
Supplemental Figure 10. Network estimates of BP-lowering drugs effects on serum potassium.
Supplemental Figure 11. Network of treatment comparison of BP-lowering agents effects on diastolic BP, heart rate, and potassium concentration outcomes.
Supplemental Figure 12. Network estimates of BP-lowering drugs effects on systolic BP in trials with low risk of bias (n=9).
Supplemental Material. References.
Supplemental Table 1. Electronic search terms.
Supplemental Table 2. Characteristics of included studies.
Supplemental Table 3. Risk of bias assessments of all included trials.
Supplemental Table 4. Assessment of loop-specific (in closed loops of evidence) and overall inconsistency and heterogeneity.
Supplemental Table 5. Assessment of agreement between direct and indirect evidence using side-splitting approach for all outcomes.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kovesdy CP, Lavallee D, Leslie J, McCullough K, Modi Z, Molnar MZ, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Rao P, Repeck K, Rhee CM, Schrager J, Schaubel DE, Selewski DT, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Tong L, Wang D, Wang M, Woodside KJ, Xin X, Yin M, You AS, Zhou H, Shahinian V: US Renal Data System 2017 annual data report: Epidemiology of kidney disease in the United States [published correction appears in Am J Kidney Dis 71: 501, 2018]. Am J Kidney Dis 71[Suppl 1]: A7, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG: Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115: 291–297, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HJL, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V: Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: A systematic review and meta-analysis of randomised controlled trials. Lancet 373: 1009–1015, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal R, Sinha AD: Cardiovascular protection with antihypertensive drugs in dialysis patients: Systematic review and meta-analysis. Hypertension 53: 860–866, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Nigwekar SU, Hegbrant J, Strippoli GF: HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev 9: CD004289, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills EJ, Thorlund K, Ioannidis JP: Demystifying trial networks and network meta-analysis. BMJ 346: f2914, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D: The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med 162: 777–784, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP: Evaluating the quality of evidence from a network meta-analysis. PLoS One 9: e99682, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Salanti G: Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 3: 80–97, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G: Evaluation of inconsistency in networks of interventions. Int J Epidemiol 42: 332–345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias S, Welton NJ, Caldwell DM, Ades AE: Checking consistency in mixed treatment comparison meta-analysis. Stat Med 29: 932–944, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A: Meta-analyses involving cross-over trials: Methodological issues. Int J Epidemiol 31: 140–149, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White IR, Barrett JK, Jackson D, Higgins JP: Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res Synth Methods 3: 111–125, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes KM, Turner RM, Higgins JP: Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J Clin Epidemiol 68: 52–60, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP: Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 41: 818–827, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaimani A, Salanti G: Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods 3: 161–176, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JA: Metan: Fixed-and random-effects meta-analysis. Stata J 8: 3–28, 2008 [Google Scholar]
- 21.Liu Y, Wang W, Zhang AB, Bai X, Zhang S: Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: A network meta-analysis. Laryngoscope 126: 951–955, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Chaimani A, Salanti G: Visualizing assumptions and results in network meta-analysis: The network graphs package. Stata J 15: 905–950, 2015 [Google Scholar]
- 23.London GM, Marchais SJ, Guerin AP, Metivier F, Safar ME, Fabiani F, Froment L: Salt and water retention and calcium blockade in uremia. Circulation 82: 105–113, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Man in ’t Veld AJ, Schicht IM, Derkx FH, de Bruyn JH, Schalekamp MA: Effects of an angiotensin-converting enzyme inhibitor (captopril) on blood pressure in anephric subjects. BMJ 280: 288–290, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leslie BR, Case DB, Sullivan JF, Vaughan ED Jr.: Absence of blood-pressure lowering effect of captopril in anephric patients. BMJ 280: 1067–1068, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung SC, Lin YP, Huang HL, Pu HF, Tarng DC: Aldosterone and mortality in hemodialysis patients: Role of volume overload. PLoS One 8: e57511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Converse RL Jr., Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG: Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Bomback AS: Mineralocorticoid receptor antagonists in end-stage renal disease: Efficacy and safety. Blood Purif 41: 166–170, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Charytan DM, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, Anderson AH, Hung AM, Mehrotra R, Sharma S, Weiner DE, Williams M, DiCarli M, Skali H, Kimmel PL, Kliger AS, Dember LM; Hemodialysis Novel Therapies Consortium : Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): A randomized, placebo-controlled, multiple dosage trial. Kidney Int 95: 973–982, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammer F, Malzahn U, Donhauser J, Betz C, Schneider MP, Grupp C, Pollak N, Störk S, Wanner C, Krane V; MiREnDa Study Group : A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int 95: 983–991, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Weir MA, Herzog CA: Beta blockers in patients with end-stage renal disease: Evidence-based recommendations. Semin Dial 31: 219–225, 2018. [DOI] [PubMed] [Google Scholar]
- 32.Weir MA, Dixon SN, Fleet JL, Roberts MA, Hackam DG, Oliver MJ, Suri RS, Quinn RR, Ozair S, Beyea MM, Kitchlu A, Garg AX: β-Blocker dialyzability and mortality in older patients receiving hemodialysis. J Am Soc Nephrol 26: 987–996, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal R, Alborzi P, Satyan S, Light RP: Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension 53: 500–507, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocco MV, Lockridge RS Jr., Beck GJ, Eggers PW, Gassman JJ, Greene T, Larive B, Chan CT, Chertow GM, Copland M, Hoy CD, Lindsay RM, Levin NW, Ornt DB, Pierratos A, Pipkin MF, Rajagopalan S, Stokes JB, Unruh ML, Star RA, Kliger AS, Kliger A, Eggers P, Briggs J, Hostetter T, Narva A, Star R, Augustine B, Mohr P, Beck G, Fu Z, Gassman J, Greene T, Daugirdas J, Hunsicker L, Larive B, Li M, Mackrell J, Wiggins K, Sherer S, Weiss B, Rajagopalan S, Sanz J, Dellagrottaglie S, Kariisa M, Tran T, West J, Unruh M, Keene R, Schlarb J, Chan C, McGrath-Chong M, Frome R, Higgins H, Ke S, Mandaci O, Owens C, Snell C, Eknoyan G, Appel L, Cheung A, Derse A, Kramer C, Geller N, Grimm R, Henderson L, Prichard S, Roecker E, Rocco M, Miller B, Riley J, Schuessler R, Lockridge R, Pipkin M, Peterson C, Hoy C, Fensterer A, Steigerwald D, Stokes J, Somers D, Hilkin A, Lilli K, Wallace W, Franzwa B, Waterman E, Chan C, McGrath-Chong M, Copland M, Levin A, Sioson L, Cabezon E, Kwan S, Roger D, Lindsay R, Suri R, Champagne J, Bullas R, Garg A, Mazzorato A, Spanner E, Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T, Pierratos A, Chan W, Regozo K, Kwok S; Frequent Hemodialysis Network (FHN) Trial Group : The effects of frequent nocturnal home hemodialysis: The frequent hemodialysis network nocturnal trial. Kidney Int 80: 1080–1091, 2011. 21775973 [Google Scholar]
- 35.Jardine MJ, Zuo L, Gray NA, de Zoysa JR, Chan CT, Gallagher MP, Monaghan H, Grieve SM, Puranik R, Lin H, Eris JM, Zhang L, Xu J, Howard K, Lo S, Cass A, Perkovic V; ACTIVE Dialysis Steering Committee; Paul : A trial of extending hemodialysis hours and quality of life. J Am Soc Nephrol 28: 1898–1911, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS, Kliger AS; FHN Trial Group : In-center hemodialysis six times per week versus three times per week [published correction appears in N Engl J Med 364: 93, 2011]. N Engl J Med 363: 2287–2300, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 298: 1291–1299, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Shroff GR, Herzog CA: β-Blockers in dialysis patients: A nephrocardiology perspective. J Am Soc Nephrol 26: 774–776, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V: Effects of beta-adrenergic antagonists in patients with chronic kidney disease: A systematic review and meta-analysis. J Am Coll Cardiol 58: 1152–1161, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Stefánsson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, Stålhammar NO: Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 9: 2124–2132, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karaboyas A, Zee J, Brunelli SM, Usvyat LA, Weiner DE, Maddux FW, Nissenson AR, Jadoul M, Locatelli F, Winkelmayer WC, Port FK, Robinson BM, Tentori F: Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 69: 266–277, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, Ma J, White WB, Williams B: Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 394: 1540–1550, 2019. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG: Hypertension in hemodialysis patients treated with atenolol or lisinopril: A randomized controlled trial. Nephrol Dial Transplant 29: 672–681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quach K, Lvtvyn L, Baigent C, Bueti J, Garg AX, Hawley C, Haynes R, Manns B, Perkovic V, Rabbat CG, Wald R, Walsh M: The safety and efficacy of mineralocorticoid receptor antagonists in patients who require dialysis: A systematic review and meta-analysis. Am J Kidney Dis 68: 591–598, 2016. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Ma X, Zheng J, Jia J, Yan T: Effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on cardiovascular events and residual renal function in dialysis patients: A meta-analysis of randomised controlled trials. BMC Nephrol 18: 206, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brignardello-Petersen R, Murad MH, Walter SD, McLeod S, Carrasco-Labra A, Rochwerg B, Schünemann HJ, Tomlinson G, Guyatt GH; GRADE Working Group : GRADE approach to rate the certainty from a network meta-analysis: Avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol 105: 60–67, 2019. [DOI] [PubMed] [Google Scholar]
- 47.Sarafidis PA, Persu A, Agarwal R, Burnier M, de Leeuw P, Ferro C, Halimi JM, Heine G, Jadoul M, Jarraya F, Kanbay M, Mallamaci F, Mark PB, Ortiz A, Parati G, Pontremoli R, Rossignol P, Ruilope L, Van der Niepen P, Vanholder R, Verharr MC, Wiecek A, Wuerzner G, London GM, Zoccali C: Hypertension in dialysis patients: A consensus document by the European renal and cardiovascular medicine (EURECA-m) working group of the European renal association - European dialysis and transplant association (ERA-EDTA) and the hypertension and the kidney working group of the European society of hypertension (ESH). J Hypertens 35: 657–676, 2017. [DOI] [PubMed] [Google Scholar]
- 48.Sarafidis PA, Loutradis C, Karpetas A, Tzanis G, Bikos A, Raptis V, Syrgkanis C, Liakopoulos V, Papagianni A, Bakris G, Parati G: The association of interdialytic blood pressure variability with cardiovascular events and all-cause mortality in haemodialysis patients. Nephrol Dial Transplant 34: 515–523, 2019. [DOI] [PubMed] [Google Scholar]
- 49.Georgianos PI, Agarwal R: Pharmacotherapy of hypertension in chronic dialysis patients. Clin J Am Soc Nephrol 11: 2062–2075, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.