Graphical abstract
Keywords: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Coronavirus disease 2019 (COVID-19), Venous thromboembolism, Deep vein thrombosis
Highlights
-
•
An association between COVID-19 and venous thromboembolism (VTE) is now recognized.
-
•
The prevalence of VTE is high in COVID-19 patients hospitalized in standard care units.
-
•
The prevalence of VTE is high even though thromboprophylaxis and in patients estimated at low risk.
-
•
A high index of suspicion for VTE is crucial in patients with SARS-CoV-2 infection.
1. Introduction
The outbreak of the coronavirus disease 2019 (COVID-19) spread rapidly throughout the world during the first months of 2020 reaching the pandemic status as a major global health issue. The main clinical and therapeutic concerns due to COVID-19 relate to the respiratory distress and failure caused by pneumonia due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. COVID-19, however, is not just a pulmonary disease. While respiratory complications and systemic inflammation unquestionably result in significantly higher morbidity and mortality rates, cardiovascular complications are also common [2].
A number of cohort studies described an association with coagulopathy and venous thromboembolism (VTE) among SARS-CoV-2 infected patients [3]. SARS-CoV-2 predisposes to thrombotic events by means of different mechanisms such as hypoxia, inflammation, prolonged immobilization occurring in the context of different and various alterations of hemostatic pathways (e.g. from endothelial dysfunction to platelet activation, from inflammation-related and consumptive coagulopathy to thrombotic microangiopathy) [4]. Alterations of prothrombin time, fibrinogen, and D-dimer are often observed in COVID-19 patients and are associated with a detrimental outcome [5]. D-dimer has been specifically proposed as a potential prognostic marker, where high D-dimer levels predict higher in-hospital mortality in COVID-19 patients [6]. Consistently, anticoagulant and fibrinolytic drugs have been proposed as possible therapeutic tools [5]. Two studies in Chinese and Dutch populations showed a remarkably high cumulative incidence of symptomatic VTE in patients admitted to the Intensive Care Units (ICU) [7,8]. The prevalence of deep vein thrombosis (DVT) in patients hospitalized for SARS-CoV-2 infection remains, however, not well defined, especially for those admitted to COVID-19 Standard Care Units (SCU). Therefore, we designed a study with the aims of assessing the prevalence of DVT among subjects with SARS-CoV-2 pneumonia in the setting of SCU and investigating the clinical and laboratory characteristics associated with DVT in COVID-19 patients.
2. Subjects and methods
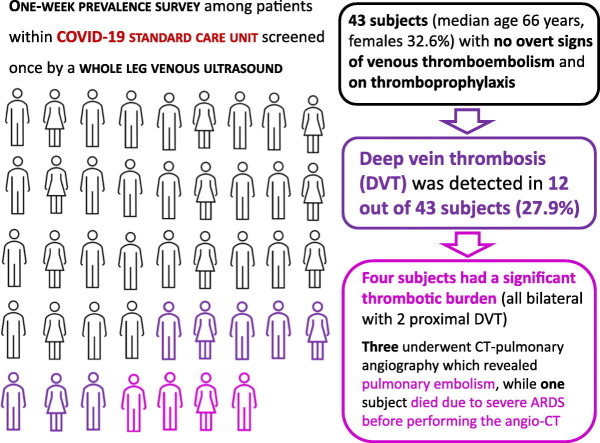
In April 2020, we performed a one-week prevalence survey among in-patients within COVID-19 SCU at the Verona University Hospital, Italy. Forty-three subjects (median age 66 years with interquartile range of 18 years and minimum-maximum range 28–96 years, females 32.6%, main duration of hospitalization 8 days with interquartile range of 17 days and minimum-maximum range of 1–43 days, 10 (23.3%) admitted from ICU, no previous DVT history) were screened once by a whole leg venous ultrasound (examining both femoral veins, popliteal veins and calf veins). All of them, with proven SARS-CoV-2 pneumonia, had no overt signs and/or symptoms of VTE at physical examination and were on thromboprophylaxis at standard dose with low-molecular-weight heparin (enoxaparin, 4000–6000 U/die) or heparin‑calcium (5000 U × 2–3/die). Four subjects taking full-dose anticoagulant therapy because of atrial fibrillation were excluded from the study. Clinical information, laboratory data and Padua Prediction Score calculation were collected consensually to the ultrasonography examination. The study was approved by the Ethics Committee of our Institution (Azienda Ospedaliera Universitaria Integrata, Verona, Italy) and witnessed oral consent was obtained.
All calculations were performed using the IBM SPSS 23.0 (IBM Inc., Armonk, NY, USA) statistical package. Distributions of continuous variables in groups were expressed as median value with minimum-maximum range and analyzed by Mann-Whitney test. Qualitative data were expressed as number and percentage with estimated 95% confidence interval (CI) and analyzed with use of the χ2 test or Fisher-exact test, when appropriate. All the variables showing an association with DVT at univariate analysis (P < 0.05) were included in a logistic regression model with backward stepwise selection of variables and the strength of association was estimated by calculating odds ratios (OR) with 95%CI. A P value <0.05 was considered significant.
3. Results
DVT was detected in 12 out of 43 (27.9% with 95%CI 14.5–41.3%) subjects. Table 1 summarizes the clinical and laboratory characteristics of the patients affected by DTV as compared to those without DVT. As expected, the 95%CI for the prevalence estimates were wide due the small sample size of this study cohort. Patients with DVT were older than those without DVT. In the subgroup of subjects below 60 years of age only 1 out of 12 subjects (8.3% with 95%CI 0–24.0%) had DVT, while in the subgroup of those above 75 years of age, the prevalence of DVT was 41.7% with 95%CI 13.8–69.6%. Patients with DVT had a higher prevalence of diabetes, higher total white blood cell and neutrophil counts, while platelet count showed a trend toward the highest range but not statistically significant. The Padua Prediction Score also tended to be higher in DVT group but a substantial number of patients with DVT (n = 7) were considered at low risk on the basis of this risk assessment model. Subjects in the study cohort were stratified according to D-dimer plasma concentration in 3 subgroups, i.e. <500, 500–1499, and ≥ 1500 μg/L. These threshold levels were predefined on the basis of prior scientific literature. While D-dimer <500 μg/L is the usually accepted threshold value to exclude VTE in low-risk patients, D-dimer ≥1500 μg/L has been proposed as the cut-off value to predicting VTE in COVID-19 patients [7]. Very high plasma concentrations of D-dimer (≥1500 μg/L) was associated with DVT (75% with 95%CI 50.5–99.5% versus 25.8% with 95%CI 10.4–41.2%, comparing patients with or without DVT respectively). However, DVT was observed also in 2 subjects with D-dimer concentrations within the normal range value (<500 μg/L). By including all the variables showing an association with DVT at univariate analysis (P < 0.05) in a logistic regression model with backward stepwise selection of variables, only high D-dimer levels (≥1500 μg/L) maintained an association with DVT (OR 8.63 with 95%CI 1.86–40.01, P = 0.006). In this regard, our results are consistent with those from previous studies proposing high D-dimer levels as a potential useful tool for identifying COVID-19 patients with poor prognosis [3,6] and/or with VTE complications [7]. On the other hand, no differences between DVT and non-DVT subjects were found for fibrinogen, PT and aPTT, the inflammatory marker C-reactive protein, markers of renal and liver function, as well as the other clinical characteristics.
Table 1.
Clinical and laboratory characteristics of the study population admitted to COVID-19 Standard Care Units, as a whole and subdivided in subjects with or without deep vein thrombosis (DVT), symptomatic or asymptomatic (P < 0.10 have been specified). Continuous variables are expressed as median value with minimum-maximum range. Qualitative data are expressed as number and percentage with estimated 95% confidence interval.
| Whole study sample (n = 43) |
DVT-free (n = 31) |
DVT (n = 12) |
P ⁎ | |
|---|---|---|---|---|
| Age (years) | 66 (28–96) | 63 (28–91) | 71 (59–96) | 0.046 |
| Age category (n /%) | ||||
| <60 years | 12/27.9 (14.5–41.3) | 11/35.5 (18.6–52.3) | 1/8.3 (0–24.0) | 0.072 |
| 60–74 | 19/44.2 (29.3–59.0) | 13/41.9 (24.6–59.3) | 6/50.0 (21.7–78.3) | |
| ≥75 years | 12/27.9 (14.5–41.3) | 7/22.6 (7.9–37.3) | 5/41.7 (13.8–69.6) | |
| Female sex (n /%) | 14/32.6 (18.6–46.6) | 10/32.3 (15.8–48.7) | 4/33.3 (6.7–60.0) | NS |
| BMI (kg/m2) | 25.6 (18.9–37.9) | 25.7 (19.6–37.9) | 25.1 (18.9–32.4) | NS |
| Time interval since hospitalization (days) | 8 (1–43) | 8 (1–43) | 9 (1–26) | NS |
| Admitted from ICU (n/%) | 10/23.3 (10.6–35.9) | 6/19.4 (5.4–33.3) | 4/33.3 (6.7–60.0) | NS |
| Previous VTE (n/%) | 0/0 | 0/0 | 0/0 | NS |
| Previous CVD (n/%) | 8/18.6 (7.0–30.2) | 6/19.4 (5.4–33.3) | 2/16.7 (0–37.8) | NS |
| Active cancer (n/%) | 4/9.3 (0.6–18.0) | 2/6.5 (0–15.1) | 2/16.7 (0–37.8) | NS |
| Hypertension (n/%) | 23/53.5 (38.6–68.4) | 16/51.6 (34.0–69.2) | 7/58.3 (30.4–86.2) | NS |
| Diabetes (n/%) | 6/14.0 (3.6–24.3) | 2/6.5 (0–15.1) | 4/33.3 (6.7–60.0) | 0.042 |
| Dyslipidemia (n/%) | 8/18.6 (7.0–30.2) | 6/19.4 (5.4–33.3) | 2/16.7 (0–37.8) | NS |
| Characteristics of DVT(n/%) | ||||
| Bilateral | – | 7/58.3 (30.4–86.2) | – | |
| Proximal | -- | 2/16.7 (0–37.8) | ||
| Padua prediction score | ||||
| Score | 2 (1–8) | 2 (1–6) | 3 (1–8) | 0.052 |
| Low risk – score < 4 (n/%) | 33/76.7 (64.1–89.4) | 26/83.9 (70.9–96.8) | 7/58.3 (30.4–86.2) | 0.087 |
| High risk – score ≥ 4 (n/%) | 10/23.3 (10.6–35.9) | 5/16.1 (3.2–29.1) | 5/41.7 (13.8–69.6) | |
| Laboratory examinations | ||||
| White blood cell count (per mm3) | 7500 (1370-28,970) |
7080 (1370-24,190) |
9370 (3940-28,970) |
0.035 |
| Neutrophils (per mm3) | 5490 (1030-22,270) |
5180 (1030-22,270) |
8140 (2130-20,800) |
0.021 |
| Lymphocytes (per mm3) | 910 (230−2130) |
860 (230–2130) |
1020 (250–1590) |
NS |
| Platelets (per mm3) | 200,000 (42,000-515,000) |
182,000 (42,000-515,000) |
243,000 (118,000-461,000) |
0.071 |
| Hemoglobin (g/dL) | 13.1 (8.7–17.4) | 13.1 (8.7–16.7) | 13.8 (9.5–17.4) | NS |
| C-reactive protein (mg/L) | 80 (3–298) | 83 (3–298) | 59 (6–280) | NS |
| Creatinine (μmol/L) | 0.98 (0.45–3.17) | 0.96 (0.52–3.17) | 1.00 (0.45–1.81) | NS |
| eGFR (mL/min) | 79 (19–117) | 83 (19–117) | 68 (24–96) | NS |
| Alanine amino-transferase (U/L) | 36 (12–117) | 36 (15–117) | 35 (12–81) | NS |
| Aspartate amino-transferase (U/L) | 38 (17–177) | 40 (17–177) | 34 (22–92) | NS |
| Albumin (g/L) | 35 (21–45) | 35 (22–45) | 32 (21–45) | NS |
| Fibrinogen (g/L) | 627 (192–1014) | 636 (192–1014) | 601 (341–891) | NS |
| Activated partial thromboplastin time (ratio) | 0.98 (0.71–1.50) | 1.00 (0.78–1.30) | 0.94 (0.71–1.50) | NS |
| Prothrombin time (ratio) | 1.10 (0.86–2.15) | 1.12 (0.86–2.15) | 1.05 (0.98–1.28) | NS |
| D-dimer (mcg/L) | 1279 (248–6753) | 935 (248–6520) | 1878 (418–6753) | 0.081 |
| D-dimer category (n /%) | ||||
| <500 μg/L | 4/9.3 (0.6–18.0) | 2/6.5 (0–15.1) | 2/16.7 (0–37.8) | 0.002 |
| 500–1499 μg/L | 22/51.2 (36.2–66.1) | 21/67.7 (51.3–84.2) | 1/8.3 (0–24.0) | |
| ≥1500 μg/L | 17/39.5 (24.9–54.1) | 8/25.8 (10.4–41.2) | 9/75.0 (50.5–99.5) | |
BMI: body mass index; CVD: cardiovascular disease; DVT: deep vein thrombosis.
By Mann-Whitney test or chi-square test or Fisher-exact test, when appropriate.
Of the 12 DVT diagnosed, 8 were limited and distal, while four patients had a significant thrombotic burden (all bilateral with 2 proximal DVT). Three of them underwent CT-pulmonary angiography which revealed pulmonary embolism, while one patient died due to severe acute respiratory distress syndrome (ARDS) before performing the angio-CT. These findings thereby support the usefulness of ultrasound examination for a prompt shift to full anticoagulant therapy to prevent further VTE complications.
4. Discussion
Our data suggest that patients hospitalized for COVID-19 have substantially high prevalence of venous thrombotic events regardless of a thromboprophylaxis regimen at standard dosage. This prevalence is impressively higher than that observed in acute medical ill patients [9], i.e. lower than 1%, and appears even comparable with that reported in previous studies of patients with severe SARS-CoV-2 pneumonia in ICU [7,8]. In the study on Chinese patients, for whom no thromboprophylactic therapy was reported, Cui and colleagues described a VTE prevalence of 25% [7]. In the Dutch study on patients undergoing an adequate thromboprophylaxis with standard doses of nadroparin, Klok and colleagues observed a VTE cumulative incidence of 27% [8]. It should be noted, however, that both those studies identified symptomatic thrombotic complications without intent of a systematic screening by way of performing a lower limb venous ultrasonography. Therefore, we speculated that the real prevalence of total, symptomatic and asymptomatic, venous thrombotic complications in COVID-19 patients in ICU could be even higher than what was described, so far. In line with our hypothesis, a very recent study showed an extremely high prevalence of asymptomatic DVT by screening with compression ultrasonography of the lower extremities COVID-19 patients in ICU (41/48–85.4%) [10]. In our study sample, although 10 out of 43 subjects were admitted from ICU, the prevalence of prior VTE was low. However, it should be taken into account that all the screened subjects were asymptomatic for DVT and no systematic screening with leg venous ultrasound was performed in ICU.
Those results and ours are consistent with recent evidences supporting the concept of a strong prothrombotic diathesis in COVID-19, which is characterized by severe hypercoagulability rather than consumptive coagulopathy [4,11]. SARS-CoV-2 infection may influence hemostatic pathways by different biological mechanisms at several levels. Recent observations by autopsies in COVID-19 patients documented pulmonary vascular endothelialitis and a high prevalence of in situ microthrombi in alveolar capillary vessels, consistent with thrombotic microangiopathy [12]. The coagulopathy of COVID-19 has been suggestively described as a perfect storm of immune-, cytokine-, and coagulation-driven processes that conspire to create a prothrombotic diathesis [4]. However, the crucial drivers of all these alterations in the hemostatic balance, remain still uncertain as it does so the optimal management of COVID-19-associated VTE risk. Further studies are, therefore, needed to clarify such issues. The findings of a high prevalence of DVT in patients taking the usual thromboprophylaxis and estimated at low risk of thrombotic complications according to the traditional risk assessment model (such as the Padua Prediction Score), emphasize some unsolved issues: i) potential SARS-CoV-2-related hypercoagulable state, ii) appropriate VTE risk stratification for hospitalized COVID-19 patients, and iii) the choice of anticoagulant agents and relative doses, which require further investigations [2]. It should be noted that there is a great debate on such issues with some controversial results [7,8,13]. These crucial questions will be solved only by the ongoing randomized clinical trials. Meanwhile, we can argue that early detection of DVT in COVID-19 patients may allow a more appropriate management, for example by shifting rapidly from standard thromboprophylaxis to full anticoagulant therapy in patients with high thrombotic burden, like the reported 3 subjects in whom CT-pulmonary angiography revealed pulmonary embolism.
This study has some limitations owing to the small sample size, and its results should be then considered as hypothesis-generating. Nonetheless, our results support the concept that a high index of suspicion for VTE is crucial in patients with SARS-CoV-2 infection. Further investigations with prospective clinical trials are urgently needed to define both clinical and laboratory predictors of VTE and the possible role of anticoagulant therapy for the outcome of patients with COVID-19.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Caprini J.A., Tafur A.J., Burton J.R., Francese D.P., Wang E.Y., Falanga A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Steg P.G., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., Lip G.Y.H. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spyropoulos A.C., Weitz J.I. Hospitalized COVID-19 patients and venous thromboembolism: a perfect storm. Circulation. 2020;142:129–132. doi: 10.1161/CIRCULATIONAHA.120.048020. [DOI] [PubMed] [Google Scholar]
- 5.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed]
- 9.Loffredo L., Arienti V., Vidili G. Low rate of intrahospital deep venous thrombosis in acutely ill medical patients: results from the AURELIO study. Mayo Clin. Proc. 2019;94:37–43. doi: 10.1016/j.mayocp.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Ren B., Yan F., Deng Z., Zhang S., Xiao L., Wu M., Cai L. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020;142:181–183. doi: 10.1161/CIRCULATIONAHA.120.047407. [DOI] [PubMed] [Google Scholar]
- 11.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., Navalesi P., Simioni P. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, Muscarella G, Orlandi M. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb. Haemost. 2020 Apr 29 doi: 10.1055/s-0040-1712097. (Online ahead of print). [DOI] [PMC free article] [PubMed]