Abstract
Background
Several underlying conditions have been associated with severe acute respiratory syndrome coronavirus 2 illness, but it remains unclear whether underlying asthma is associated with worse coronavirus disease 2019 (COVID-19) outcomes.
Objective
Given the high prevalence of asthma in the New York City area, our objective was to determine whether underlying asthma was associated with poor outcomes among hospitalized patients with severe COVID-19 compared with patients without asthma.
Methods
Electronic heath records were reviewed for 1298 sequential patients 65 years or younger without chronic obstructive pulmonary disease who were admitted to our hospital system with a confirmed positive severe acute respiratory syndrome coronavirus 2 test result.
Results
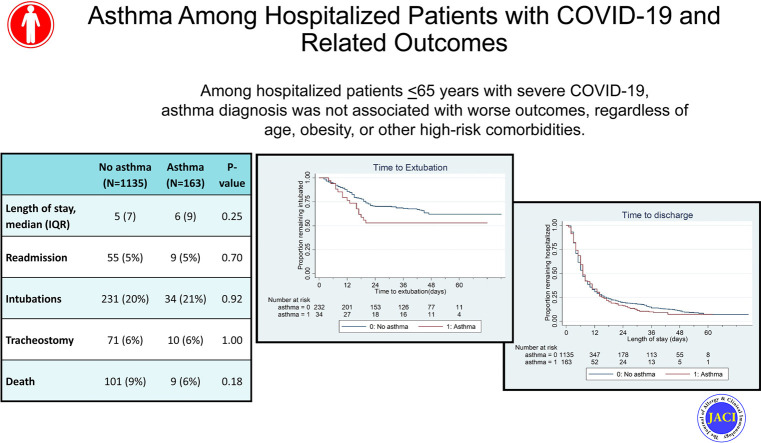
The overall prevalence of asthma among all hospitalized patients with COVID-19 was 12.6%, yet a higher prevalence (23.6%) was observed in the subset of 55 patients younger than 21 years. There was no significant difference in hospital length of stay, need for intubation, length of intubation, tracheostomy tube placement, hospital readmission, or mortality between patients with and without asthma. Observations between patients with and without asthma were similar when stratified by obesity, other comorbid conditions (ie, hypertension, hyperlipidemia, and diabetes), use of controller asthma medication, and absolute eosinophil count.
Conclusions
Among hospitalized patients 65 years or younger with severe COVID-19, asthma diagnosis was not associated with worse outcomes, regardless of age, obesity, or other high-risk comorbidities. Future population-based studies are needed to investigate the risk of developing COVID-19 among patients with asthma once universal testing becomes readily available.
Key words: SARS-CoV-2, coronavirus, New York City, respiratory disease
Abbreviations used: ACE2, Angiotensin-converting enzyme-2; AEC, Absolute eosinophil count; COPD, Chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; EHR, Electronic health record; NYC, New York City; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Graphical abstract
First reported in Wuhan, China, in November 2019, coronavirus disease 2019 (COVID-19) has now spread to nearly every country, with more than 14.5 million cases worldwide and more than 606,000 deaths. The first case in the United States was reported in January 2020, and the number has progressed to more than 3.8 million cases across the country, with New York City (NYC) at the epicenter.1 The precipitous progression of disease, high mortality rate, and swift spread across communities has made this global pandemic a public health emergency. Given the profound global implications of this acute phase of the COVID-19 pandemic, there is a need to better characterize those most at risk of severe infection in an effort to develop preventive measures to protect vulnerable populations.
The Centers for Disease Control and Prevention and the American Academy of Allergy, Asthma & Immunology consider asthma a risk factor for severe COVID-19.1 , 2 Coronavirus is among the top 5 isolated viruses during acute asthma exacerbations, with a higher prevalence in adults.3 However, the previous severe acute respiratory syndrome coronavirus outbreak of 2003 was not associated with increase in asthma exacerbations.4 , 5 A handful of early studies from China reported underlying respiratory diseases as a comorbidity among hospitalized patients with COVID-19,6, 7, 8, 9, 10 with a few specifically mentioning an underrepresentation of asthma in hospitalized patients. In the United States, where asthma prevalence is twice that of China,11 , 12 several reports have documented wide ranges of asthma prevalence in association with COVID-19, ranging from 9% to 25% of hospitalized patients.13, 14, 15, 16, 17, 18, 19, 20 However, it is unclear from these studies whether underlying asthma was associated with worse COVID-19 outcomes.6 , 10 , 20 , 21
Given the paucity and variability of data in different reports analyzing the impact of an underlying diagnosis of asthma on severity of COVID-19, there is a need for a more comprehensive review to better characterize the relationship between asthma and severe COVID-19 among hospitalized patients. The high incidence and mortality associated with COVID-19 and the high, well-defined prevalence of asthma in NYC makes this the ideal population to investigate the impact of asthma on severe COVID-19 infections. Thus, our objective was to characterize the clinical outcomes of patients with severe COVID-19 who required hospitalization in NYC who have underlying asthma compared with patients without asthma.
Methods
Data sources
This study was conducted within the New York Presbyterian hospital network, which includes a quaternary acute care hospital, a community hospital, and a children’s hospital in NYC. Data were collected from 1330 sequential patients 65 years or younger with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) who were hospitalized or died in the emergency department between February 11, 2020, and May 7, 2020. Demographic information, hospitalization dates, laboratory values, and International Classification of Diseases, Tenth Revision codes for asthma diagnosis (J45.XX) were extracted from the New York Presbyterian Clinical Data Warehouse, which stores all electronic health record (EHR) data. Subsequently, EHR data were reviewed for each of the 1330 patients to verify admission, discharge, intubation, death dates, comorbidities including asthma, smoking status, and medications prescribed to treat COVID-19. Patients were included in the analysis if they had confirmed positive nasopharyngeal swab SARS-CoV-2 PCR test result during hospitalization, initially measured by the New York State Department of Health until the hospital system developed internal PCR testing protocols (Roche Cobas SARS-CoV-2 platform) on March 11, 2020. The study was approved by the Institutional Review Board of Columbia University Irving Medical Center (CUIMC) under expedited review with a waiver of consent.
Key variables of interest
Asthma was defined as having any 1 of the following: asthma-related International Classification of Diseases, Tenth Revision code (J45.XX) within the preceding 5 years (collected from New York Presbyterian Clinical Data Warehouse), asthma diagnosis recorded in the problem list of the EHR or documented in physician notes (COVID-19 hospital admission or discharge summary). Analysis was limited to patients 65 years or younger to reduce the influence of asthma-chronic obstructive pulmonary disease (COPD) overlap that occurs more frequently in older adults and is associated with worse prognosis than the individual diseases alone.22
Severe COVID-19 was defined as hospitalization with a confirmed positive SARS-CoV-2 PCR test result. In addition, we included patients who died in the emergency department because their precipitous death was an indication of severe disease. Primary clinical outcomes of disease severity included length of stay, hospital readmission, intubation, duration of mechanical ventilation, tracheostomy tube placement, and death. Secondary outcomes included initial laboratory measures collected on admission and medications used to treat COVID-19.
Statistical analysis
Demographic characteristics were compared using Kruskal-Wallis tests for continuous variables and Fisher exact tests for categorical variables. To characterize the association between subgroups of patients with asthma and clinical outcomes of severity, data were stratified by obesity, comorbidities, use of controller asthma medication (inhaled corticosteroids, combined inhaled corticosteroid and long-acting beta-agonists, or montelukast), and pre-COVID absolute eosinophil count (AEC) as an indicator of allergic disease (dichotomized at the upper tertile). Obesity was defined as body mass index more than 30 (data available for N = 950) or documentation of obesity in physician admission note or discharge summary (N = 60). Comorbidities were defined on the basis of the top 3 comorbidities identified in our cohort (presence of hypertension [38% of sample], diabetes [30%], or hyperlipidemia [19%]). Pre-COVID AEC was defined as last measured value, at least 2 weeks before positive COVID-19 diagnosis (available for N = 111 patients with asthma).
Cox proportional hazard models were used to estimate the association between asthma and time to extubation and time to hospital discharge. Models were adjusted for age, biological sex, obesity, black race, Latinx ethnicity, and comorbidities (presence of either hypertension, hyperlipidemia, or diabetes). Follow-up time was censored at May 20, 2020, for those who were still hospitalized or intubated. Discharge included to home, rehabilitation, or hospice facility and deaths. For time to extubation, individuals who were extubated or decannulated (if tracheostomy was performed) or returned to baseline if previously tracheostomized were considered as extubations and individuals who were still intubated or with tracheostomy were censored on the basis of the duration of follow-up. All analyses were conducted in SAS 7.4 (Cary, NC) and STATA 11 (College Station, Tex).
Asthma prevalence for adults 18 years or older for each ZIP code in NYC was calculated using data from the Centers for Disease Control and Prevention 500 Cities Project.23 Underlying geographic asthma prevalence was compared with the proportion of COVID-19 cases with asthma seen at CUIMC by the patients’ ZIP code of residence. All spatial visualizations were created in R v3.6.1 using the sp package (Vienna, Austria).
Results
Characteristics of patients with versus without asthma
Of the 1330 patients 65 years or older who were hospitalized with COVID-19, 32 had COPD and were excluded from analysis. Demographic characteristics for the remaining 1298 patients, of which 163 (12.6%) had an underlying asthma diagnosis, are included in Table I . Eight patients died in the emergency department, 1 of whom had asthma. There was no difference in length of stay, hospital readmission, intubation, tracheostomy tube placement, or mortality between patients with and without asthma (P > .05; Table I).
Table I.
Characteristics of patients with and without asthma stratified by age groups
| Characteristic | Overall (N = 1298) |
Age < 21 y (N=55) |
Age 21-39 y (N = 300) |
Age 40-65 y (N = 943) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No asthma (N = 1135) | Asthma (N = 163) | P value∗ | No asthma (N = 42) | Asthma (N = 13) | P value∗ | No asthma (N=261) | Asthma (N = 39) | P value∗ | No asthma (N = 832) | Asthma (N = 111) | P value∗ | |
| Age (y), median (IQR) | 52 (21) | 51 (27) | .26 | 15 (9) | 14 (9) | .76 | 32 (7) | 31 (7) | .12 | 56 (11) | 58 (10) | .60 |
| Sex: female, n (%) | 440 (39) | 96 (59) | <.01 | 22 (52) | 7 (54) | 1.00 | 140 (54) | 19 (51) | .61 | 278 (33) | 70 (63) | <.01 |
| Race, n (%)† | .07 | .26 | .48 | .11 | ||||||||
| Black | 238 (21) | 42 (26) | 9 (21) | 1 (8) | 49 (19) | 11 (28) | 180 (22) | 30 (27) | ||||
| White | 218 (19) | 34 (21) | 14 (33) | 8 (62) | 57 (22) | 6 (15) | 147 (18) | 20 (18) | ||||
| Asian | 14 (1) | 3 (2) | 2 (5) | 4 (31) | 3 (1) | 1 (3) | 9 (1) | 2 (2) | ||||
| Other | 384 (34) | 59 (36) | 10 (24) | 4 (31) | 100 (38) | 14 (36) | 274 (33) | 41 (37) | ||||
| Latinx ethnicity, n (%)‡ | 567 (50) | 76 (47) | .64 | 17 (40) | 10 (77) | .08 | 150 (57) | 20 (51) | .67 | 400 (48) | 46 (41) | .34 |
| Smoker, n (%)§ | .06 | .10 | .90 | .07 | ||||||||
| Never | 671 (59) | 103 (63) | 31 (74) | 11 (85) | 186 (71) | 30 (77) | 454 (55) | 62 (56) | ||||
| Current | 51 (4) | 4 (2) | 0 (0) | 0 (0) | 6 (2) | 1 (3) | 45 (5) | 3 (3) | ||||
| Former | 119 (10) | 25 (15) | 0 (0) | 1 (8) | 12 (5) | 1 (3) | 107 (13) | 23 (21) | ||||
| Obese, n (%) | 445 (39) | 85 (52) | <.01 | 6 (14) | 4 (31) | .22 | 116 (44) | 21 (54) | .30 | 323 (39) | 60 (54) | <.01 |
| Comorbidities, n (%) | 593 (52) | 85 (52) | 1.00 | 5 (12) | 2 (15) | .66 | 60 (23) | 9 (23) | 1.00 | 528 (63) | 74 (67) | .53 |
| Length of stay (d),‖ median (IQR) | 5 (7) | 6 (9) | .25 | 3 (7) | 9 (14) | .17 | 4 (4) | 6 (6) | .10 | 6 (9) | 6 (9) | .92 |
| Readmission, n (%) | 55 (5) | 9 (5) | .70 | 3 (7) | 0 (0) | 1.00 | 12 (5) | 4 (10) | .14 | 40 (5) | 5 (5) | 1.00 |
| Intubations, n (%) | 231 (20) | 34 (21) | .92 | 7 (17) | 3 (23) | .69 | 28 (11) | 6 (15) | .42 | 196 (24) | 25 (23) | .91 |
| Tracheostomy, n (%) | 71 (6) | 10 (6) | 1.00 | 1 (2) | 1 (8) | .42 | 5 (2) | 0 (0) | 1.00 | 65 (8) | 9 (8) | .85 |
| Death, n (%) | 101 (9) | 9 (6) | .18 | 1 (2) | 0 (0) | 1.00 | 7 (3) | 0 (0) | .60 | 93 (11) | 9 (8) | .42 |
IQR, Interquartile range.
Boldface indicates P < .05.
P value represents Kruskal-Wallis test for continuous variables and Fisher exact test for categorical variables.
Other category includes patients self-identified as American Indian/Alaskan, Native Hawaiian/Pacific Islander, or Other; Race identification declined in N = 281 (25%) from “No Asthma” and N = 25 (15%) from “Asthma” groups.
Ethnicity: Missing N = 257 (23%) from “No asthma” and N = 37 (23%) from “Asthma” groups.
Smoker: Missing N = 294 (26%) from “No asthma” and N = 31 (19%) from “Asthma” groups.
Length of stay: 78 patients with no asthma and 10 patients with asthma remain hospitalized.
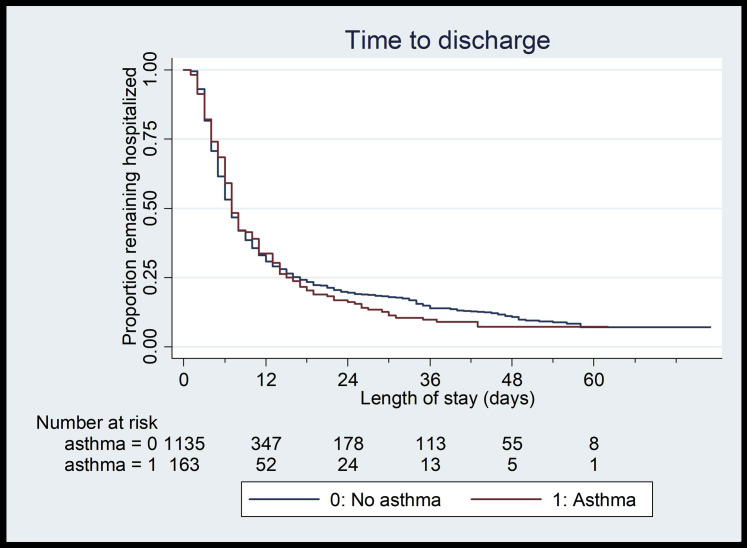
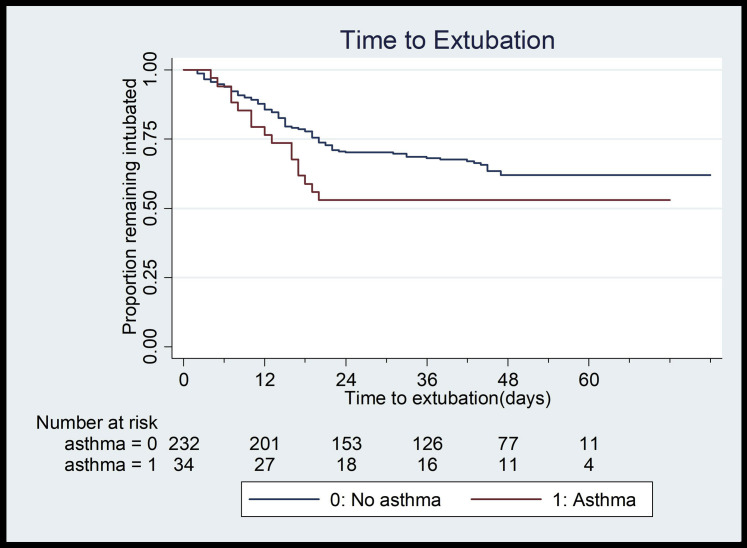
Among the 55 children younger than 21 years hospitalized with COVID-19, 24% (N = 13) had asthma, whereas 13% of patients aged 21 to 39 years (N = 39) and 13% of patients aged 40 to 65 years (N = 125) had underlying asthma (Table I). Only among older patients (age 40-65 years) were there more females and more obesity in patients with asthma compared with patients without asthma (females 63% vs 33%, P < .01; obese 54% vs 39%, P < .01) (Table I). There were no differences in length of stay, readmission, intubation, tracheostomy, or death between patients with asthma and patients without asthma across any age category (P > .05; Table I). Most notably, there were no deaths in patients with asthma younger than 40 years (Table I). In multivariable Cox proportional hazard models, there were no differences in length of stay (hazard ratio, 0.94; 95% CI, 0.78-1.12; Fig 1 ) or length of intubation between patients with and without asthma (hazard ratio, 1.33; 95% CI, 0.74-2.40; Fig 2 ).
Fig 1.
Kaplan-Meier curve demonstrating no significant difference in hospital length of stay between patients with (N = 163) and without (N = 1135) asthma.
Fig 2.
Kaplan-Meier curve demonstrating no significant difference in time to extubation between patients with (N = 163) and without (N = 1135) asthma.
Given that obesity has been reported as a risk factor for severe COVID-19, we compared outcomes of length of stay, readmission, intubation, tracheostomy, and death between patients with and without asthma, stratified by obesity, and observed no difference (P > .05; see Table E1 in this article’s Online Repository at www.jacionline.org). Similarly, there were no differences in outcomes comparing patients with and without asthma with or without the most prevalent other comorbidities (ie, diabetes mellitus, hypertension, or hyperlipidemia) (P > .05; Table E1). As an indicator of more severe asthma, we compared patients with asthma who were on controller medications versus not on controllers and again there was no difference in outcomes (P > .05; see Table E2 in this article’s Online Repository at www.jacionline.org). Likewise, there was no difference in outcomes on comparing patients with asthma with higher versus lower pre-COVID AEC (P > .05; Table E2) except for a greater frequency of readmission among patients with higher AEC (P = .04).
Laboratory values and medications administered
Laboratory values at initial presentation for COVID-19 hospitalization were available for a subset of the sample (see Table E3 in this article’s Online Repository at www.jacionline.org). C-reactive protein (CRP), D-dimer, ferritin, and glucose levels were lower in hospitalized patients with asthma versus patients without asthma (mean ± SD, CRP: 89 ± 72.8 vs 107 ± 79.7, P = .02; D-dimer: 1.8 ± 2.3 vs 2.4 ± 3.1, P = .02; ferritin: 619 ± 1130 vs 1111 ± 2696, P < .01; glucose: 135 ± 68 vs 168 ± 99, P = .02). Similar trends were observed when evaluating differences in CRP, D-dimer, ferritin, and glucose levels between patients with and without asthma who were not treated with systemic steroids or inhaled corticosteroids (data not shown), suggesting that the differences in laboratory values were not driven by the use of steroids. All other laboratory values were similar in patients with and without asthma (Table E3). Hospitalized COVID-19 patients with asthma were more frequently treated with systemic steroids compared with those without asthma (27% vs 17%; P < .01). However, there was no difference in frequency of treatment with azithromycin, hydroxychloroquine, tocilizumab, or remdesivir among patients with versus without asthma (P > .05; see Table E4 in this article’s Online Repository at www.jacionline.org).
Comparisons of patients hospitalized with COVID-19 and underlying asthma with overall asthma prevalence
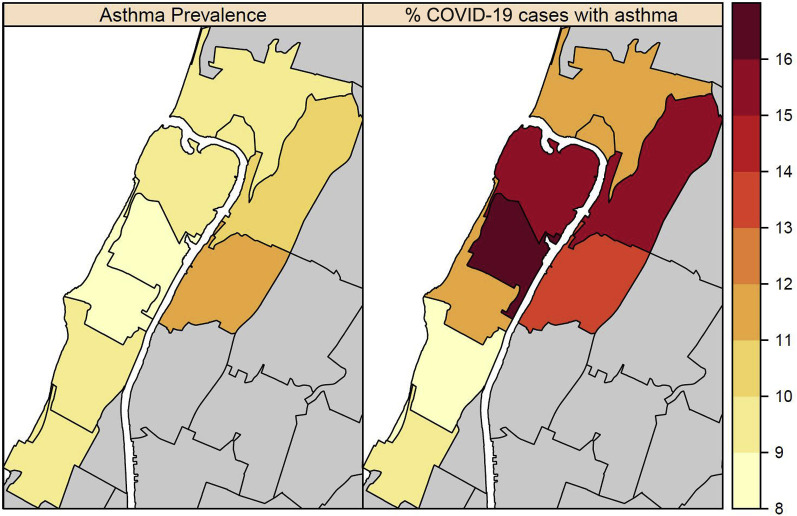
Within ZIP codes where most patients lived (>50 COVID-19 cases per ZIP code), underlying adult asthma prevalence ranges from 8.9% to 11.6% (Fig 3 ), with marginally higher prevalence among children. Across all except 1 ZIP code, the percentage of patients hospitalized with COVID-19 who had underlying asthma (range, 8.1%-17.2% per zip code) was greater than the overall asthma prevalence in that ZIP code (Fig 3).
Fig 3.
Relationship between ZIP code asthma prevalence in adults and proportion of adult COVID-19 cases with asthma in ZIP codes with more than 50 COVID-19 cases at CUIMC.
Discussion
In our sample of 1298 patients, 65 years or younger with severe COVID-19 admitted across our quaternary acute care, community, and pediatric hospitals in NYC, the epicenter of the COVID-19 pandemic, 12.6% of patients had asthma. There was no difference in length of stay, intubation, tracheostomy placement, hospital readmission, or death on comparing patients with versus without asthma. Outcomes were similar between patients with and without asthma regardless of obesity, comorbidities, and controller medication prescription. Overall, our findings suggest that although more patients with asthma may be hospitalized for COVID-19 compared with the prevalence of asthma in their neighborhood, history of asthma is not associated with worse COVID-19 outcomes among hospitalized patients with severe disease.
The rationale for exploring asthma as an underlying risk factor for hospitalization and severe disease in COVID-19 is multifold. Asthma exacerbations in both adults and children are often triggered by viral respiratory infections including many coronaviruses,24 , 25 and hospitalizations increase in seasons when viral illnesses are more common.26 , 27 Yet, clinical data and animal models of severe acute respiratory syndrome and Middle East respiratory syndrome, the 2 other strains of coronavirus known to cause severe respiratory illness, do not suggest that asthma plays a significant role in severe disease.4 , 28 Similarly, our data do not support that asthma alone contributes to increased morbidity among patients with severe COVID-19.
This is the largest study to date to investigate the association between asthma-specific morbidity and mortality, independent of COPD, in hospitalized patients with severe COVID-19. Our study is strengthened by the inclusion of patients from different types of hospitals within the COVID-19 epicenter including quaternary acute care, community, and pediatric hospitals. We specifically chose to investigate hospitalized patients because of the interest in understanding morbidity in severe disease and the lack of widespread availability of COVID-19 testing that could bias overall prevalence estimates. In a recent report from Chicago, length of intubation was reportedly longer among patients with asthma than among patients without asthma.19 However, this was a much smaller sample of only 297 hospitalized patients. Also, the reported prevalence of asthma in that adult-only sample was much higher than reported in any other study to date that could be explained by significant overlap with COPD or other respiratory diseases, or a difference in asthma phenotypes. In fact, a recent study in Russia demonstrated a trend toward worse COVID-19 disease course among patients with COPD in their intensive care units compared with patients with underlying asthma.29 Nonetheless, our studies highlight the need for further population-based studies to assess the risk for developing severe COVID-19 once universal testing becomes available.
In our study, asthma prevalence was higher specifically among pediatric hospitalized patients with COVID-19 compared with adults. Our findings are consistent with a reported asthma prevalence of 24% among hospitalized pediatric patients from a different NYC hospital.17 The smaller number and greater geographic distribution of pediatric patients with COVID-19 in our sample prevented a ZIP code–based comparison with underlying asthma prevalence in children. In 2017, the NYC Department of Health estimated that approximately 11.2% of NYC children younger than 13 years had ever been diagnosed with asthma, while approximately 16% of public high school students reported an asthma attack in the previous 12 months.30 Both these estimates of asthma prevalence are lower than what we observed among the pediatric patients with COVID-19 reporting to CUIMC. In another Centers for Disease Control and Prevention report of 7162 SARS-CoV-2–positive cases, 9.2% had a history of chronic lung disease, a term inclusive of asthma, COPD, and emphysema.13 Of note, the prevalence of chronic lung disease appears to increase with a higher level of care, with a rate of 7% in those not requiring hospitalization and rates of 15% and 21% in hospitalized patients without versus with intensive care unit level care, respectively.
We noted similar morbidity and mortality among patients with asthma with and without coexistent high-risk conditions such as diabetes, hypertension, and hyperlipidemia. Thus, the prevalence of asthma as a comorbidity in severe COVID-19 appears to be similar to that of other previously reported comorbidities.6 , 7 , 10 , 15 , 16 , 31 , 32 SARS-CoV-2 targets the respiratory system through binding with angiotensin-converting enzyme 2 (ACE2) receptors in the alveolar epithelium.33 , 34 Although some conditions such as old age, smoking, obesity, cancers, COPD, and interstitial lung disease may be associated with higher ACE2 expression, conditions such as diabetes, hypertension, and kidney disease are associated with downregulation of these receptors.35 , 36 Given findings of an inverse relationship of ACE2 expression in patients with respiratory allergies and with various controlled allergen exposures, there have been speculations that asthma may not be as prevalent in COVID-19–related illness, though related outcomes or different phenotypes of asthma have not been investigated.37 In a recent small ex vivo study, administration of IL-13, a type 2 cytokine that is elevated in asthma, was shown to downregulate ACE2 expression in airway epithelial cells.38 Although ACE2 plays an essential role in alveolar epithelial cellular entry, its role in the pathogenesis and prognosis of COVID-19 is complex and double-edged. A dual role of ACE2 in viral entry and multiplication, as well as in lung protection, has been shown.35 , 39 ACE2 has an inverse relationship with age, but our younger patients hospitalized with COVID-19 had a higher prevalence of asthma than did adults, suggesting asthma may be a comorbidity for severe COVID-19 among the pediatric population normally not compromised by other cardiovascular risk factors.
Obesity has been highlighted as a comorbidity with increased risk of severe illness and fatal outcomes in COVID-19.16 , 40, 41, 42, 43 Several plausible mechanisms include associated endothelial dysfunction, metabolic syndrome, proinflammatory state, increased ACE2 expression in adipose tissue, and poor chest wall and lung mechanics.44 , 45 In our study, outcomes did not differ in obese and nonobese patients with and without asthma, again suggesting no change in risk attributed to asthma alone. Although we were unable to further characterize asthma phenotypes in our patients, eosinophil counts did not differ among the 2 groups, which could be partially explained by masking effects of eosinopenia, a known finding in severe illnesses that is documented in severe SARS-CoV-2 infection and a known effect of systemic steroids.46 , 47
Another postulated mechanism for similar disease severity among COVID-19 patients with asthma is use of inhaled steroids, which can suppress coronavirus replication and cytokine production and may be associated with reduced ACE2 expression.5 , 37 , 48, 49, 50 Although theoretically this could be one explanation for lower levels of inflammatory and laboratory markers such as CRP, D-dimer, ferritin, and glucose among our patients with asthma, differences in laboratory markers were noted even among patients who were not treated with inhaled or systemic steroids. Alternatively, patients with asthma may have presented early in the inflammatory cascade due to earlier development of respiratory compromise in asthma. There was a significantly higher use of systemic steroids among our patients with asthma compared with patients without asthma. The implications of this are uncertain at this time and may have favorably or adversely affected outcomes in these patients. Although challenging to separate entirely, it is possible that among a subset of patients including children, the morbidity in asthma may be related to a viral illness–triggered asthma exacerbation with or without the proinflammatory acute respiratory distress syndrome phase that is clearly described in COVID-19.
We are limited in our ability to ascertain the risk of developing severe COVID-19 among patients with asthma given the observational nature of our study. However, our findings are strengthened by our approach of manually reviewing EHR data to confirm asthma history rather than relying solely on electronic data extractions, which could result in misclassification of asthma.51 Another limitation of our retrospective review is the inability to completely characterize phenotypes of asthma although we were able to capture obesity and pre-COVID eosinophil counts.
Conclusions
Overall, asthma was not associated with adverse outcomes in severe COVID-19, regardless of age, obesity, or other comorbidities. Associations between COVID-19 and asthma should be investigated further in a larger pediatric population. Also, population-based studies are needed to determine whether asthma is a risk factor for developing COVID-19 once universal testing becomes readily available.
Clinical implications.
The lack of association between asthma and severe COVID-19 outcomes among hospitalized patients 65 years or younger provides insight that can be used in determining prognosis for patients with severe COVID-19.
Acknowledgments
We acknowledge Alla Babina from the Columbia University Clinical Data Warehouse for her assistance with retrieving data.
Footnotes
We disclose the following funding sources that played no role in the writing of the manuscript or in the decision to submit it for publication: National Institutes of Health - K01 HL140216, Robert Wood Johnson Foundation – Amos Medical Faculty Development Award Program.
Disclosure of potential conflict of interest: D R. Deshpande’s spouse is employed by Bristol Myers Squibb. The rest of the authors declare that they have no relevant conflicts of interest.
Appendix
Table E1.
Stratification of clinical outcomes in patients with and without asthma stratified by obesity and comorbidities
| Clinical outcomes | No obesity (N = 768) |
With obesity (N = 530) |
||||
|---|---|---|---|---|---|---|
| No asthma (N = 690) | Asthma (N = 78) | P value∗ | No asthma (N = 445) | Asthma (N = 85) | P value∗ | |
| Length of stay (d), median (IQR) | 5 (7) | 5 (6) | .67 | 6 (7) | 7 (9) | .06 |
| Readmission, n (%) | 39 (6) | 4 (5) | 1.00 | 16 (4) | 5 (6) | .36 |
| Intubation, n (%) | 125 (18) | 14 (18) | 1.00 | 106 (24) | 20 (24) | 1.00 |
| Tracheostomy, n (%) | 36 (5) | 5 (6) | .60 | 35 (8) | 5 (6) | .66 |
| Death, n (%) | 52 (8) | 3 (4) | .35 | 49 (11) | 6 (7) | .33 |
| No comorbidities† (N = 641) |
With comorbidities† (N = 657) |
|||||
|---|---|---|---|---|---|---|
| No asthma (N = 561) | Asthma (N = 80) | P value∗ | No asthma (N = 574) | Asthma (N = 83) | P value∗ | |
| Length of stay (d), median (IQR) | 5 (7) | 6 (7) | .32 | 6 (9) | 6 (8) | .55 |
| Readmission, n (%) | 24 (4) | 4 (5) | .77 | 30 (5) | 5 (6) | .79 |
| Intubation, n (%) | 91 (16) | 16 (20) | .42 | 142 (24) | 19 (22) | .68 |
| Tracheostomy, n (%) | 30 (5) | 3 (4) | .79 | 41 (7) | 7 (8) | .65 |
| Death, n (%) | 33 (6) | 2 (3) | .30 | 68 (12) | 7 (8) | .46 |
IQR, Interquartile range.
Kruskal-Wallis test for length of stay and Fisher exact test for all other outcomes.
Comorbidities include diabetes mellitus, hypertension, and hyperlipidemia.
Table E2.
Stratification of clinical outcomes among individuals with asthma by controller asthma medication use and pre-COVID AEC
| Clinical outcomes | Controller medication use |
P value∗ | |
|---|---|---|---|
| No controller (N = 104) | Controller (N = 59) | ||
| Length of stay (d), median (IQR) | 6 (9) | 6 (8) | .61 |
| Readmission, n (%) | 8 (8) | 1 (2) | .16 |
| Intubation, n (%) | 21 (20) | 13 (22) | .84 |
| Tracheostomy, n (%) | 5 (5) | 5 (8) | .50 |
| Death, n (%) | 5 (5) | 4 (7) | .72 |
| Pre-COVID AEC |
P value∗ | ||
|---|---|---|---|
| Lower AEC† (N = 75) | Higher AEC† (N = 36) | ||
| Length of stay (d), median (IQR) | 5 (8) | 7 (8) | .12 |
| Readmission, n (%) | 2 (3) | 5 (14) | .04 |
| Intubation, n (%) | 13 (17) | 8 (22) | .61 |
| Tracheostomy, n (%) | 5 (7) | 3 (8) | .71 |
| Death, n (%) | 4 (5) | 0 (0) | .30 |
IQR, Interquartile range.
Boldface indicates P < .05.
Kruskal-Wallis test for length of stay and Fisher exact test for all other outcomes.
AEC dichotomized at the upper tertile, ≥0.23 × 103 cells/ μL.
Table E3.
Laboratory values during initial presentation for hospitalization among patients with versus without asthma
| Laboratory value | No asthma (N = 1135) |
Asthma (N = 163) |
P value | ||
|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | ||
| WBC (×103 cells/μL) | 968 | 8.4 ± 5.1 | 145 | 7.7 ± 3.9 | .08 |
| CRP (mg/L) | 754 | 107 ± 79.7 | 125 | 89 ± 72.8 | .02 |
| ESR (mm/h) | 765 | 70.8 ± 31.5 | 113 | 65.5 ± 30.5 | .09 |
| ANC (×103 cells/μL) | 895 | 6.5 ± 5.2 | 143 | 5.8 ± 4.0 | .05 |
| ALC (×103 cells/μL) | 895 | 1.3 ± 1.0 | 143 | 1.3 ± 0.7 | .69 |
| AEC (×103 cells/μL) | 847 | 0.06 ± 0.13 | 141 | 0.07 ± 0.13 | .49 |
| Hemoglobin (g/dL) | 968 | 12.3 ± 2.3 | 145 | 12.2 ± 2.3 | .61 |
| D-dimer (μg/mL FEU) | 704 | 2.4 ± 3.1 | 111 | 1.8 ± 2.3 | .02 |
| Hemoglobin A1C (%) | 286 | 8.4 ± 2.7 | 31 | 7.4 ± 2.3 | .07 |
| Procalcitonin (ng/mL) | 820 | 2.5 ± 17.4 | 126 | 3.8 ± 35.8 | .68 |
| Platelets (×103 cells/μL) | 968 | 250 ± 116 | 145 | 257 ± 119 | .46 |
| IL-6 (pg/mL) | 654 | 54.4 ± 84.3 | 99 | 42.5 ± 77.5 | .19 |
| Ferritin (ng/mL) | 1041 | 1111 ± 2696 | 150 | 619 ± 1130 | <.01 |
| Fibrinogen (mg/dL) | 203 | 593 ± 216 | 29 | 575 ± 214 | .68 |
| Glucose (mg/dL) | 172 | 168 ± 99 | 38 | 135 ± 68 | .02 |
ALC, Absolute lymphocyte count; ANC, absolute neutrophil count; ESR, erythrocyte sedimentation rate; WBC, white blood count.
Boldface indicates P < .05.
Normal levels for laboratory values: CRP high sensitivity 0-10; D-dimer ≤0.80; procalcitonin ≤0.08 ng/mL; IL-6 ≤5 pg/mL; ferritin 30-400 ng/mL; fibrinogen 191-430 mg/dL.
Table E4.
Medications administered for treatment of COVID-19 among hospitalized patients with versus without asthma
| Medicine | No asthma (N = 1135) | Asthma (N = 163) | P value∗ |
|---|---|---|---|
| Azithromycin, n (%) | 422 (37) | 72 (44) | .10 |
| Systemic corticosteroids, n (%) | 196 (17) | 44 (27) | <.01 |
| Hydroxychloroquine, n (%) | 578 (51) | 89 (55) | .40 |
| Tocilizumab, n (%) | 83 (7) | 12 (7) | 1.00 |
| Remdesivir, n (%) | 31 (3) | 8 (5) | .14 |
Boldface indicates P < .05.
Fisher exact test.
References
- 1.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) 2020.. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html Available at:
- 2.Abrams EM, Szefler SJ. Managing asthma during COVID-19: an example for other chronic conditions in children and adolescents [published online ahead of print April 21, 2020]. J Pediatr. https://doi.org/10.1016/j.jpeds.2020.04.049. [DOI] [PMC free article] [PubMed]
- 3.Zheng X.-Y., Xu Y.-J., Guan W.-J., Lin L.-F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol. 2018;163:845–853. doi: 10.1007/s00705-017-3700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Bever H.P., Chng S.Y., Goh D.Y. Childhood severe acute respiratory syndrome, coronavirus infections and asthma. Pediatr Allergy Immunol. 2004;15:206–209. doi: 10.1111/j.1399-3038.2004.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halpin D.M.G., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8:436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Xu S, Yu M, Wng K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan [published online ahead of print April 12, 2020]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed]
- 10.Zhang J-J, Dong X, Cao Y-Y, Yuan Y-B, Yan Y-Q, Akdis CA, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [published online ahead of print February 19, 2020]. Allergy. https://doi.org/10.1111/all.14238. [DOI] [PubMed]
- 11.Centers for Disease Control and Prevention Asthma’s effect on the nation. 2020.. https://www.cdc.gov/asthma/asthmadata.htm Available at:
- 12.Huang K., Yang T., Xu J., Yang L., Zhao J., Zhang X. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394:407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 13.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City [published online ahead of print April 17, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed]
- 16.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published online ahead of print April 22, 2020]. JAMA. https://doi.org/10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed]
- 17.Chao J.Y., Derespina K.R., Herold B.C., Goldman D.L., Aldrich M., Weingarten J. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 (COVID-19) at a tertiary care medical center in New York City. J Pediatr. 2020;223:14–19.e2. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahdavinia M., Foster K.J., Jauregui E., Moore D., Adnan D., Andy-Nweye A.B. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. 2020;8:2388–2391. doi: 10.1016/j.jaip.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E. Prevalence and characterization of asthma in hospitalized and non-hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L., Wang B., Yuan T., Cen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendy A., Forno E., Niyonsenga T., Carnahan R., Gasana J. Prevalence and features of asthma-COPD overlap in the United States 2007-2012. Clin Respir J. 2018;12:2369–2377. doi: 10.1111/crj.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Health Outcomes | 500 Cities. 2019.. https://www.cdc.gov/500cities/definitions/health-outcomes.htm Available at:
- 24.Papadopoulos N.G., Christodoulou I., Rohde G., Agache I., Almqvist C., Bruno A. Viruses and bacteria in acute asthma exacerbations--a GA2 LEN-DARE systematic review. Allergy. 2011;66:458–468. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurai D., Saraya T., Ishii H., Takizawa H. Virus-induced exacerbations in asthma and COPD. Front Microbiol. 2013;4:293. doi: 10.3389/fmicb.2013.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss K.B. Seasonal trends in US asthma hospitalizations and mortality. JAMA. 1990;263:2323–2328. [PubMed] [Google Scholar]
- 27.Kimes D., Levine E., Timmins S., Weiss S.R., Bollinger M.E., Blaisdell C. Temporal dynamics of emergency department and hospital admissions of pediatric asthmatics. Environ Res. 2004;94:7–17. doi: 10.1016/s0013-9351(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 28.Han M., Rajput C., Ishikawa T., Jarman C.R., Lee J., Hershenson M.B. Small animal models of respiratory viral infection related to asthma. Viruses. 2018;10:682. doi: 10.3390/v10120682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avdeev S, Moiseev S, Brovko M, Yavorovskiy A, Umbetova K, Akulkina L, et al. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19 [publishd online ahead of print May 26, 2020]. Allergy. https://doi.org/10.1111/all.14420. [DOI] [PMC free article] [PubMed]
- 30.Environment & Health Data Portal http://a816-dohbesp.nyc.gov/IndicatorPublic/Subtopic.aspx?theme_code=2,3&subtopic_id=11 Available at:
- 31.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. Correction in: Lancet Respir Med 2020;8:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J., Wang X., Jia X., Li J., Hu K., Chen G. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26:767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Zhou W., Yang L., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J Clin Med. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–88.e8. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan T., Xiao R., Lin G. Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: a double-edged sword? FASEB J. 2020;34:6017–6026. doi: 10.1096/fj.202000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with COVID-19 [published online ahead of print April 30, 2020]. Obesity (Silver Spring). https://doi.org/10.1002/oby.22859. [DOI] [PMC free article] [PubMed]
- 41.Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID-19 [published online ahead of print May 21, 2020]. Obesity (Silver Spring). https://doi.org/10.1002/oby.22842. [DOI] [PMC free article] [PubMed]
- 42.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity is associated with higher in-hospital mortality in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michalakis K., Ilias I. SARS-CoV-2 infection and obesity: common inflammatory and metabolic aspects. Diabetes Metab Syndr. 2020;14:469–471. doi: 10.1016/j.dsx.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tibiriçá E, Lorenzo AD. Increased severity of COVID-19 in people with obesity: are we overlooking plausible biological mechanisms? [published online ahead of print May 13, 2020]. Obesity (Silver Spring). https://doi.org/10.1002/oby.22887. [DOI] [PMC free article] [PubMed]
- 46.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallen N., Kita H., Weiler D., Gleich G.J. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991;147:3490–3495. [PubMed] [Google Scholar]
- 48.Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15 [published online ahead of print March 12, 2020]. bioRxiv. doi: 10.1101/2020.03.11.987016. [DOI]
- 49.Yamaya M., Nishimura H., Deng X., Sugawara M., Watanabe O., Nomura K. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58:155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maes T., Bracke K., Brusselle G.G. COVID-19, asthma, and inhaled corticosteroids: another beneficial effect of inhaled corticosteroids? Am J Respir Crit Care Med. 2020;202:8–10. doi: 10.1164/rccm.202005-1651ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieberman-Cribbin W, Rapp J, Alpert N, Tuminello S, Taioli E. The impact of asthma on mortality in patients with COVID-19 [published online ahead of print June 6, 2020]. Chest. https://doi.org/10.1016/j.chest.2020.05.575. [DOI] [PMC free article] [PubMed]