Abstract
The surface stability and resulting transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), specifically in indoor environments, have been identified as a potential pandemic challenge requiring investigation. This novel virus can be found on various surfaces in contaminated sites such as clinical places; however, the behavior and molecular interactions of the virus with respect to the surfaces are poorly understood. Regarding this, the virus adsorption onto solid surfaces can play a critical role in transmission and survival in various environments. In this article, we first give an overview of existing knowledge concerning viral spread, molecular structure of SARS-CoV-2, and the virus surface stability is presented. Then, we highlight potential drivers of the SARS-CoV-2 surface adsorption and stability in various environmental conditions. This theoretical analysis shows that different surface and environmental conditions including temperature, humidity, and pH are crucial considerations in building fundamental understanding of the virus transmission and thereby improving safety practices.
Keywords: SARS-CoV-2, surface chemistry, virus adsorption onto the surfaces, environmental conditions, molecular interactions, nonlinear optical techniques, bond and ion specific probes
Graphical Abstract
The Bigger Picture
Challenges and opportunities
-
•
The novel SARS-CoV-2 can be recognized on various surfaces in a contaminated site, and its stability in different environmental conditions has been reported.
-
•
The literature suffers from the lack of fundamental understanding of molecular drivers of the virus-surface interactions, and the chemistry that occurs on the solid surfaces and its effects on the virus adsorption and stability is still in its nascent stages because of the complexity of the phenomena.
-
•
The roles of fluids pH values, surface chemistry, relative humidity, and temperature in the virus adsorption and desorption phenomena and persistence of SARS-CoV-2 on surfaces should be explored, and experimental scientists need to unravel the molecular drivers implicated in this new coronavirus transmission from the surfaces in different environmental conditions.
The main drivers of the SARS-CoV-2 adsorption onto the solid surfaces include surface active moieties of the viral proteins, hydrophilic/hydrophobic characteristics of the surface, pH of the bulk fluid, relative humidity, and temperature of the environment. The highlighted findings shed light onto the potential molecular interactions between the virus and solid surfaces, which leads to further virus adsorption, viability, and transmission processes, and can help the scientific community take necessary measures to tackle associated challenges.
Molecular Structure of Novel SARS-CoV-2 and Its Surface-Active Species
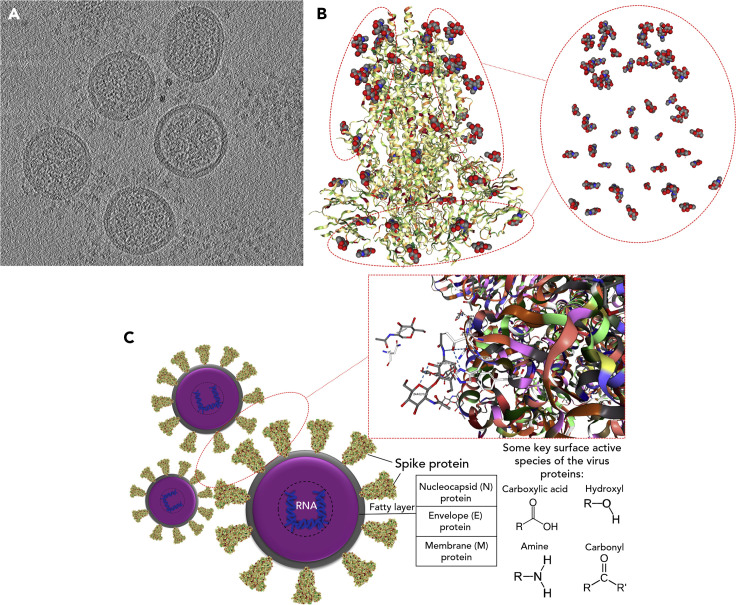
Coronavirus genomes are comparatively large for RNA viruses and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) encodes an extensive complement of non-structural proteins (e.g., 3-chymotrypsin-like [3CL] protease, papain-like protease, etc.) as well as structural proteins as follows: spike (S) glycoprotein, envelope (E) glycoprotein, membrane (M) glycoprotein, and nucleocapsid (N) phosphoprotein.1 The SARS-CoV-2 spike (S) glycoprotein exhibits ∼76% amino acid sequence identity with the SARS-CoV S (Urbani strain) and ∼80% identity with S proteins of bat SARSr-CoV ZXC21 and ZC45.1 , 2 CoV S glycoproteins form club-shaped trimers and decorate the viral membrane,3 giving coronavirus virions their characteristic morphology. As a substantial component of the outer surface of the virion, S likely plays a critical role in adsorption of viruses onto the solid surfaces under various environmental conditions.4 For further clarification, Figure 1 A depicts a central slice through an electron micrograph of mouse hepatitis virus, which exhibits the presence of S on the virion surface. Figures 1B and 1C also illustrate the structure of the SARS-CoV-2 S glycoprotein, its surface-active species, and potential intermolecular interactions among them during virus assembly.
Figure 1.
Structural Chemistry and Identifying Surface-Active Species of the Virus
(A) A central slice through a cryo-EM tomogram of mouse hepatitis virus for showing the existence of S on the outer surface of virions.
(B) Structure of the SARS-CoV-2 spike glycoprotein with highlighting corresponding functional groups (protein database [PDB]: 6VXX). Grey, blue, and red spheres are carbon, nitrogen, and oxygen atoms, respectively. The molecular structure is colored based on hydrophobicity; colored from red (hydrophilic) to green (hydrophobic). It utilizes the experimentally attained hydrophobicity scale that relied on whole-residue free energies of transfer ΔG (kcal/mol) from water to 1-Palmitoyl-2-oleoylphosphatidylcholine (POPC) interface.
(C) Model representation of SARS-CoV-2 with respective proteins assembly. Key molecular interactions among proteins on the surface of the virus particle are shown as gray dash lines, “hydrophobic interactions,” and blue dash lines, “hydrogen bonding (–O–H···O),” (PDB: 6VYB). Some key surface-active moieties of SARS-CoV-2 are denoted as hydroxyl, amine, carbonyl, and carboxylic acid functional groups.
Determination of the adsorption and stability of SARS-CoV-2 on various surfaces and under environmental conditions relevant to outbreak settings and zones is vital for amending safety measures for health care professionals, along with responding to the questions about SARS-CoV-2 public transmission. In the following sections we discuss mechanisms of virus adsorption onto different solid surfaces considering the aforementioned knowledge of the structure of viral surface glycoproteins in which amino acid functional groups play a critical role in the adhesion process.
Surface Stability of Novel Coronavirus on Various Surfaces—Adsorption Perspective
Investigations of the stability of both SARS-CoV-2 and SARS-CoV-1 in air and on different solid surfaces including stainless steel, plastic, copper, and cardboard have calculated their persistence kinetics utilizing a Bayesian regression model.5 It has been shown that SARS-CoV-2 was more stable on plastic and stainless steel surfaces than on copper and cardboard pieces, and the presence of virus was detected up to ∼72 h on certain solid surfaces. The longest persistence of viability for SARS-CoV-2 was found to be on stainless steel and plastic, which showed low-level viability after 72 h. No viable SARS-CoV-2 was detected after ∼4 and ∼24 h on copper and cardboard, respectively. After 3 h, viable virus was still detected in aerosols. These experimental data imply that fomite transmission of SARS-CoV-2 is likely, given that the virus can stay viable and infectious for h or up to several days on solid surfaces.
Generally, some viruses can retain their activities and transmission in the environment for a long period of time, possibly because of the adsorption process on surfaces.6 , 7 It can be inferred that building a fundamental understanding of the molecular interactions between viruses, i.e., the outer surface of proteins, and solid surfaces is crucial for controlling environmental transmission and designing removal processes and treatment strategies. The quantity of adsorbed virus is influenced by multiple factors, including characteristics of the virus’s outer surface proteins such as surface charge, size, stability, and steric conformation.8 These are properties of the amino acid composition and post-translational modifications such as addition of carbohydrate moieties.9 Likewise, substrate surface chemistry and environmental conditions are also key. Therefore, to determine the ability of the virus to adsorb to a surface, both the virus and the surface need to be well characterized and an understanding of how these change with environmental conditions is key.
Viruses adsorb to surfaces through two main mechanisms, van der Waals (mainly mineral surfaces10) and, more importantly, electrostatic interactions (charged surfaces in the presence of ions and or not neutral pH11, 12, 13). These two forces dictate the adsorption of viruses to surfaces. Although the interplay between these two forces is difficult to separate, indications of the interactions can be determined from past data. Viruses tend to be more hydrophobic than proteins,14 thus they are attracted to metal surfaces because of mainly van der Waals interactions as well as hydrophobic effects.15 However, their ability to maintain the virus’s viability and allow it to remain infectious is more of a function of the humidity and temperature,16 thus the surface energy of the water molecules plays a large role in the interaction between a virus particle and a surface.
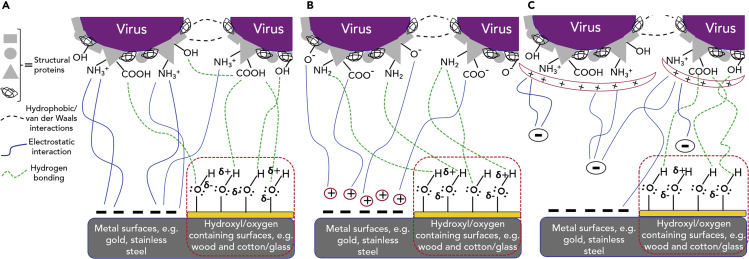
SARS-CoV-2 virions can be adsorbed onto metal surfaces (e.g., gold and stainless steel) in addition to hydroxyl functional group- and oxygen-containing substrates (e.g., wood, cotton, paper, and glass) depending on the surface chemistry and environmental conditions (e.g., bulk fluid pH, surface charge, temperature, etc).17 Hydrogen bonding plays a key role in the adsorption of viruses to the hydroxyl-containing surfaces and in the presence of an aqueous phase thin film layer.17 The strength of the bond to the surface would be high in the presence of –O–H···O bonding, particularly in pH environments where the carboxylic acid on the virus is deprotonated (typically above a pH of 418). At neutral pH, most viral particles have a net negative charge because they have an isoelectric point below 7.19 However, due to the large size of virus particles and their large variety of surface proteins, there are still multiple patches of positive and negative charge in the pH range where viruses are stable (typically from pH 5–820). Therefore, −NH2, −NH3 +, −COOH, and −COO− groups of amino acids in the SARS-CoV2 S protein drive adsorption onto the solid surfaces through double electrostatic interactions between the virion’s ionized surface-active species and the oppositely charged surfaces, as well as hydrogen bonding based on the surface characteristics. For example, at neutral pH values, the negatively charged virus particles would be adsorbed significantly less on a stainless-steel surface because of electrostatic repulsion,21 given that both virion and substrate surface have negative charges. With augmentation of the cation concentration, however, the repulsion would be decreased,22 and the quantity of adsorbed viruses would increase. Figure 2 presents the potential molecular interactions between the SARS-CoV-2 viral proteins and solid surfaces at different pH values and fluid chemistries. As denoted in Figure 2A, at pH values below the isoelectric point, the overall charge of SARS-CoV-2 could be positive, given that both the carboxylate and amine groups on the outer surface are protonated, and hydrogen bonding would be formed to hydroxyl-containing surfaces such as wood, cotton, or paper. At pH environments above the isoelectric point (Figure 2B), the outer surface of virions would be deprotonated and therefore negatively charged and cannot be adsorbed on the surface with the same charge. Accordingly, lower virus adsorption onto the surfaces would occur at higher pH values. Instead, they can interact strongly with divalent and/or monovalent cations23 if they existed in a brine electrolyte solution (more details are presented in the following section). The charge and counterions from the electrolyte could lead to thinner double layer and lower repulsion forces, and again hydrogen bonding formed to surface hydroxyl groups,24 which results in promoting the virus adsorption process. The gold surface of an electrode in the quartz crystal microbalance (QCM) biosensor, which works on the basis of the oscillating frequency alteration,25 could be employed for monitoring of the virus surface adsorption and desorption phenomena with or without the presence of negatively charged surface active species in the liquid phase.
Figure 2.
Molecular Interactions at SARS-CoV-2 Viral Interfaces in Different Environmental Conditions
Model of the potential molecular interactions among viruses and between virus and different solid surfaces having negative surface charge and/or hydroxyl functional groups at (A) relatively low pH environment, below the isoelectric point; (B) relatively high pH condition, above isoelectric point in presence of external ions (e.g., salts); and (C) way below the isoelectric point in the presence of potential chemistries (for removal from surface purposes) with negative surface charge.
3- The Effects of Humidity and Temperature on the Virus Adsorption Phenomenon
The functionality of viruses is not only based on their host cell but also on the different environments faced before and during the transmission process.26 Coronaviruses have been shown to have different inactivation kinetics at different humidities.27 Therefore, an understanding of the effect of humidity on surface stability and adsorption of this novel virus is crucial. Water molecules in the liquid phase condense from its vapor phase on various surfaces (e.g., between virus particles and solid substrates) and create liquid bridges with a curvature, which is related to the relative humidity “h” expressed by the Kelvin equation28 (Equation 1). When the solid surfaces are fixed in one dimension, as for structures with a high aspect ratio, the interface curvature radii “r” is determined by:
| (Equation 1) |
where γ is the water surface tension, V m is the water molar volume, R is the gas constant, T is the temperature. The water roundel radius |r| increments with h and would be infinite at full hydration with relative humidity of 100%.
On the water-coated surfaces, the virus particles would establish strong interactions with the hydrophilic surface in the presence of a thin film water layer, mainly through hydrogen bonding29 between water and the virus outer surface protein molecules. Water molecules can also fill the gaps between the virus particles that are spaced closer than the |r| value defined by the relative humidity. On hydrophobic surfaces, the roundel expands less. Therefore, a thin layer of water can be created around the virions; however, the lunule might not be unified to bridge the gap between two virus particles. Figure 3 illustrates this phenomenon.
Figure 3.
The Effects of Humidity and Temperature on SARS-CoV-2 Surface Stability
(A and B) The schematic diagram of the SARS-CoV-2 particles onto the (A) hydrophilic and (B) hydrophobic surfaces at environments with high and low relative humidity. The detailed molecular structure of S glycoproteins on the outer surface of the virus are not depicted for the sake of lucidity.
(C) The effect of temperature on exhaled virus-laden microdroplets that change to solid residues because of the temperature increase.
(D) Potential application of SFG spectroscopy for monitoring of changes in the hydrogen-bonding network strength due to the changes in ionic strength of the aqueous phase.
Capillary forces are also present at high relative humidity, which might vary on the bare substrate and on the virus. Thus, both the solid surface and the virus could be separated by one or multiple water stratums. The presence of either mono- or di-valent cations in the liquid phase30 (linked by formation of cationic complexes with the hydroxyl groups of the solid surfaces) can result in substitution of the remaining half shell of water ligands at mono- and/or divalent cations by the hydroxyl and carboxylate functional groups31 of the virus surface, completion of the cation bridging process, and augmentation of adsorbed amount of virus on the surface accordingly. The hydrogen-bond interactions of the interfacial water molecules with the aforementioned surface-active species of the virions, which can be strengthened or weakened by changing the aqueous phase ionic strength, can be monitored by using vibrational sum-frequency generation (vSFG) spectroscopy.32 , 33 vSFG spectroscopy is a reliable technique for molecular-level characterization of aqueous interfaces,34 , 35 including viral interfaces. This tool can probe the C–H stretches of the alkyl tails as well as the O–H stretching continuum of the hydrogen-bond network in the electrical double layer medium in the presence of the ions.36
On the other hand, as discussed, ion-specific interactions at charged interfaces could greatly affect the virus surface adsorption, protonation and/or deprotonation of the surface-active moieties of the virions; the charge densities and potentials of the viral interface, and the structuring of interfacial components in the electrical double layer. These ion-specific interactions can be probed by utilizing the second harmonic generation (SHG) spectroscopy technique,37 a non-linear optical facility like SFG spectroscopy. The interdependence of the SHG response on the electrostatic potential has resulted in the application of this powerful technique as an optical voltmeter,38 , 39 which can be employed for monitoring of the electrical double layer at the solid surface-aqueous phase-virus interfaces as well as quantifying the relative permittivity in the virus-surface gap. Figure 3D depicts potential application of SFG spectroscopy for probing alterations in the strength of hydrogen bonding network because of the variations in the aqueous phase ionic strength.
According to Equation 1, the water roundel radii |r| decreases as temperature increases, which means that at higher temperatures the described complexes and molecular interactions are disturbed, lower water bridging would occur, and a reduced quantity of virus would be adsorbed onto solid surfaces. This theoretical analysis might explain previous observations27 that higher temperature inactivates coronaviruses on stainless steel. In low temperature environments, the enveloped virions stay infectious for a number of days, as denoted in laboratory experiments.27 Human lungs acting as reservoirs of respiratory viral infections can disperse microdroplets through sneezing and coughing, and the virions can even be exhaled during the speaking and breathing.40, 41, 42 The bigger virus-laden microdroplets precipitate and adsorb onto the solid surface; however, the water contained within these virus-laden microdroplets can vaporize and form residues as solid phase or droplet nuclei at higher temperature conditions16 , 43 (Figure 3C).
It is worth noting that conditions of too-high humidity can create a greater water network around the virus,44 thus providing interactions with the proteins and the lipid bilayer that promote degradation. As the temperature increases, the movement of the water molecules increase and therefore also promote interaction and degradation.
Potential Applications of Various Experimental Techniques in SARS-CoV-2 Characterization and Understanding of Virus Transmission from Surfaces
Atomic force microscopy (AFM) and AFM-based methods, including AFM-infrared-spectroscopy (AFM-IR), are powerful techniques for characterization of a wide range of biological species and can help us to understand molecular interactions that occur at viral interfaces.45 They can monitor the molecular interactions among the biomolecules including protein-protein interaction, antibody-antigen interaction,46 and ligand-receptor interactions47 without disrupting the virion.
Nanoindentation measures the rupture force of a virus capsid under force and gives information on the chemical bond strength between viral capsid proteins.48 To understand the chemical force between a virus particle and a cell, AFM tips have been functionalized with a single viral particle and then used to probe the interaction of the probe and a cell.49 AFM can also be used to measure how viral chemistry changes in different liquid environments.45
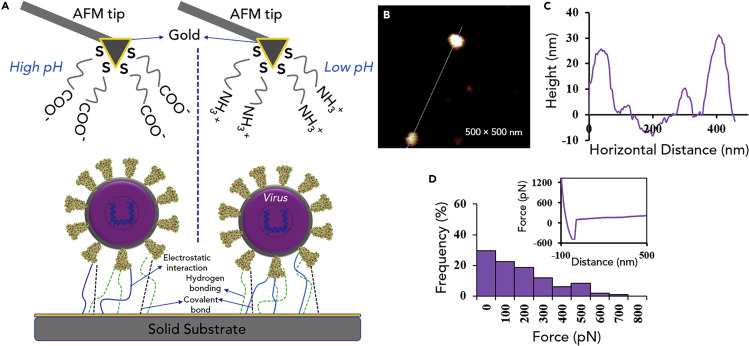
A practical example of using the AFM technique for studying of interactions of an enveloped virus with a solid surface is presented in Figure 4 . Figure 4A shows how modified AFM tips can be functionalized with different chemistries to determine the viral isoelectric point and other surface chemistry interactions at the viral interface. Shown in Figure 4B, virus particles are covalently bound to a surface. The virus particles maintain their size and shape (Figure 4C), demonstrating that their structure is maintained through the chemical bonding process. The virus is pulled with functionalized AFM tips and the rupture force is compared to measure how different chemistries (i.e., functionalization) interact with the viral surface. The charge of the viral surface changes with solution conditions,50 as already discussed, and surface hydrophobicity can also be explored (Figure 4D).
Figure 4.
The AFM Technique to Investigate the Interactions of the Virus with a Solid Surface
(A) Overview of AFM probe functionalization and virus adsorption onto a solid surface. AFM tips can be negatively or positively charged through either deprotonation of carboxyl group leading to carboxylate anion formation at high pH environment or protonation of amine functional group at low pH environment, respectively.
(B) AFM to measure virus surface chemistry. AFM image of bovine viral diarrhea virus (BVDV) attached to a glass slide and the hydrophobicity was measured with a methyl-terminated AFM tip.
(C) Corresponding height image of the line in (B). The virus was found to be the size of known BVDV particles.
(D) The frequency of different forces found from pulling on BVDV particles with a methyl-terminated AFM tip. The histogram represents >300 force-distance curves using a combination of 3 different functionalized tips and 3 different batches of virus. The spring constant for the tip was 0.07–0.15 N/m. The mean force was 221 pN. The insert is a representative force distance curve. BVDV is an enveloped virus with size range of 40–60 nm and related to the hepatitis C human virus.
Cryogenic-electron microscopy (cryo-EM) and cryo-electron tomography (cryo-ET) are other revolutionary techniques for structural biologists that can be used to inform our understanding of virus structure and the drivers of viral self-assembly.51, 52, 53, 54 In the last decade, cryo-EM and cryo-ET have been employed to determine coronavirus S glycoprotein ectodomain structures in both the pre- and post-fusion conformations, generating snapshots of the fusion reaction at the beginning and end points of the process.55, 56, 57 The atomic models generated by these techniques can inform our deeper understanding of the charge-distribution of the outer surface of SARS-COV-2 and describe structural changes to proteins that take place at different pH and ionic strength conditions,58 which play a crucial role in the virus molecular interactions with solid surfaces. Furthermore, a newly developed methodology enables us to employ liquid-cell transmission electron microscopy (LCTEM) to complement the conventional Cryogenic transmission electron microscopy (cryo-TEM) for characterization of nanoscale solvated biological soft matter as well as illustration of dynamics, growth kinetics, and reactivity of nanomaterials.59 Staining approaches, which can overcome the contrast issues because of the ultra-low electron doses,60 make LCTEM an adequate technique with potential utility in monitoring of virus interactions with surfaces.
In addition to vSFG and SHG spectroscopies described in section 3, which are recently developed non-linear optical techniques, new windows into the quantification of the hydrogen-bonding network strengths in the viral aqueous phase might be opened by employing Raman spectroscopy to realize the inter- and intramolecular details of water dynamics61 to complement our understanding of virus surface adsorption under high relative humidity conditions. Moreover, surface-enhanced Raman spectroscopy (SERS) can be used to identify the proteins of enveloped viruses, like SARS-CoV-2, that are adsorbed onto solid surfaces.62
As a newly developed approach for high relative humidity conditions, integration of the second-order non-linear spectroscopic technique SHG with quantified adsorbed mass in nanoscale from piezoelectric QCM measurements with dissipation monitoring (QCM-D)63 could be used for simultaneously determination of the charge per virus adsorbed onto the solid surface and probing the relative permittivity in the viral interfacial area of the electrical double layer.
We need to consider that spectroscopy methods might be a challenge with viral solutions because of their low concentration and difficulty in obtaining high-purity viral stocks. Viral concentrations are often measured in infectious particles per milliliter and laboratory samples typically range from 103–1010 particles per mL. These are attomolar to subnanomolar concentrations, and some analytical techniques64 struggle to detect these low viral concentrations. The other major challenge is virus purity. Viruses used for study are either grown in lab cell cultures or derived from natural sources. Regardless, the viruses are a very small percentage of the organic matter in the original sample. Purification techniques need to maintain viral viability, which requires both the structural integrity of the capsid and envelope to remain intact, as well as minimal changes to the chemical surface of the virus.65 The most common purification technique, ultracentrifugation, exposes the virus to either a high-ionic-strength cesium chloride solution or a high-osmotic-strength sucrose solution. An alternative option is to use iodixanol gradients, given that iodixanol is iso-tonic and non-toxic. However, almost all ultracentrifugation steps have a low yield. Most other laboratory purification techniques struggle to maintain viable virus with the high yield and high purity needed for many spectroscopic techniques to measure virus adhesion.
Figure 5 presents the recently developed techniques discussed in this article for study of virus structure and assembly, molecular interactions at viral interfaces, and direct analysis of solid surfaces.
Figure 5.
Some Emerging Techniques That Can Be Employed for SARS-CoV-2 Structural Characterization and Surface Interactions Analysis
(A) Schematic diagram of the LCTEM technique and its application for imaging of the virions.
(B) QCM-D technique, quantity of adsorbed virions and rigidity of binding can be determined through alterations in frequency and dissipation, respectively.
(C) Cryo-EM and cryo-ET techniques workflow, from data acquisition to 3D model, reprinted from Luque et al.51 Copyright 2020 Springer Nature.
(D) SERS to identify cells infected with newly emerging viruses (top), adapted from Lim et al.62 Copyright 2019 American Chemical Society. SERS on self-assembled monolayer (SAM)-functionalized Ag electrode (bottom left), infrared absorption spectro-electrochemistry on SAM-coated Au electrode (bottom right), reprinted from Kornienko et al.66 Copyright 2019 American Chemical Society.
(E) Mapping of virus-binding events on living cells by using AFM technique, adapted from Alsteens et al.45 Copyright 2017 Springer Nature.
(F) Overview of SFG (top left) and SHG (top right) spectroscopies for viral interface studies, and argand diagram for ion-specific SHG responses (bottom), modified from Boamah et al.37 Copyright 2019 American Chemical Society.
Conclusions and Outlook
In this Perspective, we have highlighted the adsorption characteristics and molecular interactions of the SARS-CoV-2 outer surface proteins on solid surfaces from different points of view, which is critical to understanding the virus transmission process and taking necessary actions to tackle it. As discussed, some important factors influencing the virus adsorption phenomenon include the following: surface-active moieties of the viral proteins, hydrophilic or hydrophobic characteristic of the solid surface, pH of the bulk fluid, relative humidity, and temperature of the environment.
Our theoretical analysis throughout this study has demonstrated that the SARS-CoV-2 can be adsorbed onto surfaces and remain stable within a range of pH values from acidic to basic environments at moderate temperature. On the basis of the Kelvin equation, it is expected that the SARS-CoV-2 would be less stable in higher temperature conditions. Accordingly, it is anticipated that the rate of transmission and infection will be lower during the summer months than in winter months. Further experimental research studies on this topic are needed to confirm our hypothesis and propositions we have made. The preservation of the virus shape and structure after condensation and/or evaporation processes in the air and/or water and the viral electrostatic surface properties of this new virus should also be explored.
Moreover, application of certain biophysical and biochemical techniques to the structural analysis of viral capsid proteins could be of significant utility for identification of their molecular assembly main drivers as well as development of enhanced viral assemblies. Attaining this valuable information on the SARS-CoV-2 structure and assembly is necessary for helping scientists globally with antiviral drug and vaccine design to combat against COVID-19, given that the development of antiviral drugs is a promising direction for mitigating this new worldwide health threat.
Acknowledgments
Professor David Bhella at MRC-University of Glasgow Centre for Virus Research (CVR) is acknowledged for his critical feedback on the initial draft of the manuscript. The authors would also like to thank Dr. Mairi Clarke; Dr. James Streetley of the Scottish Centre for Macromolecular Imaging ([SCMI] University of Glasgow); Liushu Wu; David Anderson and Dr. Michaela J. Conley (CVR, University of Glasgow); Professor Stanley G. Sawicki (University of Toledo), and Professor Stuart G. Siddell (University of Bristol) for providing the image of a cryo-EM tomogram of mouse hepatitis virus presented in this article. Dr. Tracy Brown, Head of Life Sciences and Healthcare-TÜV SÜD UK National Engineering Laboratory, is greatly acknowledged for her valuable comments on the manuscript. C.L.A. and O.A. acknowledge funding from NSF CAREER award (1451959) for supporting the AFM work.
Author Contributions
E.J. conceived the idea presented in the manuscript, undertook the theoretical analyses, and wrote the manuscript. A.H. contributed to the writing of the manuscript and the theoretical analyses and edited the final manuscript. C.L.H. contributed to the writing of the manuscript and supervised the AFM experiments. O.A. conducted the AFM experiments. All the authors discussed the results and contributed to the final manuscript.
References
- 1.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tortorici M.A., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D., Boons G.-J., Bosch B.-J., Rey F.A., de Groot R.J., Veesler D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dömling A., Gao L. Chemistry and Biology of SARS-CoV-2. Chem. 2020 doi: 10.1016/j.chempr.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choppin P.W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev. Infect. Dis. 1980;2:40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- 5.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerba C.P. Oceans and Health: Pathogens in the Marine Environment. Springer; 2005. Survival of viruses in the marine environment; pp. 133–142. [Google Scholar]
- 7.Bitton G. Adsorption of viruses onto surfaces in soil and water. Water Res. 1975;9:473–484. [Google Scholar]
- 8.Dika C., Ly-Chatain M.H., Francius G., Duval J.F.L., Gantzer C. Non-DLVO adhesion of F-specific RNA bacteriophages to abiotic surfaces: importance of surface roughness, hydrophobic and electrostatic interactions. Colloids Surf. A Physicochem. Eng. Asp. 2013;435:178–187. [Google Scholar]
- 9.Nakanishi K., Sakiyama T., Imamura K. On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon. J. Biosci. Bioeng. 2001;91:233–244. doi: 10.1263/jbb.91.233. [DOI] [PubMed] [Google Scholar]
- 10.Fuhs G.W., Chen M., Sturman L.S., Moore R.S. Virus adsorption to mineral surfaces is reduced by microbial overgrowth and organic coatings. Microb. Ecol. 1985;11:25–39. doi: 10.1007/BF02015106. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez L., Li X., Wang J., Nangmenyi G., Economy J., Kuhlenschmidt T.B., Kuhlenschmidt M.S., Nguyen T.H. Adsorption of rotavirus and bacteriophage MS2 using glass fiber coated with hematite nanoparticles. Water Res. 2009;43:5198–5208. doi: 10.1016/j.watres.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Joshi P.U., Turpeinen D.G., Weiss M., Escalante-Corbin G., Schroeder M., Heldt C.L. Tie line framework to optimize non-enveloped virus recovery in aqueous two-phase systems. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019;1126-1127:121744. doi: 10.1016/j.jchromb.2019.121744. [DOI] [PubMed] [Google Scholar]
- 13.Shi H., Tarabara V.V. Charge, size distribution and hydrophobicity of viruses: Effect of propagation and purification methods. J. Virol. Methods. 2018;256:123–132. doi: 10.1016/j.jviromet.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Heldt C.L., Zahid A., Vijayaragavan K.S., Mi X. Experimental and computational surface hydrophobicity analysis of a non-enveloped virus and proteins. Colloids Surf. B Biointerfaces. 2017;153:77–84. doi: 10.1016/j.colsurfb.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Langlet J., Gaboriaud F., Duval J.F.L., Gantzer C. Aggregation and surface properties of F-specific RNA phages: implication for membrane filtration processes. Water Res. 2008;42:2769–2777. doi: 10.1016/j.watres.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Hosseini V. SARS-CoV-2 Virulence: Interplay of Floating Virus-Laden Particles, Climate, and Humans. Adv. Biosyst. 2020:2000105. doi: 10.1002/adbi.202000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castaño N., Cordts S., Jalil M.K., Zhang K., Koppaka S., Bick A., Paul R., Tang S.K.Y. Fomite transmission and disinfection strategies for SARS-CoV-2 and related viruses. arXiv Prepr. 2020 doi: 10.1021/acsomega.0c06335. arXiv2005.11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cookson J.T., Jr. Mechanism of virus adsorption on activated carbon. Journal-American Water Work. Assoc. 1969;61:52–56. [Google Scholar]
- 19.Michen B., Graule T. Isoelectric points of viruses. J. Appl. Microbiol. 2010;109:388–397. doi: 10.1111/j.1365-2672.2010.04663.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakoda A., Sakai Y., Hayakawa K., Suzuki M. Adsorption of viruses in water environment onto solid surfaces. Water Sci. Technol. 1997;35:107–114. [Google Scholar]
- 21.Zerda K.S., Gerba C.P., Hou K.C., Goyal S.M. Adsorption of viruses to charge-modified silica. Appl. Environ. Microbiol. 1985;49:91–95. doi: 10.1128/aem.49.1.91-95.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuang J., Jin Y. Virus retention and transport through Al-oxide coated sand columns: effects of ionic strength and composition. J. Contam. Hydrol. 2003;60:193–209. doi: 10.1016/s0169-7722(02)00087-6. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez L., Mylon S.E., Nash B., Nguyen T.H. Deposition and aggregation kinetics of rotavirus in divalent cation solutions. Environ. Sci. Technol. 2010;44:4552–4557. doi: 10.1021/es100120k. [DOI] [PubMed] [Google Scholar]
- 24.Lipson S.M., Stotzky G. Adsorption of reovirus to clay minerals: effects of cation-exchange capacity, cation saturation, and surface area. Appl. Environ. Microbiol. 1983;46:673–682. doi: 10.1128/aem.46.3.673-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joonaki E., Buckman J., Burgass R., Tohidi B. Water versus Asphaltenes; Liquid-Liquid and Solid-Liquid Molecular Interactions Unravel the Mechanisms behind an Improved Oil Recovery Methodology. Sci. Rep. 2019;9:11369. doi: 10.1038/s41598-019-47782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao M., Zhang L., Ma J., Zhou L. On airborne transmission and control of SARS-Cov-2. Sci. Total Environ. 2020;731:139178. doi: 10.1016/j.scitotenv.2020.139178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso J.M., Tatti F., Chuvilin A., Mam K., Ondarçuhu T., Bittner A.M. The condensation of water on adsorbed viruses. Langmuir. 2013;29:14580–14587. doi: 10.1021/la4019376. [DOI] [PubMed] [Google Scholar]
- 29.Gerba C.P. Advances in applied microbiology. Elsevier; 1984. Applied and theoretical aspects of virus adsorption to surfaces; pp. 133–168. [DOI] [PubMed] [Google Scholar]
- 30.Pham M., Mintz E.A., Nguyen T.H. Deposition kinetics of bacteriophage MS2 to natural organic matter: role of divalent cations. J. Colloid Interface Sci. 2009;338:1–9. doi: 10.1016/j.jcis.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Schweiss R., Welzel P.B., Werner C., Knoll W. Dissociation of surface functional groups and preferential adsorption of ions on self-assembled monolayers assessed by streaming potential and streaming current measurements. Langmuir. 2001;17:4304–4311. [Google Scholar]
- 32.Reddy S.K., Thiraux R., Rudd B.A.W., Lin L., Adel T., Joutsuka T., Geiger F.M., Allen H.C., Morita A., Paesani F. Bulk contributions modulate the sum-frequency generation spectra of water on model sea-spray aerosols. Chem. 2018;4:1629–1644. [Google Scholar]
- 33.Eftekhari-Bafrooei A., Borguet E. Effect of electric fields on the ultrafast vibrational relaxation of water at a charged solid–liquid interface as probed by vibrational sum frequency generation. J. Phys. Chem. Lett. 2011;2:1353–1358. [Google Scholar]
- 34.Johnson C.M., Baldelli S. Vibrational sum frequency spectroscopy studies of the influence of solutes and phospholipids at vapor/water interfaces relevant to biological and environmental systems. Chem. Rev. 2014;114:8416–8446. doi: 10.1021/cr4004902. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y.R., Ostroverkhov V. Sum-frequency vibrational spectroscopy on water interfaces: polar orientation of water molecules at interfaces. Chem. Rev. 2006;106:1140–1154. doi: 10.1021/cr040377d. [DOI] [PubMed] [Google Scholar]
- 36.Doǧangün M., Ohno P.E., Liang D., McGeachy A.C., Bé A.G., Dalchand N., Li T., Cui Q., Geiger F.M. Hydrogen-bond networks near supported lipid bilayers from vibrational sum frequency generation experiments and atomistic simulations. J. Phys. Chem. B. 2018;122:4870–4879. doi: 10.1021/acs.jpcb.8b02138. [DOI] [PubMed] [Google Scholar]
- 37.Boamah M.D., Ohno P.E., Lozier E., Van Ardenne J., Geiger F.M. Specifics about specific ion adsorption from heterodyne-detected second harmonic generation. J. Phys. Chem. B. 2019;123:5848–5856. doi: 10.1021/acs.jpcb.9b04425. [DOI] [PubMed] [Google Scholar]
- 38.Macias-Romero C., Nahalka I., Okur H.I., Roke S. Optical imaging of surface chemistry and dynamics in confinement. Science. 2017;357:784–788. doi: 10.1126/science.aal4346. [DOI] [PubMed] [Google Scholar]
- 39.Boamah M.D., Ohno P.E., Geiger F.M., Eisenthal K.B. Relative permittivity in the electrical double layer from nonlinear optics. J. Chem. Phys. 2018;148:222808. doi: 10.1063/1.5011977. [DOI] [PubMed] [Google Scholar]
- 40.Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., Zhou L., Liu J. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect. Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan J., Grantham M., Pantelic J., Bueno de Mesquita P.J., Albert B., Liu F., Ehrman S., Milton D.K., Consortium E., EMIT Consortium Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA. 2018;115:1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 44.Pitol A.K., Bischel H.N., Kohn T., Julian T.R. Virus Transfer at the Skin-Liquid Interface. Environ. Sci. Technol. 2017;51:14417–14425. doi: 10.1021/acs.est.7b04949. [DOI] [PubMed] [Google Scholar]
- 45.Alsteens D., Gaub H.E., Newton R., Pfreundschuh M., Gerber C., Müller D.J. Atomic force microscopy-based characterization and design of biointerfaces. Nat. Rev. Mater. 2017;2:1–16. [Google Scholar]
- 46.Kaur J., Singh K.V., Schmid A.H., Varshney G.C., Suri C.R., Raje M. Atomic force spectroscopy-based study of antibody pesticide interactions for characterization of immunosensor surface. Biosens. Bioelectron. 2004;20:284–293. doi: 10.1016/j.bios.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Tsai H., Chen Z., Deng H., Tsai S., Fuh C.B. Estimation of molecular interaction force using atomic force microscopy for bioapplication. J. Phys. Chem. B. 2016;120:10932–10935. doi: 10.1021/acs.jpcb.6b06985. [DOI] [PubMed] [Google Scholar]
- 48.Castellanos M., Pérez R., Carrillo P.J.P., de Pablo P.J., Mateu M.G. Mechanical disassembly of single virus particles reveals kinetic intermediates predicted by theory. Biophys. J. 2012;102:2615–2624. doi: 10.1016/j.bpj.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koehler M., Aravamudhan P., Guzman-Cardozo C., Dumitru A.C., Yang J., Gargiulo S., Soumillion P., Dermody T.S., Alsteens D. Glycan-mediated enhancement of reovirus receptor binding. Nat. Commun. 2019;10:4460. doi: 10.1038/s41467-019-12411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mi X., Bromley E., Joshi P.U., Long F., Heldt C.L. Virus isoelectric point determination using single-particle chemical force microscopy. Langmuir. 2019 doi: 10.1021/acs.langmuir.9b03070. [DOI] [PubMed] [Google Scholar]
- 51.Luque D., Castón J.R. Cryo-electron microscopy for the study of virus assembly. Nat. Chem. Biol. 2020;16:231–239. doi: 10.1038/s41589-020-0477-1. [DOI] [PubMed] [Google Scholar]
- 52.Merk A., Bartesaghi A., Banerjee S., Falconieri V., Rao P., Davis M.I., Pragani R., Boxer M.B., Earl L.A., Milne J.L.S., Subramaniam S. Breaking cryo-EM resolution barriers to facilitate drug discovery. Cell. 2016;165:1698–1707. doi: 10.1016/j.cell.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walls A.C., Tortorici M.A., Frenz B., Snijder J., Li W., Rey F.A., DiMaio F., Bosch B.-J., Veesler D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2016;23:899–905. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirchdoerfer R.N., Wang N., Pallesen J., Wrapp D., Turner H.L., Cottrell C.A., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-34171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hover S., King B., Hall B., Loundras E.-A., Taqi H., Daly J., Dallas M., Peers C., Schnettler E., McKimmie C. Modulation of potassium channels inhibits bunyavirus infection. J. Biol. Chem. 2016;291:3411–3422. doi: 10.1074/jbc.M115.692673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross F.M. Opportunities and challenges in liquid cell electron microscopy. Science. 2015;350 doi: 10.1126/science.aaa9886. [DOI] [PubMed] [Google Scholar]
- 60.Gnanasekaran K., Chang H., Smeets P.J.M., Korpanty J., Geiger F.M., Gianneschi N.C. Nano Lett; 2020. In Situ Ni2+ Stain for Liposome Imaging by Liquid-Cell Transmission Electron Microscopy. [DOI] [PubMed] [Google Scholar]
- 61.Yagasaki T., Saito S. Molecular dynamics simulation of nonlinear spectroscopies of intermolecular motions in liquid water. Acc. Chem. Res. 2009;42:1250–1258. doi: 10.1021/ar900007s. [DOI] [PubMed] [Google Scholar]
- 62.Lim J.Y., Nam J.S., Shin H., Park J., Song H.I., Kang M., Lim K.I., Choi Y. Identification of newly emerging influenza viruses by detecting the virally infected cells based on surface enhanced Raman spectroscopy and principal component analysis. Anal. Chem. 2019;91:5677–5684. doi: 10.1021/acs.analchem.8b05533. [DOI] [PubMed] [Google Scholar]
- 63.Dalchand N., Cui Q., Geiger F.M. Electrostatics, Hydrogen Bonding, and Molecular Structure at Polycation and Peptide:Lipid Membrane Interfaces. ACS Appl. Mater. Interfaces. 2020;12:21149–21158. doi: 10.1021/acsami.9b17431. [DOI] [PubMed] [Google Scholar]
- 64.Pirich C.L., de Freitas R.A., Torresi R.M., Picheth G.F., Sierakowski M.R. Piezoelectric immunochip coated with thin films of bacterial cellulose nanocrystals for dengue detection. Biosens. Bioelectron. 2017;92:47–53. doi: 10.1016/j.bios.2017.01.068. [DOI] [PubMed] [Google Scholar]
- 65.Dika C., Gantzer C., Perrin A., Duval J.F.L. Impact of the virus purification protocol on aggregation and electrokinetics of MS2 phages and corresponding virus-like particles. Phys. Chem. Chem. Phys. 2013;15:5691–5700. doi: 10.1039/c3cp44128h. [DOI] [PubMed] [Google Scholar]
- 66.Kornienko N., Ly K.H., Robinson W.E., Heidary N., Zhang J.Z., Reisner E. Advancing techniques for investigating the enzyme–electrode interface. Acc. Chem. Res. 2019;52:1439–1448. doi: 10.1021/acs.accounts.9b00087. [DOI] [PMC free article] [PubMed] [Google Scholar]