Abstract
In December 2019, reports of an unknown pneumonia not responsive to traditional treatments arose in Wuhan, China. The pathogen was subsequently identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), known to be responsible for the coronavirus disease-2019 (COVID-19) illness, and public health emergency of international concern was declared by the World Health Organization. There is increasing awareness of the cardiovascular manifestations of COVID-19 disease, and the adverse impact of cardiovascular involvement on its prognosis. In this setting, the electrocardiogram (ECG) is one of the leading tools to assess the extent of cardiac involvement in COVID-19 patients, due to its wide disponibility, low cost, and the possibility of remote evaluation. In this article, we review the role of the ECG in the identification of cardiac involvement in COVID-19, highlighting relevant clinical implications.
Keywords: COVID-19, Arrhythmia, Electrocardiogram, Pandemics, QT interval, SARS-CoV-2
Introduction
In December 2019, a novel viral infection arose in Wuhan, China, which then spread worldwide within several weeks. The infection was subsequently termed coronavirus disease-2019 (COVID-19) and declared a pandemic by the World Health Organization by March 2020. Most infected patients are asymptomatic or mild symptomatic, but approximately 15–20% develop acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The effects of SARS-CoV-2 on the heart are variable, but cardiac damage confers a worse prognosis, whether in the presence or absence of pre-existing cardiovascular disease [1,2]. Cardiac complications related to COVID-19 can be categorized into five types: a) cardiac injury (mainly due to ischemia or myocarditis); b) arrhythmia; c) new-onset or worsening of pre-existing heart failure; d) thromboembolic disease; and e) cardiac abnormalities induced by medical treatment [3].
In this setting, the electrocardiogram (ECG) is one of the leading tools to assess the extent of cardiac involvement in COVID-19 patients and the effect of medications, due to its wide accessibility, low cost, and the possibility of remote evaluation. Therefore, we proposed a review on the role of the ECG in the identification of cardiac involvement in COVID-19 and highlighted relevant clinical implications.
COVID-19 and markers of myocardial damage
Overview
Cardiac involvement in patients with COVID-19 is reflected in ECG alterations, such as ST changes, QT prolongation, conduction disturbances, and ventricular arrhythmias [4]. Therefore, patients presenting with cardiac symptoms and ECG changes should be carefully assessed in order to diagnose COVID-19 related cardiac complications such as myocarditis, brady- and tachyarrhythmias (Fig. 1 ).
Fig. 1.
Postulated cardiovascular involvement in COVID-19.
In the era of the COVID-19 pandemic, a high clinical suspicion should be maintained even in patients who present with atypical symptoms or signs. Furthermore, cardiovascular disease has been found to be associated with a worse prognosis [[5], [6], [7], [8]]. It should be stressed that the virus should not be considered as the cause of all cardiovascular complications, but may exacerbate or reveal underlying conditions [9,10]. However, more studies are needed to further clarify the role of the cardiovascular system in the COVID-19 pandemic [11].
QRST-abnormalities
Non-specific ECG findings reported in COVID-19 patients have been attributed to hypoxia or inflammatory damage. This includes a patient with SI, QIII, TIII pattern followed by a reversible but near-complete atrioventricular block, ST-segment elevation accompanied with multifocal ventricular tachycardia [4], and flattening of the T-waves in the inferior leads with right axis deviation. The SIQIIITIII pattern was observed in another patient whose infection was complicated by pulmonary embolism [12]. It should be noted that the SIQIIITIII pattern suggests acute right ventricular overload. In a case series of patients with COVID-19 related complications, premature atrial complexes, lateral T wave inversions, and a QTc interval of 528 ms were noted in a patient who presented with decompensated heart failure [13]. In a heart transplant recipient, sinus rhythm with new nonspecific T-wave inversions in the inferior and lateral precordial leads was seen [13]. In light of these published case reports and with the lack of further evidence, we propose that ST-T wave abnormalities, especially in the context of a cardiac-related clinical presentation, should lead to further investigations to exclude COVID-19 related cardiac complications during the current pandemic. ST-T wave abnormalities are useful especially when they develop during the course of a febrile disease and not to exclude but to demonstrate cardiac involvement, and especially without an evident context of cardiac-related clinical presentation.
Conduction disorders
Exacerbation of new-onset high degree atrioventricular block or bradyarrhythmic side-effects of antiviral therapy is possible in patients with COVID-19. A case of a transient complete heart block in a 54-year-old man with critical COVID-19 was recently reported [14]. Atrial tachycardia or atypical atrial flutter with 2:1 conduction and a concomitant wide QRS morphology in a COVID-19 positive patient was also reported [15].
Myocarditis and pericarditis
In the case of fulminant myocarditis, sinus tachycardia and right bundle branch block pattern without significant ST-T wave abnormalities were observed [16]. Other cases of myocarditis demonstrated nonspecific intraventricular conduction delay and premature ventricular beats [17], or ST-segment elevations in leads III and aVF [18]. Furthermore, in a patient with acute myopericarditis, low voltage limb leads, diffuse ST-segment elevation (especially in the inferior and lateral leads), and ST-segment depression with a T-wave inversion in leads V1 and aVR were the reported ECG findings [19]. In another patient with COVID-19 myopericarditis without other symptoms of infection, sinus tachycardia, low voltage QRS complexes in the limb leads, ST-segment elevations in leads I, II, aVL, V2-V6, PR elevation, and ST depressions in lead aVR were observed [13].
COVID-19 and cardiac arrhythmias
Cardiac arrhythmias have been reported in 16.7% of COVID-19 patients, while malignant arrhythmias have been reported in 11.5% of patients [1,20]. In a recent study of 138 hospitalized patients with COVID-19, cardiac arrhythmias represented a leading complication and were more common among critically ill patients [20]. Another study found a higher incidence of arrhythmias in patients with severe disease than those with mild disease (44.4% versus 6.9%, p < .001) [21]. There have also been reports of critically ill patients with COVID-19 experiencing cardiac arrest with pulseless electrical activity or ventricular arrhythmias during the recovery phase of their pulmonary condition [22]. Among 187 hospitalized patients with confirmed COVID-19, 5.9% of the patients experienced malignant arrhythmias, including ventricular tachycardia and fibrillation [1]. Additionally, critically ill COVID-19 patients with fever were observed to have a slower heart rate than expected [20,23]. Bradycardia prolongs the QT interval and could facilitate Torsades de Pointes (TdP). Furthermore, QT prolongation secondary to antiviral therapies can also predispose patients to ventricular arrhythmias [24]. Although arrhythmias cannot be considered as a marker of COVID-19 infection, they can be a useful prognostic marker. Of note, patients with preexisting cardiovascular disease admitted to the intensive care unit for COVID-19-related illnesses may have a worse prognosis [20].
Given the preliminary nature of the available literature, the difference in the incidence of arrhythmia among recovering critical patients, and patients with mild disease, has not yet been well delineated. As more data become available, an improved understanding of the pathophysiology and significance of arrhythmia in patients with COVID-19 will guide the recommendations for possible additional rhythm monitoring in an outpatient setting.
Proper ECG diagnosis of atrial fibrillation (AF) is important for COVID-19 patients. It is not clear whether the presence of AF will alter the prognosis of COVID-19 patients. However, there have been speculations on the mechanism of AF in COVID-19. For instance, hypoxemia caused by COVID-19 may bring about AF and could be refractory under impaired pulmonary function. A plausible mechanism of AF reduction is the inhibition of IK1 and IKACh channels [25].
COVID-19 and QT prolongation
QT interval measurement
Leads II or V5-V6 are recommended for the measurement of the QT interval. The QT interval should always be corrected according to the heart rate — employ Bazett's formula for correction if the heart rate is less than 90 beats per minute or Fridericia's in the case of higher heart rates. The end of the T-wave should be taken as the intersection between the tangent extrapolated from the point of maximum downslope and the isoelectric line.
COVID-19 related medications
QT prolongation and subsequent ventricular arrhythmias have been associated with the use of hydroxychloroquine/chloroquine (HCQ/CQ), azithromycin (AZ), and antivirals such as lopinavir/ritonavir (Table 1 ), or in COVID-19 patients with pre-existing hepatic disease or renal failure [[26], [27], [28]]. Although cases of QT prolongation and TdP due to HCQ/CQ have been reported, data on QT prolongation due to HCQ/AZ are contradictory. Chang et al. found that of 117 patients with COVID-19, only one patient experienced QT prolongation, in which case the medication was promptly discontinued [29]. However, in another cohort, 11% of patients developed QT prolongation, among which half of those patients had normal QT level at baseline [30]. This discrepancy can be explained by the heterogeneity of the patient cohort, such as the presence of comorbidities and varying disease severity. In the largest reported cohort of COVID-19 patients to date treated with HCQ/CQ ± AZ, no instances of TdP or arrhythmogenic death were reported. Although the use of these medications resulted in QT prolongation, clinicians seldom needed to discontinue therapy [31]. AZ is also known to have an increased risk of QT prolongation, TdP, and sudden cardiac death; however, the absolute risk is low [32,33].
Table 1.
Observational and randomized studies evaluating the risk of QT prolongation and ventricular arrhythmias with short courses of potential COVID-19 treatments.
| Study | Sample size (n) | Setting | Study design | Age (yrs.) | Baseline comorbidities | Drugs administered | Treatment duration | ECG monitoring | ECG outcomes | Arrhythmia outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. [51] | 30 moderate hospitalized COVID-19 patients | Shanghai, China | RCT | 48.6 | HTN (33.3%); DM (6.7%) | HCQ | 7d | Not available | Not available | No serious adverse events |
| Chorin et al. [30] | 84 hospitalized COVID-19 patients | New York, USA | Cohort study | 63.0 | HTN (65%); DM (20%); CAD (11%); COPD (8%); CKD (7%); Acute renal failure (6%); CHF (2%) | HCQ and AZ | 5d | Baseline ECG daily |
|
No arrhythmias |
| Gautret et al. [52] | 80 mild hospitalized COVID-19 patients | Marseille, France | Cohort study | 52.5 | HTN (16.3%); DM (11.2%); Chronic respiratory diseases (10%); CAD (7.5%); Obesity (5.0%); immunosuppression (5%) | HCQ and AZ | 3d | Baseline ECG and on day 2 | Not available | No serious adverse events |
| Huang et al. [53] | 22 moderate and severe hospitalized COVID-19 patients | China | RCT | 44.0 | HTN (10%); DM (10%) | Chloroquine and Lopinavir/Ritonavir (control) | 10d | Not available | Not available | No serious adverse events |
| Molina et al. [54] | 11 hospitalized COVID-19 patients |
Paris, France | Case series | 58.7 | Solid cancer (27%); hematologic cancer (18%); Obesity (18%); HIV (9%) | HCQ and AZ | HCQ: 10d AZ: 500 mg day 1 and 250 mg days 2 to 5 |
Not available | Excessive QT prolongation on in 1 patient (from 405 ms to 460 and 470 ms) | Not reported |
| Perinel et al. [55] | 13 COVID-19 patients in the critical care unit | Saint Etienne, France | Cohort study | 68.0 | Moderate or severe renal failure (30.7%); mechanically ventilated (92%) | HCQ | Various dosing regimens | Not available | QT prolongation >500 ms in 2 of 13 patients (381 to 510 ms and 432 to 550 ms) |
Not reported |
| Mercuro et al. [56] | 90 hospitalized COVID-19 patients | Boston, USA | Cohort study | 60.1 | HTN (53.3%); DM (28.9%); COPD/asthma (20.0%); AF (13.3%); CAD (11.1%); CHF (10.0) | HCQ ± AZ | 5d | Not available |
|
1 case of TdP |
| Saleh et al. [31] | 201 hospitalized COVID-19 patients | USA | Cohort study | 58.5 | HTN (60.2%); Hyperlipidemia (41.8%); DM (32.3%); AF (7.0%); CAD (11.4%); COPD/asthma (14.9%); CKD ≥ stage III (5.0%); HF | Chloroquine/HCQ ± AZ | Various dosing regimens | Twice daily ECGs or MCOT Patch |
|
No serious adverse events |
| Ramireddy et al. [57] | 98 hospitalized COVID-19 patients | Los Angeles, USA | Case series | 62.3 | HTN (60%); DM (22%); COPD (26%); HF (20%); CKD (14%) | HCQ, AZ, or combination | Various dosing regimens | Baseline and post-medication ECG (up to 24 h) |
|
No TdP observed |
| Rosenberg et al. [58] | 1438 hospitalized COVID-19 patients | New York City, USA | Cohort study | 63 | Obesity (46.6%); Cancer (4.0%); any kidney disease (12.0%); any chronic lung conditions (17.6%); diabetes (36.6%); any CVDs (29.1%); CHF (6.3%) | HCQ + AZ, HCQ alone, AZ, or neither | Various dosing regimens | Not available |
|
|
Abbreviations: AF = atrial fibrillation; AZ = azithromycin; CAD = coronary artery disease; CKD = chronic kidney disease; CHF = congestive heart failure; COPD = Chronic obstructive pulmonary disease; COVID-19 = coronavirus disease 2019; CVD = cardiovascular disease; DM = diabetes mellitus; HCQ = hydroxychloroquine; HIV = human immunodeficiency virus; HF = heart failure; HTN = hypertension; MCOT = Mobile Cardiac Outpatient Telemetry; RCT = randomized controlled trial; TdP = Torsades de pointes.
Other drugs that are being investigated for the treatment of COVID-19, including remdesivir, favipiravir, ribavirin, sarilumab, and baricitinib, have limited data available regarding their effects on QT prolongation and cardiac arrhythmias. Kumagai et al. found no effect of favipiravir on the QT interval among healthy Japanese adults after the administration of single oral doses of 1200 and 2400 mg [34]. However, studies conducted with prolonged use of favipiravir have reported side effects such as increased uric acid levels, diarrhea, reduced neutrophil counts and abnormal liver function tests [35].
QT monitoring recommendations
Several scientific societies [22,[36], [37], [38], [39]] and hospitals [40,41] across the globe have published protocols for QT interval monitoring in COVID-19 patients (Table 2 ). Taken together, they have the following points in common:
-
•
Before considering any treatment, conduct a clinical history focused on a prior history of heart disease, syncope, sudden cardiac death, comorbidities, and generate a list of home medications.
-
•
Identify and correct potentially modifiable risk factors for the prolongation of the QT interval (Table 3 ).
-
•
Discontinue unnecessary conflicting drugs related to the prolongation QT interval
Table 2.
Cardiovascular societies' recommendations on QT-interval monitoring in patients with COVID-19.
| Society/Guideline | QT monitoring recommendations |
|---|---|
| American College of Cardiology [59] | Baseline:
|
| European Society of Cardiology [36] |
|
| HRS COVID-19 Task Force, ACC Electrophysiology Section and AHA EP and Arrhythmias Committee [22] |
|
| Latin American Heart Rhythm Society [38] |
|
| Canadian Cardiovascular Society [44] |
|
Abbreviations: AADs = Antiarrhythmic Drugs; AZ = azithromycin; ACC = American College of Cardiology; AHA = American Heart Association; COVID-19 = coronavirus disease 2019; ECG = electrocardiogram; HCQ = Hydroxychloroquine; HRS = Heart Rhythm Society; TdP = Torsades de Pointes; VT = ventricular tachycardia.
Table 3.
Risk factors for inducible QT prolongation and arrhythmias.
Modifiable risk factors
|
Non-modifiable risk factors
|
It should be noted that these guidance documents vary in their ECG-related recommendations. Some recommend that all patients receive a baseline and repeat ECG [36], whereas others have reserved this recommendation for higher-risk populations [22,38].
COVID-19 and treatment guidance
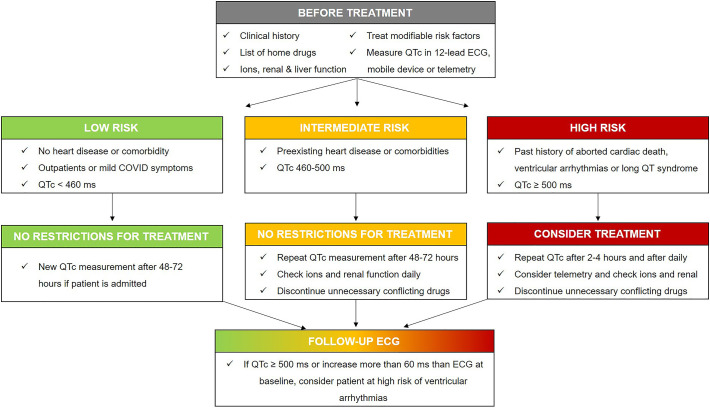
The risk stratification of COVID-19 patients should be performed based on their preexisting diseases since their prognosis varies greatly based on their underlying comorbidities. High-risk patients should be monitored more closely, in particular through the use of an ECG, than those who are otherwise healthy (Fig. 2 ). We propose the following ECG-guided recommendations:
-
•
Patients with inherited arrhythmic syndrome (long QT, Brugada syndrome, ARVC and hypertrophic cardiomyopathy): It is well known that some patients, notably those with inherited long QT syndrome, may be at an elevated risk for drug-induced ventricular arrhythmia [42]. COVID-19 has also been reported to unmask inherited arrhythmias, such as Brugada syndrome in the setting of syncope [9]. Hypertrophic cardiomyopathy, being the most common inherited cardiomyopathy with risk for sudden death, particular caution is required. Therefore, an expert opinion of a cardiologist/electrophysiologist may be essential in determining how to best minimize the risk of malignant arrhythmias in patients with inherited arrhythmic syndrome [43].
-
•
Patients with prolonged QTc intervals at baseline: If baseline ECG testing reveals a moderately prolonged QTc (above normal upper limit both for men and women until QTc = 500 ms), optimization of medications and electrolytes may permit therapy. If the QTc is markedly prolonged (QTc above 500 ms), drugs with potential QT lengthening effects should be avoided or modified, or expert consultation may permit administration with mitigating precautions [44].
-
•
Patients on multiple drugs that may cause QT prolongation and an increased risk of malignant arrhythmias: It is important to note that combining more than one proarrhythmic medication is known to increase the risk of significant QT prolongation [45]. Therefore, medications should be reviewed, and unnecessary medications with QT-prolonging effects should be discontinued. Interestingly, amiodarone — as a medication that can potentially prolong the QT interval — has been suggested as a possible inhibitor against the spreading of SARS-CoV-2 due to its ability to interfere with the endocytic pathway [46]. Therefore, some experts recommend the administration of prophylactic intravenous amiodarone to mitigate the risk of sudden cardiac arrest among patients with COVID-19. However, given the increased risk of ventricular tachyarrhythmia, we recommend very close monitoring of the QTc interval in patients on regular amiodarone. Although amiodarone causes QT prolongation, it rarely leads to ventricular arrhythmias, specifically TdP.
-
•
Vulnerable patients with multiple comorbidities and a high frailty status: Drug-induced QT prolongation in frail older patients may be exacerbated with pre-existing cardiac conditions such as cardiomyopathy, ischemia, heart failure, or bradycardia; and by other conditions such as diabetes, electrolyte abnormalities, hypoglycemia, or renal failure. Critically ill COVID-19 patients will likely be at a higher clinical risk of drug-induced arrhythmia, in which case ECG monitoring will more likely be indicated for supportive medical care. Patients with pre-existing structural heart disease pose a high risk of developing malignant arrhythmia; therefore, ECG should be assessed and monitored regularly before and during the initiation of COVID-19 related pharmacotherapy.
Fig. 2.
Proposed flow diagram concerning the arrhythmic vulnerability related to QTc prolongation from potential QTc-prolonging drugs.
COVID-19 and ECG monitoring
ECG monitoring is advisable especially when patients experience electrolyte disturbances and use concomitant QTc-prolonging drugs [47]. Therefore, ECG monitoring upholds a critical role in patient safety during the dose adjustment of medications used in the management of COVID-19. In an outpatient setting, mobile devices such as the KardiaMobile 6 L (AliveCor, Mountain View, California) and the Apple Watch ECG (Apple, Cupertino, California) have shown to be effective in monitoring the QTc interval [48,49]. In a recent study, the QTc interval in leads I and II from a handheld ECG device and 12‑lead ECG were compared across 99 healthy volunteers, and 20 hospitalized patients in sinus rhythm treated with dofetilide or sotalol [50]. The handheld ECG device was accurate in the measurement of QTc interval for both patients with sinus rhythm and QT prolongation [50]. In cases with limited resources or quarantine, the Kardia6L system — which has received expedited US Food and Drugs Administration clearance — could be used to deduce the risk status before initiation of drug therapy.
Conclusion
In the era of the SARS-CoV-2 pandemic, COVID-19 should be considered as a differential diagnosis for new or presumably new electrocardiographic abnormalities accompanied by a clinical presentation indicative of potential cardiac involvement. However, further studies with a systematic approach in the measurement of ECG parameters are needed to elucidate the potential role of ECG in myocardial injury diagnosis and risk stratification of COVID-19 patients.
References
- 1.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of Cardiac Injury with Mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.He J., Wu B., Chen Y., Tang J., Liu Q., Zhou S., et al. Characteristic electrocardiographic manifestations in patients with COVID-19. Can J Cardiol. 2020;36(6):966.e1–966.e4. doi: 10.1016/j.cjca.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Fang J., Zhu Y., Chen L., Ding F., Zhou R., et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang hospital. Clin Microbiol Infect. 2020;26(8):1063–1068. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang D., Saleh M., Garcia-Bengo Y., Choi E., Epstein L., Willner J. COVID-19 infection unmasking Brugada syndrome. HeartRhythm Case Rep. 2020;6:237–240. doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:2540–2541. doi: 10.1056/NEJMc1805679. [DOI] [PubMed] [Google Scholar]
- 11.Linschoten, Asselbergs F.W. CAPACITY-COVID: a European Registry to determine the role of cardiovascular disease in the COVID-19 pandemic. Eur Heart J. 2020;41:1795–1796. doi: 10.1093/eurheartj/ehaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey K., Iteen A., Nicolini R., Auten J. COVID-19 pneumonia with hemoptysis: acute segmental pulmonary emboli associated with novel coronavirus infection. Am J Emerg Med. 2020;38(7):1544.e1–1544.e3. doi: 10.1016/j.ajem.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azarkish M., Laleh Far V., Eslami M., Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J. 2020;41:2131. doi: 10.1093/eurheartj/ehaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy V., Reddy V., Mangat S., Shokr M., Kundumadam S., Laharwani H. Wide complex tachycardia in a COVID-19 patient: what is the mechanism? J Electrocardiol. 2020;60:200–202. doi: 10.1016/j.jelectrocard.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J.H., Liu Y.X., Yuan J., Wang F.X., Wu W.B., Li J.X., et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;60:200–202. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim I.C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. ehaa190, [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;41(19):1859. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakkireddy D.R., Chung M.K., Gopinathannair R., Patton K.K., Gluckman T.J., Turagam M., et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the heart rhythm society COVID-19 task force; electrophysiology section of the American college of cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020;141:e823–e831. doi: 10.1161/CIRCULATIONAHA.120.047063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szekely Y., Lichter Y., Shrkihe B.A., Bruck H., Oster H.S., Viskin S. Chloroquine-induced torsades de pointes in a patient with coronavirus disease 2019. Heart Rhythm. 2020;S1547-5271(20):30420–30423. doi: 10.1016/j.hrthm.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobon C., Palacio L.C., Chidipi B., Slough D.P., Tran T., Tran N., et al. The antimalarial chloroquine reduces the burden of persistent atrial fibrillation. Front Pharmacol. 2019;10:1392. doi: 10.3389/fphar.2019.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., Wang Y., Agostinis P., et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11:512. doi: 10.1038/s41419-020-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jankelson L., Karam G., Becker M.L., Chinitz L.A., Tsai M.C. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.008. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Chang D., Saleh M., Gabriels J., Ismail H., Goldner B., Willner J., et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992–2993. doi: 10.1016/j.jacc.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chorin E., Dai M., Shulman E., Wadhwani L., Bar-Cohen R., Barbhaiya C., et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020 doi: 10.1038/s41591-020-0888-2. [Ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Saleh M., Gabriels J., Chang D., Kim B.S., Mansoor A., Mahmood E., et al. The effect of Chloroquine, Hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020;13(6):e008662. doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi Y., Lim H.S., Chung D., Choi J.G., Yoon D. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. Biomed Res Int. 2018;2018:1574806. doi: 10.1155/2018/1574806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumagai Y., Murakawa Y., Hasunuma T., Aso M., Yuji W., Sakurai T., et al. Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults. Int J Clin Pharmacol Ther. 2015;53:866–874. doi: 10.5414/CP202388. [DOI] [PubMed] [Google Scholar]
- 35.Chinello P., Petrosillo N., Pittalis S., Biava G., Ippolito G., Nicastri E. INMI Ebola Team. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. Case Rep Dermatol. 2017;11 doi: 10.1371/journal.pntd.0006034. e0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardiology . 2020. TESf. ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. [Google Scholar]
- 37.Sacher F., Fauchier L., Boveda S., de Chillou C., Defaye P., Deharo J.C., et al. Use of drugs with potential cardiac effect in the setting of SARS-CoV-2 infection. Arch Cardiovasc Dis. 2020;113:293–296. doi: 10.1016/j.acvd.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saenz L.C., Miranda A., Speranza R., Texeira R.A., Rojel U., Enriquez A., et al. Recommendations for the organization of electrophysiology and cardiac pacing services during the COVID-19 pandemic: Latin American Heart Rhythm Society (LAHRS) in collaboration with: Colombian College Of Electrophysiology, Argentinian Society of Cardiac Electrophysiology (SADEC), Brazilian Society Of Cardiac Arrhythmias (SOBRAC), Mexican Society Of Cardiac Electrophysiology (SOMEEC) J Interv Card Electrophysiol. 2020 doi: 10.1007/s10840-020-00747-5. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapoor A., Pandurangi U., Arora V., Gupta A., Jaswal A., Nabar A., et al. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19 patients: a scientific statement from the Indian Heart Rhythm Society. Indian Pacing Electrophysiol J. 2020;20:117–120. doi: 10.1016/j.ipej.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitra R.L., Greenstein S.A., Epstein L.M. An algorithm for managing QT prolongation in coronavirus disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: possible benefits of intravenous lidocaine. HeartRhythm Case Rep. 2020;6(5):244–248. doi: 10.1016/j.hrcr.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and Torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roden D.M. Predicting drug-induced QT prolongation and torsades de pointes. J Physiol. 2016;594:2459–2468. doi: 10.1113/JP270526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C.I., Postema P.G., Arbelo E., Behr E.R., Bezzina C.R., Napolitano C., et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.024. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sapp J.L., Alqarawi W., MacIntyre C.J., Tadros R., Steinberg C., Roberts J.D., et al. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: a statement from the Canadian Heart Rhythm Society. Can J Cardiol. 2020;36(6):948–951. doi: 10.1016/j.cjca.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tisdale J.E., Jaynes H.A., Kingery J.R., Mourad N.A., Trujillo T.N., Overholser B.R., et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stadler K., Ha H.R., Ciminale V., Spirli C., Saletti G., Schiavon M., et al. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am J Respir Cell Mol Biol. 2008;39:142–149. doi: 10.1165/rcmb.2007-0217OC. [DOI] [PubMed] [Google Scholar]
- 47.Chinello P., Petrosillo N., Pittalis S., Biava G., Ippolito G., Nicastri E., et al. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung E.H., Guise K.D. QTC intervals can be assessed with the AliveCor heart monitor in patients on dofetilide for atrial fibrillation. J Electrocardiol. 2015;48:8–9. doi: 10.1016/j.jelectrocard.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Strik M., Caillol T., Ramirez F.D., Abu-Alrub S., Marchand H., Welte N., et al. Validating QT-interval measurement using the apple watch ECG to enable remote monitoring during the COVID-19 pandemic. Circulation. 2020;142(4):416–418. doi: 10.1161/CIRCULATIONAHA.120.048253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garabelli P., Stavrakis S., Albert M., Koomson E., Parwani P., Chohan J., et al. Comparison of QT interval readings in Normal sinus rhythm between a smartphone heart monitor and a 12-Lead ECG for healthy volunteers and inpatients receiving Sotalol or Dofetilide. J Cardiovasc Electrophysiol. 2016;27:827–832. doi: 10.1111/jce.12976. [DOI] [PubMed] [Google Scholar]
- 51.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Sevestre J., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang M., Tang T., Pang P., Li M., Ma R., Lu J., et al. Treating COVID-19 with Chloroquine. J Mol Cell Biol. 2020;12:322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perinel S., Launay M., Botelho-Nevers E., Diconne E., Louf-Durier A., Lachand R., et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa394. ciaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020:e201834. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramireddy A., Chugh H., Reinier K., Ebinger J., Park E., Thompson M., et al. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.120.017144. e017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardiology ACo . Cardiology magazine. 2020. Ventricular arrhythmia risk due to Hydroxychloroquine-azithromycin treatment for COVID-19. [Google Scholar]