Abstract
In this paper, we raise the hypothesis that Methylene Blue may be a treatment option for Corona Virus Disease of 2019 specially when combined with Non Steroid Anti-Inflammatory Drugs. In previous publications including ours, the role of kininogen system has been postulated. A correlation between clinical findings of the disease and this mechanism has been drawn to denote a pivotal role of kininogen-kallikrein system in pathophysiology of the disease. Therein the possible role of Icatibant, Ecallantide and Aprotinin in the treatment of this disease has been raised. Here we want to emphasize on an important post-receptor mechanism of bradykinin that is Nitric Oxide. We came to this aim because we found out how access to these novel treatment nominees may be expensive and unaffordable. For this reason we are focusing on possible role of an old albeit “mysterious” drug namely Methylene Blue. This medication may abort effects of Bradykinin by inhibition of Nitric Oxide synthase inhibitor and promote oxygen saturation while it is inexpensive and ubiquitously accessible. Clinical studies cannot be over emphasized.
Keywords: Methylene blue, Aprotinin, Icatibant, Angiotensin-converting enzyme 2, Ecallantide, NSAID, Bradykinin, SARS-CoV-2, COVID-19
Hypothesis
Since emergence and wide spread dissemination of Corona Virus Disease of 2019 (COVID19) all efforts in the scientific community have been focused on understanding the pathophysiology of the disease, alongside considering all aspects of a global health problem and its complexities. The scientific community is trying to accelerate the velocity of knowledge broadcast beyond that of the virus transcontinental spread by various strategies of fast track, green open access and preprints and early disclosure of nascent ideas to corroborate third parties into clinical trials for more valid outcomes. At the same time, we must try to avoid any enticement for exoneration in accuracy and methodological soundness of the studies.
The hypothesis presented here explores the role of Methylene Blue(MB) as a new repurposed or under noticed medication for this disease based on published manuscript of lead author of this manuscript and some fortunately, other independent researchers [1], [2], [3].
Traditionally there are well known indications for MB in medicine. MB is a derivative of phenothiazine. It converts ferric iron in methemoglobin to ferrous iron of normal hemoglobin and is a well-known medication for methemoglobinemia at doses of 1–2 mg/kg in repeatable single dose infusion shots [4]. Administered intravenously, it is turned into leucomethylene blue in the body which can reduce the heme group from methemoglobin to haemoglobin [5].
Also, MB has been used for the treatment of Ifosfamide neuropsychiatric toxicity. Chloracetaldehyde is a noxious metabolite of Ifosfamide, which MB prevents its formation by inhibiting multiple amine oxidase activities [6]. MB is administered up to six intravenous doses per day for treatment purpose and three times daily as prophylaxis [7]. Interestingly MB is a Monoamine Oxidase Inhibitor(MAOI) which raises serious hazard of serotonin syndrome when used with antidepressants especially Selective Serotonin Reuptake Inhibitor(SSRI)s, concomitantly [8], [9]
MB is used in septic shock and anaphylaxis by a recommended dosing of 1–2 mg/kg, which results in consistent increase of blood pressure [10].
Individuals with Glucose-6-Phosphate Dehydrogenase(G6PD) deficiency are at risk for hemolytic anemia, while receiving MB [5], [11].
MB doses higher than 4 mg/kg may cause abdominal pain, nausea, vomiting, reversible staining of skin, discoloration of urine and feces, precordial pain and dyspnea [5], [12].
Discussion
Hitherto three main territories of physio-pathogenesis of the Severe Acute Respiratory Syndrome-Corona Virus2(SARS-CoV2) causing COVID19 have been targeted.
-
(A)
The virus and its mechanism of replication and envelope integrity which have been aimed as potential targets of antiviral drugs like Remdesivir, Favipiravir and Kaletra [13], [14], [15], [16].
-
(B)
The receptor of the virus in the body, the most notorious of them known as Angiotensin-Converting Enzyme 2(ACE2) which has been addressed by actually distracting the virus by a recombinant soluble ACE2 product [17]. The intriguing part of the story is that the indigenous ACE2 is a functional receptor/enzyme in the body so it cannot be innocuously antagonized in an irreversible manner, without serious adverse consequences [18], [19].
-
(C)
The immunologic cascade known to ensue in a phenomenon known as “cytokine storm” which has previously been observed in other viral diseases like Influenza [20], [21].
The terminology “cytokine storm” frequently used in COVID19 may be misleading in conveying this implicit presumption as a “proved fact” that no cytokine or autacoid can be impeached as the starter or the main player of the game. This presumption has not been approved yet, although it may subsequently unveil as true.
Some authors including authors of this article raised theoretical pivotal role of kininogen system in SARS-CoV-2 scenario [2], [3], [22]. In our previous article, we tried to imply that Kininogen-kallikrein system, may be not “a bean in the bean pot” among all the events of the disease. There we tried to show how a derangement in ACE2 receptor/enzyme may disinhibit kininogen- kallikrein system and the increased Bradykinin activity may be a key factor in COVID19 clinical picture. ACE2 directly metabolizes Bradykinin into inactive forms [23]. Furthermore, a tendency of Angiotensin 2 increase happens because ACE2 is no longer functional. This exerts an increased load of activity on ACE even when Angiotensin2 does not show an apparent increase. Some forms of ACE gene polymorphism have resulted in increased rate and severity of essential hypertension which denotes that enzyme activity is not the same in different individuals [24]. This is congruent with findings that pose a higher risk of SARS-COV2 infection in patients with hypertension [25].
Whatsoever in our previous paper we tried to substantiate relationship and theoretical congruence of the special clinical features of this disease with frame of this hypothesis aka dry cough, lung vascular leakage syndrome, ameliorating effect of zinc, deleterious role of ACE inhibitors and early drop in saturation (Table 1 ).
Table 1.
Explanation of distinct COVID19 signs and symptoms by the proposed theory of Bradykinin/Nitric Oxide pathway.
| COVID19 distinct findings | Explanation by proposed hypothesis |
|---|---|
| Dry cough | Increase in BRK activity |
| Oxygen saturation drop |
|
| Coagulopathy | Increased BRK activity occupying Kallikrein activity. This may result in distraction of functional capacity of Kallikrein from its parallel role in coagulation cascade |
| Ameliorating effect of Zinc supplement | The backdoor pathway of BRK inactivation are amino-peptidase and carboxypeptidase both having a Zinc core limited by availability of total body Zinc reserve |
| Prevalence in hypertensive patients | ACE gene polymorphisms produce less active and less abundant enzyme which in turn is better exhausted out of capacity, therefore increasing BRK |
| Deleterious effect of ACEIs like Captopril, Lisinopril | ACEI decreases ACE function that is already weak due to SARS-CoV-2(as explained in the text). As a result active BRK increases |
| ARB less detrimental and even protective | Causes an compensatory increase in ACE activity (after previous chronic use) which in turn neutralize BRK into inactive forms |
| Reported effect of NO aerosol | It conjugates the NO in the lung making Dinitrogen dioxide which is exhaled by expiration. Therefore NO is eliminated from the whole body as an exhaust |
| A smooth prodrome of a weak or so and a rapid flare up afterwards | Amino-peptidase enzyme keeps BRK tissue level low till it runs out of Zinc supply and at that time a burst of BRK trigger all other inflammatory agents-the so called-cytokine storm. White lungs happen due to massive vascular leakage |
| Disorder of taste and smell | Has been ascribed to increased BRK activity as a side effect of ACEI confirming the BRK role in this symptom |
| Diarrhea | Carboxypeptidase is recruited for BRK inactivation. It is also an important intestinal brush border enzyme which may fail this important function in this setting |
ACE: Angiotensin Converting Enzyme, ACEI:Angiotensin Converting Enzyme Inhibitor, ARB: Angiotensin2 Receptor Blocker, BRK: Bradykinin, NO: Nitric Oxide.
Dry cough has been ascribed to increased BRK activity as a well-known side effect of ACE inhibitors like Captopril. A characteristic course of SARS-COV2 infection is rapid aggravation of respiratory function after a week or two. We speculated this may be explained by a sudden running out of amino-peptidase capacity which is an alternative pathway of Bradykinin inactivation. Zinc supplies can increase amino-peptid ase activity and interestingly ameliorating effects of zinc on SARS-COV2 infection has been observed [26]. More unpublished and/or non-peer reviewed data are being propagated [27] to hypothesize mechanism of hemoglobin distraction from efficient oxygen exchange. In this article we will try to demonstrate prominent role of Nitric Oxide(NO) in this process and relationship with clinical events.
In above mentioned papers about kininogen-kallikrein implication in COVID19 scenario, some medications have been proposed: Icatibant as a B2 Bradykinin receptor antagonist, Ecallantide and Aprotinin.
All these medications have favorable and unfavorable profiles. Icatibant and Ecallantide which have formally been approved for treatment of hereditary angio-edema, are classified as orphan drugs and are quite expensive and unavailable in many settings. On the other hand, Aprotinin has received a black box warning due to hypersensitivity and its effects on coagulation system are somewhat concerning for clinicians [28].
It is noteworthy that none of these medications have been reported to be used in COVID19 and there are no studies performed yet to prove pre-requisites of these theories comprising assessment of Bradykinin levels in sampled lung tissue. As far we know that Bradykinin plasma level may not be trustworthy due to autacoid and transient nature of the molecule.
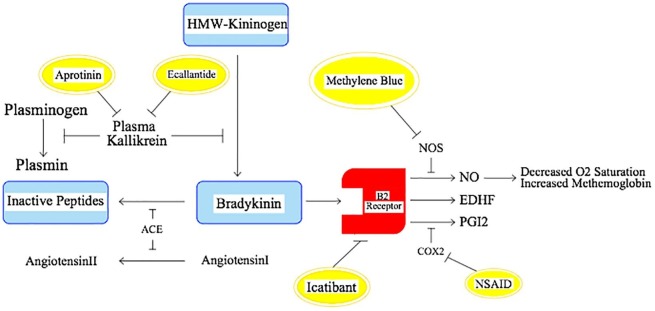
A re-contemplation on mechanism of Bradykinin action in the body and literature search, led us to the notion that several Bradykinin functions were exerted via NO; a factor previously named as Endothelial Derived Relaxation Factor [29] Fig. 1 . Having read the theories and widespread authentic observation about hemoglobin drop out of oxygen exchange process [27], the consideration of NO as an allosteric ligand of hemoglobin [30], [31] seems sound. One does not expect to detect this volatile substance with very short half-life in blood as all of the vicious process takes place in lungs as a physiologic trap. Interestingly, the role of hyperbaric oxygen [32], [33] and inhaled NO have been shown [34]. Hyperbaric oxygen can force hemoglobin to bind up to the oxygen. Inhaled NO paradoxically may turn soluble NO into gas and let it to be exhaled.
Fig. 1.
Schematic illustration of Bradykinin/NO pathway involved in SARS-CoV-2 infection (COX2: Cyclooxygenase2; EDHF: Endothelium-Derived Hyper-polarizing Factor; NO: Nitric Oxide; NOS: Nitric Oxide Synthase; NSAID: Non-Steroid Anti-Inflammatory Drug; PGI2: Prostaglandin I2).
NO synthase inhibitors and remarkably MB have been shown to neutralize effects of Bradykinin [35]. MB, a ubiquitously available NO Synthase inhibitor, may seem to be effective in impediment of disease process and promote hemoglobin recruitment and oxygen saturation [35], [36].
There are reports and presumptions about deteriorating effects of Non-Steroid Anti-Inflammatory Drug(NSAID)s on disease process [37]. We presume inhibition of cyclooxygenase2 by NSAIDs may up-regulate B2 activity and over-produce NO instead, as a shift of duty. Hence a lack of response to MB, theoretically, may prompt us to addition of NSAIDs and inhibit both pathways simultaneously.
Comparing to Icatibant, regardless of price or availability, MB is post receptor, does not induce complex cascade coagulopathies and while we know Bradykinin is unstable and may harbor in the lung as a trench, MB may prevail all through the body and seize the affected hemoglobin. MB have been used as a sustained infusion in some indication which guarantees a real time and ongoing neutralization of NO production in the body as the disease process continues relentlessly. This may also be an important advantage. As a bona fide proximal kininogen cascade adjoining hemostasis axis is least affected. All these speculations must be clinically assessed (Table 2 ).
Table 2.
Comparison of kininogen-kallikrein system targeting drugs and methylene blue.
| Kininogen- Kallikrein system targeting drugs | Methylene Blue |
|---|---|
| Far more expensive(Icatibant, Ecallantide) | Affordable and inexpensive |
| Orphan drugs(Icatibant, Ecallantide) | Ubiquitous |
| Receptor targeted(Icatibant), Enzyme targeted(Ecallantide, Aprotinin) | Post receptor |
| Coagulation pathways interference(Aprotinin) | Least effect on coagulation pathways |
| Effect dependent on receptor regional distribution | Effects through out the vascular system in the whole body |
| No direct effect on hemoglobin oxygen transport | Direct effect on hemoglobin oxygen transport |
| Subcutaneous shots(Icatibant) | Continuous infusion or repeated shots and real time neutralization of ongoing NO production is feasible |
Interestingly there are reports of MBl role in COVID19 story [38], [39]. However these studies are considering MB in context of Photodynamic therapy which has been the nearest pre-existing scenario on the counter of clinicians already used for the treatment of other diseases and remarkably not MB per se [39].
Moreover, MB has been deemed effective in sepsis in numerous reports [40], [41] and some vascular leakage syndromes[42] which supports our notion that MB must not go unnoticed as a potential treatment of advanced stages of COVID19.
Furthermore, some direct antiviral effects have been reported for MB [43] some focusing on photodynamic treatment. We have not meticulously explored these properties in our consideration yet to see whether it has any direct effect on this kind of Ribonucleic Acid(RNA) virus without photodynamic therapy.
Conclusion
We think that MB as an inexpensive ubiquitously available medication, may have a considerable potential role in the treatment of COVID19. We could not prompt to set-up a clinical study swiftly and it was prudent to share the idea with scientific community as soon as possible. This medication can be considered in this critical health emergency, either as compassionate/informed voluntary treatment for individuals or preferably through clinical trials.
Paradoxical and unexpected effects have been observed in MB administration in other diseases if not meticulously done with proper dosage. Adverse reaction may happen in patients with a history of serotonergic medications as anti-depressants which may specially be prevalent these days [44] Also a condition of G6PD deficiency must be ruled out before MB administration. Concomitant NSAID administration may block alternative pathways of BRK activity. In the condition of NO synthesis block, these pathways may be over activated and continue the disease process. Hence NSAIDs may add a benefit to MB. any clinical step must be taken cautiously. On the other hand, we must know that we are in a scrambling global health emergency and too much obsessive and procrastinating methodology may leave us with many dead patients to treat at the end of the day.
Funding sources
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.van de Veerdonk F.L., Netea M.G., van Deuren M. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. Preprints. 2020 doi: 10.20944/preprints202004.0023.v1. 2020040023. [DOI] [Google Scholar]
- 2.Tolouian R., Vahed S.Z., Ghiyasvand S., Tolouian A., Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J Renal Inj Prev. 2020;9(2) doi: 10.34172/jrip.2020.19. [DOI] [Google Scholar]
- 3.Ghahestani S.M., Mahmoudi J., Hajebrahimi S. Bradykinin as a probable aspect in SARS-Cov-2 scenarios: is bradykinin sneaking out of our sight? Iranian J Allergy Asthma Immunol. 2020;25(4):1–4. doi: 10.18502/ijaai.v19i(s1.r1).2850. [DOI] [PubMed] [Google Scholar]
- 4.Boylston M., Beer D. Methemoglobinemia: a case study. Crit Care Nurse. 2002;22:50–55. [PubMed] [Google Scholar]
- 5.Clifton J., Leikin J.B. Methylene blue. Am J There. 2003;10:289–291. doi: 10.1097/00045391-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Alici-Evcimen Y., Breitbart W.S. Ifosfamide neuropsychiatric toxicity in patients with cancer. Psychooncology. 2007;16:956–960. doi: 10.1002/pon.1161. [DOI] [PubMed] [Google Scholar]
- 7.Pelgrims J., De Vos F., Van den Brande J., Schrijvers D., Prove A., Vermorken J.B. Methylene blue in the treatment and prevention of Ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br J Cancer. 2000;82:291–294. doi: 10.1054/bjoc.1999.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillman P.K. Methylene blue implicated in potentially fatal serotonin toxicity. Anaesthesia. 2006;61:1013–1014. doi: 10.1111/j.1365-2044.2006.04808.x. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay R.R., Dunford C., Gillman P.K. Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A(MAO A) confirms a theoretical prediction. Br J Pharmacol. 2007;152:946–951. doi: 10.1038/sj.bjp.0707430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo J.C.Y., Darracq M.A., Clark R.F. A review of methylene blue treatment for cardiovascular collapse. J Emer Med. 2014;46:670–679. doi: 10.1016/j.jemermed.2013.08.102. [DOI] [PubMed] [Google Scholar]
- 11.Paciullo C.A., McMahon Horner D., Hatton K.W., Flynn J.D. Methylene blue for the treatment of septic shock. Pharmacotherapy. 2010;30:702–715. doi: 10.1592/phco.30.7.702. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimons M.G., Gaudette R.R., Hereford W.E. Critical rebound methemoglobinemia after methylene blue treatment: case report. Pharmacotherapy. 2004;24:538–540. doi: 10.1592/phco.24.5.538.33356. [DOI] [PubMed] [Google Scholar]
- 13.Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020;5 doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko W.C., Rolain J.M., Lee N.Y. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Q., Yang M., Liu D. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J., Jeon S., Shin H.Y. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(6) doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhenc-Gelas F., Drueke T.B. Blockade of SARS-CoV-2 infection by recombinant soluble ACE2. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos R., Ferreira A.J., Verano-Braga T., Bader M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216(2):R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q., Zhou Y.-h., Yang Z.-Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13(1):3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Veerdonk F.L., Netea M.G., van Deuren M. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife. 2020;9:e57555. doi: 10.7554/eLife.57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oudit G.Y., Crackower M.A., Backx P.H., Penninger J.M. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13(3):93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 24.Mondry A., Loh M., Liu P., Zhu A.-L., Nagel M. Polymorphisms of the insertion/deletion ACE and M235T AGT genes and hypertension: surprising new findings and meta-analysis of data. BMC Nephrol. 2005;6(1):1. doi: 10.1186/1471-2369-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skalny A.V., Rink L., Ajsuvakova O.P. Zinc and respiratory tract infections: Perspectives for COVID-19. Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read R. Flawed methods in “COVID-19: attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism”. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12120912.v1. [DOI] [Google Scholar]
- 28.Siehr S., Stuth E., Tweddell J. Hypersensitivity reactions to aprotinin re-exposure in pediatric surgery. Eur J Cardiothorac Surg. 2010;37(2):307–311. doi: 10.1016/j.ejcts.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 29.Linz W., Wohlfart P., Schölkens B.A., Malinski T., Wiemer G. Interactions among ACE, kinins and NO. Cardiovasc Res. 1999;43(3):549–561. doi: 10.1016/s0008-6363(99)00091-7. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z., Shiva S., Kim-Shapiro D.B. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115(8):2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim-Shapiro D.B., Schechter A.N., Gladwin M.T. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol. 2006;26(4):697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 32.Harch PaulG. Hyperbaric oxygen treatment of novel coronavirus (COVID-19) respiratory failure. Med Gas Res. 2020;10(2):61. doi: 10.4103/2045-9912.282177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R, Zhong X, Tang Y, et al. The Outcomes of Hyperbaric Oxygen Therapy to severe and critically ill patients with COVID-19 pneumonia. 2020. https://oxycamaras.com.br/wp-content/uploads/2020/04/Outcome-of-HBOT-to-COVID19.pdf.
- 34.Martel J, Ko Y-F, Young JD, Ojcius DM. Could nitric oxide help to prevent or treat COVID-19? Microbes Infect. 2020; Epub ahead of print. doi: 10.1016/j.micinf.2020.05.002. [DOI] [PMC free article] [PubMed]
- 35.Rhaleb N.E., Dion S., Barabé J. Receptors for kinins in isolated arterial vessels of dogs. Eur J Pharmacol. 1989;162(3):419–427. doi: 10.1016/0014-2999(89)90332-4. [DOI] [PubMed] [Google Scholar]
- 36.Mayer B., Brunner F., Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol. 1993;45(2):367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- 37.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368 doi: 10.1136/bmj.m1185. [DOI] [PubMed] [Google Scholar]
- 38.Yu B, Jin C, Zhang J, et al. Methylene blue photochemical treatment as a reliable SARS-CoV-2 plasma virus inactivation method for blood safety and convalescent plasma therapy for the COVID-19 outbreak. 2020. doi: 10.21203/rs.3.rs-17718/v1. [DOI] [PMC free article] [PubMed] [Retracted]
- 39.Henry M., Summa M., Patrick L., Schwartz L. A cohort of cancer patients with no reported cases of SARS-CoV-2 infection: the possible preventive role of Methylene Blue. Substantia. 2020;4(1):888. doi: 10.13128/Substantia-888. [DOI] [Google Scholar]
- 40.Galili Y., Kluger Y., Mianski Z. Methylene blue–a promising treatment modality in sepsis induced by bowel perforation. Eur Surg Res. 1997;29(5):390–395. doi: 10.1159/000129548. [DOI] [PubMed] [Google Scholar]
- 41.Kwok E.S., Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med. 2006;21(6):359–363. doi: 10.1177/0885066606290671. [DOI] [PubMed] [Google Scholar]
- 42.Umbrello M., Gardinali M., Ottolina D., Zanforlin G., Iapichino G. Systemic capillary leak syndrome: is methylene blue the silver bullet? Case Rep Crit Care. 2014;2014 doi: 10.1155/2014/141670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Floyd RA, Schinazi RF. Antiviral therapy using thiazine dyes. Google Patents; 2002:6346529 http://www.freepatentsonline.com/6346529.html.
- 44.Hencken L., To L., Ly N., Morgan J.A. Serotonin syndrome following methylene blue administration for vasoplegic syndrome. J Cardiac Surg. 2016;31(4):208–210. doi: 10.1111/jocs.12705. [DOI] [PubMed] [Google Scholar]