Abstract
The number of people infected with severe acute respiratory syndrome coronavirus 2 is increasing globally, and some patients have a fatal clinical course. In light of this situation, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) a pandemic on March 11, 2020. While clinical studies and basic research on a treatment for COVID-19 are ongoing around the world, no treatment has yet been proven to be effective. Several clinical studies have demonstrated the efficacy of chloroquine phosphate and nafamostat mesylate with COVID-19. Here, we report the case of a Japanese patient with COVID-19 with severe respiratory failure who improved following the administration of hydroxychloroquine and continuous hemodiafiltlation with nafamostat mesylate. Hence, hydroxychloroquine with nafamostat mesylate might be a treatment option for severe COVID-19.
Keywords: SARS-CoV-2, COVID-19, Hydroxychloroquine, Nafamostat mesylate, Continuous hemodiafiltlation
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread from China to the other Asian countries as well as to North and South America and Europe [1] over the past six months since the end of 2019, when SARS-CoV-2 was first discovered as the etiological agent for pneumonia of an unknown cause in China [2]. In Japan, there have been 4768 confirmed cases and 85 deaths as of April 9, 2020 (excluding the cases from the cruise liner outbreak), and of these, 109 patients reportedly required hospitalization in an intensive care unit and/or a ventilator [3]. However, there is no established treatment protocol for this new infectious disease yet, and countries around the world are seeking to develop an effective treatment. The Japanese Association for Infectious Diseases submitted a proposal (first edition) on February 26, 2020, for treating COVID-19 using antiviral medications, with lopinavir/ritonavir (LPV/r) and favipiravir listed as the two drugs for consideration. Here, we have reported the case of a patient who did not respond to treatment with LPV/r and experienced respiratory failure requiring ventilation. Consequently, hydroxychloroquine and continuous hemodiafiltlation with nafamostat mesylate were administered as other treatment approach, resulting in an improvement in the patient's condition.
2. Case presentation
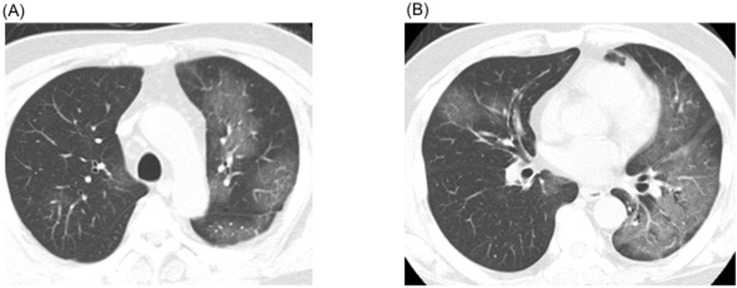
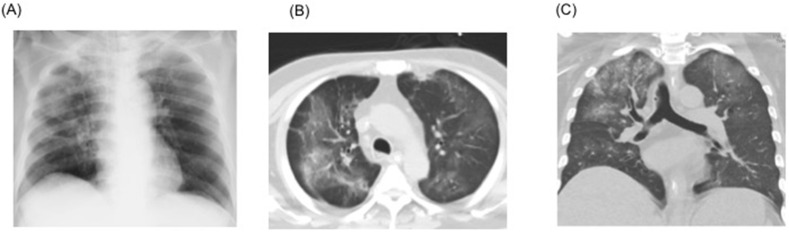
A 69-year-old Japanese man was admitted to a designated hospital for infectious diseases through a referral by the public health center on February 19, 2020. The patient had no history of recent overseas travel nor any recent contact with anyone who had come from abroad. The only medical history was hypertension and type 2 diabetes, along with a history of smoking (20 cigarettes/day for 30 years, between the ages of 20 and 50 years). The patient had visited a general practitioner after experiencing symptoms of fatigue and a nighttime fever >38.0 °C that persisted for a week. Subsequently, a chest X-ray of the patient showed left lung pneumonia, thereby strongly suggesting COVID-19; therefore, he was referred to the public health center. On the same day, the patient's oropharyngeal swab tested positive for SARS-CoV-2 using real time reverse transcriptase polymerase chain reaction (rRT-PCR), and he was subsequently hospitalized in the medical institution designated for infectious diseases. At the time of hospitalization, the patient was overall in a good condition; however, both lungs (predominantly the left) exhibited ground glass opacities on chest computed tomography (CT) (Fig. 1 ). Despite the immediate administration of LPV/r (800 mg/day) on the day after hospitalization, the pneumonia spread to the right lung on the second day of hospitalization and resulted in respiratory failure. The oxygen requirement increased up to 7 L/min with a reservoir mask on day 2 and up to 10 L/min on day 3 of admission. Finally, the patient also received mechanical ventilation on day 3. On the same day, the patient was transported to our hospital to receive extracorporeal membrane oxygenation (ECMO) as swiftly as possible in an isolated negative pressure room.
Fig. 1.
Chest computed tomography (CT) findings on admission at the former institution. A: CT shows ground glass opacities spread over in the left lobe of lung. B: Patchy ground glass opacities in the right lung.
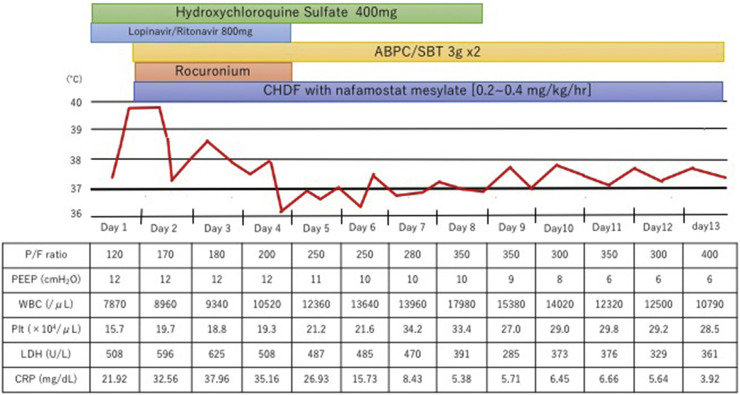
Although the patient was sedated during transport, he retained clear consciousness prior to intubation. The patient's body temperature was 39.5 °C; blood pressure, 144/56 mmHg; pulse rate, 77 beats per minute; and spontaneous respiratory rate, 20/min. The ventilator was set to synchronized intermittent mechanical ventilation mode, with inspired oxygen fraction (FiO2), 0.7; positive end-expiratory pressure (PEEP), 12 cmH2O; and pressure support (PS), 10 cmH2O. The arterial blood gas test revealed that the arterial partial pressure of oxygen (PaO2) was 82.3 mmHg, arterial partial pressure of carbon dioxide (PaCO2) was 43.1 mmHg, and the PaO2/FiO2 (P/F) ratio was 117 (Fig. 2 ).
Fig. 2.
Timeline of the disease course since intensive care unit admission in our hospital. Clinical data and ventilator settings are shown. Hydroxychloroquine started from the day of admission. We began CHDF with nafamostat mesylate (0.2–0.4 mg/kg/hour) from day 2. The fever went down from 2 day of admission. The P/F ratio increased day by day up to 300 on day 7 of admission. White blood cell count increased after day 8 and body temperature elevated again after day 9. Gram staining of endotracheal aspirates reveal several morphotypes and neutrophils, but few pathogens cultured. Ventilator-associated pneumonia considered as the cause of the patient's fever.
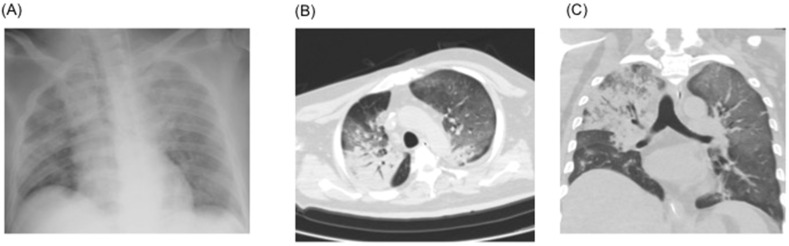
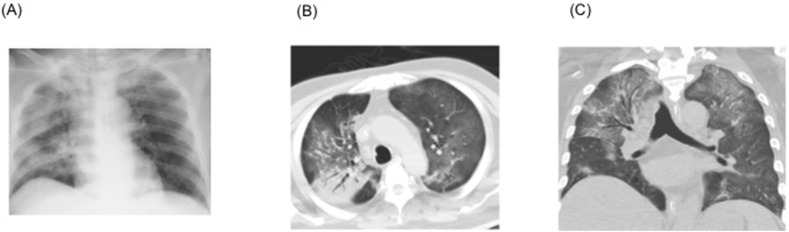
Although ECMO was first considered when the patient was transferred, after interdepartmental discussion, ECMO was not initiated as the case did not meet the recommended indications of the Extracorporeal Life Support Organization guidelines [4]. We began treatment initially with ventilation in accordance with the lung-protective ventilation strategies for acute respiratory distress syndrome (ARDS) [5]. Although LPV/r had been administered for 3 days, it proved to be ineffective. Therefore, we started the patient on the antimalarial medication, hydroxychloroquine, at a dose of 400 mg once a day (the same dosage as is prescribed for systemic lupus erythematosus [SLE]). The CT performed on the second day of hospitalization (Fig. 3 ) showed extensive consolidation of the right lung and ground glass opacities spread over the left lung. The respiratory rate increased to more than 30 breaths/min, indicating overventilation, and the tidal volume was over 8 mL/kg. Therefore, we began the administration of a muscle relaxant (rocuronium) on the same day to lower the tidal volume and reduce the respiratory rate. To address the progressive renal insufficiency, we also began renal replacement therapy using continuous hemodiafiltration (CHDF) with nafamostat mesylate as an anticoagulant [0.2–0.4 mg/kg/hr]. As shown in Fig. 2, the patient began to show some improvement in oxygenation starting from day 3 of hospitalization in our hospital, and on day 4, the P/F ratio improved to surpass the >200 threshold. In addition, chest CT showed improvement in the right lobe pneumonia (Fig. 4 ). On the same day, we discontinued the administration of the muscle relaxant. As an increase in bilirubin starting from day 5 was observed, we discontinued the administration of LPV/r considering the side effects. Respiratory status and imaging results continued to show improvement (Fig. 5 ). With improvement in the inflammation indicators and respiratory condition, we completed the course of hydroxychloroquine on day 9. However, a SARS-CoV-2 RT-PCR test of the sputum sample on days 3 and 9 of hospitalization still yielded positive results.
Fig. 3.
Imaging at the time of admission and on the second day of admission. A: Chest radiographs show diffuse pulmonary infiltrates. B&C: Chest computed tomography shows spreading of consolidation over the upper lobe in the right lung and ground glass opacities in the left lung. Volume reduction and traction bronchiectasis also observed in one part of the right upper lobe of the lung.
Fig. 4.
Imaging at the time of admission and on the fourth day of admission. A: Chest radiographs show partial resolution after starting treatment. B&C: Chest computed tomography shows improvement of consolidation over the upper lobe of the right lung and ground glass opacities in the left lung.
Fig. 5.
Imaging at the time of admission and on the seventh day of admission. A: Chest radiographs show resolution when compared with the chest radiograph taken day 4. B&C: Chest computed tomography shows improvement of one part of the right lung consolidation.
On day 13, the P/F ratio significantly improved with a ratio of 400 (FiO2: 0.4 and PEEP: 6 cmH2O). Based on the ventilatory settings, extubation was possible. However, extubation was postponed because the patient displayed insufficient awakening. Considering the difficulty in management after extubation in our surgical intensive care unit (ICU), the patient was transferred to the former hospital on day 14. The patient was extubated after transfer, and SARS-CoV-2 RT-PCR result was confirmed to be negative with a pharyngeal sample. Although it took approximately one month, the patient was discharged from the former hospital.
3. Discussion
In this case report, we have described the case of a critically ill patient with COVID-19 who was administered both of hydroxychloroquine and nafamostat mesylate after his condition continued to worsen despite early administration of LPV/r. After two days of hydroxychloroquine administration, the patient's severe pneumonia condition began to improve, suggesting the potential of hydroxychloroquine with nafamostat mesylate as a possible treatment in patients with COVID-19.
In a recently published randomized controlled trial, LPV/r treatment did not demonstrate any benefit in patients with severe COVID-19 infection [6]. In a post hoc analysis of the trial, clinical recovery and reduced mortality were observed in the subgroup of patients who were treated within 12 days after the onset of symptoms. In our case, although LPV/r treatment was administered early (7 days after onset), it proved to be ineffective.
While the antimalarial drug chloroquine phosphate is recommended in the treatment guidelines for the novel coronavirus according to the National Health Commission of the People's Republic of China [7] at that time, chloroquine phosphate is not currently manufactured in Japan. However, hydroxychloroquine, which has the same pharmacokinetic profile as chloroquine phosphate in addition to reduced toxicity, is already approved in Japan and various other nations for treatment of SLE. Treatment with chloroquine phosphate resulted in the improvement of pneumonia symptoms in over 100 patients, along with significant improvement in clinical measures such as a higher viral clearance rate and a shorter subjective symptom period [8]. In addition, in vitro studies have demonstrated that the efficacy of hydroxychloroquine was comparable to that of chloroquine phosphate [9,10].
Chloroquine impairs the replication of several viruses by increasing the endosomal pH and inhibiting glycosylation [11]. Hydroxychloroquine with azithromycin was also reported to be effective in early viral clearance in an open-label non-randomized trial, although we should be careful of QT prolongation when both of them are administered simultaneously [12].
Therefore, several clinical studies have been performed to confirm whether chloroquine or hydroxychloroquine is effective. Now most studies concluded hydroxychloroquine is not useful in mild-to-moderate COVID-19 patients [13,14] and WHO discontinued hydroxychloroquine treatment trial for COVID-19 because of little or no reduction in the mortality of the hospitalized COVID-19 patients.
This time we started not only hydroxychloroquine but also nafamostat mesylate (0.2–0.4 mg/kg/hr) as an anticoagulant for CHDF. Nafamostat mesylate, protease inhibitor, can inhibit the fusion of SARS-CoV-2 to cell membranes by preventing viral protease activity at small blood concentration [15]. Thus nafamostat mesylate was also reported as candidate drugs for COVID-19 and some case series show nafamostat mesylate might be effective [16].
Doi et al. reported the case series of combination therapy of nafamostat mesylate and favipiravir for severe COVID-19 patients [17]. This report showed the possibility of combination therapy with nafamostat mesylate for severe COVID-19 patients. For that reason, combination therapy of hydroxychloroquine and nafamostat mesylate might affect our patient's improvement.
In addition, COVID-19 also occurs multiorgan coagulopathy what is called disseminated intravascular coagulation (DIC) [18] and nafamostat mesylate is used for DIC in Japan. D-dimer of our patient increased up to 32.6 μg/mL and anticoagulant treatment reported to decrease mortality in severe COVID-19 patients with coagulopathy [19]. So, nafamostat mesylate may also affect the clinical course by improving DIC.
Chloroquine is known to have an immunomodulatory effect via Toll-like receptor signaling pathways, which stimulate the activation of various inflammatory cytokines and T cell receptors in the downstream cascade [20].
Xu et al. reported the case of a patient who died from SARS-CoV-2 infection. Postmortem biopsy samples taken from the lungs exhibited pneumocyte desquamation and hyaline membrane formation, which were compatible with ARDS. Notably, there were no obvious intranuclear or intracytoplasmic viral inclusions. In addition, they also demonstrated the hyperactivation of CD4 and CD8 T cells using flow cytometry of the peripheral blood, which implied a high cytotoxic cell response [21].
Therefore, regulating the cytokine release syndrome is critical in patients with severe COVID-19, and some immunomodulatory drugs, such as tocilizumab and steroids, have been investigated for their role as potential therapeutic agents [22]. Hydroxychloroquine immunomodulatory effect may be benefit for COVID-19 patients with severe condition.
Although the mortality rates for COVID-19 have varied widely in different geographic locations, recent report have suggested the mortality rate to be approximately 1.4% [23]. However, the mortality rate in patients aged >80 years is as high as 14% in China [24], and the 28-day mortality rate for severe cases requiring ICU and ventilation is as high as 61.5% [23]. As such, COVID-19 has a high mortality rate for specific demographics. Our case report focuses on the possibility that hydroxychloroquine with nafamostat mesylate might aid in the recovery of patients with severe COVID-19 in a clinical setting. However, our findings derived from a single case cannot be generalized; therefore, further case studies and clinical trials on combination treatment with nafamostat mesylate for COVID-19 are needed.
Funding
No funding was obtained from external sources of this study.
Ethics approval
Informed consent was obtained from the patient for publication of this case report.
Authors’ contributions
All authors meet the ICMJE authorship criteria. SI managed and wrote the case report. SY and KT contributed to patient management and writing the case report. KN, YY and JM helped in patient management. YN and NS planed the care of the patient. TA revised the manuscript for important intellectual content. TT is the guarantor for this article. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare no conflicts of interest in association with the present study.
References
- 1.World Health Organization. reportCoronavirus disease (COVID-2019) situation reports. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/)[accessed March 30, 2020].
- 2.World Health Organization . 2020. Pneumonia of unknown case – China.https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ [Google Scholar]
- 3.Ministry of health, Labour, and Welfare, Japan. Coronavirus disease 2019 (COVID-19) situation within and outside the country. (https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/newpage_00032.html)[accessed April 8, 2020].
- 4.Extracorporeal Life Support Organization. ELSO guidelines general v1.4 (https://www.elso.org/Portals/0/ELSO%20Guidelines%20General%20All%20ECLS%20Version%201_4.pdf) [accessed March 30, 2020].
- 5.Fan E., Del Sorbo L., Goligher E.C., Hodgson C.L., Munshi L., Walkey A.J., et al. An official American thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir and Crit Care Med. 2017 May 1;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. PMID:28459336. [DOI] [PubMed] [Google Scholar]
- 6.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020 Mar 18 doi: 10.1056/NEJMoa2001282. [Epub ahead of print] PMID:32187464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notice on issuance of the sixth edition of the guidance for COVID-19: prevention, control, diagnosis, and management. National health commission. (http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml) [accessed May 10, 2020]. [in Chinese].
- 8.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020 Feb 19;14(1):72–73. doi: 10.5582/bst.2020.01047. PMID:32074550. [DOI] [PubMed] [Google Scholar]
- 9.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020 Mar 18;6(16) doi: 10.1038/s41421-020-0156-0. PMID:32194981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. [Epub ahead of print] PMID:32150618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003 Nov;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. PMID:14592603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautret P., Lagler J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int Antimicrob Agents. 2020 March 20 doi: 10.1016/j.ijantimicag.2020.105949. [Epub ahead of print] PMID:32205204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020 Jul 23 doi: 10.1056/NEJMoa2019014. [Epub ahead of print] PMID:32706953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitjà O., Corbacho-Monné M., Ubals M., Tebe C., Peñafiel J., Tobias A., et al. Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial. Clin Infect Dis. 2020 Jul 16 doi: 10.1093/cid/ciaa1009. [Epub ahead of print] PMID:32674126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020 Apr 20 doi: 10.1128/AAC.00754-20. [Epub ahead of print] PMID:323127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang S., Rhee J.Y. Three cases of treatment with nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy. Int J Infect Dis. 2020 May 26 doi: 10.1016/j.ijid.2020.05.072. [Epub ahead of print] PMID:32470602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi K., Ikeda M., Hayase N., Moriya K., Morimura N., COVID-UTH Study Group Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series. Crit Care. 2020 Jul 3 doi: 10.1186/s13054-020-03078-z. PMID:32620147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wichmann D., Sperhake J.D., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020 May 6 doi: 10.7326/M20-2003. [Epub ahead of print] PMID:32374815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemostasis. 2020 May;18(5):1094–1099. doi: 10.1111/jth.14817. PMID:32220112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace D.J., Gudsoorkar V.S., Weisman M.H., Venuturupalli S.R. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol. 2012 Sep;8(9):522–533. doi: 10.1038/nrrheum.2012.106. PMID:22801982. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z., Shi L., Zhang J., Zhang C., Liu S., Zhao P., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Feb 18 doi: 10.1016/S2213-2600(20)30076-X. [Epub ahead of print] PMID:32085846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020 Apr 14;18(1):164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 Feb 24 doi: 10.1016/S2213-2600(20)30079-5. [Epub ahead of print] PMID:32105632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z., McGoogan J.M. JAMA; 2020 Feb 24. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. [Epub ahead of print] PMID:32091533. [DOI] [PubMed] [Google Scholar]