Abstract
In this study, it was aimed to investigate the effects of both using curcumin and microencapsulation method on in vitro release behaivour of chia seed oil and its antioxidant potential during simulated gastrointestinal (GI) tract. Maltodextrin (MD) and gum Arabic (GA) was used as wall materials for freeze dried capsules. Sample 6, having 1:3 MD to GA ratio, 1:5 chia seed oil to wall material ratio and 40% total dry matter content, was found to have the optimum results in terms of emulsion stability (CI% = 0), zeta potential (−32.2 ± 0.8 mV) and size distribution (600 ± 8 nm). Moreover, release profiles of encapsulated chia seed oil samples were evaluated to determine if curcumin addition has any significant effect. The results revealed that curcumin addition decreased the release of chia seed oil from 44.6% to 37.2%. On contrary, it increased total phenolic content of in fraction of intestine to 22 mg gallic acid equivalents (GAE)/L.
Keywords: Encapsulation, Chia seed oil, Curcumin, Omega-3 fatty acids, In-vitro release
Highlights
-
•
Digestion behaivour and in vitro antioxidant capacity of chia seed oil was studied.
-
•
Oil was encapsulated in maltodextrin and gum Arabic by freeze-drying method.
-
•
Most of oil was released in intestinal stage due to the wall material composition.
-
•
Curcumin addition increased total phenolic content of oil microcapsules.
1. Introduction
In recent years, people have become more aware of the impact of diet on health (Us-Medina, Julio, Segura-Campos, Ixtaina, & Tomás et al., 2018). Therefore, they are in search of food products enriched with compounds which are good for health (Julio et al., 2015). Polyunsaturated fatty acids (PUFAs) are well-known to have health-promoting effects (Timilsena, Adhikari, Barrow, & Adhikari, 2017). Of the PUFA family, omega-3 and omega-6 essential fatty acids (EFAs) need to be taken by diet since they cannot be synthesized in the human body (Timilsena, Adhikari, Barrow, & Adhikari, 2016; Us-Medina, Julio, Segura-Campos, Ixtaina, & Tomás, 2018). Fish oils are commonly consumed as omega-3 fatty acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), source. Vegetable oils having high EFA content are increasing in popularity compared to fish oils since there are controversies about fish oil usage and vegan people cannot consume fish oils (Timilsena, Adhikari, et al., 2017). For this reason, fortification of functional foods with plant based-omega-3 fatty acids are becoming an attractive option in the food industry (Julio et al., 2015). As a possible source of these components, chia seed contains satisfactorily high amounts of omega-3 PUFAs compared to other plant sources (Marineli et al., 2014).
Salvia hispanica L., widely known as chia, is an annual plant (Marineli et al., 2014). Chia seeds have promising bioactive components for human health. Chia seed is composed of approximately 30% of oil (Özcan, Al-Juhaimi, Ahmed, Osman, & Gassem, 2019), 85% of which is PUFAs (Timilsena et al., 2016). α-linolenic acid (ALA) is the main fatty acids with a ratio of 68% of total fatty acids in chia seed (Özcan et al., 2019). As stated above, chia seed oil contains high amount of α-linolenic (omega-3) and α-linoleic acids (omega-6) which are essential nutrients since human being cannot synthesize them (Silva et al., 2016). Omega-3 to omega-6 ratio is also, an important parameter for healthy diet; thus, chia seed oil consumption will be beneficial since its ratio is between 3.18 and 4.18 providing a good equilibrium (Ayerza, 1995; Us-Medina et al., 2018). This high concentration of omega-3 is associated with the reduced risk of coronary artery disease, cardiovascular diseases, being antithrombotic, antiinflammatory, antiarrhythmic and favoring plaque stabilization, hypertension, diabetes, rheumatoid arthritis, autoimmune diseases, immune response disorders and cancer (Mcclements, Decker, & Weiss, 2007; Meyer & Groot, 2017). Chia seeds can lower blood concentrations of triglycerides (TG), total cholesterol (TC), low density lipoproteins (LDL) and very low density lipoproteins (VLDL) induce oxidative stress also increase plasma and hepatic antioxidant capacity (Silva et al., 2016). Owing to its high PUFA content, chia seed oil is susceptible to oxidative deteriorations arising from environmental and storage factors, such as heat, light and moisture. Encapsulation of chia seed oil is a promising alternative to protect the oil against lipid oxidation and improve solubility and stability. In the interest of preventing lipid oxidation and providing lipid stability, encapsulation application is used in food products (Campo et al., 2017). In order to protect EFA content and bioavailability of oil, encapsulation of chia seed oil is employed (Timilsena et al., 2016).
Chia seed oil has natural antioxidants such as tocopherols, phytosterols, carotenoids and phenolic compounds which makes the oil quite stable against its high PUFA content. There's a great interest in natural antioxidants that have protective effect against the reactive oxygen and nitrogen species damages (Martínez-Cruz & Paredes-López, 2014; Taga, Miller, & Pratt, 1984).
Curcuma longa L., widely known as turmeric, has gained interest due to its antioxidant capacity (Almeida, Sampaio, Bastos, & Villavicencio, 2018). Curcumin, the main bioactive compound of turmeric, is easily disintegrated and encapsulation application can be used to protect it from degradation. Furthermore, studies show that direct curcumin addition has a negative impact on food products, whereas, encapsulated curcumin improves product characteristics in terms of sensory features (Rafiee, Nejatian, Daeihamed, & Jafari, 2019). Curcumin, as stated above component of turmeric, is a compound that has considerable attention for therapeutic, anticarcinogenic, antiinflammatory and antioxidant properties against many diseases such as neurodegenerative disorders, oxido-nitrosative stress (Pulido-Moran, Moreno-Fernandez, Ramirez-Tortosa, & Ramirez-Tortosa, 2016). However curcumin has many important pharmacological properties, high rate of degradation, limited blood–brain barrier diffusion, poor intestinal absorption, low solubility in aqueous solutions, and chemical instability limit its potential as a therapeutic agent. Therefore, encapsulation studies have being attempted to improve its stability and bioavailability (Ullah et al., 2017).
Microencapsulation process protects bioactive compounds from deteriorations by capturing them in a matrix (Us-Medina et al., 2018). In addition, their application into the food matrix become much easier, since liquid compounds are entrapped into a solid matrix allowing them to become more applicable (Timilsena, Adhikari, et al., 2017). The core/wall ratio, wall material, and encapsulation technique are important factors affecting the encapsulation efficiency (Copado, Diehl, Ixtaina, & Tomás, 2017). In the food industry, the wall material varieties are limited compared to the pharmaceutical industry due to their GRAS status. Proteins, natural gums, waxes, carbohydrates having low molecular weight and gelatins can be used for food products (Us-Medina et al., 2018), whereas whey protein concentrate, gum Arabic, and soy protein isolate can be used for chia seed oil (Campo et al., 2017). Although spray drying is more commonly used method for encapsulation, freeze drying is better than any methods in the quality aspects (Chen, Zhong, Wen, McGillivray, & Quek, 2013). The structural stability of chia seed oil was improved and the functional properties were enhanced by encapsulating it in emulsions. In this study, freeze drying technique was used to obtain microencapsulated chia seed oil with curcumin. Freeze drying, also known as lyophilisation, is simple dehydration process that involves freezing and sublimation at low temperatures (Laokuldilok & Kanha, 2015). This process improves antioxidant activities and protects oils against oxidative reactions. For heat sensitive compounds such as fatty acids, tocopherols and phenolics; this process is more suitable (Fang & Bhandari, 2012).
The use of different matrices and techniques for the encapsulation of oils has varying influences on the bioavailability of their bioactive chemical constituents. In recent years, in vitro digestion models have been used for assessment of bioavailability of microencapsulated PUFAs. Bioavailability of oils are generally assessed with percentage of free fatty acid (FFA) release during digestion assay. Joung et al. (2016) mentioned that diminished droplet size affects FFA release in a positive manner.
The aim of this study was to encapsulate curcumin and chia seed oil into gum Arabic (GA) and maltodextrin (MD) by freeze drying. On the other hand, an improved understanding of the release behaivour and antioxidant potential of encapsulated chia seed oil containing curcumin have been studied during in vitro digestion studies.
2. Materials and methods
2.1. Materials
Cold-press chia seed oil (Salvia hispanica L.) was purchased from Neva Food Co. (Istanbul, Turkey); curcumin (95% purity) from Herbaturk Co. (Istanbul, Turkey) and gum Arabic from acacia tree from Nexira Co. (Rouen, France). Maltodextrin was provided from Tate&Lyle (Mold, Flintshire, UK). All other reagents and solvents were purchased from Sigma Chemical Co. (St. Louis, MO) and Merck (Whitehouse, NJ) and they were of analytical or chromatographic grade.
2.2. Methods
2.2.1. Emulsion formation
Emulsions were prepared using the wall material MD and GA in different ratios as stated in Table 1 . Emulsions containing different ratios of MD, GA, chia seed oil and curcumin were prepared with a rotor-stator homogenizer (Ultra-Turrax, IKA T18, Germany) for 5 min operating at 18,000 rpm (Carneiro, Tonon, Grosso, & Hubinger, 2013). MD to GA ratio was used as 1:1, 3:1 and 1:3. The total dry matter of the emulsion was adjusted to 30 and 40% by Brix° measurement in which the oil to wall material ratios were changed as 1:3 and 1:5, respectively.
Table 1.
Experiment plan for emulsion studies.
| MD/GA ratio | Total dry matter (40%) |
Total dry matter (30%) |
||
|---|---|---|---|---|
| Oil/Wall material ratio (1:3) | Oil/Wall material ratio (1:5) | Oil/Wall material ratio (1:3) | Oil/Wall material ratio (1:5) | |
| 1:1 | Sample 1 | Sample 2 | Sample 7 | Sample 8 |
| 3:1 | Sample 3 | Sample 4 | Sample 9 | Sample 10 |
| 1:3 | Sample 5 | Sample 6 | Sample 11 | Sample 12 |
In order to determine curcumin amount in curcumin included emulsions, oil ratios of emulsions were calculated. After solubility assay, the highest amount of curcumin that can be solubilized in chia seed oil was added to the emulsion. Sample 6 was chosen according to stability assays, which had 6.67% oil. Thus, 5.12 mg curcumin/100 mL emulsion was added to the sample.
2.2.2. Emulsion stability test
Immediately after emulsion preparation, each emulsion was poured into a 10 mL cylindrical graduated glass tube, sealed and stored at 4 °C for one day. Emulsion stability was analyzed by the volume of the upper phase measured after 24 h (Noello, Carvalho, Silva, & Hubinger, 2016). The phase separation was calculated according to Equation (1).
| CI % = (V/V0) × 100 | (1) |
where CI is the creaming index, V0 represents the initial emulsion volume and V is the upper phase volume.
2.2.3. pH and zeta-potential measurements
In order to measure pH of the emulsions, they were homogenized with vortex. The pH was determined electrometrically using a glass electrode. pH meter was calibrated using a series of standard buffer solutions having a pH of 4.0 and 7.0 (Teo, Misran, & Low, 2012).
To determine the electrical charge on the surface of the oil droplets, freshly prepared emulsions were diluted to the concentration of 0.1% (v/v) in water. The particle size measurement was performed in a microelectrophoresis chamber of a Nano ZS-90 Zetasizer (Malvern Instruments Ltd., Worcestershire UK) according to Etzler and Deanne (1997). Physical characterization studies showed that chia seed oil has a refraction index of 1.4761 (Segura-Campos, Ciau-Solís, Rosado-Rubio, Chel-Guerrero, & Betancur-Ancona, 2014).
2.2.4. Solubility measurements of curcumin in chia seed oil
Solubility of curcumin in chia seed oil was measured spectrophotometrically. Curcumin at an amount of 10 mg was mixed with 10 mL chia seed oil by vortex and centrifuged at 400 rpm for 30 min. Solubility of curcumin was measured at a wavelength of 428 nm (Cunico, Acosta, & Turner, 2017).
2.2.5. Microencapsulation by freeze-drying
Freeze-drying method was used for microencapsulation of chia seed oil with curcumin. Prepared emulsions were frozen at −80 °C then dried in Alpha 1–2 LD plus lyophilizer (Christ, Osterode am Harz, Germany) at 50 °C, 0.1 mbar pressure for 36 h (González, Martínez, Paredes, León, & Ribotta, 2016).
2.2.6. Microcapsule characterization
Moisture content and water activity, total and surface oil determination, and encapsulation efficiency of the microcapsules were investigated in the study.
2.2.6.1. Moisture content and aw analysis
Moisture content was measured with infrared moisture analyzer (MOC63u, Shimadzu, Kyoto, Japan), which has a temperature of 120 °C. Water activity of encapsulated samples was measured using moisture meter, aw device (Protimeter Surveymaster, GE, Billerica, MA) (Campo et al., 2017).
2.2.6.2. Total and surface oil analysis
Total oil (TO) determination and surface oil (SO) analysis were carried out according to a previous methodology of González et al. (2016) with some modifications. Briefly, an aliquot of each microcapsule (10.0 ± 0.1 g) was placed in the Soxhlet extraction apparatus and refluxed with n-hexane (200 mL) for 6 h. The total oil extracted was weighed and expressed as a percentage of oil with respect to the weight (dry basis) of the microcapsules. For surface oil determination, an aliquot of each microcapsule (3.0 ± 0.1 g) was mixed with n-hexane (20 mL) for 1 min. The solvent is then evaporated and the remaining oil was weighed and expressed as a percentage of oil with respect to the weight.
2.2.6.3. Determination of encapsulation efficiency
The encapsulation efficiency (EE) was determined by calculating the ratio of the total oil contained in the microcapsules (TO) and the surface oil (SO) located on its surface, which were determined previously, according to Eq. (2).
| EE = [(TO − SO)/TO] × 100 | (2) |
2.2.7. Characteristics of microcapsules during simulated GI tract conditions
A static in-vitro digestive system model that simulates digestion in the mouth, stomach and intestine was prepared. Microcapsules (12.5 g) equal to 1 g of oil were kept in the mouth, stomach and intestine fluid for 2 min, 2 h and 2 h, respectively. Oral and gastric fluid solutions were prepared according to the methods mentioned by Timilsena, Adhikari, Barrow, and Adhikari (2017). Intestinal fluid solution was composed of 1% pancreatin, 0.9% sodium chloride, and 0.3% bile salts as mentioned in the study performed by Yüksel-Bilsel and Şahin-Yeşilçubuk (2019). An aliquot of oral solution (10 mL) was added to microcapsules having 1 g of oil. This mixture was placed in an orbital shaker (IKA, KS4000i, Germany), and the temperature of the equipment was adjusted as 37 °C. At the end of oral digestion period, 10 mL of gastric fluid solution was added to digested solution, and shaking procedure was continued for 2 h 20 mL of intestinal fluid solution and 96 mg of pancreatic lipase were added to gastric digested solution, after pH of the intestinal solution was set to 7.8.
2.2.7.1. Oil release
In order to determine oil release during simulated digestion, samples were taken at 30 min intervals. Each sample underwent filtration through Whatman No.1 filter paper and washing with 10 mL of hexane. Hexane layer was collected for further spectrophotometric analysis and absorbances were evaluated at 450 nm. Calibration curve was drawn according to known amount of chia seed oil - hexane mixtures (Timilsena, Adhikari, et al., 2017).
2.2.7.2. Antioxidant potential
Total phenolics were analyzed colorimetrically according to the method of Spanos and Wrolstad (1990). Briefly, sample solution at an amount of 100 μL was filtered using 0.45-μm Millipore membrane. It was completed to 1 mL by the addition of distilled water. Afterwards, 5 mL of Folin Ciocalteau reagent (0.2 N) and 4 mL of saturated sodium carbonate (75 g/L) solutions were added. The mixture was homogenized by vortex. The obtained solution was waited for 2 h. At the end of 2 h period, absorbances were read at 765 nm using spectrophotometer. The standard calibration curve of gallic acid was prepared according to known amount of gallic acid mixtures.
The antioxidant (AOX) activity of samples, based on the scavenging activity of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, was determined by the method described by Braca et al. (2001). Antioxidant activity was also determined by cupric reducing AOX capacity (CUPRAC) method, standardized by Apak, Guclu, Ozyurek, and Karademir (2004). It measures the copper (II) or cupric ion reducing ability of polyphenols. The standard calibration curve of each AOX compound was prepared as absorbance versus concentration. The molar absorptivity of the CUPRAC method for each AOX was found from the slope of the calibration line concerned and the AOX activity was expressed as mg Trolox per liter (Murali, Kar, Mohapatra, & Kalia, 2014).
2.2.8. Statistical analysis
The results are expressed as means ± standard deviation of three independent repetitions and two experimental repetitions, and they were analyzed using one factor analysis of variance (ANOVA) test at the 95% level (p < 0.05) of significance. Emulsion preparation analysis were evaluated by full factorial design method, and in vitro release behaviour analysis were evaluated by partial t-test. When ANOVA indicated significant differences between mean values, means were compared using t-test. For statistical studies MINITAB software (version 16.1.0) program was used.
3. Results and discussion
3.1. Emulsion formation
pH values of emulsions prepared using chia seed oil, MD and GA in different ratios are given in Table 2 pH values varied between 4.37 and 4.74. There was no significant difference between the results of all samples (p < 0.05).
Table 2.
pH values, zeta potential and zeta size values of emulsions prepared with chia seed oil and wall materials at different ratiosa.
| Sample | MD/GA ratio | Oil/wall material ratio | pH valueb | Zeta potential (mV)c | Droplet size (nm)c |
|---|---|---|---|---|---|
| 1 | 1:1 | 1:3 | 4.60 ± 0.28 | −32.8 ± 0.6de | 649 ± 16bc |
| 2 | 1:1 | 1:5 | 4.52 ± 0.11 | −32.5 ± 1.5e | 766±7bc |
| 3 | 3:1 | 1:3 | 4.65 ± 0.13 | −37.1 ± 0.9b | 1574 ± 84a |
| 4 | 3:1 | 1:5 | 4.66 ± 0.06 | −37.9 ± 2.0b | 1541 ± 66a |
| 5 | 1:3 | 1:3 | 4.47 ± 0.04 | −33.7 ± 0.5de | 908±4bc |
| 6 | 1:3 | 1:5 | 4.44 ± 0.02 | −32.2 ± 0.8e | 600±8bc |
| 7 | 1:1 | 1:3 | 4.37 ± 0.23 | −32.5 ± 1.0e | 725±2bc |
| 8 | 1:1 | 1:5 | 4.50 ± 0.00 | −35.9 ± 0.6bc | 453±7c |
| 9 | 3:1 | 1:3 | 4.67 ± 0.03 | −40.6 ± 2.0a | 1002 ± 168b |
| 10 | 3:1 | 1:5 | 4.74 ± 0.06 | −40.4 ± 0.6a | 805±2bc |
| 11 | 1:3 | 1:3 | 4.51 ± 0.01 | −34.1 ± 0.5cde | 607±0bc |
| 12 | 1:3 | 1:5 | 4.46 ± 0.01 | −34.7 ± 0.9cd | 408 ± 18c |
Differences between zeta potential and zeta sizes are indicated with lettering according to the results of statistical experiments for each column.
Values are presented as mean ± SD indicate the replicates of two experimental repetitions.
Values are presented as mean ± SD indicate the replicates of three independent and two experimental repetitions.
3.2. Emulsion stability test
Observations showed that there were no phase separations in samples 2, 5, 6 and 12; they remained stable (CI% = 0) for a 24 h period (data not shown). When the stability results were evaluated, it was seen that emulsions having a higher wall material and GA contents were more stable than others. To conclude, emulsion stability of samples is said to be provided primarily by the emulsifying characteristics of GA.
The choice of wall material is one of the most important properties for forming a stable emulsion. Most commonly used carrier agents for oil encapsulation are MD, GA and whey protein concentrates (WPC). MD is hydrolyzed starch and has low cost, low viscosity at higher concentration and provides protection against oxidation, but because of its low emulsifying capacity, it is preferred only in combination with other carrier agents such as GA and WPC (Premi & Sharma, 2017). Moreover, the combination of MD and GA is cheap and effective (Gallardo et al., 2013), and the stability of the emulsion prepared with MD and GA does not deteriorate at 45 °C (Premi & Sharma, 2017).
GA is often preferred in encapsulation methods due to its film forming and emulsifying properties. GA forms stable emulsions with different oils between wide pH ranges. Another feature of this gum is its slow dispersion in water at room temperature. With this feature, it is thought that microcapsules help to strengthen the food structure. GA can be used alone or in combination with other polymers in different proportions as wall material. Since it is not effective as a sole wall material, in many studies, the use of GA with other polymers has benefited from its synergistic effects (Gallardo et al., 2013).
3.3. pH and zeta-potential measurements
pH value, zeta potential and zeta size measurements were done for all the emulsions. Results are given in Table 2. It can be seen from Table 2 that emulsions having higher MD contents tended to have higher droplet sizes and zeta potential measurements. Thus, to obtain a more stable product, it was more appropriate to select emulsions composed of lower MD/GA ratios.
Zeta potential value is one of the important factors that provide the interpretation of the stability of emulsions. The zeta potential value measures the charge gravitations or repulsions between the emulsifying particles. (Premi & Sharma, 2017). When zeta potential and CI results were taken into consideration, it was shown that Sample 6 was the most stable one amongst the samples investigated.
According to Campo et al. (2017) the gums have negative zeta potential because they contain carboxylic acid groups. In emulsions with a pH below 2, positive loads increase in zeta potential measurements, and when the pH value is above 2, negative loads increase in measurements. In our study, the pH value of all 12 emulsion samples varied between 4.37 and 4.74 and it is suitable for zeta potential measurements since the pH value is over 2.
Moringa oleifera plant was encapsulated with MD and GA as wall material. Premi and Sharma (2017) stated that GA increased emulsion stability because of its natural emulsion formation capacity and stabilization nature. GA can form stable emulsions and can be utilized at maximum amount leaving very less amount of uncovered oil droplets. Zeta potential measurements of MD/GA emulsions were lower which represented higher stability.
In another study, fish oil was encapsulated using MD, mesquite gum and GA by spray-drying method. The MD/GA ratio of 50:50 indicated the highest thermo-oxidative stability (Pedroza Islas, Macías-Bravo, & Vernon-Carter, 2002). This shows the high stability of the emulsions when MD and GA are used together. In our study, MD and GA were used together as wall materials.
3.4. Solubility measurements of curcumin in chia seed oil
Solubility of curcumin in chia seed oil was measured spectrophotometrically. Curcumin and chia seed oil mixture was diluted by 1/200 and absorption was measured at 428 nm. Curcumin/chia oil solutions prepared at different concentrations and the maximum solubility amount of curcumin in chia seed oil was calculated as 0.77 mg curcumin/mL chia seed oil based on the absorption curve.
Diverse methods exist in the aspect of finding curcumin solubility in oils. Joung et al. (2016) studied curcumin solubility of different food grade oils. Medium chain triglyceride (MCT) oil, composed of 45% capric acid and 55% caprylic acid, was found to have the best solubility with 0.25 mg curcumin/mL oil. (Takenaka et al., 2013) performed a research similar to Joung et al. (2016) and they also concluded that MCT oils (Panacet 800 and Panacet 810) had a better solubility among tested edible oils. After MCT oils, corn oil has the best result with a solubility of 0.53 mg curcumin/g oil. In another study, ultrasonication took place and linseed oil was found as a better solvent (0.035% solubility) than other investigated edible oils (Cretu, Dima, Bahrim, & Dima, 2011).
Solubility assays of mentioned researches were not the same as the procedure applied in this study; therefore, there could be slight differences between the results. However, when oil solubilities were compared, curcumin solubility was higher in chia seed oil. Accordingly, chia seed oil can be a better carrier for curcumin than other food grade oils.
3.5. Microcapsule characterization
The most stable emulsion in terms of creaming index and zeta potential values (sample 6), prepared with chia seed oil and curcumin, was freeze dried and the obtained microcapsules were characterized by their moisture content, aw value, total and surface oil, and encapsulation efficiency. The results of characterization studies are given in Table 3 .
Table 3.
Moisture content, aw value, total oil, surface oil, and encapsulation efficiency results of microcapsules.
| Sample | Moisture Content (%) | aw value | Total oil (%) | Surface oil (%) | Encapsulation efficiency (%) |
|---|---|---|---|---|---|
| MECO | 3.47 ± 0.057 | 0.05 ± 0.001 | 7.50 ± 0.092 | 2.45 ± 0.031 | 67.36 ± 0.040 |
| MECCO | 3.56 ± 0.042 | 0.04 ± 0.001 | 7.53 ± 0.084 | 2.51 ± 0.043 | 66.69 ± 0.20 |
MECO, Microencapsulated chia seed oil; MECCO, Microencapsulated chia seed oil with curcumin.
3.5.1. Moisture content and aw analysis
Since moisture contents of microencapsulated chia seed oil (MECO) and microencapsulated chia seed oil with curcumin (MECCO) were not higher than 3–4% and aw values were lower than 0.6, it can be said that microcapsules can maintain their stability during shelf life and they are microbiologically stable according to Copado et al. (2017). Chuyen, Roach, Golding, Parks, and Nguyen (2019) also found similar results and concluded that their encapsulated powder was considered to be stable during storage.
In another study in which sodium caseinate and lactose were used as wall materials of chia oil microcapsules, moisture contents of capsules were measured as 1.48–3.52 g/100 g. It was indicated that powders used in food industry should have a maximum moisture specification between 3% and 4% (Ixtaina, Julio, Wagner, Nolasco, & Tomás, 2015).
Copado et al. (2017) also worked with sodium caseinate and lactose for chia oil microcapsules. In their study, moisture contents were found as 0.31–2.23%, which were lower than 3–4%. Thus, they concluded that they could maintain their stability during shelf life since heat treatment may improved the protection of chia oil against lipid oxidation due to the antioxidant properties of the Maillard reaction products.
3.5.2. Determination of total and surface oil, and encapsulation efficiency
Encapsulation efficiency is a ratio calculated to find the protection of encapsulated material from environmental disturbances (González et al., 2016). In order to determine encapsulation efficiency, firstly, the percentages of total and surface oil were found. Efficiencies were calculated as 67.36% and 66.69% for MECO and MECCO, respectively. It can be said that curcumin addition did not have any effect on the encapsulation efficiency of microcapsules. Encapsulation efficiencies together with the percentages of total oil and surface oil are given in Table 3.
Copado et al. (2017) used sodium caseinate and lactose as wall materials for encapsulation of chia seed oil by freeze drying process. Encapsulation efficiency was found to vary between 41.4 and 83.9% for this process. In another study, chia protein isolate and MD were used for encapsulation of chia seed oil and freeze drying process was applied. Efficiency of encapsulation was found between 59.6 and 65.5% (González et al., 2016) which was close to our efficiency results.
3.6. Characteristics of microcapsules during simulated GI tract conditions
3.6.1. Oil release
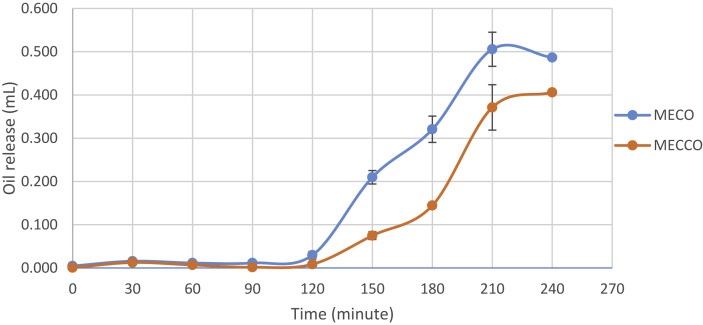
In order to determine the release behaivour of MECO and MECCO and to investigate the release behaivour of chia seed oil, in vitro release study of microcapsules were performed by simulating GI tract. Samples were collected for 4 h at 30 min intervals and chia seed oil release was observed (Fig. 1 ).
Fig. 1.
Chia seed oil release from microcapsules during in vitro digestion*.
MECO, Microencapsulated chia seed oil; MECCO, Microencapsulated chia seed oil with curcumin. *Values are presented as mean values indicate the replicates of three independent and two experimental repetitions.
During 120 min period, oral and gastric digestion were completed. Since the wall materials were not composed of protein structured materials, oil release was low and this protected capsule degradation before intestinal phase. Timilsena, Adhikari, et al. (2017) encapsulated chia seed oil into chia seed protein-gum complex, and subjected the capsules to in-vitro digestion. Oil release in the gastric phase was found to be high, which was interpretted to the enzymatic protein decomposition during digestion.
After 240 min, total enteric release of oil from MECO was 487 μL, and from MECCO was 406 μL. Taking these values into consideration, total oil release from MECO was 44.6%, and from MECCO was 37.2%. Released oil contents were found significantly different (p < 0.05). Curcumin addition decreased the release of microencapsulated chia seed oil. As a result, it can be concluded that the addition of curcumin may have increased the physical stability during the encapsulation process. Curcumin addition could increase the interactions between chia seed oil and wall materials or it can enhance the durability of wall materials during digestion. Thus, this might decrease oil release from the capsules. Furthermore, since it was a spectrophotometric method, curcumin could interrupt absorbance results.
Release test studies in the literature are generally focused on in vitro emulsified oil release; thus, studies focused on microencapsulated oil release are respectively less than others. In a study, chia protein isolate and chia gum was used as wall materials for encapsulation of chia seed oil and the highest release in gastric phase was shown in chia protein isolate encapsulant (Timilsena, Vongsvivut, Adhikari, & Adhikari, 2017).
3.6.2. Antioxidant potential
Total phenolic compounds and total antioxidant capacity of MECO and MECCO are given in Table 4 . MECCO was said to have higher values than MECO for total phenolic content. Contrary to expectations, MECO had a higher antioxidant potential than MECCO according to DPPH and CUPRAC antioxidant assays. It can be interpretted that because of the low durability of curcumin, antioxidant potential was not differed between two samples during in vitro digestion. On the other hand, curcumin has a prominent yellow color and antioxidant tests were spectrophotometric methods. Thus, color of curcumin could interfere with absorbances, causing a lower measured antioxidant potential of MECCO.
Table 4.
Total phenolic compounds and total antioxidant capacity analysis (by DPPH and CUPRAC methods) of MECO and MECCO in intestine in fractiona.
| Sample | Total phenolic compounds (mg GAE/L)b | DPPH (mg Trolox/L)b | CUPRAC (mgTrolox/L)b |
|---|---|---|---|
| MECO | 14 ± 3 b | 11±1a | 59 ± 16a |
| MECCO | 22 ± 4 a | 12±1a | 51 ± 14a |
MECO, Microencapsulated chia seed oil; MECCO, Microencapsulated chia seed oil with curcumin; GAE, Gallic acid equivalent.
Differences between measurements are indicated with lettering according to the results of statistical experiments for each column.
Values are presented as mean ± SD indicate the replicates of three independent repetitions and two experimental repetitions.
Recovery values of total phenolic compounds in intestinal fraction were calculated as 38.9% for MECO and 22.6% for MECCO. Recovery values found by CUPRAC method were 38.6% and 30.6%, respectively. Recoveries obtained by DPPH method for MECO and MECCO were 27.3% and 25.5%, respectively. Taking all results into consideration, recovery ratio from intestine in fraction of MECO was higher compared to the ratio of MECCO.
Kumavat et al. (2013) studied the stability of curcumin in aqueous solutions in different pH ranges and presence and absence of light at 37 °C. Higher pH values were found to be more destructive for curcumin, especially in the presence of light. When our study is considered, in vitro digestion took place in an aqueous solution at 37 °C. Moreover, intestinal digestion had a pH value of 7.8. Fig. 1 showed that most of the oil released in intestinal phase of the digestion. When the lipid-soluble property of curcumin was taken into consideration, it could be understood that curcumin release, also, occurred mostly in the intestinal stage. To conclude, curcumin could have been degraded under aqueous and basic environment; thus, this could lead to the lower antioxidant results of MECCO.
4. Conclusions
Chia seed oil, with and without curcumin, was encapsulated into MD and GA by freeze drying method. The effect of encapsulation process and curcumin addition on in vitro release behaivour and antioxidant capacity of chia seed oil were investigated. Emulsion having the best stability, which had total dry matter contents of 40%, 1:3 MD to GA ratio in wall material, and 1:5, oil to wall material ratios were used for the encapsulation process.
Curcumin is a promising bioactive compound due to its therapeutic and antioxidant properties. However, its high rate of degradation, poor intestinal absorption, low solubility in aqueous solutions and chemical instability limit its potential usage. In this study, according to the findings, some of these problems were overcome. Solubility of curcumin was improved with the use of emulsification technique in chia seed oil. Moreover, MD and GA enhanced the stability of microcapsules, which leads to enhancement of curcumin stability. Finally, incorporation of curcumin in chia seed oil provided significant increment of total phenolic content of microcapsules.
Obtained data will serve as a basis for further studies in order to scale up production for functional products. Nowadays, consumer demands for immune-boosting products have increased significantly especially because of Covid-19 pandemic. Also potential intake of antioxidants and omega-3 fatty acid supplements were boosted due to high bioavailability of these components when used together. It's thought that this research will be a precursor for further studies. In near future, there might be more research about in vivo studies and clinical trials. Moreover nano-encapsulation techniques can also be employed.
CRediT authorship contribution statement
Burcu Fırtın: Defined an idea, Designed and performed experiments, analyzed and interpreted data. Hande Yenipazar: Analyzed and interpreted the data, co-wrote the paper. Ayşe Saygün: Helped to interpret and writing results parts, co-wrote the paper. Neşe Şahin-Yeşilçubuk: Supervised reviewed, interpreted and revised the research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lwt.2020.109947.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Almeida M.C., Sampaio G.R., Bastos D.H., Villavicencio A.L. Effect of gamma radiation processing on turmeric: Antioxidant activity and curcumin content. Radiation Physics and Chemistry. 2018;152:12–16. doi: 10.1016/j.radphyschem.2018.07.008. [DOI] [Google Scholar]
- Apak R., Guclu K., Ozyurek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E using their cupric ion reducing capabilities in the presence of neocuproine, CUPRAC method. Journal of Agricultural and Food Chemistry. 2004;52:7970–7981. doi: 10.1016/10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Ayerza R., Jr. Oil content and fatty acid composition of chia (Salvia hispanica L.) from five northwestern locations in Argentina. Journal of American Oil Chemists’ Society. 1995;72:1079–1081. [Google Scholar]
- Braca A., DeTommasi N., Di Bari L., Pizza C., Politi M., Morelli I. Antioxidant principles from Bauhinia tarapotensis. Journal of Natural Products. 2001;64:892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- Campo C.D., Santos P.P., Costa T.M., Paese K., Guterres S.S., Rios A.D. Nanoencapsulation of chia seed oil with chia mucilage ( Salvia hispanica L.) as wall material: Characterization and stability evaluation. Food Chemistry. 2017;234:1–9. doi: 10.1016/j.foodchem.2017.04.153. [DOI] [PubMed] [Google Scholar]
- Carneiro H.C.F., Tonon R.V., Grosso C.R.F., Hubinger M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. Journal of Food Engineering. 2013;115:443–451. doi: 10.1016/j.jfoodeng.2012.03.033. [DOI] [Google Scholar]
- Chen Q., Zhong F., Wen J., McGillivray D., Quek S.Y. Properties and stability of spray-dried and freeze-dried microcapsules co-encapsulated with fish oil, phytosterol esters, and limonene. Drying Technology. 2013;31(6):707–716. doi: 10.1080/07373937.2012.755541. [DOI] [Google Scholar]
- Chuyen H.V., Roach P.D., Golding J.B., Parks S.E., Nguyen M.H. Encapsulation of carotenoid-rich oil from Gac peel: Optimisation of the encapsulating process using a spray drier and the storage stability of encapsulated powder. Powder Technology. 2019;344:373–379. doi: 10.1016/j.powtec.2018.12.012. [DOI] [Google Scholar]
- Copado C.N., Diehl B.W., Ixtaina V.Y., Tomás M.C. Application of Maillard reaction products on chia seed oil microcapsules with different core/wall ratios. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2017;86:408–417. doi: 10.1016/j.lwt.2017.08.010. [DOI] [Google Scholar]
- Cretu R., Dima C., Bahrim G., Dima S. Improved solubilization of curcumin with a microemulsification formulation. The Annals of the University of Dunarea de Jos of Galati. Fascicle VI. Food Technology. 2011;35(2):46. [Google Scholar]
- Cunico L.P., Acosta M.C., Turner C. Experimental measurements and modeling of curcumin solubility in CO2-expanded ethanol. The Journal of Supercritical Fluids. 2017;130:381–388. doi: 10.1016/j.supflu.2017.06.018. [DOI] [Google Scholar]
- Etzler F.M., Deanne R. Particle size analysis: A comparison of various methods II. Particle and Particle System Characterization. 1997;14:278–282. doi: 10.1002/ppsc.19970140604. [DOI] [Google Scholar]
- Fang Z., Bhandari B. Spray drying, freeze drying and related processes for food ingredient and nutraceutical encapsulation. In: Garti N., Mc Clements D.J., editors. Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Woodhead Publishing Limited; Cambridge, UK: 2012. pp. 73–109. [DOI] [Google Scholar]
- Gallardo G.L., Guida L., Martinez V., Lopez M.C., Bernhardt D.C., Blasco R. Microencapsulation of linseed oil by spray drying for functional food application. Food Research International. 2013;52(2):473–482. doi: 10.1016/j.foodres.2013.01.020. [DOI] [Google Scholar]
- González A., Martínez M.L., Paredes A.J., León A.E., Ribotta P.D. Study of the preparation process and variation of wall components in chia (Salvia hispanica L.) oil microencapsulation. Powder Technology. 2016;301:868–875. doi: 10.1016/j.powtec.2016.07.02. [DOI] [Google Scholar]
- Ixtaina V.Y., Julio L.M., Wagner J.R., Nolasco S.M., Tomás M.C. Physicochemical characterization and stability of chia oil microencapsulated with sodium caseinate and lactose by spray-drying. Powder Technology. 2015;271:26–34. doi: 10.1016/j.powtec.2014.11.006. [DOI] [Google Scholar]
- Joung H., Choi M.J., Kim J.T., Park S.H., Park H.J., Shin G.H. Development of food-grade curcumin nanoemulsion and its potential application to food beverage system: Antioxidant property and in vitro digestion. Journal of Food Science. 2016;81(3):745–753. doi: 10.1111/1750-3841.13224. [DOI] [PubMed] [Google Scholar]
- Julio L.M., Ixtaina V.Y., Fernández M.A., Sánchez R.M., Wagner J.R., Nolasco S.M. Chia seed oil-in-water emulsions as potential delivery systems of ω-3 fatty acids. Journal of Food Engineering. 2015;162:48–55. doi: 10.1016/j.jfoodeng.2015.04.005. [DOI] [Google Scholar]
- Kumavat S.D., Chaudhari Y.S., Borole P., Mishra P., Shenghani K., Duvvuri P. Degradation studies of curcumin. International Journal of Pharmacy Review and Research. 2013;3(2):50–55. [Google Scholar]
- Laokuldilok T., Kanha N. Effects of processing conditions on powder properties of black glutinous rice (Oryza sativa L.) bran anthocyanins produced by spray drying and freeze drying. LWT- Food Science and Technology. 2015;64(1):405–411. [Google Scholar]
- Marineli R.D., Moraes É.A., Lenquiste S.A., Godoy A.T., Eberlin M.N., Jr M.R. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.) Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2014;59(2):1304–1310. doi: 10.1016/j.lwt.2014.04.014. [DOI] [Google Scholar]
- Martínez-Cruz O., Paredes-López O. Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra high performance liquid chromatography. Journal of Chromatography A. 2014;1346:43–48. doi: 10.1016/j.chroma.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Mcclements D.J., Decker E.A., Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. Journal of Food Science. 2007;72:109–124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Meyer B., Groot R. Effects of omega-3 long chain polyunsaturated fatty acid supplementation on cardiovascular mortality: The importance of the dose of DHA. Nutrients. 2017;9(12):1305. doi: 10.3390/nu9121305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali S., Kar A., Mohapatra D., Kalia P. Encapsulation of black carrot juice using spray and freeze drying. Food Science and Technology International. 2014;21(8):604–612. doi: 10.1177/1082013214557843. [DOI] [PubMed] [Google Scholar]
- Noello C., Carvalho A.G.S., Silva V.M., Hubinger M.D. Spray dried microparticles of chia oil using emulsion stabilized by whey protein concentrate and pectin by electrostatic deposition. Food Research International. 2016;89:549–557. doi: 10.1016/j.foodres.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Özcan M.M., Al-Juhaimi F.Y., Ahmed I.A., Osman M.A., Gassem M.A. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chemistry. 2019;278:190–196. doi: 10.1016/j.foodchem.2018.11.048. [DOI] [PubMed] [Google Scholar]
- Pedroza Islas R., Macías-Bravo S., Vernon-Carter E.J. Oil thermo-oxidative stability and surface oil determination of biopolymer microcapsules. Revista Mexicana de Ingeniería Química. 2002;1:37–44. [Google Scholar]
- Premi M., Sharma H.K. Effect of different combinations of maltodextrin, gum Arabic and whey protein concentrate on the encapsulation behaivour and oxidative stability of spray dried drumstick (Moringa oleifera) oil. International Journal of Biological Macromolecules. 2017;105:1232–1240. doi: 10.1016/j.ijbiomac.2017.07.160. [DOI] [PubMed] [Google Scholar]
- Pulido-Moran M., Moreno-Fernandez J., Ramirez-Tortosa C., Ramirez-Tortosa M. Curcumin and health. Molecules. 2016;21:264. doi: 10.3390/molecules21030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee Z., Nejatian M., Daeihamed M., Jafari S.M. Application of different nanocarriers for encapsulation of curcumin. Critical Reviews in Food Science and Nutrition. 2019;59(21):3468–3497. doi: 10.1080/10408398.2018.1495174. [DOI] [PubMed] [Google Scholar]
- Segura-Campos M.R., Ciau-Solís N., Rosado-Rubio G., Chel-Guerrero L., Betancur-Ancona D. Physicochemical characterization of chia (Salvia hispanica) seed oil from Yucatán, México. Agricultural Sciences. 2014;5(3):220–226. doi: 10.4236/as.2014.53025. [DOI] [Google Scholar]
- Silva B.P., Dias D.M., de Castro Moreira M.E., Toledo R.C., da Matt S.L., Lucia C.M. Chia seed shows good protein quality, hypoglycemic effect and improves the lipid profile and liver and intestinal morphology of wistar rats. Plant Foods for Human Nutrition. 2016;71:225–230. doi: 10.1007/s11130-016-0543-8. [DOI] [PubMed] [Google Scholar]
- Spanos G.A., Wrolstad R.E. Influence of processing and storage on the phenolic composition of thompson seedless grape juice. Journal of Agricultural and Food Chemistry. 1990;38:1565–1571. doi: 10.1021/jf00097a030. [DOI] [Google Scholar]
- Taga M.S., Miller E.E., Pratt D.E. Chia seeds as a source of natural lipid antioxidants. Journal of American Oil Chemists’ Society. 1984;61:928–931. [Google Scholar]
- Takenaka M., Ohkubo T., Okadome H., Sotome I., Itoh T., Isobe S. Effective extraction of curcuminoids by grinding turmeric (Curcuma longa) with medium-chain triacylglycerols. Food Science and Technology Research. 2013;19(4):655–659. doi: 10.3136/fstr.19.655. [DOI] [Google Scholar]
- Teo Y.Y., Misran M., Low K.H. Effect of pH on physicochemical properties and encapsulation efficiency of PEGylated linolenic acid vesicles. Journal of Chemistry. 2012;9(2):729–738. doi: 10.1155/2012/421286. [DOI] [Google Scholar]
- Timilsena Y.P., Adhikari R., Barrow C.J., Adhikari B. Microencapsulation of chia seed oil using chia seed protein isolate-chia seed gum complex coacervates. International Journal of Biological Macromolecules. 2016;91:347–357. doi: 10.1016/j.ijbiomac.2016.05.058. [DOI] [PubMed] [Google Scholar]
- Timilsena Y.P., Adhikari R., Barrow C.J., Adhikari B. Digestion behaivour of chia seed oil encapsulated in chia seed protein-gum complex coacervates. Food Hydrocolloids. 2017;66:71–81. doi: 10.1016/j.foodhyd.2016.12.017. [DOI] [Google Scholar]
- Timilsena Y.P., Vongsvivut J., Adhikari R., Adhikari B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chemistry. 2017;228:394–402. doi: 10.1016/j.foodchem.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Ullah F., Liang A., Rangel A., Gyengesi E., Niedermayer G., Münch G. High bioavailability curcumin: An anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch Toxicol. 2017;91:1623–1634. doi: 10.1007/s00204-017-1939-4. [DOI] [PubMed] [Google Scholar]
- Us-Medina U., Julio L.M., Segura-Campos M.R., Ixtaina V.Y., Tomás M.C. Development and characterization of spray-dried chia oil microcapsules using by-products from chia as wall material. Powder Technology. 2018;334:1–8. doi: 10.1016/j.powtec.2018.04.060. [DOI] [Google Scholar]
- Yüksel-Bilsel A., Şahin-Yeşilçubuk N. Production of probiotic kefir fortified with encapsulated structured lipids and investigation of matrix effects by means of oxidation and in vitro digestion studies. Food Chemistry. 2019;296:17–22. doi: 10.1016/j.foodchem.2019.05.181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.