Abstract
Urbanization induced habitat loss and alteration causes significant challenges for the survival of many species. Identifying how species respond to urbanization can yield insights for the conservation of wildlife, but research on reptiles has been narrowly-focused. We compared morphology among four populations of western fence lizards (Sceloporus occidentalis) to determine whether a common native species affected by urbanization exhibits morphological differences consistent with habitat use. We quantified habitat differences across four sites in Los Angeles County, California, USA that varied in level of urbanization, measured how lizards used microhabitats, and assessed variation in body size, limb length, toe length, and scalation of lizards collected from each site. Urban and suburban populations of fence lizards mostly used human-made substrates while lizards from more natural areas mostly used natural woody substrates. Lizards from the most urban site also exhibited the widest breadth of substrates used, indicating that urban sites might offer more variable microhabitats. Urban lizards had reduced limb lengths and toe lengths consistent with how they used microhabitats and other habitat characteristics (e.g., percent impervious surface cover). Urban lizards also had fewer dorsal scales, which might be associated with changes in ambient temperature (e.g., urban heat island effect), given that lizards with fewer and larger scales typically have reduced evaporative water loss. Our results uniquely differ from past studies on lizard responses to urbanization, indicating that work on diverse taxa is necessary to assess the potential varied pathways of morphological adaptations to urban environments.
Keywords: behavior, phenotypic change, HIREC, urban evolution, reptile, thermal ecology
Introduction
Urbanization induced habitat loss and alteration causes significant challenges for the survival of many native species. A major conservation concern, therefore, is understanding how animals survive in altered habitats. To be successful, individuals or species must respond appropriately to the novel conditions associated with human-induced rapid environmental change (HIREC). Compared to nearby natural areas and to historical conditions, urban areas often have different types of predators, less connected habitat patches, reduced vegetation, altered water availability, and novel structures and substrates such as buildings and roads (Pickett et al. 2001; Shochat et al. 2006b; Fischer et al. 2012). Because these habitat characteristics may be different from the conditions in which species evolved, animals in urban environments may experience an ecological or evolutionary mismatch that has fitness consequences (Kokko and Sutherland 2001; Sih 2013). Thus, there should be strong selection on urban populations for phenotypes better suited to the urban environment (Johnson and Munshi-South 2017). This may be especially true for animals that cannot easily move away from the disturbance.
Anthropogenically-driven habitat changes do indeed induce high rates of phenotypic change (Hendry et al. 2008; Hendry et al. 2017). These changes can be rapid (e.g., Atwell et al. 2012) and some have even been shown to correlate with genetic changes, essentially demonstrating many species’ capacity for rapid evolution (reviewed by Johnson and Munshi-South 2017). While behaviors are likely the first suite of traits to change in response to anthropogenic-habitat alterations (Tuomainen and Candolin 2011; Lowry et al. 2013), selection pressures on morphological traits could be great in urban habitats, especially for species whose performance (and fitness) is directly linked to specific morphological traits. For example, bill morphology of urban finch populations has diverged from natural populations as a response to feeding on large, hard sunflower seeds provided at bird feeders (Badyaev et al. 2008). Lizards also have the potential to show strong morphological divergence with urbanization as many studies have shown that performance is linked to morphology, which associates strongly with structural habitat (i.e., ecomorphological specialization; Losos 1990, 2009, Luxbacher and Knouft 2009, Kaliontzopoulou et al. 2010). However, even though squamates (lizards and snakes) comprise the second largest order of extant vertebrates, studies on morphological responses to urbanization have narrowly focused on a few species.
Because urban environments consist of human-made structures, urban lizards might switch from using natural substrates to human-made substrates such as buildings, walls, and sidewalks. Use of such substrates could correspond to changes in limb and toe lengths because these morphological traits affect lizards’ climbing and sprint performance. Past studies have found that arboreal anoles, which used narrow branches and vegetation in natural environments, switched to using broad and smooth substrates (e.g., walls) in urban environments, and these differences in habitat use corresponded with urban anoles having longer limbs and more toe lamellae than non-urban anoles (Marnocha et al. 2011, Winchell et al. 2016). Lizards with longer limbs run faster on flat surfaces, anoles with more toe lamellae have stronger clinging performance, and anoles from urban populations perform better on human-made substrates than those from natural populations (Winchell et al. 2018). However, for species that are not ecologically similar to anoles in habitat use, responses to human-induced habitat alterations are likely to differ. For example, a recent study on western fence lizards, a species that commonly uses rocks and broad woody substrates (logs and tree trunks) in natural environments, found that females on urbanized college campuses had shorter limbs than non-urban females (Sparkman et al. 2018). However, this study did not determine whether these morphological changes were associated with changes in habitat use.
In addition to changes in habitat structure, urban areas can substantially differ in thermal environments compared to adjacent natural habitat. The reduced vegetation and increased hardscape common in urban areas can make them warmer than natural areas, creating an urban heat island (UHI) effect (Arnfield 2003). As a result, urban inhabitants may experience increased thermal stress in warmer months, but they also may experience an expanded annual active season and/or growing season into colder months because winters are less severe (Sukopp and Wurzel 2003; Kuttler 2008). The effects of UHI have been shown to reduce available activity time for lizards in hot climates of the U.S. Southwest (Ackley et al. 2015). However, urban areas could also be cooler than natural areas in arid regions because of supplemental watering by humans. For instance, in Arizona, some urban areas are cooler than the natural desert habitat, with a 0.3 °C reduction in mean surface temperature for every $10,000 increase in neighborhood median annual household income (Jenerette et al. 2007), presumably due to increased water usage and increased vegetative cover. Ectotherms living in urban habitats might exhibit phenotypic responses to changes in the thermal environment because their physiological performance relies on body temperature. Rapid changes in physiological heat tolerance have already been described in urban ectothermic invertebrates (Angilletta et al. 2007; Brans et al. 2017; Diamond et al. 2017). Morphological responses to the thermal environment, however, could also be important for urban ectotherms if specific traits enhance or reduce thermoregulatory performance, including evaporative water loss.
We focused our study on western fence lizards (Sceloporus occidentalis) to test morphology-habitat relationships in urban environments. We quantified habitat differences across four sites in Los Angeles County, California, USA that varied in history of urban development and asked whether free-ranging lizards differed in their use of microhabitats at these sites. We then measured morphological traits of lizards collected at these sites, looking at whether limb and toe lengths and numbers of dorsal and ventral scales associated with differences in microhabitat use and with differences in site thermal attributes, respectively. Fence lizards from natural environments are variable in their microhabitat use, but in general, they are rarely found on narrow branches or vegetation. Instead, they tend to perch upon broader substrates such as cliffs, rocks, logs, and tree trunks. Previous research examining inter-population differences in morphology in western fence lizards and another phrynosomatid, ornate tree lizards (Urosaurus ornatus), found that lizards from populations that utilized trees exhibited longer limbs compared to lizards from more open, rocky habitats (Sinervo et al. 1991; Sinervo and Losos 1991; Herrel et al. 2001). Herrel et al. (2001), also found that tree lizards that climb smooth, vertical cliffs are more likely to have short limbs (humerus and femur) and hind toes, suggesting that the type of substrate used (trees vs. cliffs) associates with different morphological responses, such that tree-climbers have long limbs and cliff-climbers have short limbs.
Consistent with expectations from the above-mentioned ecomorphology research on phrynosomatid lizards, we predicted that fence lizards from sites with higher percent impervious surface cover would exhibit shorter limbs and shorter hind toes because they will use more human-made substrates (e.g., walls and buildings), which would be analogous to using open-rocky habitats or vertical cliffs (Herrel et al. 2001). Fence lizards from more natural habitats were expected to be more tree-dwelling and would therefore exhibit longer limbs compared to lizards from more urban populations (Sinervo and Losos 1991). In terms of scalation, we examined whether fence lizards exhibited changes consistent with thermal differences at sites that vary in urban development. Previous studies show that Uta, Anolis, and Liolaemus lizards inhabiting areas with hotter and drier climatic conditions have fewer, but larger scales (Hellmich 1951; Soulé 1966; Calsbeek et al. 2006; Wegener et al. 2014), and this is typically interpreted as an adaptive response to reduce evaporative water loss by minimizing the surface area of skin between scales (Bogert 1949). There is evidence that scale size in Sceloporus lizards varies with climatic variables as well (Bogert 1949; Oufiero et al. 2011). If more-urban populations experience a warmer thermal environment, we predicted a decrease in scale counts.
Methods
Study sites
We selected four sites within Los Angeles County that had historical differences in urban development. We used the 2011 National Land Cover Database (NLCD) to determine land cover classifications for each site and the Los Angeles County Assessor Parcels 2016 Tax Roll to determine the history of urban development at each site (i.e., year built of parcel). The sites are geographically isolated from each other with at least 15 km of urban development between them. Recent species occurrence data generated through the Reptiles and Amphibians of Southern California (RASCals; www.nhm.org/rascals; Spear et al. 2017) citizen science project hosted on the iNaturalist platform demonstrate that although fence lizards can be found in some urban neighborhoods in the Los Angeles region, they tend to be found in or within a few house lots of corridors containing woody vegetation; fence lizards are not found in higher density residential and commercial areas. Sites are also associated with different mountain ranges or physiographic features surrounding the Los Angeles Basin. Translocations of individuals between any of our sites is unlikely. Thus, there is likely minimal or no modern gene flow among lizard populations at these four sites. The sites we chose to work at essentially form a gradient of urbanization, in terms of extent and intensity of urban development and in time since development. We describe below the habitat characteristics of each site, introducing them from most natural to most urban, and we label them with an abbreviation and a number from one to four, with one being least and four being most urbanized.
Whitney Canyon (WC-1; 34.366123, −118.494705) is a 442-acre wilderness park at the edge of the Angeles National Forest on the northwest end of the San Gabriel Mountains. Although there is a history of grazing and some dirt roads through the park, the closest human structures include a parking lot, some buildings at the park entrance, and wooden posts running along hiking trails. It is considered shrubland by the NLCD, and specifically shrub/scrub, an area dominated by shrubs less than 5 m tall and with a scrub canopy greater than 20% of total vegetation. Sage Hill (SH-2; 34.073582, −118.454758) is a small remnant plot of oak-grassland within the University of California, Los Angeles campus; this site abuts the foothills of the Santa Monica Mountains, is considered a mixed forest (an area dominated by trees greater than 5 m tall and greater than 20% of total vegetation cover) by the NLCD, and it is surrounded by developed open space and developed low intensity land. The plot became increasingly isolated in the 1960s with campus expansions, and by 1980, it was completely surrounded by houses, a parking lot, and various campus buildings. The Palos Verdes (PV-3; 33.780280, −118.349140) site consists of the Rolling Hills Estates on the Palos Verdes Peninsula at the southern tip of the Los Angeles Basin. The site is a suburban neighborhood with sizable homes on large lots, a nearby botanical garden, and equestrian trails throughout the neighborhood. It is categorized as developed, low intensity land by the NLCD. Significant development of this neighborhood began in the late 1960s. The Long Beach site (LB-4; 33.846027; −118.200241) is on the floor of the Los Angeles Basin along the current Los Angeles River channel. This urban neighborhood has small housing lots developed in the late 1950s, and it is categorized as developed medium intensity land. The LB-4 site also has a vacant plot of land (which used to be a single-story school) and is bordered on its west side by a bike trail and the Los Angeles River, which runs within a concrete-lined channel.
To further quantify habitat differences among the sites, we calculated percent impervious surface, percent tree cover, and accessed temperature data from WorldClim (Fick and Hijmans 2017). We chose these habitat characteristics because they should be associated with morphology-performance relationships in lizards. A higher percentage of impervious surface and lower percentage of tree cover should reduce availability of natural climbing substrates, increase availability of human-made substrates, and increase temperature (UHI effect). For lizards found at each site (see below), we created a 100-m radius buffer around the geographic points of their location and quantified percent impervious surface and percent tree cover within the buffer using the ‘sf’ package in R (v3.4.2). We chose 100 m because previous studies have shown movement and dispersal in adult Sceloporus occidentalis rarely exceed this distance (Davis and Ford 1983; Sheldahl and Martins 2000; Massot et al. 2003). We used the 2011 NLCD tree canopy and impervious surface datasets, which are the most recent data available for these habitat values. We downloaded WorldClim data for each site, including mean annual temperature and maximum temperature during the warmest month, at a spatial resolution of 1 km2.
Lizard microhabitat use
We visited the sites in March 2017 to quantify the characteristics of the microhabitats used by lizards (n = 20–24 per site). We took body, substrate, and ambient temperature measurements to determine whether lizards at the sites with high impervious surface and low tree cover experience a UHI effect during our period of study. We visited sites between 1000 and 1500 h, the times of day when lizards are most active at these locations (personal observation). We generally followed the methods of Winchell et al. (2016) to measure microhabitat use. We walked around slowly looking for adult lizards scanning at all potential heights. Once spotted, we attempted to capture the lizard using a lasso. Immediately following capture, we measured the lizard’s temperature using a non-contact infrared temperature gun (model eT650D, ennoLogic). We then recorded its position on the substrate (vertical or horizontal), perch height and diameter, substrate type (natural or human-made), substrate temperature, ambient temperature, and time of day. Substrate temperature was measured using the temperature gun. We also noted when lizards were found not on a perch (i.e., on the ground), and distinguished between natural ground (e.g., dirt) or human-made ground (e.g., concrete sidewalk). Ambient temperature was taken in a shaded area next to where the lizard was found using a Kestrel weather meter (model 5500). We recorded the lizard’s geographic position in latitude and longitude using the Solocator smart phone application (± 5 m; iOS v2.3, Civi Corp Pty Ltd). Perch height was measured to the nearest centimeter with a measuring tape. Because many perches have widths that are logistically immeasurable (e.g., walls), we binned perch widths into 1 cm categories up to 10 cm. Any perch wider than 8 cm should be functionally equivalent for lizards (Irschick and Losos 1999; Winchell et al. 2016), and we choose a more conservative value of 10 cm to analyze differences in widths used among sites. All the above methods were approved by the University of California Los Angeles Institutional Animal Care and Use Committee (ARC # 2016-051-03).
Morphological measurements
We measured morphometric traits of museum specimens collected from the four sites in March 2015 and deposited at the Natural History Museum of Los Angeles County (n = 8–10 lizards per site). All specimens were adults (SVL > 49 mm). For each, we measured eight morphological traits (detailed in Table 1). We measured lower limb lengths (i.e., tibia/fibula and metatarsals, and radius/ulna and metacarpals) because the elbow/knee provides a repeatable location from which to take measurements. Measurements of upper limb lengths (i.e., femur and humerus) can be more variable and so we elected not to take these to reduce error in our dataset. To reduce variation among measurements, one person measured all specimens (MG). Before measuring the specimens, the recorder was trained to take consistent measurements (< 5% error).
Table 1.
List of the morphological traits quantified for Sceloporus occidentalis at four different sites in Los Angeles County, USA
| Trait | Measurement definition |
|---|---|
| Snout-vent-length (SVL) | Tip of snout to posterior edge of the cloaca |
| Lower forelimb length | Tip of the fourth digit excluding the claw to the elbow |
| Lower hind limb length | Tip of the fourth digit excluding the claw to the knee |
| Forelimb fourth digit length | Tip of the fourth toe excluding the claw to the point of insertion of the toe at the footpad |
| Hind limb fourth digit length | Tip of the fourth toe excluding the claw to the point of insertion of the toe at the footpad |
| Dorsal scale count | Along midline, from the parietal scale to the posterior end of the hind limb insertion points |
| Ventral scale count | Along midline, from the post-mental scale to the anterior edge of the cloaca |
Morphological measurements were quantified using a dial caliper with graduations of 0.05 mm. Scale counts were quantified using an optical microscope. The recorder was blind to specimen ID (i.e., site of collection), and made each measurement at least twice, recording the average of the two.
Statistical analyses
To examine differences in percent impervious surface and tree cover among sites, we used Kruskal-Wallis nonparametric ANOVA because transformations failed to normalize distributions and homogenize variances. For lizard microhabitat use, we used ANOVA, with site as a factor, to test for differences in square-root transformed lizard perch height among sites. We used a Kruskal-Wallis nonparametic ANOVA to test for differences in perch width. We used Chi-squared tests to examine differences in lizards’ likelihood of being found vertically on substrates and differences in use of human-made substrates among sites. We further divided substrate type into sub-categories based on the substrate’s material: ‘Woody’ (branches, logs, fences), ‘Hardscape’ (rocks, bricks, concrete, sidewalk), ‘Natural Ground’ (found on dirt or leaf litter), or ‘Other’ (any substrates that did not fall under the previous categories). We calculated Levins’ measure of niche breadth to estimate the diversity of substrate types used by lizards at each site (Levins 1968; Pianka 1986). Levins’ B is defined as the uniformity of distribution of individuals among the resource states. B ranges from 1 to n, where n is the total number of resource states and would indicate the highest breadth:
Where = Levins' measure of niche breadth, and = the proportion of individuals found using the resource state.
Following the methods of Winchell et al. (2016), we fitted an ANCOVA, with site as a factor, and time of day as a covariate to examine differences in ambient temperature; an ANCOVA with site as a factor and ambient temperature as a covariate to examine differences in square-root transformed substrate temperature; and an ANCOVA with site as a factor and substrate temperature and lizard mass as covariates to examine differences in lizard temperature.
We tested for differences in morphological traits among sites by fitting general linear models. First, we looked for differences in body size, measured as SVL, among sites by fitting a model with site and sex as factors. Then, we fitted models on all other morphological traits with site, sex, and SVL as factors (to account for allometric scaling). All morphometric traits and SVL were log transformed for these tests. We include sex in our models merely to account for sexual dimorphism in this species, given that males tend to be larger than females. For all statistical analyses, we used either Tukey post-hoc tests (parametric) or Mann-Whitney U tests with Bonferroni corrections (nonparametric) to examine differences between sites. We set our alpha to 0.05.
Results
Habitat characteristics
We found that sites differed in percent impervious surface (Kruskal-Wallis X2=70.55, df=3, P<0.001, Table 2) and tree cover (Kruskal-Wallis X2=67.97, df=3, P<0.001, Table 2), with the most urban site, LB-4, having the most amount of impervious surface and least amount of tree cover. Worldclim data showed that WC-1 had the highest maximum annual temperature (Table 2), consistent with an increase in high temperatures during summer months from the coast to inland valleys (WC-1 is the most inland site). However, the mean annual temperature was highest at LB-4 and lowest at WC-1, suggesting that LB-4 is on average warmer than WC-1 over the course of a year.
Table 2.
Habitat characteristics of each site
| Site | Land Cover Category |
Mean ± SE % Tree Cover |
Mean ± SE % Impervious Surface |
Mean Annual Temp (°C) |
Max Annual Temp (°C) |
|---|---|---|---|---|---|
| Whitney Canyon (WC-1) | Shrub/Scrub | 11.0 ± 0.79 | 1.1 ± 0.30 | 16.3 | 32.2 |
| Sage Hill (SH-2) | Mixed Forest | 19.6 ± 0.31 | 32.4 ± 0.48 | 17.5 | 27.3 |
| Palos Verdes (PV-3) | Developed, Low Intensity |
10.7 ± 0.38 | 36.0 ± 1.50 | 17.4 | 27.4 |
| Long Beach (LB-4) | Developed, Medium Intensity |
3.7 ± 019 | 55.4 ± 1.42 | 17.9 | 28.3 |
Lizard microhabitat use
Lizards were found at different perch heights among the sites (F3,79=4.54, P=0.005), with LB-4 lizards using significantly lower perches compared to PV-3 and WC-1 lizards (Fig. 1a). Perch widths used by lizards did not differ among sites (Kruskal-Wallis X2=0.933, df=3, P=0.817). Widths used were nearly all larger than 10 cm at every site, effectively representing flat surfaces for lizards (Fig. 2). Lizards were typically found horizontally (>60% horizontal at each site), and no population was using vertical structures more than another (X2=2.70, df=3, P=0.441).
Fig. 1.
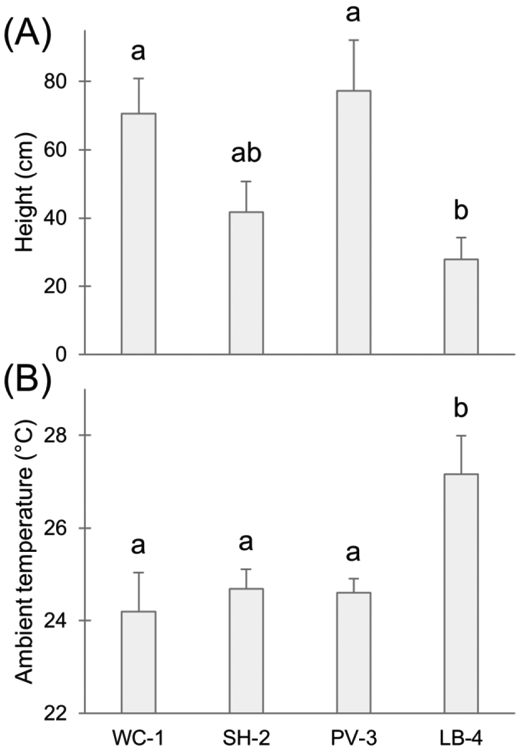
Differences in lizard microhabitat use among sites: (A) average ± SE heights at which lizards were found, (B) average ± SE ambient temperature recorded at each lizard location. Different letters illustrate statistically different mean values
Fig. 2.
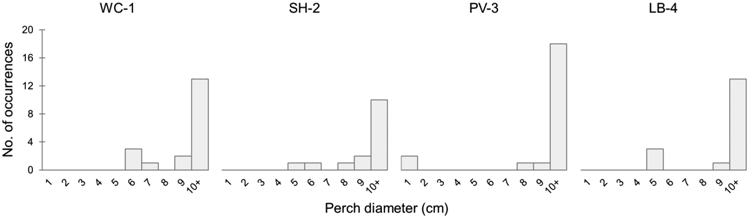
The frequencies of occurrence of perch width on which lizards were found at each site
The majority of lizards found at LB-4 and PV-3 were on human-made substrates including both vertical structures (walls) and human-made ground (sidewalks; Fig. 3). Lizards at WC-1 and SH-2 differed from lizards at LB-4 and PV-3 in their habitat use (X2=18.05, df=3, P<0.001, Fig. 3), rarely being found on human-made substrates, and when they were, they tended to be on wooden posts or telephone poles. WC-1 and SH-2 lizards were most often found on natural woody substrates such as tree trunks and fallen logs. The breadth of substrates (Levins’ B) used by lizards increased with urbanization (Fig. 3), and this might be due to differences in substrate availability among sites with urban areas providing more options than natural areas.
Fig. 3.
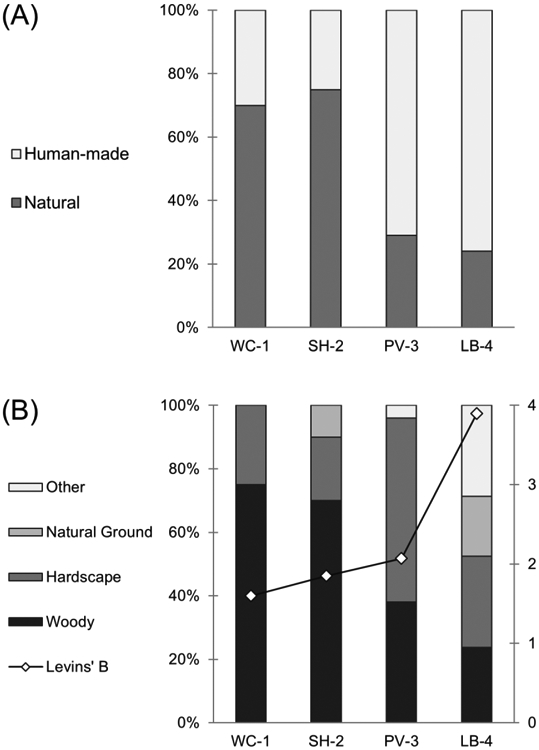
Substrate types used by lizards: (A) percent of natural and human-made substrates on which lizards were found at each site, (B) percent of sub-categories on which lizards were found (left y-axis) and Levins’ B (right y-axis) for each site. Sub-categories include both natural and human-made substrates (e.g., woody encompasses natural trees and logs and human-made wooden posts). Levin’s B is a measure of niche breadth and was calculated using the proportion of lizards found in each substrate sub-category
Ambient temperature near the microhabitat used by lizards differed among sites (F3,79=6.34, P=0.001) and varied by time of day (F1,79=32.86, P<0.001) with LB-4 lizards experiencing warmer temperatures in March (the month of our survey) than lizards at other sites (Fig. 1b). Our most urban site was approximately 2–3 degrees warmer on average (27.2 °C) than the other three sites (all between 24–25 °C) during our March sampling trips. Neither temperature of the substrates used by lizards nor lizard body temperature differed among sites (substrate: F3,80=1.76, P=0.161; lizard: F3,41=0.408, P=0.748).
Morphological traits
A number of morphological traits varied as a function of site. Average body size differed among sites (Table 3, Fig. 4), but did not associate with the urbanization gradient. Population differences in limb lengths and toe lengths (Table 3, Fig. 4) appeared to associate with differences in urban development among sites (Table 2); as predicted, lizards inhabiting more urban areas had shorter limbs and hind toes. Dorsal scale counts decreased with urbanization as predicted for the UHI effect (Fig. 4). Ventral scale count did not differ among the sites, and site differences in forelimb fourth digit length failed to reach statistical significance after implementing Tukey posthoc tests.
Table 3.
Results from GLMs examining differences in morphological traits among four sites that differ in level of urbanization. Significant outcomes are shown in bold
| Model Factors |
N | X2 | df | P | |
|---|---|---|---|---|---|
| Model (a): ~ Site + Sex | |||||
| SVL | Site | 35 | 15.392 | 3 | 0.002 |
| Sex | 35 | 3.399 | 1 | 0.065 | |
| Model (b): ~ Site + Sex + SVL | |||||
| Lower Forelimb Length | Site | 35 | 17.319 | 3 | 0.001 |
| Sex | 35 | 0.178 | 1 | 0.673 | |
| SVL | 35 | 188.252 | 1 | <0.001 | |
| Lower Hind Limb Length | Site | 35 | 19.780 | 3 | <0.001 |
| Sex | 35 | 7.430 | 1 | 0.006 | |
| SVL | 35 | 191.960 | 1 | <0.001 | |
| Forelimb 4th Toe Length | Site | 35 | 9.012 | 3 | 0.029 |
| Sex | 35 | 0.662 | 1 | 0.416 | |
| SVL | 35 | 44.078 | 1 | <0.001 | |
| Hind limb 4th Toe Length | Site | 35 | 60.264 | 3 | <0.001 |
| Sex | 35 | 4.135 | 1 | 0.042 | |
| SVL | 35 | 218.613 | 1 | <0.001 | |
| Dorsal Scale Count | Site | 35 | 16.388 | 3 | <0.001 |
| Sex | 35 | 0.028 | 1 | 0.866 | |
| SVL | 35 | 0.286 | 1 | 0.593 | |
| Ventral Scale Count | Site | 35 | 1.123 | 3 | 0.772 |
| Sex | 35 | 0.047 | 1 | 0.828 | |
| SVL | 35 | 0.161 | 1 | 0.688 |
Fig. 4.
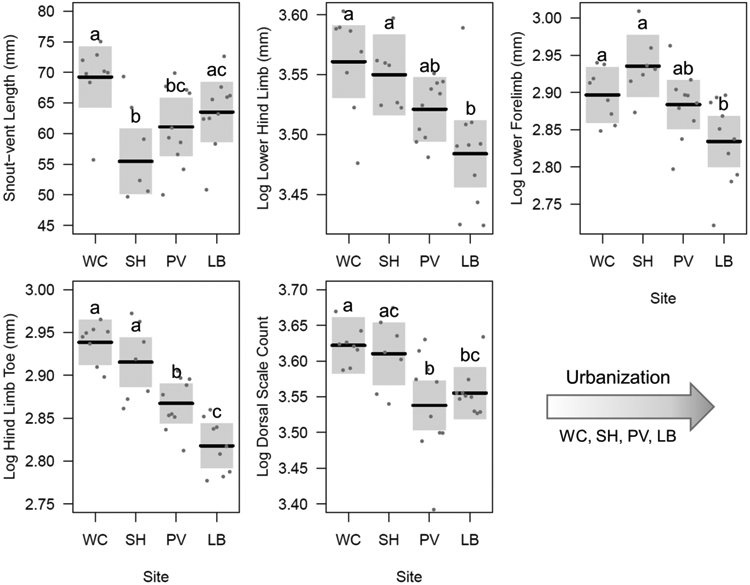
Conditional plots visualizing results of the regression models comparing morphological traits of western fence lizards among sites (increasing in level of urbanization from left to right) while holding all other explanatory variables constant. Lines are plotted to the partial residuals of the models (accounting for SVL, when appropriate, and sex), and confidence intervals are shown for each. Different letters illustrate statistically different mean values after Tukey posthoc tests
Discussion
We found that western fence lizards have detectable population differences in limb and toe lengths and scale counts that are consistent with differences in their habitat. Limb lengths (both lower forelimb and lower hind limb) and hind toe lengths decreased as sites became more urbanized consistent with our predictions regarding greater use of human-made substrates at more urban sites. Counts of dorsal scales also decreased with urbanization. We found that the most urban site, Long Beach (LB-4), which had the most impervious surface, the least amount of tree cover, and highest annual temperature, had the most phenotypic differentiation from the most natural site, Whitney Canyon (WC-1). Our results on microhabitat use and associated limb/toe morphologies largely differ from past studies on urban lizards, which have been focused on other taxa with different morphological and ecological specializations, illustrating that research on various species is required to generate meaningful predictions on how species, populations, and individuals will respond to environmental change.
Limb and toe lengths decrease with urbanization
We predicted that populations of fence lizards spanning an urban-to-rural gradient would exhibit a similar morphological trend as other phrynosomatid lizards inhabiting vertical cliff habitats or open rocky habitats relative to those inhabiting woody environments as reported by Sinervo and Losos (1991) and Herrel et al. (2001). These past studies on intraspecific variation found that rock-dwellers have shorter limbs than tree-dwelling populations. We found similar results: fence lizards from populations that more often use natural woody substrates (i.e., tree-dwellers) had longer limbs than populations that more often use human-made substrates. Limb length was shortest in the population that occupied the site with the highest percentage of impervious surface cover, Long Beach (LB-4), although these values were not statistically different from those of the suburban population, Palos Verdes (PV-3). The above differences are for lower limb lengths, and future work using x-rays (a method to acquire more precise measurements) could determine whether differences in upper limb lengths (i.e., the humerus and femur) are consistent with these results. We also predicted that populations in which lizards climbed on human-made structures would exhibit shorter toes compared to populations in which lizards used rougher, woody substrates because past studies found that phrynosomatid lizards that use cliffs have shorter toes compared to those that use trees. Indeed, hind toe length drastically decreased as level of urban development increased.
The pattern in limb and toe length differences we found does not correlate with perch height differences among sites (Fig. 1a), but does correlate with percent impervious surface cover, use of human-made substrates, and breadth of substrates used (Levin’s B). Lizards across all sites mostly used horizontal surfaces, and at LB-4, rarely climbed to high perches. Thus, climbing by lizards might not greatly influence these traits. Perhaps the use of various types of structures (regardless of perch height) selects for shorter-limbed individuals, but more work will be necessary to determine this. Yet, previous research shows that longer toes increase propulsion and acceleration across horizontal surfaces (Losos 1990; Bels et al. 1992). Fence lizards with longer limbs also sprint faster than those with shorter limbs (Sinervo and Losos 1991). Most morphology-performance relationship studies in lizards have not been conducted using human-made substrates, but a few have assessed this in anoles (Kolbe et al. 2016; Winchell et al. 2018). These past studies have found that lizards perform poorly on smooth human-made substrates compared to natural rough substrates, but longer limbs (especially the hind limb) aid in sprint performance on smooth substrates. However, competing demands in the urban environment might influence limb and toe lengths, as we and others (see Sparkman et al. 2018) have found different effects of urbanization on these traits. Littleford-Colquhoun et al. (2017) even found strong morphological differentiation among city park populations of water dragons within a single city. Further research on performance across various urban substrates and in various taxonomic groups is clearly warranted.
It also could be the case that differences in predator regimes across sites influence limb and toe traits. A loss of predator diversity is common in urban habitats (Shochat et al. 2006; Eötvös et al. 2018) and this could relax selection on sprint performance if long limbs and toes are no longer important for sprinting away from major threats (e.g., snakes are typically uncommon or absent in urban habitats; Shea 2010). Indeed, Sparkman et al. (2018) found that fence lizards on college campuses (urban populations) exhibited reduced antipredator responses, shorter limbs (in females), and lower sprint speeds, presumably as a response to changes in predation pressure and/or human activity. On the other hand, urban lizard morphological traits could respond to an increase in domestic and feral animal abundances (e.g., cats). Shorter limbs presumably enhance climbing performance by placing the center of gravity closer to the substrate (Losos 1990), and this might help lizards avoid domestic animal attacks if they can climb out of reach of these predators. This idea deserves further testing.
Notably, our results on morphological responses to urban environments in fence lizards are distinctly different from past urban lizard research. Lizards as a taxonomic group are extremely diverse in their ecologies, and differences in habitat use in natural populations should lead to different outcomes as they adapt to urban environments. For example, work on anoles has found that urban populations have longer limbs and toes than rural populations (Marnocha et al. 2011; Winchell et al. 2016), but anoles are generally arboreal and are switching from use of narrow branches and vegetation to hard, flat vertical substrates as they filter into urban environments. The Agamid lizard, Lophognathus temporalis, which is also arboreal, and is from the wet-dry tropics of Australia, also has longer front and hind limbs in urban populations than rural populations (Iglesias et al. 2012). Yet, garden skinks (Lampropholis guichenoti), which are ground-dwelling, do not exhibit morphological changes with urbanization (Prosser et al. 2006). This highlights that not all lizards respond to urban habitats in the same ways and therefore more research on multiple species and from different types of natural habitats will be necessary to generate meaningful predictions for squamates as a whole. Sceloporus lizards could be an ideal alternative system from Anolis for studying urban adaptation in squamate reptiles, as ours and a previous study (Sparkman et al. 2018) found strong phenotypic differentiation among populations that vary in urban development.
Dorsal scale counts decrease with urbanization
We found that dorsal scale counts in fence lizards decreased as populations became more urbanized. Past work on invertebrates has shown that reductions in body size are associated with differences in thermal tolerance in urban populations (Brans et al. 2017), but ours is the first study on a vertebrate, to our knowledge, to test whether changes to a morphological trait associated with the thermal environment associates with urbanization. We did not have an a priori assumption as to whether the urban environment was warmer than surrounding natural habitat, because Southern California is an arid region with Mediterranean climate and supplemental watering by humans could reduce the UHI effect (Jenerette et al. 2007). Additionally, onshore breezes could reduce the UHI effect given that our most urban sites are also the most coastal. However, our results are consistent with lizards experiencing greater thermal stress at more urban sites because suburban Palos Verdes (PV-3) lizards and urban Long Beach (LB-4) lizards had fewer, but larger dorsal scales compared to lizards from the most natural population. This change in morphology should reduce evaporative water loss, and overall heat load, because it reduces the surface area of living skin cells between the scales (Soulé 1966). We did not find differences in ventral scale counts.
Even though Whitney Canyon is inland and experiences the highest maximum temperature during the summer, the more-urban sites had higher mean annual temperatures, suggesting that lizards at these sites experience warmer conditions during colder months (i.e., winter). Long Beach (LB-4), which was the most urban site, had the highest ambient temperature during our visits, the highest mean annual temperature (based on WorldClim data), the least amount of canopy cover, and the most impervious surface, and thus lizards likely experience a strong UHI effect at this site. If urban habitats are on average warmer than natural habitats, this may affect the thermoregulatory behaviors of lizards. During our time of study, lizards did not differ in the temperature of perches on which they were found and in average body temperature, providing some evidence that urban lizards are not using warmer perches. Our temperature measurements, however, were only collected over a short time period; long-term monitoring of the temperatures available to lizards at these sites (i.e., operative temperatures) would be worthwhile.
Conclusion
We show that western fence lizard populations across four Southern California sites have significant differences in morphological characters that associate with urbanization and the thermal environment. Limb lengths and hind toe length decreased with urbanization, suggesting urban lizards are more robust with shorter and stouter limbs. Dorsal scale counts also decreased with urbanization, which suggests that urban lizards are more vulnerable to evaporative water loss even though urban areas are irrigated by humans. Although our results could be caused by various factors, future studies are required to tease these apart. But importantly, this study shows that work on various lizard taxa is useful for studying gradients in urbanization. Fence lizards (Sceloporus), in particular, use a variety of habitats from rocky outcrops in grassland habitat, to areas with woody vegetation, to heavily urbanized areas where walls and fences provide vertical structure. Given their occurrence from natural to heavily urbanized areas, this genus makes an excellent group for ecological and evolutionary studies examining responses to urbanization and can help provide information towards a generalized understanding of how squamates have and will respond to anthropogenically-driven habitat changes.
Acknowledgments
We thank Lauren Chan, Maria Florencia-Caruso, Kris Kaiser, and Stevie Kennedy-Gold for help in collecting specimens, Jane Li for geospatial analyses, and Kristin Winchell, Lindsey Swierk, and members of the Urban Nature Research Center at the Natural History Museum of Los Angeles County for comments on earlier drafts. This work was funded by a Postdoctoral Research Fellowship in Biology from the National Science Foundation (DBI-1611562 to BJP), the National Institute of General Medical Sciences of the National Institutes of Health (R25GM055052 awarded to T. Hasson and MG), and the Urban Nature Research Center.
References
- Ackley JW, Angilletta MJ, DeNardo D, Sullivan B, Wu J (2015) Urban heat island mitigation strategies and lizard thermal ecology: landscaping can quadruple potential activity time in an arid city. Urban Ecosyst 18:1447–1459. doi: 10.1007/s11252-015-0460-x [DOI] [Google Scholar]
- Angilletta MJ, Wilson RS, Niehaus AC, Sears MW, Navas CA, Ribeiro PL (2007) Urban physiology: City ants possess high heat tolerance. PLoS ONE. doi: 10.1371/journal.pone.0000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnfield AJ (2003) Two decades of urban climate research: A review of turbulence, exchanges of energy and water, and the urban heat island. Int J Climatol 23:1–26. doi: 10.1002/joc.859 [DOI] [Google Scholar]
- Atwell JW, Cardoso C, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 5:960–969. doi: 10.1093/beheco/ars059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev AV., Young RL, Oh KP, Addison C (2008) Evolution on a local scale: Developmental, functional, and genetic bases of divergence in bill form and associated changes in song structure between adjacent habitats. Evolution 62:1951–1964. doi: 10.1111/j.1558-5646.2008.00428.x [DOI] [PubMed] [Google Scholar]
- Bels VL, Theys J-P, Bennett MR, Legrand L (1992) Biomechanical analysis of jumping in Anolis carolinensis (Reptilia: Iguanidae). Copeia 1992:492–504. doi: 10.2307/1446210 [DOI] [Google Scholar]
- Bogert CM (1949) Thermoregulation in reptiles, a factor in evolution. Evolution 3:195–211. doi: 10.2307/2405558 [DOI] [PubMed] [Google Scholar]
- Brans KI, Jansen M, Vanoverbeke J, Tüzün N, Stoks R, De Meester L (2017) The heat is on: Genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob Chang Biol 23:5218–5227. doi: 10.1111/gcb.13784 [DOI] [PubMed] [Google Scholar]
- Calsbeek R, Knouft JH, Smith TB (2006) Variation in scale numbers is consistent with ecologically based natural selection acting within and between lizard species. Evol Ecol 20:377–394. doi: 10.1007/s10682-006-0007-y [DOI] [Google Scholar]
- Davis J, Ford RG (1983) Home range in the Western Fence Lizard (Sceloporus occidentalis occidentalis). Copeia 1983:933–940. doi: 10.2307/1445094 [DOI] [Google Scholar]
- Diamond SE, Chick L, Perez A, Strickler SA, Martin RA (2017) Rapid evolution of ant thermal tolerance across an urban-rural temperature cline. Biol J Linn Soc 121:248–257. doi: 10.1093/biolinnean/blw047 [DOI] [Google Scholar]
- Eötvös CB, Magura T, Lövei GL (2018) A meta-analysis indicates reduced predation pressure with increasing urbanization. Landsc Urban Plan 180:54–59. doi: 10.1016/j.landurbplan.2018.08.010 [DOI] [Google Scholar]
- Fick SE, Hijmans RJ (2017) Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas.
- Fischer JD, Cleeton SH, Lyons TP, Miller JR (2012) Urbanization and the predation paradox: The role of trophic dynamics in structuring vertebrate communities. Bioscience 62:809–818. doi: 10.1525/bio.2012.62.9.6 [DOI] [Google Scholar]
- Hellmich WC (1951) On ecotypic and autotypic characters, a contribution to the knowledge of the evolution of the genus Liolaemus (Iguanidae). Evolution 5:359–369. doi: 10.1111/j.1558-5646.1951.tb02794.x [DOI] [Google Scholar]
- Hendry AP, Farrugia TJ, Kinnison MT (2008) Human influences on rates of phenotypic change in wild animal populations. Mol Ecol 17:20–29. doi: 10.1111/j.1365-294X.2007.03428.x [DOI] [PubMed] [Google Scholar]
- Hendry AP, Gotanda KM, Svensson EI (2017) Human influences on evolution, and the ecological and societal consequences. Phil. Trans. R. Soc. B 372:20160028. doi: 10.1098/rstb.2016.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrel A, Meyers JJ, Vanhooydonck B (2001) Correlations between habitat use and body shape in a phrynosomatid lizard (Urosaurus ornatus): a population-level analysis. Biol J Linn Soc 74:305–314. doi: 10.1006/bijl.2001.0579 [DOI] [Google Scholar]
- Iglesias S, Tracy C, Bedford G, Christian K (2012) Habitat differences in body size and shape of the Australian agamid lizard, Lophognathus temporalis. J Herpetol 46:297–303. doi: 10.1670/11-084 [DOI] [Google Scholar]
- Irschick DJ, Losos JB (1999) Do lizards avoid habitats in which performance is submaximal? The relationship between sprinting capabilities and structural habitat use in Caribbean anoles. Am Nat 154:293–305. doi: 10.1086/303239 [DOI] [PubMed] [Google Scholar]
- Jenerette GD, Harlan SL, Brazel A, Jones N, Larsen L, Stefanov WL (2007) Regional relationships between surface temperature, vegetation, and human settlement in a rapidly urbanizing ecosystem. Landsc Ecol 22:353–365. doi: 10.1007/s10980-006-9032-z [DOI] [Google Scholar]
- Johnson MTJ, Munshi-South J (2017) Evolution of life in urban environments. Science 358:eaam8327. doi: 10.1126/science.aam8327 [DOI] [PubMed] [Google Scholar]
- Kaliontzopoulou A, Carretero MA, Llorente GA (2010) Intraspecific ecomorphological variation: Linear and geometric morphometrics reveal habitat-related patterns within Podarcis bocagei wall lizards. J Evol Biol 23:1234–1244. doi: 10.1111/j.1420-9101.2010.01984.x [DOI] [PubMed] [Google Scholar]
- Kokko H, Sutherland WJ (2001) Ecological traps in changing environments: Ecological and evolutionary consequences of a behaviourally mediated Allee effect. Evol Ecol Res 3:537–551. [Google Scholar]
- Kolbe JJ, Battles AC, Avilés-Rodríguez KJ (2016) City slickers: poor performance does not deter Anolis lizards from using artificial substrates in human-modified habitats. Funct Ecol 30:1418–1429. doi: 10.1111/1365-2435.12607 [DOI] [Google Scholar]
- Kuttler W (2008) The urban climate – basic and applied aspects. In: Marzluff JM (ed) Urban Ecology: an International Perspective on the Interaction between Humans and Nature. Springer, New York, pp 233–248. doi: 10.1007/978-0-387-73412-5 [DOI] [Google Scholar]
- Levins R (1968) Evolution in changing environments: some theoretical explorations. Princeton University Press, Princeton [Google Scholar]
- Littleford-Colquhoun BL, Clemente C, Whiting MJ, Ortiz-Barrientos D, Frére CH (2017) Archipelagos of the Anthropocene: rapid and extensive differentiation of native terrestrial vertebrates in a single metropolis. Mol Ecol 26:2466–2481. doi: 10.1111/mec.14042 [DOI] [PubMed] [Google Scholar]
- Losos JB (1990) Ecomorphology, performance capability, and scaling of West Indian Anolis lizards: An evolutionary analysis. Ecol Monogr 60:369–388. doi: 10.2307/1943062 [DOI] [Google Scholar]
- Losos JB (2009) Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. California University Press, Berkeley, CA [Google Scholar]
- Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549. doi: 10.1111/brv.12012 [DOI] [PubMed] [Google Scholar]
- Luxbacher AM, Knouft JH (2009) Assessing concurrent patterns of environmental niche and morphological evolution among species of horned lizards (Phrynosoma). J Evol Biol 22:1669–1678. doi: 10.1111/j.1420-9101.2009.01779.x [DOI] [PubMed] [Google Scholar]
- Marnocha E, Pollinger J, Smith TB (2011) Human-induced morphological shifts in an island lizard. Evol Appl 4:388–396. doi: 10.1111/j.1752-4571.2010.00170.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massot M, Huey RB, Tsuji J, Van Berkum FH (2003) Genetic, prenatal, and postnatal correlates of dispersal in hatchling fence lizards (Sceloporus occidentalis). Behav Ecol 14:650–655. doi: 10.1093/beheco/arg056 [DOI] [Google Scholar]
- Oufiero CE, Gartner GEA, Adolph SC, Garland T (2011) Latitudinal and climatic variation in body size and dorsal scale counts in Sceloporus lizards: a phylogenetic perspective. Evolution 65:3590–3607. doi: 10.1111/j.1558-5646.2011.01405.x [DOI] [PubMed] [Google Scholar]
- Pianka ER (1986) Ecology and natural history of desert lizards; analyses of the ecological niche and community structure. Princeton University Press, Princeton [Google Scholar]
- Pickett STA, Cadenasso ML, Grove JM, et al. (2001) Urban ecological systems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu. Rev. Ecol. Syst doi: 10.1146/annurev.ecolsys.32.081501.114012 [DOI] [Google Scholar]
- Prosser C, Hudson S, Thompson MB (2006) Effects of urbanization on behavior, performance, and morphology of the garden skink, Lampropholis guichenoti. J Herpetol 40:151–159. [Google Scholar]
- Shea GM (2010) The suburban terrestrial reptile fauna of Sydney - winners and losers In: Lunney D, Hutchings P, Hochuli D (eds) The Natural History of Sydney. Royal Zoological Society of NSW, Mosman, pp 154–197 [Google Scholar]
- Sheldahl LA, Martins EP (2000) The territorial behavior of the western fence lizard, Sceloporus occidentalis. Herpetologica 56:469–479. [Google Scholar]
- Shochat E, Warren P, Faeth S, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191. doi: 10.1016/j.tree.2005.11.019 [DOI] [PubMed] [Google Scholar]
- Sih A (2013) Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85:1077–1088. doi: 10.1016/j.anbehav.2013.02.017 [DOI] [Google Scholar]
- Sinervo B, Hedges R, Adolph SC (1991) Decreased sprint speed as a cost of reproduction in the lizard Sceloporus occidentalis: variation among populations. J Exp Biol 336:323–336. [Google Scholar]
- Sinervo B, Losos JB (1991) Walking the tight rope: Arboreal sprint performance among Sceloporus occidentalis lizard populations. Ecology 72:1225–1233. doi: 10.2307/1941096 [DOI] [Google Scholar]
- Soule M (1966) Trends in the insular radiation of a lizard. Am Nat 100:47–64. [Google Scholar]
- Sparkman A, Howe S, Hynes S, Hobbs B, Handal K (2018) Parallel behavioral and morphological divergence in fence lizards on two college campuses. PLoS ONE 13: e0191800. doi: 10.1371/journal.pone.0191800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear DM, Pauly GB, Kaiser K (2017) Citizen science as a tool for augmenting museum collection data from urban areas. Front Ecol Evol 5:86. doi: 10.3389/fevo.2017.00086 [DOI] [Google Scholar]
- Sukopp H, Wurzel A (2003) The effects of climate change on the vegetation of central European cities. Urban Habitats 1:66–86. doi: 10.1007/978-94-007-5341-9 [DOI] [Google Scholar]
- Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657. doi: 10.1111/j.1469-185X.2010.00164.x [DOI] [PubMed] [Google Scholar]
- Wegener JE, Gartner GEA, Losos JB (2014) Lizard scales in an adaptive radiation: variation in scale number follows climatic and structural habitat diversity in Anolis lizards. Biol J Linn Soc 570–579. doi: 10.1111/bij.12380 [DOI] [Google Scholar]
- Winchell KM, Maayan I, Fredette J, Revell L (2018) Linking locomotor performance to morphological shifts in urban lizards. Proc R Soc Biol Sci. doi: 10.1098/rspb.2018.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchell KM, Reynolds RG, Prado-Irwin SR, et al. (2016) Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evolution 70:1009–1022. doi: 10.1111/evo.12925 [DOI] [PubMed] [Google Scholar]


