Abstract
ROS1-rearranged (also known as ROS1 fusion-positive) non-small-cell lung cancer is an uncommon but distinct molecular subgroup seen in approximately 1–2% of cases. Oncogene addiction due to constitutive ROS1 tyrosine kinase activation has allowed development of molecularly targeted therapies with remarkable anti-tumor activity. Both crizotinib and entrectinib, multitargeted tyrosine kinase inhibitors (TKIs) have now received approval by the FDA for treatment of patients with advanced ROS1-rearranged lung cancers; however, the clinical efficacy and safety of these drugs have been derived from expansion cohorts of single-arm phase I or basket clinical trials with relatively small populations of this clinically and molecularly distinct subgroup. Both drugs lead to high objective response rates (approximately 70–80%) and have manageable side effects, although only entrectinib has potent intracranial efficacy. Lorlatinib is an oral brain-penetrant ALK/ROS1 TKI with activity in both TKI-naïve and some crizotinib-resistant settings (albeit with limited potency against the crizotinib/entrectinib-resistant ROS1-G2032R mutation). We describe cases of advanced ROS1-rearranged lung cancer receiving crizotinib, entrectinib, and/or lorlatinib in first and later line treatment settings to dissect the current state of evidence supporting management decisions for these patients. The next generation ROS1 TKIs (repotrectinib and DS-6051b), owing to their broad activity against kinase mutations including ROS1-G2032R in preclinical studies, hold promise to transform the current treatment paradigm and permit even further gains with regards to long-term outcomes in this subset of patients.
Keywords: ROS1 fusion, rearrangement, non-small-cell lung cancer (NSCLC), crizotinib, entrectinib, lorlatinib, acquired resistance
Introduction
Rearrangements/fusions of the ROS1 gene that encodes for protooncogene receptor tyrosine kinase ROS1 characterize a distinct molecular subgroup of oncogene-addicted non-small-cell lung cancer (NSCLC) with a frequency of approximately 1–2% (1,2). These alterations are mutually exclusive with other driver alterations (i.e., EGFR mutations, ALK rearrangements, BRAF mutations, NTRK rearrangements, MET exon 14 skipping mutation, RET rearrangements, ERBB2 mutations, KRAS mutations, among others) seen in NSCLC (3,4). The retained kinase domain of the ROS1 fusion with partner genes (most commonly CD74) leads to constitutive activation of its tyrosine kinase domain and drives oncogenesis (3,5). Another uncommon molecular subgroup—ALK (anaplastic lymphoma kinase gene)-rearranged NSCLC—shares clinicopathologic characteristics and a high degree of homology in tyrosine kinase domains of the oncoproteins with ROS1 fusion-positive NSCLC. Both subgroups overwhelmingly include those patients with NSCLC occurring in the presence of limited/no tobacco history and adenocarcinoma histology (5–8). In clinical practice, ROS1 rearrangements/fusions are usually evaluated in tumor biopsy samples by either fluorescence in situ hybridization (FISH) or next-generation sequencing (NGS) panels; however, immunohistochemistry (IHC) and reverse transcription polymerase chain reaction (RT-PCR) have been utilized as well (7).
Due to the low frequency of ROS1-rearranged NSCLC, the majority of clinical evidence utilizing targeted therapies has been derived from expansion cohorts of single-arm phase I/II or basket clinical trials. Our group had previously published a comprehensive review of the literature on ROS1 fusion-positive NSCLC in 2018 (2). At that time, crizotinib—a multitargeted MET/ALK/ROS1 tyrosine kinase inhibitor (TKI)—was the only targeted therapy approved by the United States Food and Drug Administration (FDA) for management of ROS1 fusion-positive NSCLC. In the time since, another drug—entrectinib [ROS1/tropomyosin receptor kinase (TRK) inhibitor]—has received approval from the FDA for management of this subgroup of NSCLC in both treatment-naïve and chemotherapy-treated settings. In addition, emerging phase I/II trials suggest there may be a role for lorlatinib and other multi-targeted ROS1 TKIs.
Here, we describe three patient cases as a framework to dissect the evidence supporting the current use of ROS1 TKIs in the clinic. We present our approach towards management decisions for these patients in the first line treatment setting and beyond.
Case presentations (Figure 1)
Figure 1.
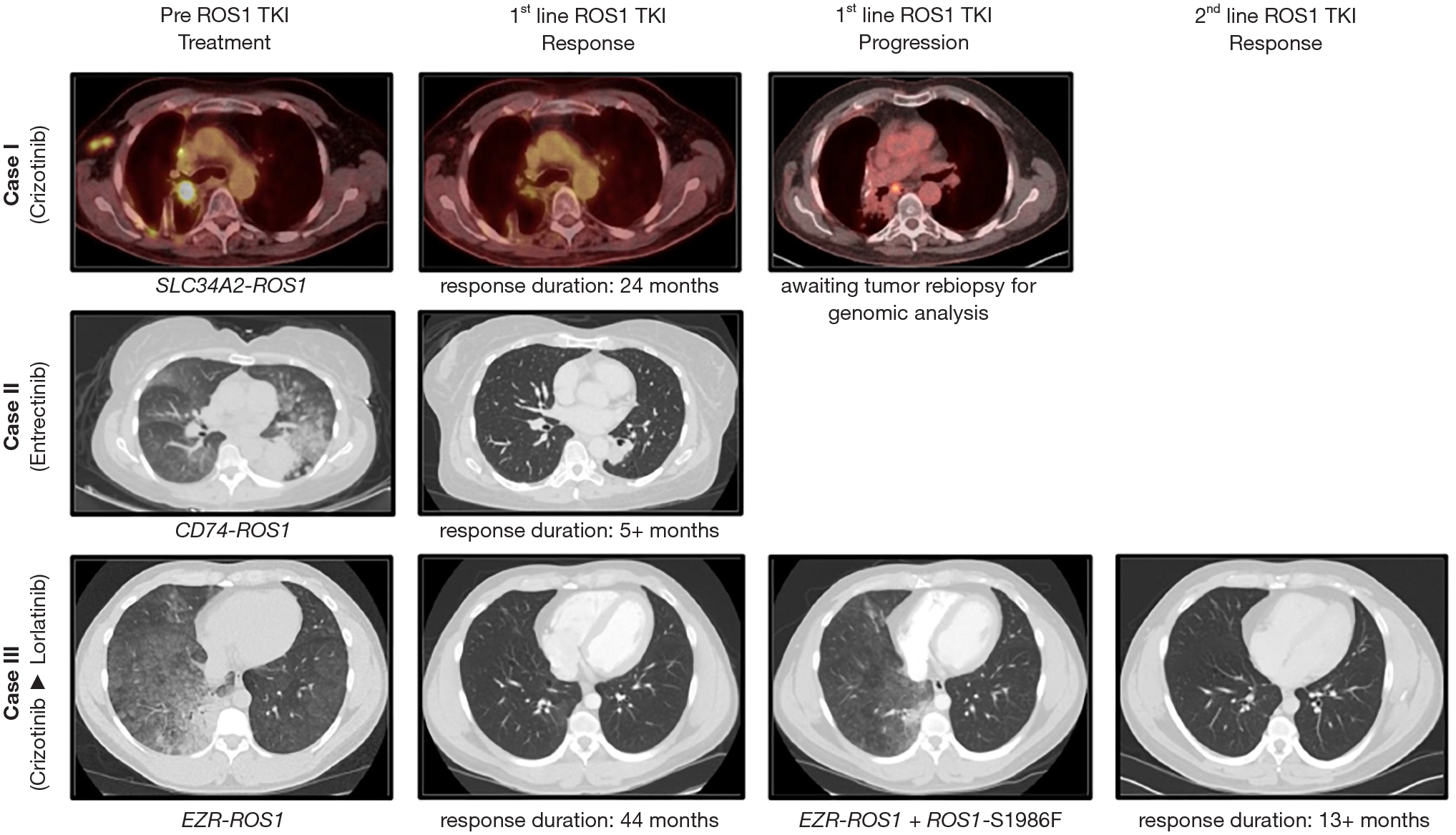
Cases of advanced ROS1-rearranged (fusion-positive) non-small-cell lung cancer (NSCLC). Case I: on-label crizotinib; Case II: on-label first line entrectinib, Case III: off-label second line lorlatinib. Representative imaging studies [either computed tomography (CT) of chest or fusion PET-CT scans] are shown. The genomic ROS1 aberration and duration of response are indicated, when available. TKI, tyrosine kinase inhibitor.
We present the following cases in accordance with the CARE guideline.
Case I
A 72-year-old white male with remote (<5 pack-years) tobacco history was diagnosed with locally advanced lung adenocarcinoma (hilar, mediastinal, and ipsilateral supraclavicular lymphadenopathy) following presentation with chronic dry cough. FISH from the original tumor biopsy was positive for a rearrangement involving ROS1 gene (37.5% of cells). He was treated with curative intent concurrent chemoradiation, with control of disease for 21 months. He was subsequently found to have recurrence of disease in the lung on follow up imaging and underwent right upper lobe wedge resection and right middle lobectomy. ROS1 gene rearrangement was confirmed in the tumor specimen this time by both FISH (90% of cells) and NGS (Solid Fusion Assay v2, Massachusetts General Hospital); the latter identified a fusion transcript involving SLC34A2 exon 13 and ROS1 exon 34. He was subsequently followed for 15 months with no evidence of recurrence on imaging studies. Eventually, both local and distant intrathoracic and nodal disease progression were noted (Figure 1). Brain imaging was negative for evidence of intracranial metastases. Biopsy of a right axillary lymph node showed lung adenocarcinoma with NGS (FoundationOne, Foundation Medicine) showing SLC34A2-ROS1 fusion, CDKN2A loss, and TET2P1617fs86 mutation. Based on these findings, he was started on first line crizotinib 250 mg twice daily. Follow up scans 2 months later showed significant reduction of disease burden. He experienced multiple crizotinib-related adverse events, including fatigue, gastrointestinal disturbances (alternating diarrhea and constipation), lower extremity edema, decreased appetite, and low testosterone levels; crizotinib was dose reduced to 250 mg daily. His disease continued to respond and remained under control for 24 months. Subsequent follow up imaging showed findings concerning for slow progression of disease in the lungs and mediastinal lymph nodes (Figure 1). Bronchial sampling showed adenocarcinoma. Liquid biopsy (FoundationOne Liquid, Foundation Medicine) from peripheral blood showed no abnormalities [i.e., failed to identify tumor-derived circulating free DNA (cfDNA)]. A repeat tissue biopsy evaluation and repeat imaging studies are pending at this time to confirm lack of histologic transformation and obtain genomic data that will help further support future decisions of systemic palliative therapy–either off-label lorlatinib or systemic chemotherapy +/− immune checkpoint inhibitor versus local therapy (such as radiotherapy) with use of crizotinib beyond progression.
Case II
A 60-year-old female with no tobacco history (0 pack-years), with history of atrial fibrillation was evaluated for worsening shortness of breath and cough and diagnosed with lung adenocarcinoma of the left lower lobe. Imaging studies did not show findings concerning for extra-thoracic metastatic disease. However, imaging of the brain revealed eight intracranial lesions consistent with metastatic disease. Evaluation of the cerebrospinal fluid was negative for presence of malignant cells. She received local brain-directed treatment with palliative stereotactic radiotherapy. Tumor NGS (FoundationOne CDx, Foundation Medicine) revealed the presence of CD74-ROS1 (C6:R33) fusion gene. She was started on treatment with entrectinib 600 mg daily due to its known central nervous system (CNS) activity in ROS1-rearranged NSCLC. She had rapid resolution of her respiratory symptoms; follow up computed tomography (CT) body imaging obtained just one week after initiation of entrectinib revealed dramatic radiographic response (Figure 1). Her treatment over the next three months was complicated by dizziness, gait instability, and diarrhea-all best attributed to the TKI. Follow up imaging of the brain showed stable disease, without new/progressive lesions. The dose of entrectinib was reduced to 400 mg daily with improvement in adverse events. Imaging studies show sustained response to therapy, ongoing five months since start of entrectinib.
Case III
A 32-year-old Hispanic male with minimal (<1 pack-year) tobacco history was evaluated for worsening dry cough and shortness of breath. He was diagnosed with advanced lung adenocarcinoma with metastatic disease in both lungs and the axilla. ROS1 gene rearrangement was detected by FISH in 67.5% of cells. He was started on first line treatment with crizotinib 250 mg twice daily, resulting in a sustained response to therapy lasting for 44 months (Figure 1). Subsequently, follow up imaging demonstrated growth in the primary right lower lobe mass with evidence of new lymphangitic spread. He concurrently experienced clinical worsening with increased cough and malaise. Tumor rebiopsy confirmed adenocarcinoma with no evidence of histologic transformation. Tumor NGS (FoundationOne, Foundation Medicine) of rebiopsied tumor showed the known EZR-ROS1 fusion gene along with acquisition of a ROS1-S1986F resistance mutation. He was transitioned to second line off-label lorlatinib at a dose of 100 mg daily. Within two months, he reported subjective improvement in his respiratory symptoms with radiographic evidence of a complete response to therapy on follow-up imaging (Figure 1). Treatment with lorlatinib was complicated by weight gain, metabolic derangements (dyslipidemia, elevation in hemoglobin A1c), fatigue, confusion, and depression. These were attributed to adverse events from lorlatinib and managed with dose reductions to 75 mg daily and subsequently to 50 mg daily with improvements in the above. A statin was initiated for hyperlipidemia. Disease response to treatment has been maintained at the last follow-up 13 months since start of lorlatinib.
Discussion
Use of on-label Crizotinib (Case I)
The earliest and most definitive evidence for clinical activity of crizotinib in ROS1 fusion-positive NSCLC was obtained from the dose expansion ROS1 cohort of the global PROFILE 1001 trial (ClinicalTrials.gov identifier: NCT00585195) (6,9). This phase I trial was amended to enroll patients with advanced NSCLC harboring ROS1 rearrangements who were treated with crizotinib at the recommended phase II dose of 250 mg orally twice daily (6,10). In an initial report of 50 patients, the primary endpoint of objective response rate (ORR) was 72% (95% CI, 58% to 84%); complete (CR) and partial responses (PR) were seen in three (6%) and 33 (66%) patients, respectively. The disease control rate (DCR) was 90%; with median duration of response (DoR) 17.6 months (95% CI, 14.5 months to not reached). Median progression-free survival (PFS) was 19.2 months (95% CI, 14.4 months to not reached) at the time of this report. This data led to the approval for use of crizotinib by the US FDA and European Union for treatment of ROS1 fusion-positive advanced NSCLC in 2016.
The updated efficacy, survival, and safety data from this ROS1 expansion cohort with 53 patients and median follow up of 62.6 months was published in 2019 (10). Similar to the initial report, ORR was 72% (95% CI, 58% to 83%), which included six (11%) CRs and 32 (60%) confirmed PRs. These responses were proven to be durable, with median DoR of 24.7 months (95% CI, 15.2 to 45.3 months). Median PFS and overall survival (OS) were 19.3 months (95% CI, 15.2 to 45.3 months) and 51.4 months (95% CI, 29.3 months to not reached), respectively. The remarkable anti-tumor activity and survival effects seen with treatment with crizotinib in ROS1 fusion-positive advanced NSCLC were confirmed in this updated analysis. The authors also performed subgroup analyses of ORR, DoR and OS for different ROS1 fusion partners and found no differences between them. Overall, treatment-related adverse events (TRAEs) were common (100% patients), but the majority were grade 1 or 2 in severity (Common Terminology Criteria for Adverse Events [CTCAE]). The most common adverse events (AEs) were consistent with previous reports of crizotinib and included: visual disturbances, nausea/vomiting, gastrointestinal disturbances (diarrhea/constipation) and peripheral edema. There were no treatment discontinuations or deaths due to TRAEs by CTCAE.
Crizotinib has been evaluated in European and East Asian populations, as well. The phase II AcSé trial (NCT02034981) from Europe reported an ORR of 69.4%; however PFS and OS were much shorter than previously published at 5.5 months (95% CI, 4.2 to 9.1 months) and 17.2 months (95% CI, 6.8 to 32.8 months), respectively (11,12). Shorter PFS and OS in the AcSé trial were attributed to a heavily pre-treated study population with higher frequency of Eastern Cooperative Oncology Group (ECOG) performance status 2 compared to PROFILE1001. In another European single-arm multicenter phase II study (EUCROSS, NCT02183870), with more similar patient demographics to the PROFILE1001 trial, ORR was 70% (95% CI, 51% to 85%) and median PFS 20 months (95% CI, 10.1 months to not reached) (13). The largest prospective phase II international study (NCT01945021) in East Asian patients with advanced ROS1-rearranged NSCLC also confirmed this data with ORR of 71.7% (95% CI, 63% to 79.3%), median DoR of 19.7 months (95% CI, 14.1 months to not reached), and median PFS of 15.9 months (95% CI, 12.9 to 24 months) (14).
Despite remarkable and often brisk responses to crizotinib in this molecularly-defined subgroup of patients, evolution of drug resistance either due to acquisition of “on-target” and/or “off-target” resistance mechanisms, histologic transformation, or development of CNS metastases is most often inevitable (2,15). The first published case of on-target crizotinib resistance described development of the ROS1 solvent front mutation (SFM) G2032R just three months following initiation of therapy in a 48-year-old woman with CD74-ROS1 fusion-positive advanced lung adenocarcinoma (16). SFMs prevents crizotinib binding through steric hinderance while retaining normal kinase function. Interestingly, autopsy sampling of multiple disease sites following progression on crizotinib in this case revealed no heterogeneity—both original and solvent front mutations were identified at all sites. Subsequently, the largest series to date investigating ROS1 TKI resistance mechanisms found this ROS1-G2032R SFM to be the most common (41%) mechanism of crizotinib resistance (17). Other identified ROS1 kinase mutations included D2033N (6%) and S1986F (6%). No clear mechanisms of resistance were identified in 47% of patients, even after additional expanded/comprehensive profiling aiming to identify gene amplifications or known bypass tracts (SNaPshot-NGS versions 1 and 2) in some of the samples. Finally, while on-target resistance tends to occur later in the course of other oncogene-addicted tumors (18), 62.5% (10/16) of cases of on-target ROS1 resistance in this series developed within 10 months of crizotinib initiation (17).
Though less common than “on-target” resistance mutations, “off-target” alterations in KRAS, EGFR, KIT, and RAS signaling have been linked to crizotinib resistance (7,19–22). As observed in other oncogene-addicted lung cancers, a subset of cases appears to demonstrate epithelial-mesenchymal transition or histologic transformation to small cell lung cancer at the time of progression (7,17,22). To date, information about these alterations at the time of progression has not been translated to a clinically proven combination therapeutic strategy. Brain or other CNS progression of disease on crizotinib is predominantly thought to be due to pharmacokinetic failure and is supported by the known poor blood-brain barrier penetration of crizotinib (23) and detection of no (0%, 0/3) on-target resistance mutations in intracranial compared to extracranial (64.3%, 9/14) specimens at disease progression (17). This has spurred efforts to design advanced generation ROS1-directed TKIs with improved brain penetrance as well as activity against a broad spectrum of crizotinib resistance mutations. The strategy of local consolidative therapy along with continuation of crizotinib beyond intracranial or systemic progression has been found to be feasible and promising in case reports/real-world studies in ROS1-rearranged NSCLC (24,25), and may be considered for a select group of patients with oligoprogressive disease.
Use of on-label Entrectinib (Case II)
Brain metastases are commonly detected in treatment-naïve stage IV ROS1-positive NSCLC, with incidence ranging from 19.4% to 36% in retrospective studies (15,17). In addition, CNS was a common and often the first site of progression of disease on crizotinib-seen in 47% of the patients in one study (15). Entrectinib is a next generation potent ROS1/ALK/pan-TRK inhibitor (26,27), approved for upfront management of advanced of ROS1-rearranged NSCLC in 2019. Its ability to penetrate the blood-brain barrier makes it especially attractive for those with intracranial metastases-including our case II. The safety profile and clinical activity of entrectinib in solid tumors have been evaluated in three prospective trials: STARTRK (Studies of Tumor Alterations Responsive to Targeting Receptor Kinases)-1 (NCT02097810), STARTRK-2 (NCT02568267), and ALKA-372–001 (EudraCT, 2012–000148–88) (28,29). STARTRK-1 was a multinational phase I dose-escalation trial which enrolled patients from United States, Spain and South Korea, while ALKA-372–001 was also a phase I dose-escalation trial conducted only in Italy. STARTRK-2 is a global phase II basket trial in 15 countries evaluating entrectinib at dose of 600 mg orally once daily.
The most definitive evidence on entrectinib so far comes from the prespecified integrated analysis of ROS1 fusion-positive patients from these phase I and II trials (29). This efficacy analysis was performed in only TKI-naïve patients with locally advanced or metastatic disease. The patients were required to have ECOG performance status of 0–2, receive at least 600 mg (one dose) of entrectinib, and have at least 12 months of follow up. The co-primary end points for this pooled analysis were ORR and DoR assessed by blinded independent central review. 53 patients were included, with ORR of 77% (95% CI, 64% to 88%) with complete and partial responses seen in three (6%) and 38 (72%) patients, respectively. The ROS1 fusion partner (i.e., CD74 versus non-CD74) did not have an impact on the response to entrectinib in a subgroup analysis. Responses were durable with median DoR 24.6 months (95% CI, 11.4 to 34.8 months). The secondary endpoints of median PFS and OS were 19.0 months (95% CI, 12.2 to 36.6 months) and not reached (95% CI, 15.1 months to not reached), respectively, at the time of the report. Due to the known CNS penetrance of this drug, intracranial efficacy was also evaluated in 20 (38%) patients with baseline brain metastases per independent central review. Objective intracranial responses were seen in 11 (55%, 95% CI, 32% to 77 %) patients, with four (20%) CRs. The median duration of intracranial response was 12.9 months (95% CI, 5.6 months to not reached), with a median intracranial PFS of 7.7 months (95% CI, 3.8 to 19.3 months). In addition, the median time to CNS progression in the entire population was not reached (95% CI, 15.1 months to not reached).
The safety analysis dataset was more broadly defined and included those patients who had received prior ROS1-targeted TKIs and those with less than 12 months of follow up. In the safety-evaluable population of 134 patients, 59% and 34% patients experienced grade 1–2 and grade 3–4 CTCAE TRAEs respectively. The most common adverse events were dysgeusia, dizziness, gastrointestinal disturbances, fatigue, weight gain (related to hyperphagia) and paresthesias. TRAEs led to treatment discontinuation in seven (5%) patients, while no treatment-related deaths were reported. Serious TRAEs were seen in 11% patients with most common being nervous system and cardiac disorders. Adverse effects related to appetite regulation and the nervous system due to entrectinib are attributed to TRK inhibition (28). Together, this data supports both the systemic and intracranial efficacy of entrectinib and with a manageable adverse event profile.
Within the limitations of cross-trial comparisons, ORR and median PFS seen with entrectinib were similar to those seen with crizotinib in the PROFILE 1001 trial (6). Rates of intracranial response and intracranial disease control, however, are notable differences between the two agents, with far more favorable results in this regard with entrectinib. The median PFS of entrectinib in this analysis was also longer than seen in the phase II crizotinib study in the East Asian trial (14). Similar results were seen in a comparative efficacy analysis of 53 entrectinib trial patients versus 69 matched real-world crizotinib patients with advanced ROS1 fusion-positive NSCLC (30). This study suggested longer time to treatment discontinuation and PFS with entrectinib. In an earlier analysis restricted to only the two phase I trials (STARTRK-1 and ALKA-372–001), no antitumor activity was seen with entrectinib in six patients with ROS1 fusion-positive NSCLC who had received prior treatment with crizotinib (28).
Taken together, these data support use of entrectinib for upfront systemic therapy in advanced stage ROS1-rearranged NSCLC, particularly in those with baseline intracranial metastases where there are pronounced benefits as compared with upfront use of crizotinib. It remains uncertain if entrectinib can be an effective treatment option for those who develop disease progression due to crizotinib resistance, although limited preclinical and clinical data available thus far argues against it.
As with other advanced generation TKIs, the underlying mechanisms of entrectinib resistance have yet to be elucidated. An in vitro study using HCC78 cells (with the SLC34A2-ROS1 fusion) demonstrated a variety of acquired mechanisms, including KRAS-G12C mutation, KRAS amplification, and FGF3 amplification; no on-target ROS1 resistance mutations were seen (31). In addition, sustained downstream pathway activation was also detected and could be overcome in vitro with combination entrectinib and selumetinib. These data were consistent with other reports that have demonstrated activation of mitogen-activated protein kinase (MAPK) pathway as mediators of innate and acquired resistance for both ROS1 and TRK fusions (32,33). In patients with tumors harboring ROS1 fusions, MAPK upregulation was also associated with worse survival (32). In a plasma-based NGS analysis of 18 paired blood samples at baseline and on disease progression on entrectinib from STARTRK-2 trial, acquired on-target ROS1 resistance mutations (G2032R and F2004C/I) were detected in four (28%) patients (34). Further studies are required to characterize acquired mechanisms of entrectinib resistance in more detail, while long-term survival data is awaited from these pivotal clinical trials.
Use of off-label Lorlatinib (Case III)
Similar to entrectinib, lorlatinib was designed as a CNS-penetrant drug, albeit originally to overcome the ALK kinase mutation ALK-G1202R (35). It is a potent third generation TKI that targets both ROS1 and ALK. The strongest evidence for clinical activity of lorlatinib in advanced ROS1 fusion-positive NSCLC comes from an ongoing open-label single-arm phase I-II trial (NCT01970865) (36). This report extended the preliminary data on anti-tumor efficacy seen with lorlatinib in the phase I portion of the study (37). Sixty-nine patients (12 from phase I portion and 47 from phase II portion) with metastatic ROS1 fusion-positive NSCLC and favorable performance status (ECOG performance status 2 or less) with or without CNS metastases were enrolled from 12 countries, with median follow up of 21.1 months. A majority of the patients had received prior treatments, with 30% patients being TKI-naïve, 58% having received prior TKI treatment with only crizotinib, and 12% having previously received either non-crizotinib TKI or more than one ROS1 TKI. The recommended phase II dose for lorlatinib was 100 mg orally once daily. In the TKI-naïve group, 13 (62%; 95% CI, 38% to 82%) out of 21 patients had objective responses as assessed by independent central radiology review. In the group previously treated with crizotinib as the only TKI, 14 (35%; 95% CI, 21% to 52%) out of 40 patients had objective responses. Intracranial response rate, a co-primary end point, was 64% (95% CI, 31% to 89%) in TKI-naïve patients with baseline brain metastases; and 50% (95% CI, 29% to 71%) in prior crizotinib-only treated patients with baseline brain metastases. Median time to intracranial progression for all patients in both groups was not reached, further supporting CNS-penetrance and intracranial activity of lorlatinib. Median PFS in TKI-naïve patients and prior crizotinib-only treated groups was 21 months (95% CI, 4.2 to 31.9 months) and 8.5 months (95% CI, 4.7 to 15.2 months), respectively. These data supported the role of lorlatinib in crizotinib-resistant disease, albeit with a lower efficacy compared to TKI-naïve patients.
Adverse events were common with lorlatinib, with 96% patients experiencing at least one TRAE. The most common grade 1–2 adverse events were: hypercholesterolemia (65% of patients), hypertriglyceridemia (42%), peripheral edema (39%), peripheral neuropathy (35%), cognitive effects (cluster of symptoms including impairment of verbal learning, psychomotor function, attention or memory; 26%), weight gain (16%), and mood effects (cluster of symptoms including depression; 16%). Grade 3 and 4 adverse reactions occurred in 43% and 6% patients, respectively, with the most common being hypertriglyceridemia, hypercholesterolemia, and weight gain. Serious TRAEs were seen in 7% of patients, while drug discontinuation due to TRAEs was needed in 1% patients. A clinical practice guideline generated with expert consensus opinion based on experience with ALK-rearranged NSCLC provides practical information on management of adverse events associated with lorlatinib (38). Most patients require at least one lipid-lowering agent, which should be chosen taking drug-drug interactions into account due to the effect of lorlatinib on CYP450 enzymes. Most adverse events can be managed with treatment interruptions and dose reductions, as was done with case III. Permanent discontinuations were required in only 1–2% patients.
Resistance mechanisms to lorlatinib have been previously characterized in ALK-rearranged NSCLC (39–42). Molecular profiling of baseline tumor tissue and plasma samples to identify biomarkers of drug response in ROS1 fusion-positive NSCLC was performed for at least one sample type in 68 patients in the above-mentioned phase I/II study (36). As expected, no ROS1 resistance mutations were detected in baseline samples in TKI-naïve patients. Among patients previously treated with any TKIs, ROS1 mutations were detected in 15% (6/41) of patients in plasma and 24% (5/24) of patients in de novo tumor specimens at baseline. These mutations included ROS1-G2032R, L2026M, S1986F, and K1991E. Durable PRs were seen with lorlatinib in one patient each with acquired K1991E and S1986F resistance mutations. However, none (0%) of the six patients with tumors harboring the most common acquired ROS1-G2032R, experienced an objective response. Stable disease (range, 2.9 to 9.6 months) was seen as the best response in five patients. This suggests limited potency of lorlatinib against ROS1-G2032R SFM and is in contrast to the activity of lorlatinib seen with the ALK-G1202R SFM (43). However, the numbers of patients with resistance mutations in this analysis was too limited to allow any definitive conclusions at this time, and further studies are warranted to explore “on-target” and “off-target” mechanisms of resistance to lorlatinib.
Conclusions and future directions
The therapeutic landscape for management of advanced ROS1 fusion-positive NSCLC continues to evolve with improvements in understanding of innate/acquired resistance mechanisms and optimization of drug pharmacokinetics, thus permitting rational drug design of next generation TKIs. We have discussed here the literature supporting the current on-label use of crizotinib and entrectinib, as well as off-label use of lorlatinib in real-world settings. Figure 2 illustrates our approach to selection of first and later-line treatment options in advanced ROS1-rearranged (fusion-positive) NSCLC. The choice between crizotinib and entrectinib for first-line therapy depends on the presence of intracranial metastases and the availability of these TKIs in a given health care system (Figure 2).
Figure 2.
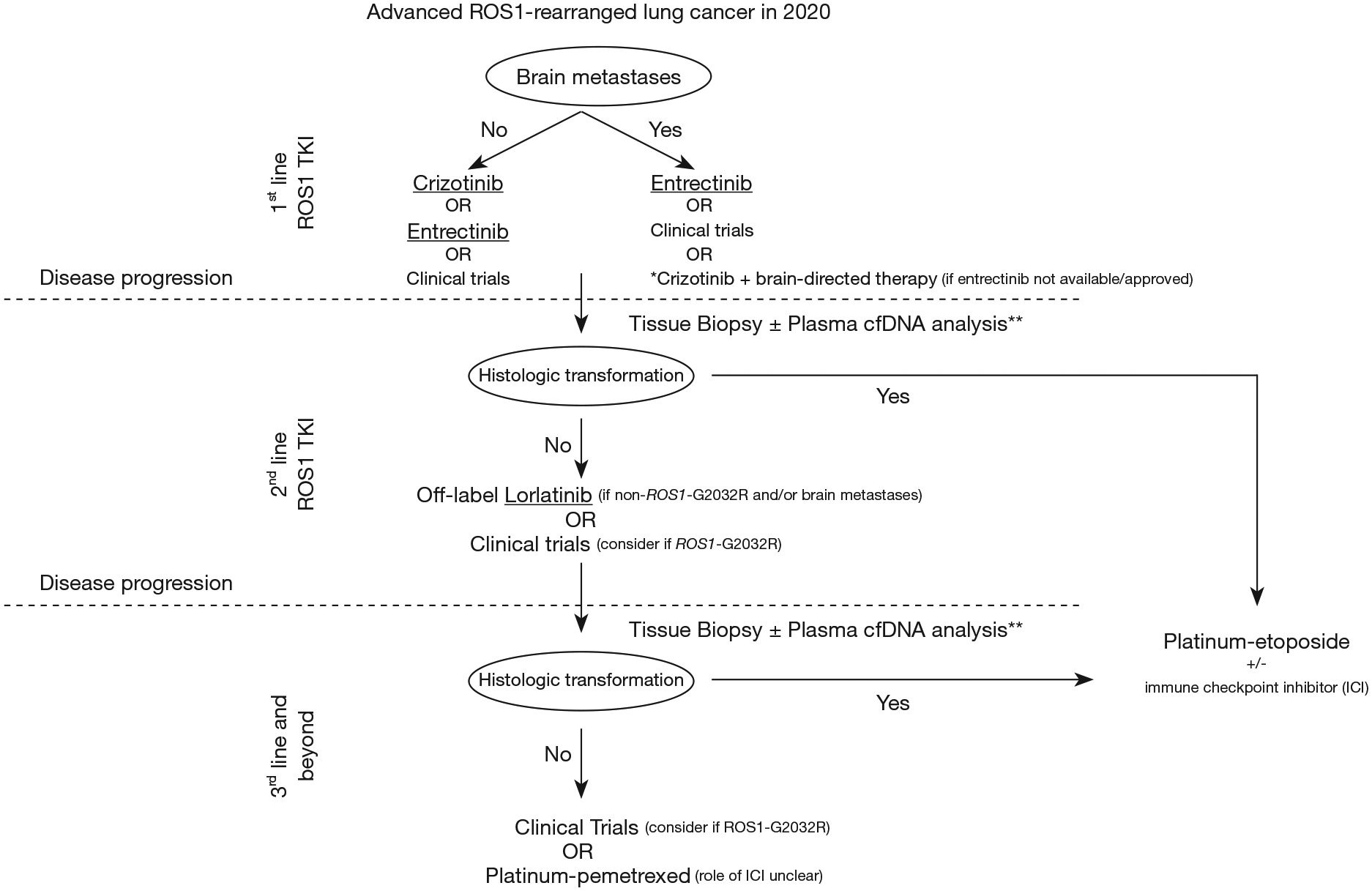
Management approach for advanced ROS1-rearranged (fusion-positive) non-small-cell lung cancer (NSCLC). TKI, tyrosine kinase inhibitor; cfDNA, circulating free DNA; ICI, immune checkpoint inhibitor. *, not preferred and to be used only if other options are not available; must involve multidisciplinary care with neuro-oncology and radiation oncology; **, sensitivity of individual cfDNA next-generation sequencing assay for detection of mutations, fusions and amplifications should be considered.
For second-line therapy, we recommend either lorlatinib (particularly if crizotinib/entrectinib resistance is mediated by on-target resistance other than ROS1-G2032R mutation) or clinical trial with an in-development or novel agent (44–48) (particularly in the presence of the ROS1-G2032R mutation) if crizotinib or entrectinib were administered in the first line setting (Figure 2).
Once all targeted therapeutic approaches have been exhausted, we recommend use of platinum-doublet chemotherapy, particularly pemetrexed-based schemes in these cases of predominantly lung adenocarcinoma histology. Platinum-pemetrexed has been associated with high ORR (40–60%) in multiple retrospective studies of ROS1-rearranged NSCLC (49–51). The role of immune checkpoint inhibitors (ICIs) in the TKI-resistant setting—either as monotherapy or in combination with chemotherapy-remains to be determined, with some series showing limited activity of ICIs in these patients with low smoking exposure (52,53). If histologic transformation to small cell lung carcinoma is detected, we would recommend consideration of platinum-etoposide +/− ICI (Figure 2).
Other ROS1 inhibitors such as repotrectinib and DS-6051b have shown promising activity (46,48), while others appear to have limited efficacy or unacceptable toxicities. Cabozantinib demonstrated preclinical activity against both ROS1 G2032R and D2033N mutations (54). However, its toxicity profile (palmar-plantar dysesthesia, gastro-intestinal adverse events, hypertension, fatigue, and anorexia) and absent clinical efficacy against ROS1-G2032R mutation have hampered its further development in this setting (55). Ceritinib and brigatinib appear to be active in TKI-naïve patients and in those with ROS1-L2026M mutations, but not amongst patients with ROS1-G2032R, D2033N, L1951N, or S1986Y mutations (2,7,17,44). None of these TKIs have yet garnered regulatory approval for regular use outside of clinical trials. This may change in the next few years with demonstration of activity of newer generation inhibitors—repotrectinib and DS-6051b—against the most common acquired solvent front ROS1-G2032R mutation in the preclinical models and proof-of-concept case report from the phase I/II clinical trial of repotrectinib (NCT03093116) (45–48).
In summary, ROS1-rearranged NSCLC is a bona fide subgroup of lung cancer in which rational use of systemic therapies (mostly in the form of oral multitargeted ROS1 TKIs) can lead to prolonged periods of survival for the majority of cases.
Acknowledgements
Funding: This work was funded in part through National Institutes of Health (NIH)/National Cancer Institute (NCI) grant R37 CA218707 (to DB Costa).
Footnotes
Provenance and Peer Review: This article was commissioned by the Editorial Office, Precision Cancer Medicine for the series “Precision Oncology Tumor Board”. The article was sent for external peer review organized by the Guest Editor Dr. Balazs Halmos and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-2020-potb-02). The Series “Precision Oncology Tumor Board” was commissioned by the editorial office without any funding or sponsorship. DBC reports personal fees (consulting fees and honoraria) and nonfinancial support (institutional research support) from Takeda/Millennium Pharmaceuticals, and AstraZeneca, and Pfizer, as well as nonfinancial support (institutional research support) from Merck Sharp and Dohme Corporation, Merrimack Pharmaceuticals, Bristol-Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals and Tesaro, all outside the submitted work. DR reports nonfinancial support (institutional research support) from Bristol-Myers Squibb, Novocure, and Abbvie/Stemcentrx, all outside the submitted work. KS serves as an unpaid editorial board member of Precision Cancer Medicine from Apr 2019 to Mar 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Patient information was collected from medical records in accordance with research protocols approved by the Beth Israel Deaconess Medical Center institutional review board.
References
- 1.Bergethon K, Shaw AT, Ou SH, Katayama R, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sehgal K, Patell R, Rangachari D, et al. Targeting ROS1 rearrangements in non-small cell lung cancer with crizotinib and other kinase inhibitors. Transl Cancer Res 2018;7:S779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 2013;18:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin JJ, Ritterhouse LL, Ali SM, et al. ROS1 Fusions Rarely Overlap with Other Oncogenic Drivers in Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378–81. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin JJ, Shaw AT. Recent Advances in Targeting ROS1 in Lung Cancer. J Thorac Oncol 2017;12:1611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go H, Kim DW, Kim D, et al. Clinicopathologic analysis of ROS1-rearranged non-small-cell lung cancer and proposal of a diagnostic algorithm. J Thorac Oncol 2013;8:1445–50. [DOI] [PubMed] [Google Scholar]
- 9.Clark JW, Camidge DR, Kwak EL, et al. Dose-escalation trial of the ALK, MET & ROS1 inhibitor, crizotinib, in patients with advanced cancer. Future Oncol 2020;16:4289–301. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol 2019;30:1121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moro-Sibilot D, Cozic N, Perol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSe phase II trial. Ann Oncol 2019;30:1985–91. [DOI] [PubMed] [Google Scholar]
- 12.Moro-Sibilot D, Faivre L, Zalcman G, et al. Crizotinib in patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC). Preliminary results of the ACSé phase II trial. J Clin Oncol 2015;33:8065. [Google Scholar]
- 13.Michels S, Massuti B, Schildhaus HU, et al. Safety and Efficacy of Crizotinib in Patients With Advanced or Metastatic ROS1-Rearranged Lung Cancer (EUCROSS): A European Phase II Clinical Trial. J Thorac Oncol 2019;14:1266–76. [DOI] [PubMed] [Google Scholar]
- 14.Wu YL, Yang JC, Kim DW, et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1405–11. [DOI] [PubMed] [Google Scholar]
- 15.Patil T, Smith DE, Bunn PA, et al. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non-Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J Thorac Oncol 2018;13:1717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad MM, Engelman JA, Shaw AT. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med 2013;369:1173. [DOI] [PubMed] [Google Scholar]
- 17.Gainor JF, Tseng D, Yoda S, et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017. doi: 10.1200/PO.17.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cargnelutti M, Corso S, Pergolizzi M, et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget 2015;6:5182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies KD, Mahale S, Astling DP, et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS One 2013;8:e82236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dziadziuszko R, Le AT, Wrona A, et al. An Activating KIT Mutation Induces Crizotinib Resistance in ROS1-Positive Lung Cancer. J Thorac Oncol 2016;11:1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song A, Kim TM, Kim DW, et al. Molecular Changes Associated with Acquired Resistance to Crizotinib in ROS1-Rearranged Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:2379–87. [DOI] [PubMed] [Google Scholar]
- 23.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443–5. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Yu H, Chang J, Chen H, et al. Crizotinib in Chinese Patients with ROS1-Rearranged Advanced NonSmall-Cell Lung Cancer in Routine Clinical Practice. Target Oncol 2019;14:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Occhipinti M, Falcone R, Onesti CE, et al. Crizotinib plus radiotherapy in brain oligoprogressive NSCLC ROS1 rearranged and PD-L1 strong. J Thorac Dis 2017;9:E985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menichincheri M, Ardini E, Magnaghi P, et al. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor. J Med Chem 2016;59:3392–408. [DOI] [PubMed] [Google Scholar]
- 27.Ardini E, Menichincheri M, Banfi P, et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol Cancer Ther 2016;15:628–39. [DOI] [PubMed] [Google Scholar]
- 28.Drilon A, Siena S, Ou SI, et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372–001 and STARTRK-1). Cancer Discov 2017;7:400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drilon A, Siena S, Dziadziuszko R, et al. Entrectinibin ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol 2020;21:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doebele R, Perez L, Trinh H, et al. P1.01–83 Comparative Efficacy Analysis Between Entrectinib Trial and Crizotinib Real-World ROS1 Fusion-Positive (ROS1+) NSCLC Patients. J Thorac Oncol 2019;14:S392. [Google Scholar]
- 31.Ku BM, Bae YH, Lee KY, et al. Entrectinib resistance mechanisms in ROS1-rearranged non-small cell lung cancer. Invest New Drugs 2020;38:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato H, Schoenfeld AJ, Siau E, et al. MAPK pathway alterations correlate with poor survival and drive resistance to therapy in patients with lung cancers driven by ROS1 fusions. Clin Cancer Res 2020. doi: 10.1158/1078-0432.CCR-19-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cocco E, Schram AM, Kulick A, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med 2019;25:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doebele RC, Dziadziuszko R, Drilon A, et al. Genomic landscape of entrectinib resistance from ctDNA analysis in STARTRK-2. Ann Oncol 2019;30:v865. [Google Scholar]
- 35.Johnson TW, Richardson PF, Bailey S, et al. Discoveryof (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h] [2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 2014;57:4720–44. [DOI] [PubMed] [Google Scholar]
- 36.Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol 2019;20:1691–701. [DOI] [PubMed] [Google Scholar]
- 37.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer TM, Felip E, Solomon BJ, et al. Clinical Management of Adverse Events Associated with Lorlatinib. Oncologist 2019;24:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Recondo G, Mezquita L, Facchinetti F, et al. Diverse Resistance Mechanisms to the Third-Generation ALK Inhibitor Lorlatinib in ALK-Rearranged Lung Cancer. Clin Cancer Res 2020;26:242–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madsen A, Jakobsen K, Demuth C, et al. P3.02–050 Mechanisms of Acquired Resistance to the ALK Inhibitor Lorlatinib in ALK-Rearranged NSCLC Cell Lines. J Thorac Oncol 2017;12:S2255. [Google Scholar]
- 41.Redaelli S, Ceccon M, Zappa M, et al. Lorlatinib Treatment Elicits Multiple On- and Off-Target Mechanisms of Resistance in ALK-Driven Cancer. Cancer Res 2018;78:6866–80. [DOI] [PubMed] [Google Scholar]
- 42.McCoach CE, Le AT, Gowan K, et al. Resistance Mechanisms to Targeted Therapies in ROS1(+) and ALK(+) Non-small Cell Lung Cancer. Clin Cancer Res 2018;24:3334–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim SM, Kim HR, Lee JS, et al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol 2017;35:2613–8. [DOI] [PubMed] [Google Scholar]
- 45.Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov 2018;8:1227–36. [DOI] [PubMed] [Google Scholar]
- 46.Cho BC, Drilon AE, Doebele RC, et al. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). J Clin Oncol 2019;37:9011. [Google Scholar]
- 47.Katayama R, Gong B, Togashi N, et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat Commun 2019;10:3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujiwara Y, Takeda M, Yamamoto N, et al. Safety and pharmacokinetics of DS-6051b in Japanese patients with non-small cell lung cancer harboring ROS1 fusions: a phase I study. Oncotarget 2018;9:23729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen YF, Hsieh MS, Wu SG, et al. Efficacy of Pemetrexed-Based Chemotherapy in Patients with ROS1 Fusion-Positive Lung Adenocarcinoma Compared with in Patients Harboring Other Driver Mutations in East Asian Populations. J Thorac Oncol 2016;11:1140–52. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Jiang T, Zhao C, et al. Efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement. Oncotarget 2016;7:75145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park S, Ahn BC, Lim SW, et al. Characteristics and Outcome of ROS1-Positive Non-Small Cell Lung Cancer Patients in Routine Clinical Practice. J Thorac Oncol 2018;13:1373–82. [DOI] [PubMed] [Google Scholar]
- 52.Berghoff AS, Bellosillo B, Caux C, et al. Immune checkpoint inhibitor treatment in patients with oncogene-addicted non-small cell lung cancer (NSCLC): summary of a multidisciplinary round-table discussion. ESMO Open 2019;4:e000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res 2015;21:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guisier F, Piton N, Salaun M, et al. ROS1-rearranged NSCLC With Secondary Resistance Mutation: Case Report and Current Perspectives. Clin Lung Cancer 2019;20:e593–6. [DOI] [PubMed] [Google Scholar]


