Abstract
The high prevalence of cardiovascular disease and worldwide diabetes epidemic has created an ever-increasing burden on the healthcare system. This calls for the creation of a new medicine subspecialty: cardiometabolic medicine. Using information from review articles listed on PubMed and professional society guidelines, the authors advocate for a cardiometabolic medicine specialization training program. The curriculum would integrate relevant knowledge and skills of cardiology and endocrinology as well as content of other disciplines essential to the optimal care of cardiometabolic patients, such as epidemiology, biostatistics, behavioral science and psychology. Cardiometabolic medicine should be seen as an opportunity for life-long learning, with core concepts introduced in medical school and continuing through CME courses for practicing physicians. To improve care for complex patients with multiple co-morbidities, a paradigm shift must occur, transforming siloed education, and treatment and training to interdisciplinary and collaborative work.
Keywords: cardiology, diabetes, endocrinology, metabolism, prevention
Introduction
We are entering an era of chronic metabolic and cardiovascular multi-comorbidities. Amongst patients with type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD) remains the main cause of mortality and morbidity [1]. Importantly, both habitually coexist against the background of obesity and insulin resistance: indeed, an extensive proportion of patients with CVD have pre-diabetes or T2DM, although these metabolic disorders are often undiagnosed [2]. Optimization and coordination of the – often complex – medical management of these patients is crucial, as it has direct implications for outcomes. By curtailing adverse CVD events, minimizing redundant testing and reducing drug-drug interactions, optimized cardiometabolic management can also have large benefits in terms of reducing medical expenditure. For example, the estimated 1-year cardiometabolic disease cost associated with a unhealthy diet in the USA amongst those aged 35–85 years is $301 per capita or a total population cost of $50.4 billion [3].
Numerous barriers will have to be overcome, however, to accomplish this goal. As of now, comprehensive primary and secondary prevention care plans addressing the complex issues of cardiometabolic patients are underdeveloped in most centers. Patients with metabolic diseases and CVD currently require the care of multiple specialists who work in parallel and with little interaction, with no ‘main’ specialist coordinating the management from an advanced, holistic standpoint. Endocrinologists may be uncomfortable prescribing drugs for CVD, and the same may be true for cardiologists when using novel drugs approved or in development for the treatment of patients with diabetes. Moreover, only a limited number of providers have extensive experience prescribing anti-obesity medication. This may result in the underuse of evidence-based, guideline-recommended class I therapies.
Indeed, recent advances in pharmacological therapy have resulted in the availability of ‘cardiometabolic’ drugs, which are FDA-approved to treat metabolic conditions such as obesity and diabetes while providing a proven CVD benefit. For instance, cardiovascular outcome trials (CVOTs) demonstrated that glucagon-like peptide-1 (GLP-1) receptor agonists as well as sodium-glucose transporter 2 (SGLT2) inhibitors significantly reduce major CVD events and are effective for glucose management. This has increased the attention towards the relevance of cardiometabolic patients, professionals, and their training.
Traditional models and silos of physician education and treatment presently in place will need to be reassessed. In this article, we review the shortcomings of current approaches and propose a training curriculum for a cardiometabolic specialist. We envision core concepts of cardiometabolic medicine to be introduced in medical school. A cardiometabolic clinical training program would include relevant parts of traditional endocrinology and cardiology programs with an important emphasis on lifestyle. In addition, in this review, we also expand the importance of other areas highly relevant to the cardiometabolic professional, such as biostatistics and epidemiology, lifestyle science with a special focus on dietary patterns, behavioral science and psychology, personalization of care, and creation of important collaborations with other specialties relevant to the cardiometabolic patient.
Recent trends in the epidemiology of cardiometabolic disease
The overall body of literature suggests that the incidence of CVD has been declining over time [4]. However, although the rates of decline of CVD as well as heart disease and stroke mortality have decelerated in the last decade [5], projections even suggest that the prevalence of CVD in the USA may escalate by 10% until 2030 [6]. These trends may be attributed to the increasing prevalence of key CVD risk factors including the metabolic syndrome that encompasses central obesity, diabetes, dyslipidemia and hypertension. Moreover, not listed under the criteria for metabolic syndrome are other insulin-resistant paradigms that relate to CVD risk including nonalcoholic fatty liver disease (NAFLD), polycystic ovarian syndrome and pro-thrombotic and pro-inflammatory states [7].
Obesity
In 2017–2018, obesity prevalence of adults in the USA reached 42.4% and severe obesity 9.2% [8]. Estimates suggest that obesity prevalence in the USA will soar to 50% by 2030. Moreover, in 20 years, approximately one in four Americans is projected to be severely obese [9]. Individuals diagnosed with obesity have a much higher risk for CVD and diabetes than non-obese persons, as well as an elevated risk of developing other comorbidities [10]. A meta-analysis showed that each 1 SD increase in BMI increased the odds of coronary artery disease by 20% and T2DM by 67% [11]. As a consequence, a large portion of patients with CVD or at high risk of CVD are obese.
Diabetes
Obesity is a major contributor to the diabetes pandemic (Fig. 1). Currently, 463 million people, comprising 8.8% of the world’s population, are diagnosed with diabetes and >90% have T2DM [12]. Estimates by the International Diabetes Federation suggest that by 2045, cases of diabetes will rise to 700 million [12]. In North America, 13.3% of adults from ages 20–75 have diabetes and this number is expected to rise to 15% by 2045. In addition, the USA has one of the highest number of diabetes-related deaths worldwide [12]. Diabetes and even pre-diabetes range glucose levels are associated with multiple cardiovascular conditions and CVD is the leading cause of death in patients with these conditions. The relative risk (RR) for CVD for patients with diabetes lies between 1.6 and 2.6 [13].
Fig. 1.
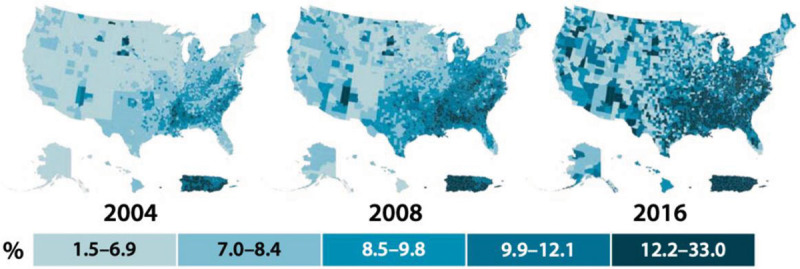
Age-adjusted, county-level prevalence of diagnosed diabetes among adults aged 20 years or older, USA, 2004, 2008, and 2016. Data were unavailable for some US territories. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020.
Apart from diabetes, the prediabetic state is also a strong predictor of CVD and is highly prevalent among cardiometabolic patients [14,15]. Patients with prediabetes have greater extent of coronary atherosclerosis and plaque vulnerability [16]. In the Multi-Ethnic Study of Atherosclerosis, a diagnosis of prediabetes was associated with a 3-fold higher prevalence of unrecognized myocardial infarction [17]. Glycated hemoglobin levels in the pre-diabetic range have been found to be associated with CVD [18].
Hypertension
Hypertension is another major risk factor for atherosclerotic CVD (ASCVD) and is closely linked to obesity and insulin resistance. Excess weight accounts for 65–78% of risk of essential hypertension [19]. It is well established that antihypertensive treatment reduces the risk of mortality and cardiovascular morbidity [20,21]. The prevalence of hypertension is rising in the USA, as is the proportion of patients receiving antihypertensive medications. However, as of 2015–2016, only 43.5% of individuals who were prescribed antihypertensive agents had controlled hypertension [22]. Hypertension is a common comorbidity of diabetes [23], with these patients being frequent recipients of multiple hypertensive drugs.
Dyslipidemia
Dyslipidemia is a key contributor in the development of atherosclerosis. Atherogenic dyslipidemia, characterized by elevated triglycerides and small dense-low density lipoprotein and reduced high-density lipoprotein cholesterol, is the most prevalent lipid abnormality among individuals with diabetes [24]. Prevalence of lipid abnormalities is higher among individuals with diabetes, contributing to their elevated cardiovascular risk [25].
Metabolic syndrome
The cluster of cardiovascular risk factors making up the metabolic syndrome and other parameters of insulin resistance are drivers in the underlying pathological processes of CVD and diabetes. Metabolic syndrome is associated with a RR for CVD of 2.88 for men and 2.25 for women, respectively [26]. The RR of T2DM, which is driven mostly by fasting plasma glucose, was also shown to be greatly increased for women (RR 6.90) as well as for men (RR 6.92) [26]. Despite prevalence of metabolic syndrome remaining stagnant, the total burden of metabolic syndrome is very high with an estimated one-third of all US adults and one-half of adults 60 years or older affected [27].
Cardiometabolic multimorbidity
While we are witnessing a rise in individual disease entities, prevalence of multimorbidity is increasing as well. A European survey on diabetes and heart disease reported that 31% of individuals with CAD were also diagnosed with diabetes [28]. A cohort study of almost two million participants showed that of individuals with T2DM, 17.9% had a manifestation of CVD, the most common being peripheral arterial disease (16.2%) and heart failure (14.1%) [29]. Evidence suggests that rates for hospitalization from HF are two-fold higher in patients with DM [30]. Moreover, recent evidence suggests that ECG evidence of silent myocardial infarction, which is more common among patients with diabetes, improves risk discrimination over and beyond traditional factors [31]. Obesity, diabetes and atrial fibrillation (AF) are closely related. Obesity-related diseases are mediated by regional fat deposits. For example, while pericardial fat is associated with CVD, it was also found to be a strong risk factor for AF [32].
Advances in cardiometabolic drugs and limited real-world uptake
We are witnessing the development of pharmaceuticals targeting the variety of conditions associated with cardiometabolic disease. CVOTs demonstrated that some pharmaceuticals used to treat diabetes reduce important cardiovascular outcomes, as well as mitigate various CVD risk factors. DPP-4 inhibitors were safe but proved not to have any cardiovascular benefits compared to placebo. In contrast, the SGLT2 inhibitors [empagliflozin (EMPA-REG OUTCOME) [33], canagliflozin (CANVAS) and dapagliflozin (DECLARE-TIMI 58) [34]] have demonstrated significant reductions in CVD [35]. Canagliflozin, empagliflozin and dapagliflozin have additionally been shown to reduce the progression of kidney disease, as well as reduce incidence of hospitalization from heart failure [36–39]. Four FDA-approved GLP-1 receptor agonists [liraglutide (LEADER) [40], semaglutide (SUSTAIN-6) [41], albiglutide (Harmony Outcomes) [42] and dulaglutide (REWIND) [43]] have demonstrated significant reduction in CVD events. GLP-1 receptor agonists were additionally found to benefit patients with macroalbuminuria [44].
Based on this emerging evidence, the 2020 American Diabetes Association’s Standards of Medical Care for patients with T2DM recommends the use of SGLT2 inhibitors as well as GLP-1 receptor agonists appropriate to mitigate cardiovascular risk in patients with diabetes [35]. An even more effective treatment of individuals with cardiometabolic disease may be offered through the development of novel, more potent GLP-1 agonists [45], In addition, patients with and without diabetes may also benefit from the further expansion of SGLT-2 inhibitors effective for individuals with heart failure with preserved ejection faction [46]. The formulation of an oral administration of semaglutide proved successful in multiple trials and will hopefully improve therapy adherence compared to injectable medication [47–49].
Despite their clear cardiovascular benefits and FDA approval of cardiovascular indication for these medications, these agents are underused in real-world clinical care. Estimates suggest less than 20% of patients with ASCVD and diabetes receive this guideline-recommended risk reduction treatment [50]. An evaluation of a large outpatient registry concluded that of potential eligible patients for SGLT2 inhibitors and GLP-1 agonists, only 5.2 and 6.0% were prescribed liraglutide and empagliflozin, respectively [51]. Broader and more targeted use of these medications would likely lead to reductions in cardiovascular events and all-cause mortality.
Current training program
While advancements in pharmacology continue to blur the lines between cardiology and endocrinology, physicians are still trained in siloed programs that are unfit for cardiometabolic patients with one or many co-morbidities that require requiring interdisciplinary style management. Even though cardiologists may seem well-positioned to take the ‘lead’ in managing cardiometabolic patients, many may be uncomfortable or uninterested in taking on patients’ glycemic management, and prescribing weight-loss interventions and drugs. The treatment of ASCVD already requires management of multiple drugs and their side effects, such as statins, PCSK9 inhibitors, angiotensin-converting enzyme inhibitors, beta-blockers or dual antiplatelet agents or other anticoagulant regimens. Cardiologists may feel overwhelmed with additionally prescribing SGLT2 inhibitors and GLP-1 agonists and monitoring related side effects. In addition, there are patients on basal or basal/bolus insulin including the increasing number of patients with T1DM with CVD or high CVD risk [52]. Moreover, insulin infusion pumps and continuous glucose monitoring (CGM) are parts of the therapeutic management of patients with or at high risk of CVD.
Ultimately, the lack of interdisciplinary patient management results in fragmented care and worse outcomes for patients. There is a timely need for professionals who can manage complex, cardiometabolic patients in a comprehensive guideline-adherent manner.
Core areas of training in cardiometabolic medicine
As cardiometabolic patients require the expertise of multiple specialists, they serve to benefit from the integration of knowledge and skills of relevant disciplines and multidisciplinary collaboration between health professionals. Therefore, and building on the notions discussed above, we strongly advocate for the development of a discrete field of cardiometabolic medicine. The cardiometabolic patient requires expertise across a wide range of specialties, including cardiology, endocrinology, primary care, nutrition, podiatry, neurology, nephrology, hepatology, pediatrics and family medicine and some key competences of these disciples should be incorporated into cardiometabolic training programs.
The following is a summary of the six core components of cardiometabolic training. Primary and secondary ASCVD prevention would be the main focus of the cardiology portion of the training program. With regard to primary ASCVD prevention, physicians would engage in advanced cardiovascular risk assessment training, including knowledge of risk calculators, risk enhancing factors and primary prevention pharmacology. Personalization of risk assessment to optimize preventive care is heavily endorsed by current guidelines, so training would include proficiency in CT imaging to obtain and analyze coronary artery calcium scores and evaluation of plaque burden [53].
To stay focused on cardiometabolic diseases, physicians would forgo training in electrophysiology, interventional cardiology, advanced heart failure, or cardiac transplantation. Instead, the program would concentrate on inpatient cardiac care, inpatient cardiology consults, preventive cardiology clinic, interpretation of electrocardiography, cardiac rehabilitation and cardiac imaging (echocardiography, stress testing, coronary computed tomography). To enhance expertise in management of severe hypertension, elective time would be spent in a multispecialty resistant hypertension clinic and vascular medicine.
The endocrinology portion of this program would cover metabolic diseases and provide trainees with sufficient clinical experience in T1DM, T2DM, obesity, lipid and lipoprotein disorders, and hypertension management. In addition, the program would include training in basal/bolus insulin administration, insulin infusion pumps and CGM. Rotations through lipid clinics to obtain expertise in handling complex lipid disorders would also be part of the endocrine component. Traditional parts of endocrinology training, such as thyroid, pituitary, reproductive endocrinology, metabolic bone disease and disorders of calcium metabolism would not be covered in the cardiometabolic training program. With ample evidence pointing to micro- and macrovascular complications in adulthood associated with childhood diabetes mellitus [54], components of pediatric diabetes management should be incorporated into the curriculum.
A substantial component of the cardiometabolic training program would be comprehensive lifestyle counseling. Trainees would gain a deep understanding of exercise physiology and nutrition including quality and quantity of diets, as well as smoking cessation, including cessation pharmacology and novel tobacco products. We also plan for behavioral intervention training to be an integral part of cardiometabolic training, with focus on physician-patient communication, motivational coaching, methods enhancing therapy adherence and mobile health strategies.
In addition, the cardiometabolic training program would incorporate the most relevant aspects of obesity medicine, that is, hepatology with focus on NAFLD and nephrology. With regard to obesity medicine, for example, physicians would rotate through multidisciplinary obesity clinics and obtain skills in individualized lifestyle management that promote weight reduction, obesity pharmacology and training and in referral to and postoperative management of patients undergoing bariatric (metabolic) surgery. Expertise of pediatricians regarding prevention of childhood obesity should also be incorporated. Childhood overweight, particularly during puberty, is associated with increased risk of T2DM in adulthood [55]. Trainees would obtain this knowledge through rotations in pediatric obesity clinics.
Enriching the breadth of cardiometabolic medicine even further
After completion of a 2- or 3-year general internal medicine program, fellowship training in the cardiometabolic specialist program would start. Besides the core training described above, we suggest adding the following components and complementary skills to enhance cardiometabolic training even further (Table 1 and Fig. 2).
Table 1.
Overview of knowledge, skills and rotations of cardiometabolic medicine during residency and specialist training

Fig. 2.
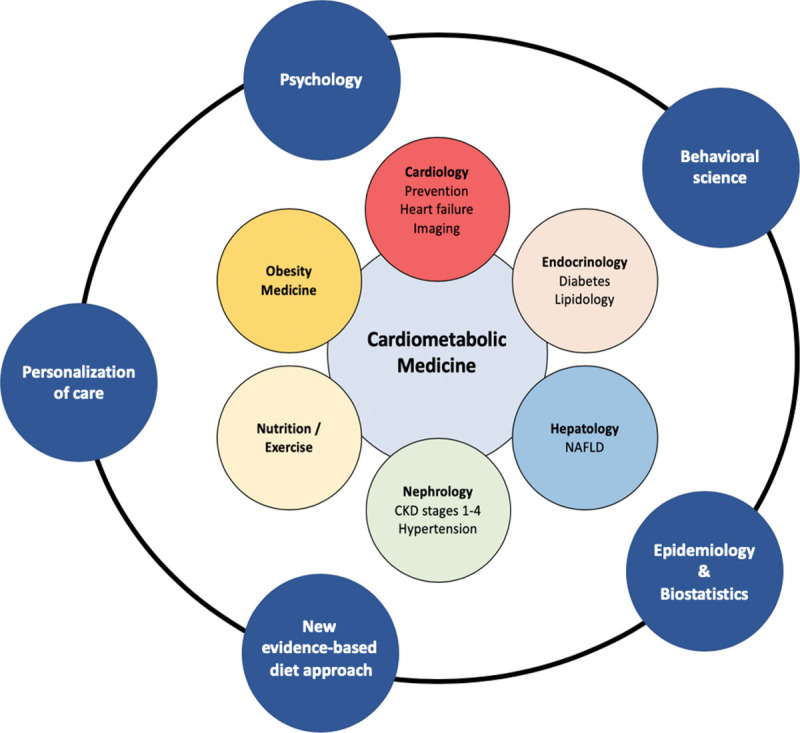
Fields of cardiometabolic medicine.
Epidemiology and biostats
Cardiometabolic disease is evolving into an ever-pressing public health problem. Current emphasis in epidemiological research on epidemiology modeling and economic endpoints will inform cardiometabolic physicians on the cost-effectiveness and health impact of prevention strategies, populations at risk and health disparities [56]. We are also witnessing the integration of ‘big data’ into CVD epidemiology. Digital technology in the form of wearable devices or in-the-body digital elements offer novel methods of epidemiological data collection [57]. This results in a much greater availability of epidemiological research information. In addition, an extensive number of biomarkers and risk scores characterizing the development of CVD has been developed. Therefore, it is vital for cardiometabolic medicine trainees to become adept at critical appraisal of scientific literature and to gain a nuanced understanding of the limitations of risk scoring methods [58]. We envision incorporating components of the Master of Public Health program into the cardiometabolic medicine training program. This would include formal epidemiology and biostatistics training, as well as instruction on principles for design and interpretation of clinical trials. Exposure to the public health system may be facilitated through clinical experience, such as rotations in community health centers or health department clinics.
Behavioral science and psychology
During the cardiometabolic training program, physicians will learn skills for behavioral change counseling and methods to implement these in clinical practice through rotations with behavioral psychologists.
CVD development and outcomes are highly dependent on psychological factors. For instance, depression is an established risk factor for coronary heart disease (CHD), recurrent CHD events, and heart failure (HF) [56]. Depression is associated with poor outcomes in CVD, postcoronary bypass, and HF [56]. Diabetes distress and depression are also an important part of comprehensive diabetes assessment and therapy [59].
Patients’ lifestyle behaviors are attributed to causing 40% of all deaths in the USA [60]. The WHO estimates that 75% of CVD could be avoided by four essential lifestyle practices: a healthy diet, physical activity, avoidance of tobacco, and moderate alcohol intake [61]. Despite clear evidence supporting the importance of a healthy lifestyle, propagation of this knowledge during medical school is minimal compared to time spent on pharmaceutical training [62].
In addition, adherence to prescribed medication is an issue, which could be addressed by behavioral change therapy. Approximately one-third of patients with and one-half of patients without a history of myocardial infarction do not adhere to prescribed medication [63].
Behavioral changes have been shown to result in robust, cost-effective improvements in outcomes [64,65]. Much evidence also suggests that weight loss counseling by physicians has a positive impact on patient’s weight loss efforts and healthy eating habits [66,67]. However, simply explaining scientific facts to patients is insufficient to change behavior [68]. To facilitate any lasting behavioral transformation, physicians require a deep understanding of behavioral science principles, as well as tools to assist patients throughout the process of their lifestyle change [68].
Nutrition
The nutrition portion of cardiometabolic medicine training should stress critical evaluation of the dietary science literature to gain a deep understanding of how to best counsel patients regarding dietary interventions to optimize cardiometabolic health. This skill is best implemented by offering rotations with registered dieticians and certified diabetes care and educators.
The importance of nutrition in medicine is undisputed. A healthy diet is a key lifestyle habit with large implications for metabolic risk factors and CVD. Guidelines clearly stress the importance of nutritional intervention as part of evidence-based clinical care, however, specialists lack the education to implement these recommendations [69]. A recent survey of 646 cardiologists stated that 90% received no or minimal education on nutrition counseling during their fellowship, even though 95% thought it was their responsibility to do so [70].
In addition, patients as well as physicians are confronted with novel ‘healthy’ diets almost on a daily basis. The list of popular diets is extensive, such as Mediterranean, DASH, Paleo, low-carb, low-fat diet and a plethora of fad diets. Physicians should emphasize that a cardioprotective diet consists of a divers inclusion of healthful foods, which permits flexibility and personal preference of patients [71].
A growing interest in dietary supplements also exists. However, evidence does not support the use of supplements to augment a cardioprotective diet with healthful foods and some supplements may even be harmful [72–74].
Personalization of care – sex and racial/ethnic-specific risk profiles of cardiometabolic diseases
In the light of a growing interest in developing a personalized approach to the prevention and treatment of cardiometabolic diseases, awareness of racial/ethnic and gender disparities should be emphasized during the cardiometabolic medicine training program. Cardiometabolic specialists should also be able to culturally adapt interventions to enhance therapy adherence.
An important heterogeneity in risk of developing cardiometabolic diseases exists within different ethnic groups. In particular, South Asian individuals have on average a heightened ASCVD risk and a four-fold risk of developing diabetes compared with white Europeans [75,76]. Evidence suggests that even though anthropometrically thin, South Asians are ‘metabolically’ obese, with greater visceral and hepatic fat even from infancy [77,78]. This may be reason for the observed development of T2DM at a much younger age and at lower levels of BMI [79].
Compared with whites and blacks, diabetes mellitus is disproportionally present in Hispanics. There is also a higher prevalence of metabolic syndrome in Mexican Americans compared to Puerto Ricans and white Americans. Hispanics are at greater risk of all-cause and CVD mortality than their white and Asian counterparts, despite similar atherosclerotic burden [80]. These differences may also be explained by socioeconomic disparities and healthcare access [80,81].
In the USA, the highest prevalence of overt diabetes exists in American Indians/Alaska Natives [82]. In addition, the highest rates of ASCVD risk factors are seen in this population compared to non-Hispanic whites. Engagement in activities that elevate risk for cardiometabolic disease may explain these observations. For example, 22.6% of Native American/Alaskan Natives adults are smokers, compared to 15% non-Hispanic white men, 14.6% of non-Hispanic black men, 9.8% of Hispanic men and 7.1% of Asian men [83]. Physical inactivity is also common. Estimates suggest that five in 10 Native Americans are physically inactive and 26.4% do not meet the FDA’s recommended physical activity participation; a rate higher than in any other racial/ethnic group [84].
The cardiometabolic specialists should be aware of the sex-specific differences regarding metabolic and CVD. For instance, a history of premature menopause as well as pregnancy associated conditions, such as preeclampsia and gestational diabetes mellitus, increase ASCVD risk for women [85]. Sex-specific atherosclerotic plaque profiles exist as well. Evidence suggests that women are subject to more diffuse and extensive atherosclerosis, increasing their CVD risk [86]. Diabetes during pregnancy confers its own maternal and fetal risks, such as spontaneous abortions, fetal anomalies, neonatal hypoglycemia, among others. Diabetes during pregnancy may also increase risk of T2DM and obesity in the offspring [87].
Research skills
We envision that a focused training program on cardiometabolic medicine inspires new research ideas as a result of close collaboration between different disciplines. At the same time, cardiometabolic medicine specialists may also take jobs in an academic setting and lead important and novel investigations in basic, preclinical, clinical and population science and public health. Some studies in this field are amongst the highest impact in science and medicine. Therefore, projects require leadership of professionals with strong training in academic research. This could be facilitated by incorporating research electives into the training program.
Cardiometabolic medicine as a longitudinal training curriculum – from medical school to lifelong learning
We envision a longitudinal training curriculum, with introduction of core concepts in medical school and the possibility of deepening this knowledge during specialization as a cardiometabolic physician. Life-long learning in this field will be ensured by offering continuing medical education (CME) programs.
Medical school
We hope that students will be interested in the cardiometabolic specialty, as many medical students strive to ‘make a difference’ in clinical care and clinical research. In this sense, cardiometabolic medicine is appealing because it focuses on conditions which affect an extremely large proportion of patients.
Medical education focuses little on prevention of cardiovascular and metabolic diseases but places disproportionally more emphasis on mechanisms behind the chronic disease states and pharmaceutical management. Further, diseases are taught as single entities, but many patients are diagnosed with multiple conditions that often have similar mechanisms, for example, insulin resistance, that all have to be managed simultaneously. A course in cardiometabolic medicine would offer a historic and holistic perspective on preventive medicine, and provide students with the skills to treat complex, multimorbid patients. In Table 2, we provide an overview of the specific skills important to the cardiometabolic specialist which are lacking in current curricula.
Table 2.
Knowledge and skills of cardiometabolic medicine in medical school

How would a cardiometabolic training program in medical school be implemented? Some argue that medical school programs are already overloaded with expanding medical knowledge. However, we propose that rather than adding cardiometabolic medicine as a completely separate specialty to the curriculum, concepts could be integrated into existing content. For example, a recent trend in medical education is restructuring education around disease entities instead of the arbitrary boundaries of organ system blocks. Existing content such as T1DM/T2DM and ASCVD classically covered within separate endocrine organ systems and cardiology, respectively, could instead be taught within a holistic cardiometabolic medicine course. Many medical universities have adopted longitudinal integrated clerkships, in which students are assigned to a preceptor and patient and train within this disciple and care for this patient for a year. Cardiometabolic patients are the optimal patients for this type of curriculum, as they are currently required to see an extensive number of specialists. This would provide students with the opportunity to learn from many different physicians by following these patients from visit to visit.
This framework of knowledge and skills would be valuable to all physicians, including students who do not choose to specialize in cardiometabolic medicine. For example, lessons on behavioral science are easily transferable to other specialties that engage in direct patient care.
Overall, we hope to offer medical students an early exposure to cardiometabolic medicine by providing students with foundation knowledge of this disciple.
Specialty training
We believe that the formulation of cardiometabolic specialist program would further enhance the ability of internal medicine physicians to manage complex patients with cardiometabolic disease. While many internal medicine residents might be interested in metabolic diseases or ASCVD, currently they still spend many months of training in catheterization or electrophysiology labs or learn about thyroid disease, bone health, or endocrinologic cancers. This time could be instead invested on specific skills and concepts truly relevant to facilitate the comprehensive treatment for the cardiometabolic patient. In addition, recognition of a cardiometabolic specialty would lead to more innovative research ideas through the closer collaboration amongst the variety disciples. More research dedicated exclusively to cardiometabolic disease would ultimately improve patient care in the long run.
Continuing medical education and lifelong learning
We believe that the skills of the cardiometabolic specialist are very much needed among professionals who already completed their specialty training. Training modules, online courses and CME training could be offered to effectively distribute these skills amongst already specialized physicians. We also envision a separate journal dedicated to cardiometabolic medicine as a medium to share the latest advancements in research in this disciple.
Gaps and future directions
To enhance the cardiometabolic education program even further, more research is needed to identify the training needs of different groups of relevant professionals seeing cardiometabolic patients. The cardiometabolic medicine training program proposed here also has to be developed in more detail to ensure adherence to guidelines and patient safety. We hope the cardiometabolic specialty will gain momentum by initially integrating it into existing cardiology and endocrinology training programs.
Finally, we envision a ‘cardiometabolic clinic’ where physicians and other specialists work together collaboratively in interdisciplinary teams. The optimal cardiometabolic team would consist of cardiometabolic specialists, dieticians, diabetes care and education specialists (formally called CDEs), exercise physiologists, specialized rehabilitation physicians and behavioral psychologists. Rehabilitation facilities as well as outpatient clinics would be an integral part of the cardiometabolic clinic.
Conclusion
The dire need to treat the cardiometabolic disease epidemic while providing cost-effective, high-quality healthcare is one of the most important health challenges of the 21st century. Introducing cardiometabolic medicine as a separate specialty with a comprehensive training program starting in medical school and extending until after specialization is the most effective method to tackle this enormous task. Prevention, treatment and management of risk factors of cardiometabolic disease are best achieved by a less siloed approach with cardiometabolic specialists working collaboratively with other specialists in interdisciplinary teams.
Acknowledgements
Conflicts of interest
R.H.E. has served on scientific advisory boards for KOWA, Novo Nordisk as well as for the cardiovascular outcome trial PROMINENT (Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes).
M.J.B. has served on scientific advisory boards for Amgen, Sanofi, Regeneron, Novartis, Novo Nordisk, Bayer and Akcea. For the remaining authors, there are no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. 2020, Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services [Google Scholar]
- 2.Lauruschkat AH, Arnrich B, Albert AA, Walter JA, Amann B, Rosendahl UP, et al. Prevalence and risks of undiagnosed diabetes mellitus in patients undergoing coronary artery bypass grafting. Circulation. 2005; 112:2397–2402 [DOI] [PubMed] [Google Scholar]
- 3.Jardim TV, Mozaffarian D, Abrahams-Gessel S, Sy S, Lee Y, Liu J, et al. Cardiometabolic disease costs associated with suboptimal diet in the United States: a cost analysis based on a microsimulation model. Plos Med. 2019; 16:e1002981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017; 135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidney S, Quesenberry CP, Jr, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016; 1:594–599 [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011; 123:933–944 [DOI] [PubMed] [Google Scholar]
- 7.Lim S, Eckel RH. Pharmacological treatment and therapeutic perspectives of metabolic syndrome. Rev Endocr Metab Disord. 2014; 15:329–341 [DOI] [PubMed] [Google Scholar]
- 8.Hales CM, Carroll MD, Fryar CD, Ogden CL.Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018 Key Findings Data from the National Health and Nutrition Examination Survey. 2020. https://www.cdc.gov/nchs/products/index.htm. Accessed March 23, 2020. [PubMed]
- 9.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019; 381:2440–2450 [DOI] [PubMed] [Google Scholar]
- 10.Guo F, Garvey WT. Trends in cardiovascular health metrics in obese adults: National Health and Nutrition Examination Survey (NHANES), 1988-2014. J Am Heart Assoc. 2016; 5:e003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riaz H, Khan MS, Siddiqi TJ, Usman MS, Shah N, Goyal A, et al. Association between obesity and cardiovascular outcomes: a systematic review and meta-analysis of mendelian randomization studies. JAMA Netw Open. 2018; 1:e183788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IDF Diabetes Atlas. Demographic and geographic outline. https://diabetesatlas.org/en/sections/demographic-and-geographic-outline.html. Published 2019. Accessed December 5, 2019
- 13.Cefalu WT, Buse JB, Tuomilehto J, Fleming GA, Ferrannini E, Gerstein HC, et al. Update and next steps for real-world translation of interventions for type 2 diabetes prevention: reflections from a diabetes care editors’ expert forum. Diabetes Care. 2016; 36:1186–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007; 116:151–157 [DOI] [PubMed] [Google Scholar]
- 15.International Diabetes Federation. IDF DIABETES ATLAS Ninth Edition 2019. 2019, Brussels: Belgium; https://www.diabetesatlas.org [Google Scholar]
- 16.Kurihara O, Takano M, Yamamoto M, Shirakabe A, Kimata N, Inami T, et al. Impact of prediabetic status on coronary atherosclerosis: a multivessel angioscopic study. Diabetes Care. 2013; 36:729–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stacey RB, Leaverton PE, Schocken DD, Peregoy JA, Bertoni AG. Prediabetes and the association with unrecognized myocardial infarction in the Multi-Ethnic Study of Atherosclerosis. Am Heart J. 2015; 170:923–928 [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010; 362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrison RJ, Kannel WB, Stokes J, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham offspring study. Prev Med. 1987; 16:235–251 [DOI] [PubMed] [Google Scholar]
- 20.Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016; 352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997; 350:757–164 [DOI] [PubMed] [Google Scholar]
- 22.Dorans KS, Mills KT, Liu Y, He J. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018; 7:e008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40:1273–1284 [DOI] [PubMed] [Google Scholar]
- 24.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009; 5:150–159 [DOI] [PubMed] [Google Scholar]
- 25.Jacobs MJ, Kleisli T, Pio JR, Malik S, L’Italien GJ, Chen RS, Wong ND. Prevalence and control of dyslipidemia among persons with diabetes in the United States. Diabetes Res Clin Pract. 2005; 70:263–269 [DOI] [PubMed] [Google Scholar]
- 26.Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005; 112:3066–3072 [DOI] [PubMed] [Google Scholar]
- 27.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015; 313:1973–1974 [DOI] [PubMed] [Google Scholar]
- 28.Bartnik M, Rydén L, Ferrari R, Malmberg K, Pyörälä K, Simoons M, et al. ; Euro Heart Survey Investigators. The prevalence of abnormal glucose regulation in patients with coronary artery disease across EuropeThe Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004; 25:1880–1890 [DOI] [PubMed] [Google Scholar]
- 29.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015; 3:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAllister DA, Read SH, Kerssens J, Livingstone S, McGurnaghan S, Jhund P, et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018; 138:2774–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singleton MJ, German CA, Bertoni AG, Ambrosius WT, Bhave PD, Soliman EZ, et al. Association of silent myocardial infarction with major cardiovascular events in diabetes: the ACCORD trial. Diabetes Care. 2020; 43:e45–e46 [DOI] [PubMed] [Google Scholar]
- 32.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009; 30:850–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 34.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357 [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020; 43:S111–S134 [DOI] [PubMed] [Google Scholar]
- 36.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 37.Wanner Ch, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016; 375:1801–1802 [DOI] [PubMed] [Google Scholar]
- 38.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019; 7:606–617 [DOI] [PubMed] [Google Scholar]
- 39.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 40.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. ; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 42.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Sr, Granger CB, Jones NP, et al. ; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018; 392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 43.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019; 394:121–130 [DOI] [PubMed] [Google Scholar]
- 44.Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: an updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. 2019; 21:2576–2580 [DOI] [PubMed] [Google Scholar]
- 45.Müller TD, Clemmensen C, Finan B, DiMarchi RD, Tschöp MH. Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacol Rev. 2018; 70:712–746 [DOI] [PubMed] [Google Scholar]
- 46.Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al. ; EMPEROR-Preserved Trial Committees and Investigators. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-preserved trial. Eur J Heart Fail. 2019; 21:1279–1287 [DOI] [PubMed] [Google Scholar]
- 47.Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Villegas ECM, et al. PIONEER 1: randomized clinical trial comparing the efficacy and safety of oral semaglutide monotherapy with placebo in patients with type 2 diabetes. Diabetes Care. 2019; 42:1724–1732 [DOI] [PubMed] [Google Scholar]
- 48.Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, et al. ; PIONEER 7 investigators. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019; 7:528–539 [DOI] [PubMed] [Google Scholar]
- 49.Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. ; PIONEER 4 investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019; 394:39–50 [DOI] [PubMed] [Google Scholar]
- 50.Arnold SV, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Mues KE, et al. ; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019; 140:618–620 [DOI] [PubMed] [Google Scholar]
- 51.Arnold SV, Inzucchi SE, Tang F, McGuire DK, Mehta SN, Maddox TM, et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR® Research to Practice project. Eur J Prev Cardiol. 2017; 24:1637–1645 [DOI] [PubMed] [Google Scholar]
- 52.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014; 130:1110–1130 [DOI] [PubMed] [Google Scholar]
- 53.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation. 2019; 140:e596–e646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dabelea D, Stafford JM, Mayer-Davis EJ, D’Agostino R, Jr, Dolan L, Imperatore G, et al. ; SEARCH for Diabetes in Youth Research Group. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017; 317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjerregaard LG, Baker JL. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med. 2018; 378:2537–2538 [DOI] [PubMed] [Google Scholar]
- 56.Bairey Merz CN, Alberts MJ, Balady GJ, Ballantyne CM, Berra K, Black HR, et al. ; American Academy of Neurology; American Association of Cardiovascular and Pulmonary Rehabilitation; American College of Preventive Medicine; American College of Sports Medicine; American Diabetes Association; American Society of Hypertension; Association of Black Cardiologists; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute; National Lipid Association; Preventive Cardiovascular Nurses Association. ACCF/AHA/ACP 2009 competence and training statement: a curriculum on prevention of cardiovascular disease: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on competence and training (writing committee to develop a competence and training statement on prevention of cardiovascular disease): developed in collaboration with the American Academy of Neurology; American Association of Cardiovascular and Pulmonary Rehabilitation; American College of Preventive Medicine; American College of Sports Medicine; American Diabetes Association; American Society of Hypertension; Association of Black Cardiologists; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute; National Lipid Association; and Preventive Cardiovascular Nurses Association. Circulation. 2009; 120:e100–e126 [DOI] [PubMed] [Google Scholar]
- 57.Vasan RS, Benjamin EJ. The future of cardiovascular epidemiology. Circulation. 2016; 133:2626–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cainzos-Achirica M, Bilal U, Kapoor K, Ayala RQ, McEvoy JW, Pladevall-Vila, et al. Methodological Issues in Nutritional Epidemiology Research—Sorting Through the Confusion. Cardiovasc Risk Rep. 2018; 12:4 [Google Scholar]
- 59.Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014; 31:764–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. J Am Med Assoc. 2004; 291:1238–1245 [DOI] [PubMed] [Google Scholar]
- 61.Cardiovascular diseases (CVDs). https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed March 20, 2020
- 62.Hauer KE, Carney PA, Chang A, Satterfield J. Behavior change counseling curricula for medical trainees: a systematic review. Acad Med. 2012; 87:956–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012; 125:882–7.e1 [DOI] [PubMed] [Google Scholar]
- 64.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. ; Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003; 289:2083–2093 [DOI] [PubMed] [Google Scholar]
- 66.Hewat C. Weight management in general practice: what do patients want? Med J Aust. 2006; 185:344. [DOI] [PubMed] [Google Scholar]
- 67.Rose SA, Poynter PS, Anderson JW, Noar SM, Conigliaro J. Physician weight loss advice and patient weight loss behavior change: a literature review and meta-analysis of survey data. Int J Obes (Lond). 2013; 37:118–128 [DOI] [PubMed] [Google Scholar]
- 68.Hivert MF, Arena R, Forman DE, Kris-Etherton PM, McBride PE, Pate RR, et al. ; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; the Behavior Change Committee, a joint committee of the Council on Lifestyle and Cardiometabolic Health and the Council on Epidemiology and Prevention; the Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; and the Council on Cardiovascular and Stroke Nursing. Medical training to achieve competency in lifestyle counseling: an essential foundation for prevention and treatment of cardiovascular diseases and other chronic medical conditions: a scientific statement from the American Heart Association. Circulation. 2016; 134:e308–e327 [DOI] [PubMed] [Google Scholar]
- 69.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014; 63:2960–2984 [DOI] [PubMed] [Google Scholar]
- 70.Devries S, Agatston A, Aggarwal M, Aspry KE, Esselstyn CB, Kris-Etherton P, et al. A deficiency of nutrition education and practice in cardiology. Am J Med. 2017; 130:1298–1305 [DOI] [PubMed] [Google Scholar]
- 71.Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, et al. The 2015 dietary guidelines advisory committee scientific report: development and major conclusions. Adv Nutr. 2016; 7:438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005; 142:37–46 [DOI] [PubMed] [Google Scholar]
- 73.Miller ER, III, Juraschek S, Pastor-Barriuso R, Bazzano LA, Appel LJ, Guallar E. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010; 106:517–527 [DOI] [PubMed] [Google Scholar]
- 74.Huang HY, Caballero B, Chang S, Alberg AJ, Semba RD, Schneyer CR, et al. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Ann Intern Med. 2006; 145:372–385 [DOI] [PubMed] [Google Scholar]
- 75.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019; 74:1376–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, et al. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and Brent Revisited) – a prospective population-based study. J Am Coll Cardiol. 2013; 61:1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003; 27:173–180 [DOI] [PubMed] [Google Scholar]
- 78.Unnikrishnan R, Gupta PK, Mohan V. Diabetes in south Asians: phenotype, clinical presentation, and natural history. Curr Diab Rep. 2018; 18:30. [DOI] [PubMed] [Google Scholar]
- 79.Misra A, Sattar N, Tandon N, Shrivastava U, Vikram NK, Khunti K, Hills AP. Clinical management of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018; 6:979–991 [DOI] [PubMed] [Google Scholar]
- 80.Orimoloye OA, Budoff MJ, Dardari ZA, Mirbolouk M, Uddin SMI, Berman DS, et al. Race/ethnicity and the prognostic implications of coronary artery calcium for all-cause and cardiovascular disease mortality: the Coronary Artery Calcium Consortium. J Am Heart Assoc. 2018; 7:e010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nanna MG, Navar AM, Zakroysky P, Xiang Q, Goldberg AC, Robinson J, et al. Association of patient perceptions of cardiovascular risk and beliefs on statin drugs with racial differences in statin use: insights from the patient and provider assessment of lipid management registry. JAMA Cardiol. 2018; 3:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Golden SH, Yajnik C, Phatak S, Hanson RL, Knowler WC. Racial/ethnic differences in the burden of type 2 diabetes over the life course: a focus on the USA and India. Diabetologia. 2019; 62:1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.CDC. Current cigarette smoking among adults in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed April 2, 2020
- 84.Schoenborn CA, Adams PF, Barnes PM, Vickerie JL, Schiller JS. Health behaviors of adults: United States, 1999-2001. Vital Heal Stat 10. 2004; 219:1–79 [PubMed] [Google Scholar]
- 85.Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016; 1:767–776 [DOI] [PubMed] [Google Scholar]
- 86.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC consortium. Eur Heart J. 2018; 39:3727–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000; 49:2208–2211 [DOI] [PubMed] [Google Scholar]


