Abstract
Retinitis pigmentosa (RP), the most common form of rod-cone dystrophy, is caused by greater than 3100 mutations in more than 71 genes, many of which are preferentially expressed in rod photoreceptors. Cone death generally follows rod loss regardless of the underlying pathogenic mutation. Preventing the secondary loss of cone photoreceptors would preserve central visual acuity and substantially improve patients’ quality of life. In this issue of the JCI, Wang et al. demonstrate that adeno-associated virus–mediated overexpression of TGF-β1 promoted cone survival and function in 3 distinct RP models with rod-specific mutations. TGF-β1 induces microglia to metabolically tune from a glycolytic phenotype (M1) to an oxidative phenotype (M2), which associates with neuroprotection and the antiinflammatory ecosystem. Consolidating the results of this study with our current understanding of how TGF-β1 regulates microglia polarization, we highlight cell-specific metabolome reprogramming as a promising non–gene-specific therapeutic avenue for inherited retinal degenerations.
Inherited retinal degenerations
Retinitis pigmentosa (RP) is a blinding disorder that can be caused by any one of over 3100 mutations in more than 71 genes (1). In RP, many of these genes exhibit rod-specific expression and, when mutated, initiate the same biphasic rod-cone progressive pathology — a wave of rod death that drives a second, partially overlapping, wave of cone death (2). Clinically, the gradual loss of rods corresponds to symptoms such as impaired night vision and peripheral vision loss. This is followed by the secondary loss of cones, which leads to the more devastating loss of day vision (2). Unexpectedly, patients with mutations in different RP genes display first-order decay, suggesting that the rate of vision loss is independent of the nature of the causative RP gene (3).
Limitations of current gene therapy strategy
The best hope for a cure lies in a genetic therapy that can either repair the mutation, supplement the affected gene, or both. Unfortunately, these precision medicine strategies require that the therapeutic components (such as guide RNA [gRNA] with repair template or cDNA supplement) be custom designed, engineered, tested, and FDA approved for each mutation or gene — a nearly impossible undertaking given the plethora of mutations known to contribute to RP (3). A more widely implementable therapeutic strategy would be to target the pathways underlying secondary cone loss, which is common to many of these genetically heterogeneous forms of RP.
Cell nonautonomous degeneration of cone photoreceptors
Mutations in genes encoding for subunits of the rod-specific enzyme cyclic guanosine monophosphate (cGMP) phosphodiesterase 6 (PDE6), are responsible for approximately 72,000 cases of RP worldwide each year (4). The Cepko laboratory has pioneered the use of Pde6 models to examine metabolic coupling and cell nonautonomous degeneration of cones in RP (5). The PDE6βRD1/RD1 mouse carries homozygous mutations in a gene encoding the β subunit of rod photoreceptor cGMP PDE6β. Rod PDE6 is a heterotetramer composed of 2 inhibitory subunits (PDE6γ) and 2 catalytic subunits (PDE6 and PDE6β) that act to hydrolyze cGMP for the closure of the cyclic nucleotide–gated (CNG) channels (4, 6). In Pde6 mutants, rod death has been attributed to toxicity from high levels of free cGMP directly or the secondary high Ca2+ influx through the CNG channels when free cGMP is elevated. Elucidation of pathways responsible for cell nonautonomous degeneration of cones has emerged as a major topic of interest in light of the potential for such pathways to serve as therapeutic targets.
Precision reprogramming in imprecision medicine
In this issue of the JCI, Wang et al. explore the role of microglia in secondary cone degeneration by using FDA-approved adeno-associated virus (AAV) serotype 8 to deliver TGF-β, a major antineuroinflammatory cytokine that inhibits microglial activation, to the cones of 3 preclinical RP models harboring rod-specific genetic defects (7). The authors achieved sustained, cone-specific overexpression of the 3 TGF-β isoforms (TGF-β1, TGF-β2, TGF-β3) with the AAV8–human red opsin (1–3). Notably, only TGF-β1, precisely, but not TGF-β2 or TGF-β3, coding AAV8 inhibited the secondary death of cones and nearly tripled the cone number in PDE6βRD1/RD1 mice and improved visual function in PDE6βRD10/RD10 mice at 2 months of age. The anatomical rescue effects were further corroborated in a third more slowly degenerating RP mouse model, Rho–/–. However, when the researchers depleted all M1 and M2 microglia in untreated PDE6βRD1/RD1 mice, cone survival failed to improve. Conversely, microglia depletion nullified AAV8–TGF-β1–mediated cone rescue, indicating the importance of precision reprogramming microglia in facilitating TGF-β1 therapeutic efficacy.
The neuroprotective contributions of microglia can be understood in terms of their metabolic tuning in many degenerative disorders (8). The resting metabolic state of M0 homeostatic microglia is oxidative phosphorylation. However, under stress, a shift from oxidative phosphorylation to aerobic glycolysis induces the M1 microglia phenotype through activation of the TAM (TYRO3, AXL, and MERTK) receptor tyrosine kinase signaling pathway (9). This metabolic switch is associated with the chronic release of neurotoxic inflammatory factors, which may underlie tissue damage and photoreceptor death. More recently, TGF-β1 has been found to precisely suppress glycolytic M1 programming while promoting oxidative M2 microglia polarization (10, 11). Interestingly, M2 polarization is known to promote neuroprotection and antiinflammatory response, which is abrogated with diminished TGF-β1. Altogether, these findings suggest that secondary cone loss is mediated by a pathological upregulation of glycolytic processes in M1 microglia, which can be countered by reestablishing M2 oxidative metabolism. Wang et al. demonstrate that AAV-mediated supplementation of TGF-β1 can counteract some aspects of neurodegenerative disorders in preclinical Pde6b and Rho models, thereby suggesting the therapeutic potential of precisely reprogramming glycolytic M1 to oxidative M2 microglia (7).
Future directions in precision reprogramming
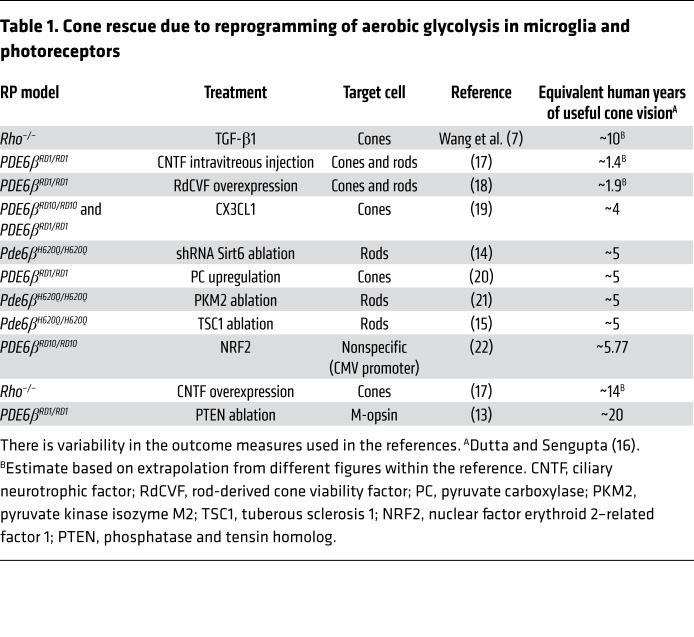
Cell type–specific metabolic reprogramming can rejuvenate aerobic glycolytic balance within the eye and rescue vision. Importantly, Wang and colleagues elegantly validate the therapeutic feasibility of using AAVs (7). AAVs have become the most widely used vector in FDA trials due to their low immunogenicity and cytotoxicity, and the ability of their serotype to be engineered for cell type–specific targeting (1). As such, they represent the ideal gene delivery vehicle to mediate metabolomic reprogramming in microglia as well as other retinal cells. As proposed by the Hurley hypothesis, different retinal cells are metabolically coupled (12), and thus how these dynamics are disrupted in dystrophic retinas may pose another critical direction for cone preservation (Table 1).
Table 1. Cone rescue due to reprogramming of aerobic glycolysis in microglia and photoreceptors.
Recently, the photoreceptors and retinal pigmented epithelial cells have been identified as other cell types that suffer from dysregulation of glycolytic and oxidative phase-shifting during RP progression. In healthy retinas, rods take up glucose from the retinal pigmented epithelium (RPE) and convert it to lactate via aerobic glycolysis, even in the presence of oxygen. Much like microglia, young RPE prefers an oxidative metabolic state and uses the generated lactate as a substrate for oxidative phosphorylation and a suppressor for RPE-specific glucose consumption. In contrast, in PDE6 dystrophies, the lactate production decreases as rods die, stagnating NAD+ consumption in the RPE. The resultant accumulation of NAD+ stimulates glycolysis in the RPE, which depletes all the available glucose from the choriocapillaris as an energy source. As the delicate mutualism between oxidative and glycolytic tissue processes fails, cones begin to die from malnutrition (5). These findings suggest that boosting aerobic glycolysis in dystrophic photoreceptors might counter photoreceptor death from RP-induced metabolic imbalance. Clinicians can apply the AAV vector delivery implemented by Wang et al. to drive diseased photoreceptors toward enhancing aerobic glycolysis (Table 1). Genetically ablating inhibitors of aerobic glycolysis, either Tsc1 (13) or Sirt6 (14), increase the number of photoreceptors in RP retinas (13–15). SIRT6 is a master promoter of aerobic glycolysis via the rate-limiting enzymes for glycolysis and GLUT1-mediated glucose transport. Future studies directed toward precision metabolome reprogramming may substantially advance the development of a gene agnostic therapy for neurodegenerative disorders.
Acknowledgments
We are grateful to James B. Hurley and Anthony T. Moore for insightful comments. Jonas Children’s Vision Care is supported by NIH grants 5P30CA013696, U01 EY030580, U54OD020351, R24 EY028758, R24EY027285, 5P30EY019007, R01EY018213, R01EY024698, and R01EY026682, New York State (SDHDOH01-C32590GG-3450000), the Foundation Fighting Blindness New York Regional Research Center Grant (PPA-1218-0751-COLU), Nancy and Kobi Karp, the Crowley Family Funds, the Rosenbaum Family Foundation, Alcon Research Institute, the Gebroe Family Foundation, the Research to Prevent Blindness (RPB) Physician-Scientist Award, and unrestricted funds from RPB.
Version 1. 07/13/2020
Electronic publication
Version 2. 08/03/2020
Print issue publication
Version 3. 03/16/2022
Updated author name
Footnotes
Conflict of interest: SHT has received financial benefits from Spark Therapeutics and research funding and support from Abeona Therapeutics Inc. SHT has a patent titled “Anabolic Enhancers for Ameliorating Neurodegeneration: WO2017201425” (2017).
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(8):3971–3973. https://doi.org/10.1172/JCI139239.
See the related article at Microglia modulation by TGF-β1 protects cones in mouse models of retinal degeneration.
Contributor Information
Salvatore Marco Caruso, Email: smc2304@columbia.edu.
Joseph Ryu, Email: jr4000@cumc.columbia.edu.
Peter M.J. Quinn, Email: pq2138@cumc.columbia.edu.
Stephen H. Tsang, Email: sht2@columbia.edu.
References
- 1.DiCarlo JE, Mahajan VB, Tsang SH. Gene therapy and genome surgery in the retina. J Clin Invest. 2018;128(6):2177–2188. doi: 10.1172/JCI120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12(1):44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright AF, et al. Lifespan and mitochondrial control of neurodegeneration. Nat Genet. 2004;36(11):1153–1158. doi: 10.1038/ng1448. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin ME, Ehrhart TL, Berson EL, Dryja TP. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA. 1995;92(8):3249–3253. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Cepko CL. HDAC4 regulates neuronal survival in normal and diseased retinas. Science. 2009;323(5911):256–259. doi: 10.1126/science.1166226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsang SH, et al. Retinal degeneration in mice lacking the gamma subunit of the rod cGMP phosphodiesterase. Science. 1996;272(5264):1026–1029. doi: 10.1126/science.272.5264.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SK, et al. Microglia modulation by TGF-β1 protects cones in mouse models of retinal degeneration. J Clin Invest. 2020;130(8):4360–4369. doi: 10.1172/JCI136160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci. 2018;19(10):622–635. doi: 10.1038/s41583-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173(4):649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mia S, Warnecke A, Zhang XM, Malmström V, Harris RA. An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-β yields a dominant immunosuppressive phenotype. Scand J Immunol. 2014;79(5):305–314. doi: 10.1111/sji.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294–52306. doi: 10.18632/oncotarget.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanow MA, et al. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife. 2017;6:e28899. doi: 10.7554/eLife.28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatesh A, Ma S, Le YZ, Hall MN, Rüegg MA, Punzo C. Activated mTORC1 promotes long-term cone survival in retinitis pigmentosa mice. J Clin Invest. 2015;125(4):1446–1458. doi: 10.1172/JCI79766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, et al. Reprogramming metabolism by targeting sirtuin 6 attenuates retinal degeneration. J Clin Invest. 2016;126(12):4659–4673. doi: 10.1172/JCI86905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, et al. Reprogramming towards anabolism impedes degeneration in a preclinical model of retinitis pigmentosa. Hum Mol Genet. 2016;25(19):4244–4255. doi: 10.1093/hmg/ddw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Lipinski DM, et al. CNTF gene therapy confers lifelong neuroprotection in a mouse model of human retinitis pigmentosa. Mol Ther. 2015;23(8):1308–1319. doi: 10.1038/mt.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne LC, et al. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J Clin Invest. 2015;125(1):105–116. doi: 10.1172/JCI65654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang SK, Xue Y, Rana P, Hong CM, Cepko CL. Soluble CX3CL1 gene therapy improves cone survival and function in mouse models of retinitis pigmentosa. Proc Natl Acad Sci USA. 2019;116(20):10140–10149. doi: 10.1073/pnas.1901787116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chinchore Y, Begaj T, Guillermeir C, Steinhauser ML, Punzo C, Cepko CL. Transduction of gluconeogenic enzymes prolongs cone photoreceptor survival function in models of retinitis pigmentosa [preprint]. Posted on bioRxiv March 6, 2019. [DOI]
- 21.Zhang E, et al. PKM2 ablation enhanced retinal function and survival in a preclinical model of retinitis pigmentosa. Mamm Genome. 2020;31(3–4):77–85. doi: 10.1007/s00335-020-09837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125(4):1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]