Abstract
Optic pathway gliomas (OPGs) occur in 15%–20% of children with neurofibromatosis type 1 (NF1), leading to visual deficits in fewer than half of these individuals. The goal of chemotherapy is to preserve vision, but vision loss in NF1-associated OPG can be unpredictable. Determining which child would benefit from chemotherapy and, equally important, which child is better observed without treatment can be difficult. Unfortunately, despite frequent imaging and ophthalmologic evaluations, some children experience progressive vision loss before treatment. Indications for chemotherapy usually are based on a comprehensive, quantitative assessment of vision, but reliable vision evaluation can be challenging in young children with NF1-OPG. The ability to identify and predict impending vision loss could potentially improve management decisions and visual outcomes. To address this challenge, ophthalmologic, electrophysiologic, and imaging biomarkers of vision in NF1-OPG have been proposed. We review current recommendations for the surveillance of children at risk for NF1-OPG, outline guidelines for initiating therapy, and describe the utility of proposed biomarkers for vision.
Optic pathway glioma (OPG) is the most common brain tumor in children with neurofibromatosis type 1 (NF1), a tumor predisposition syndrome caused by germ-line mutations in the NF1 gene (1). OPGs are low-grade glial neoplasms involving the visual pathway (optic nerve, chiasm, tracts, and radiations) and may also involve the hypothalamus. The tumor arises in approximately 15%–20% of children with NF1, and although children rarely die from NF1-OPGs, they often experience vision loss (2), endocrinopathies (3–5), impaired social interactions, and difficulties completing activities of daily living (6). Adults with NF1-OPG and vision loss are more likely to be unemployed and live with a caregiver compared with glioma survivors without vision loss (7).
Treatment for NF1-OPG is most commonly intended to reduce the visual morbidity from this disease. Chemotherapy is considered as standard first-line treatment, and radiation is used sparingly because of the risk of second malignancy as well as cognitive and neurovascular complications in this young patient population (8). Surgery is indicated only in unusual circumstances, such as disfiguring proptosis in a nearly blind eye or enlarging tumor with mass effect on critical structures (9). Unfortunately, even our best current therapies (carboplatin-based chemotherapy) do not reliably preserve vision (10,11). For example, a retrospective study of 115 previously untreated children with NF1-OPG reported improved visual acuity in 32% (at least a 2-line improvement in visual acuity testing), stable visual acuity in 40%, and worsened visual acuity in 28% (at least a 2-line deterioration in one or both eyes) after the completion of chemotherapy (12).
Many NF1-OPGs never cause symptoms, and not all patients with symptomatic NF1-OPG will require treatment (13,14). In a study of 57 children with NF1-OPG, only 32 (59%) had signs or symptoms attributable to their tumor at diagnosis (14). Twenty-six (50%) showed clinical progression over an average 8.6 years of follow-up, and 6 (11%) of those progressed after more than a year of stability. Individual tumors can show dramatic variability in their growth pattern. Numerous individual reports describe NF1-OPG in young children undergoing periods of rapid growth, prolonged quiescence, and rare spontaneous regression (15–21).
Because NF1-OPG clinical behavior and chemotherapeutic responses are difficult to predict, choosing who should receive chemotherapy and when therapy should be initiated are challenging management issues. Rigorous ophthalmology and imaging surveillance are essential to monitor for tumor progression and associated morbidity. Treatment indications should depend on current vision and the impact of potential visual decline. However, impaired cognition and attention deficits frequently seen in patients with NF1 (22) may complicate the assessment of vision and occasionally lead to unreliable or unreproducible ophthalmologic exams (23). Quantitative biomarkers of visual loss currently under development may assist in clinical decision-making by supporting visual acuity assessment and evaluating the risk of future deficits. We will review the state of prognostic factors and current recommendations for surveillance, propose indications for treatment, and describe recent studies on biomarkers of vision in NF1-OPG.
PROGNOSTIC FACTORS
Based on our current understanding of NF1-OPG, it is unclear which OPGs will cause symptoms sufficient to justify treatment. Whereas past studies focused on tumor growth, the importance of visual acuity outcomes has been highlighted recently (24,25). Numerous reports have attempted to define risk factors for symptomatic OPG. Tumors limited to the optic nerve have a less aggressive course than those involving the optic tracts and radiations (26–28), as well as possibly the chiasm (29). Recent studies suggest that female patients require treatment more frequently for tumors limited to the optic nerve than males, despite a relatively equal incidence of tumors in this region (30,31). Although young children with NF1-OPG may require treatment more frequently (32), studies have been unable to prove that young age increases the risk of visual loss (13,14). Although these characteristics inform the risk of aggressive behavior in OPG, the impact of each will require further investigation.
RECOMMENDATIONS FOR SURVEILLANCE
Without reliable clinical indicators to predict the development of symptomatic NF1-OPG, careful surveillance for early signs of vision loss is essential (Tables 1 and 2). Ideal intervals for surveillance have not been studied, and surveillance recommendations in children with NF1 are based on clinical experience and limited available evidence. In addition to annual assessments by an experienced NF clinician, children suspected or known to have NF1 should undergo yearly comprehensive ophthalmologic or neuro-ophthalmic evaluations including assessment of visual acuity, visual fields, color vision, pupillary testing, eye movements, and optic disc appearance. In children without a known OPG, vision screening should be performed annually until 8 years of age, then every other year until 18 years as vision loss is less common in older age groups (2). However, subjective symptoms of vision loss should prompt a comprehensive ophthalmologic exam at any age.
TABLE 1.
Surveillance recommendations for patients with NF1 with and without an OPG
| Evaluation | Frequency |
|---|---|
| Suspected or known NF1 without an OPG | |
| Ophthalmology* | Every year until 8 yr Then every other year until 18 yr |
| NF1-OPG confirmed by MRI Ophthalmology* | Every 3 mo for first yr Then every 6 mo for 2 yr and ≥8 yr of age Then annually until age 18 if stable |
| Neuroimaging† | Every 3 mo for first year Then every 6 mo for 2 yr Then annually for 3–5 yr Then less frequent imaging as per clinical judgment until 18 yr |
Ophthalmology (or neuro-ophthalmology) includes quantitative visual acuity, visual field assessment, color vision, and the evaluation of the optic disc. Visual acuity thought to be unreliable should be repeated within 2 weeks.
Neuroimaging includes T1-and T2-weighted MRI sequences of the brain with and without contrast. High-resolution imaging of the optic nerves and chiasm should be included.
NF1, neurofibromatosis type 1; OPG, optic pathway glioma.
TABLE 2.
Advantages and disadvantages of vision biomarkers in patients with NF1-OPG
| Biomarker | Advantages | Disadvantages |
|---|---|---|
| Visual evoked potential (VEP) | Able to identify OPG | Unable to differentiate between symptomatic and asymptomatic OPG or follow visual response to therapy |
| Optical coherence tomography (OCT) | Identifies concurrent vision loss in multiple studies | Unclear if OCT can reliably identify impending vision loss; requires sedation and specialized equipment for the youngest children; needs additional prospective validation |
| Diffusion tensor imaging (DTI) | Identifies concurrent vision loss; 2 small studies suggest that diffusion imaging may predict future visual loss | Technical considerations may limit DTI in the optic nerve and chiasm; needs additional prospective validation |
| Volumetric MRI | Anterior tumor size associated with risk of vision loss | Measures limited to the most anterior portion of visual pathway (optic nerve, chiasm, and proximal 10 mm of the optic tracts); needs additional prospective validation |
NF1, neurofibromatosis type 1; OPG, optic pathway glioma.
The most important assessment in the ophthalmologic evaluation is age-appropriate, best-corrected quantitative visual acuity (2). Methods to assess visual acuity must be quantitative, reliable, and available for a wide spectrum of age and cognitive ability (24,25). If visual acuity results are believed to be unreliable because of poor cooperation or deficits in attention and cognition, testing should be repeated within 2 weeks. Visual fields should be assessed either by confrontation or, preferably, perimetry.
When a symptomatic NF1-OPG is suspected, MRI of the brain with dedicated high-resolution sequences of the optic nerves and chiasm is the imaging technique of choice to evaluate the extent of OPG involvement and to monitor for radiographic progression or response. T1-weighted sequences with and without contrast and T2-weighted sequences are typically used. Because contrast enhancement may be heterogeneous and variable, T2-weighted sequences are often the most useful to define tumor involvement (33). However, defining tumor borders can be challenging because areas of T2 hyperintensity common in NF1 may abut the tumor, and diffuse tumors may have uncertain boundaries (Fig. 1).
FIG. 1.
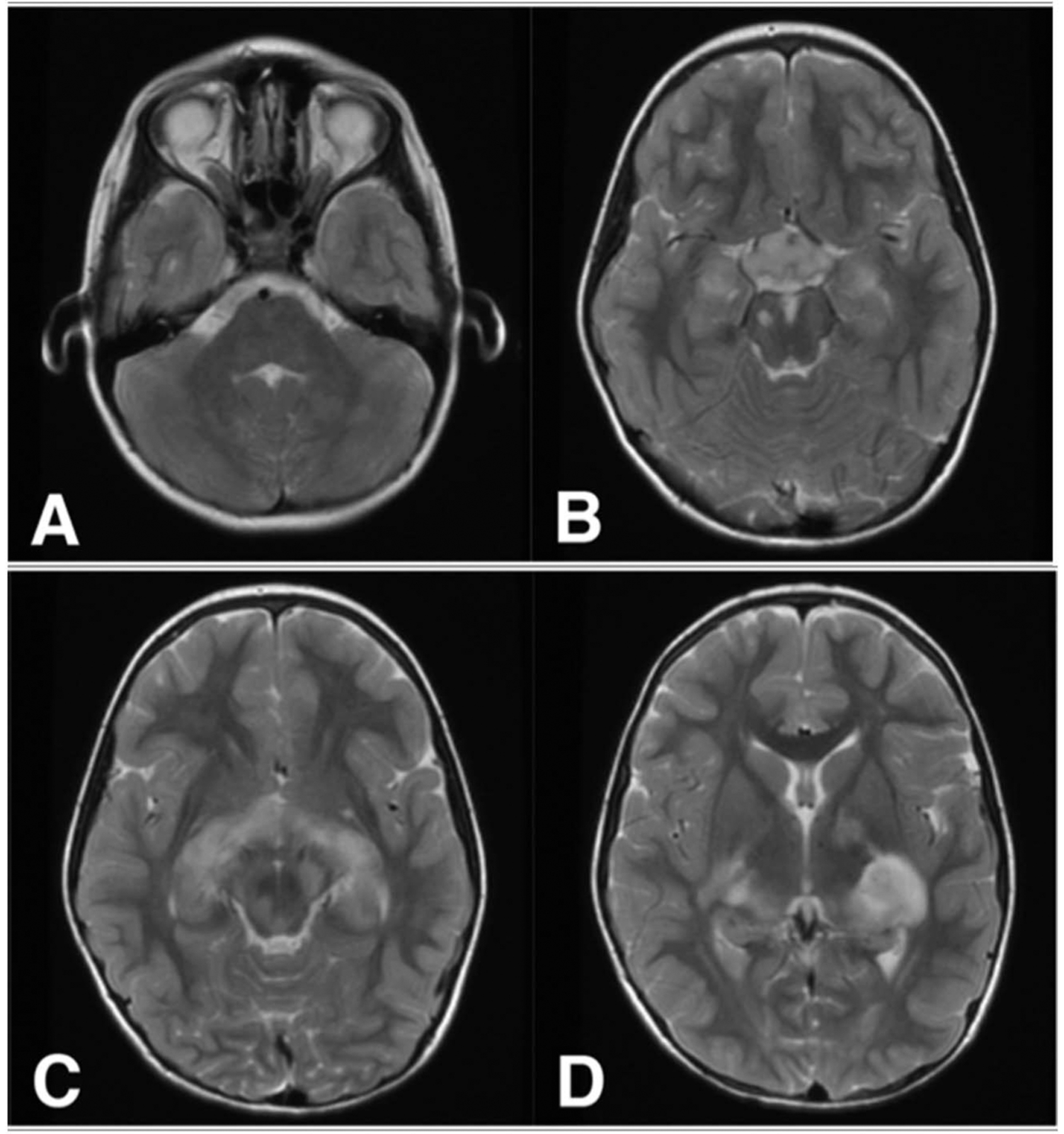
A 7-year-old girl with NF1-OPG involving the optic nerves (A), optic chiasm and hypothalamus (B), and optic tracts (C) shown on T2 sequences. Focal areas of T2 signal intensity are demonstrated in the midbrain and thalamus (B–D) and may abut tumor margins. NF1, neurofibromatosis type 1; OPG, optic pathway glioma.
Scant data exist regarding optimal intervals for imaging and ophthalmologic surveillance in children with newly diagnosed NF1-OPG. We recommend evaluations every 3 months in the first year (2), as most children treated for NF1-OPG initiated treatment within the first year (12). Once tumor size and vision are shown to be stable, the frequency of evaluations may be extended to every 6 months. Tumors that remain stable for more than 3 years are less likely to become symptomatic (12) and can be followed annually with MRI, but ophthalmology evaluations should continue every 6 months until at least 8 years of age before extending them to annually. NF1-OPGs that remain stable on ophthalmology evaluation and neuroimaging for ≥5 years may undergo imaging less frequently. Ophthalmology evaluations should continue annually until 18 years of age. Because vision in NF1-OPGs generally is stable after 18 years of age, surveillance of known NF1-OPG may be discontinued after that age if clinically stable.
INDICATIONS FOR TREATMENT
NF1-OPG rarely threatens life; therefore, the main goal of therapy is directed at reducing the risk of permanent, clinically significant, visual dysfunction. The decision to treat or observe without treatment may not be straightforward because it can be challenging or impossible to predict which tumors will cause further deficits and which of those will respond to therapy. The most comprehensive review of indications for treatment showed no clear consensus among 10 experienced NF centers, and the most frequent primary indications included visual acuity loss and radiographic tumor progression (12). More studies are needed to explore which indications for treatment are best able to prevent or mitigate vision loss in children with NF1-OPG. The following guidelines for chemotherapy are intended to reduce the functional impact of NF1-OPG, while taking into consideration the patient’s current condition, risk of further deficit, and potential for benefit (Fig. 2).
FIG. 2.
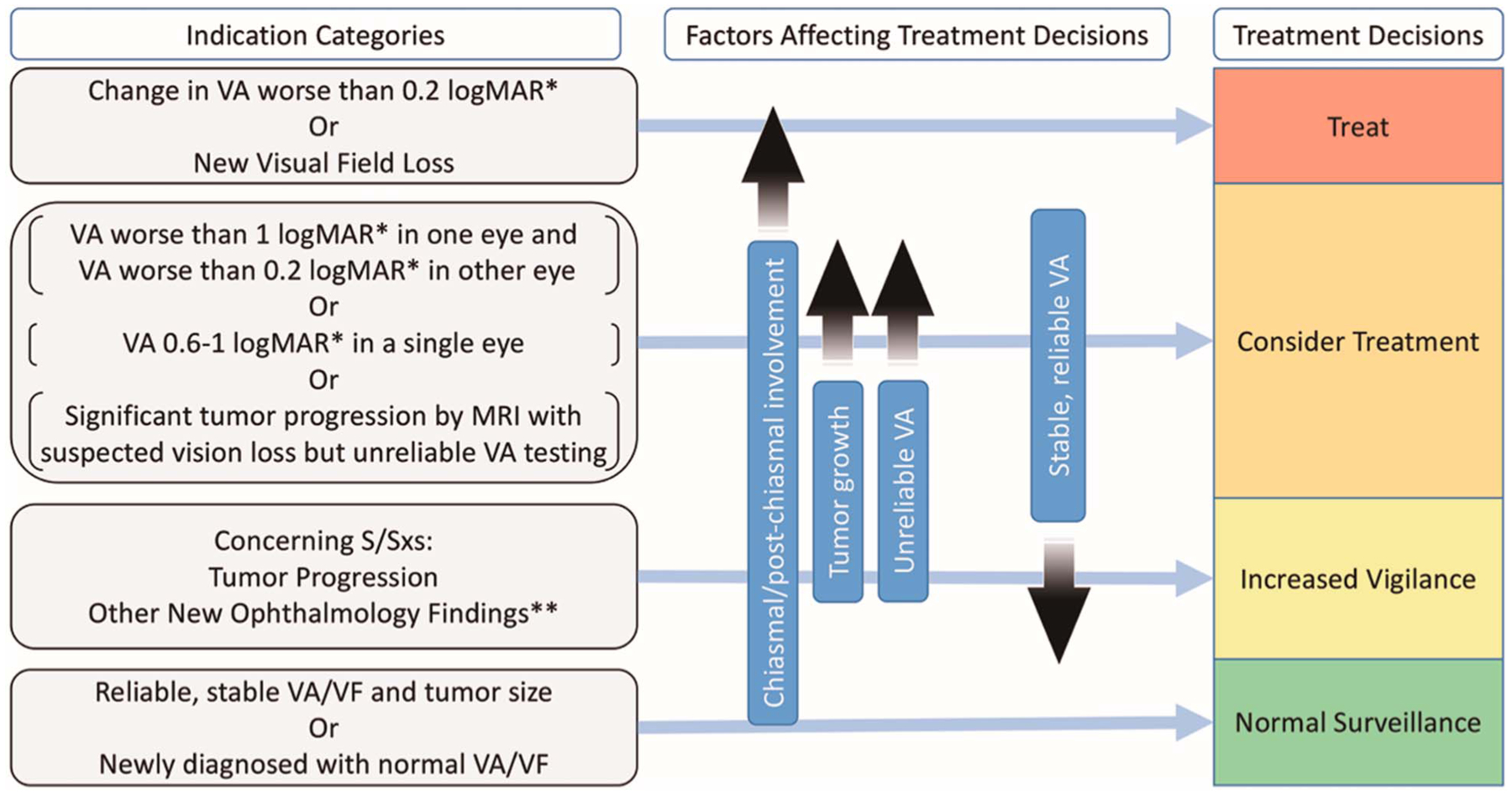
Indications for treatment and factors affecting decision-making in patients with NF1-OPG. logMAR, logarithm of the minimal angle of resolution; NF1, neurofibromatosis type 1; OPG, optic pathway glioma; S/Sx, signs and symptoms; VA, visual acuity; VF, visual fields; *VA compared with age-based norms ** including change in color vision, new afferent pupillary defect, strabismus, optic disc swelling/pallor.
Indications for Chemotherapy
The best indication for treatment is evidence of progressive, clinically significant vision loss. A difference of 0.2 logMAR or greater in visual acuity in a single eye from age-based normal values is considered abnormal, and a change in visual acuity of 0.2 logMAR or more is considered clinically significant (2,24,25). Because vision deficits due to NF1-OPG may go unnoticed and even precede a diagnosis of OPG, ophthalmologic abnormalities found at tumor presentation and thought to be chronic may not require therapy. Although precise measurement of visual field deficits can be challenging in young children with NF1-OPG, convincing evidence of new or progressive visual field loss (for instance, a new quad-rantanopia) also would constitute clinically significant vision loss and indicate treatment.
Other scenarios to consider treatment come from the combination of an increased risk of visual loss along with an increased impact of further visual deficit. Although complete or near complete vision loss in a single eye (worse than 1.0 logMAR relative to age norms [2]) is not a sufficient indication to initiate treatment by itself, it increases the importance and impact of preserving vision in the contralateral/well-seeing eye. Therefore, children who are blind or nearly blind in one eye and have either visual compromise (worse than 0.2 logMAR relative to age norms [2]) in the seeing eye or tumor progression by MRI may benefit from chemotherapy to preserve monocular vision. Similarly, when vision loss is suspected, but a reliable visual acuity is unobtainable, the impact of potential vision loss is increased because identifying modest vision loss early may be impossible. When reliable visual acuity is unobtainable, radiographic tumor progression or an accumulation of risk factors may support treatment for presumed vision loss. Children with visual acuity loss in a single eye near a functional threshold (0.6–1.0 logMAR relative to age norms [2]) may benefit from chemotherapy to preserve or improve visual acuity because of the potential impact of any visual acuity change (Fig. 2) (12).
Indications for Increased Vigilance
Although many signs and symptoms may raise concerns for tumor progression in a child with OPG, few are proven predictors of clinically significant vision loss. Although the accumulation of these risk factors in the appropriate clinical setting may support a consideration of instituting therapy, factors that may be associated with visual acuity loss or visual field deficit are reasons for increased vigilance, but are not indications for treatment.
Although the assessment of visual acuity and visual fields is fundamental to making treatment decisions for NF1-OPG, other ophthalmologic findings in isolation lack either reliability or clinical significance to be considered primary indications for treatment. Such findings include new onset color vision loss, disc pallor, optic nerve swelling, afferent pupillary defect, strabismus, or nystagmus. In the absence of new visual acuity changes or new field deficits, these findings are not an indication for treatment but should raise concern for more frequent evaluations.
Numerous previous studies have shown that radiographic tumor progression is neither necessary nor sufficient to cause vision loss (10,12,34–36). Tumor progression generally does not require treatment when visual acuity and visual fields remain stable. However, tumor growth warrants increased surveillance, may cause nonvisual morbidity if the tumor infiltrates other structures, and is particularly concerning when a reliable visual acuity is unobtainable.
MRI abnormalities, such as optic nerve tortuosity and nerve sheath thickening, are distinct from OPG but common in children with NF1. NF1 children with optic nerve tortuosity may be more likely to develop OPG, but are not more likely to require treatment than other children with NF1-OPG (37,38). Follow-up imaging may be helpful in distinguishing tumor from more benign findings (Fig. 2).
Factors That Should Not Influence Treatment Decisions
Precocious puberty or changes in growth hormone related to NF1-OPG are not indications for treatment with chemotherapy. Precocious puberty should be treated with gonadotropin-releasing hormone antagonists; therapy for growth hormone excess related to NF1-OPG is controversial (39,40). Similarly, chemotherapy may in some instances improve proptosis caused by NF1-OPG, but should not be started to manage the proptosis alone. In a nonseeing or low vision eye, therapy for proptosis should be limited to tumors causing corneal exposure or unacceptable cosmetic appearance. In these cases, a trial of chemotherapy may reduce proptosis and preserve any remaining vision before an attempt at surgical debulking (41).
BIOMARKERS OF VISION
Treatment for OPG is commonly based on progressive vision loss and the risk of future disability. However, ophthalmologic measures can be difficult in young patients and cannot predict impending vision loss or response to therapy. Imaging and quantitative measures of the visual pathway have attempted to address these challenges. Currently in development, biomarkers of vision assess the integrity of the visual pathway to provide objective and reliable measures of visual function (Table 2).
Visual Evoked Potentials
Visual evoked potentials (VEPs) measure cortical activity in response to a visual stimulus using occipital scalp electrodes similar to electroencephalography. VEP latency or decreased amplitude may indicate damage to the visual pathway. Studies conducted over the past 3 decades demonstrated that VEP has high sensitivity (90%–100%) in identifying OPGs but moderate specificity (60%–69%) (42–44). VEP requires patient cooperation and skilled staff members to successfully acquire and is not widely available in most centers. However, the most significant drawback of VEP is that, although it has been able to identify tumor, it has not been able to distinguish symptomatic from asymptomatic OPGs. In small retrospective and longitudinal studies, VEP corresponded with visual acuity only about 50% of the time (45,46). In addition, large between-visit variation in latency and amplitude, even in untreated patients, makes small changes in serial VEP difficult to interpret (36). In children treated for bilateral OPGs, there was no significant change in VEP amplitude before, during, or after therapy, and no relationship between VEP amplitude and response to treatment (47). VEP thus far has been unable to identify OPGs that require treatment or monitor the visual response to treatment. Because a clinically useful biomarker must be able to predict or identify visual acuity loss to affect treatment decisions, VEP has not yet fulfilled this role.
Optical Coherence Tomography
Optical coherence tomography (OCT) used to assess structural changes within the eye secondary to visual pathway injury has flourished as a biomarker of vision. OCT measures of the peripapillary retinal nerve fiber layer (pRNFL) thickness and the ganglion cell-inner plexiform layer complex have been associated with visual acuity and visual field defects in tumors compressing the chiasm (48,49), optic neuropathies (50), as well as multiple sclerosis and optic neuritis (51–54).
Over the past 6 years, OCT has gained considerable attention as a potential biomarker of vision in NF1-OPGs, and multiple investigations have helped demonstrate its reliability (55–58). In a cross-sectional study of 48 children and young adults with OPG, reduced pRNFL thickness (,80 mm) was associated with decreased vision (abnormal visual acuity and/or visual field defect) (55). The magnitude of visual acuity loss, as measured by low-contrast (i.e., Sloan) or high-contrast testing methods, also significantly correlated with the magnitude of pRNFL thickness. A pRNFL threshold greater than 80 mm was found in most subjects with normal visual acuity and visual fields, suggesting that a normal RNFL thickness may be reassuring in children with an OPG in whom a reliable visual acuity cannot be obtained. OCT may be most useful in young children who have difficulties with cooperation and attention. In these children, handheld OCT makes taking measurements practical during sedation with excellent reproducibility (59–61). Unfortunately, the logistics of acquiring studies during sedation for a clinically recommended MRI and the lack of native segmentation software makes it difficult for many centers to use handheld OCT.
Two longitudinal studies have investigated the usefulness of following OCT over time in children with OPG. In 23 patients with symptomatic OPG (14 with NF1-OPG) followed for 2 years, RNFL thinning was significantly greater in 5 children with either tumor growth (n = 2) or tumor growth with vision loss (n = 3) compared with those without radiographic or clinical progression (8.6 vs 2.4 mm thinning) (62). Although all children with vision loss were considered “progressive,” this OCT study was unable to separate the effect of vision loss from asymptomatic tumor progression using OCT. In another study of 46 children with OPG (31 with NF1-OPG) followed by OCT, 10 eyes from 7 patients experienced new vision loss. Using a 10% decline in pRNFL thickness confirmed by 2 consecutive visits as a threshold, OCT measures identified children with visual acuity or visual field loss with 70% sensitivity and 100% specificity (100% positive predictive value and 94% negative predictive value) (56).
Although the change in pRNFL reported in these 2 studies was able to identify concurrent vision loss, it is unclear whether early changes in pRNFL thickness will be able to identify impending vision loss or predict the risk of future vision loss. pRNFL decline seems to continue well after vision loss occurs, complicating the interpretation of longitudinal measures. A normal pRNFL thickness may reassure treating physicians that vision loss is unlikely to have occurred, supporting close surveillance, rather than immediate treatment. Despite recent advances in OCT technology to improve acquisition and reproducibility for pediatric studies (63), differences across protocols and device manufacturers should be considered when interpreting results (64). Multi-institutional longitudinal OCT studies in children with NF1 and newly diagnosed OPG may help define the utility of OCT in making informed decisions in children unable to reliably perform quantitative visual acuity testing.
Diffusion Tensor Imaging
Diffusion tensor imaging (DTI) is an MR technique that measures the diffusion of water molecules as they traverse hydrophobic white matter fibers. By following diffusion patterns in the brain, a map of white matter tracts can be created, where diffusion without a dominant direction (e.g., decreased fractional anisotropy, FA) may represent areas of white matter damage (Fig. 3).
FIG. 3.
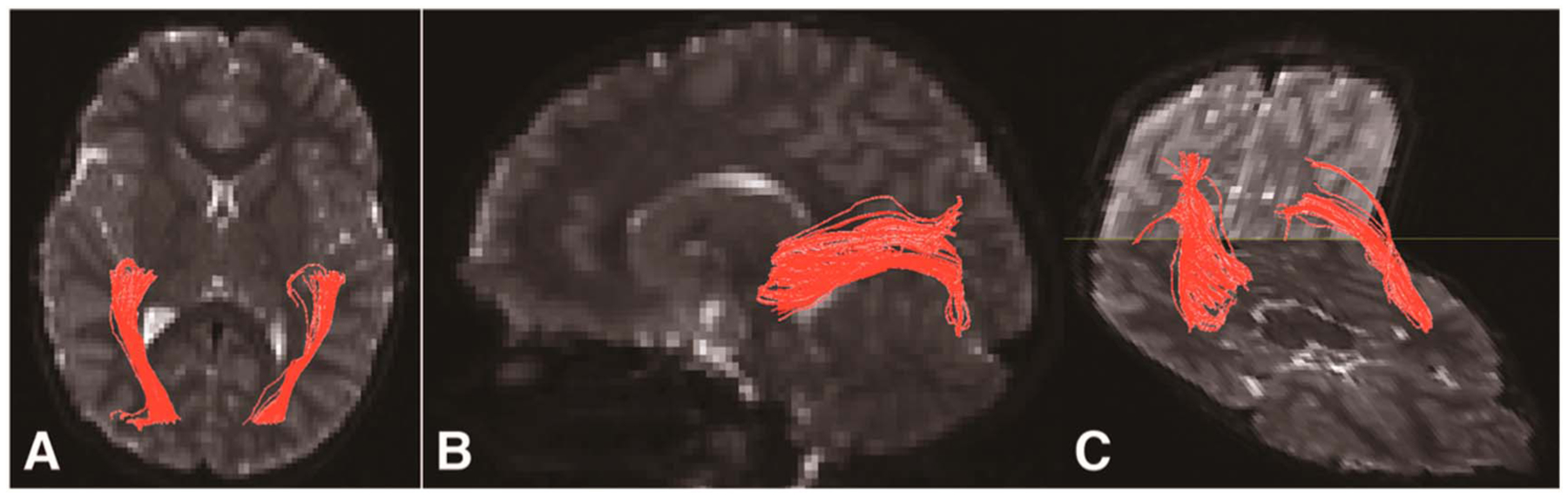
Diffusion tractography of the optic radiations (in red) superimposed on axial (A), sagittal (B), and 3-dimensional composite (C) b = 0 sec/mm2 images of a child with NF1-OPG. Decreased diffusion along these tracts may reflect white matter damage. NF1, neurofibromatosis type 1; OPG, optic pathway glioma.
In a study of 10 children with OPG (4 with NF1-OPG), visual fibers were attenuated or absent in all subjects. Although no statistical association was found between fiber tractography and visual acuity, measures of white matter damage were not investigated (65). To determine whether white matter integrity was associated with vision in OPG, DTI measures and visual acuity were compared in 50 children with NF1-OPG (66). Although no association was found in the optic nerve or optic tracts, FA of the optic radiations was significantly associated with visual acuity, even after excluding tumors located in the radiations, suggesting that damage to the radiations may reflect axonal degeneration from the anterior pathway. The association between FA and visual acuity was corroborated in 23 children with OPG followed prospectively with DTI where visual acuity was associated with FA of the optic nerves and optic radiations (67).
Increased diffusion may also be associated with the risk of future clinical progression in NF1-OPG. In a subset of 15 children from the previous study with longitudinal measures over a year, initial FA of the optic radiations predicted visual acuity change 1 year later (66). The association between increased diffusion and worse visual outcomes has been corroborated by diffusion-weighted imaging (DWI) studies of the chiasmatic OPG (68) although not all DWI studies found a significant effect (69). Although DTI offers the possibility of predicting future vision loss, these results need to be replicated in larger studies, and it is uncertain whether predicted losses can be avoided with treatment.
DTI is technically difficult in accessing the optic nerves (where neighboring fat, bone, and sinuses can cause susceptibility artifacts) and the optic chiasm (where white matter decussation can confound tractography). For this reason, DTI may be best suited to an evaluation of the posterior visual pathway and may be complementary to the evaluation of the optic nerve provided by OCT. However, the cost-effectiveness of adding DTI to MRI and recent advances in automation that eliminate reliance on the operator (70) are 2 advantages of this potentially convenient biomarker of visual acuity.
Volumetric MRI
Although traditional measures of OPG dimensions have not correlated with vision loss, volumetric magnetic resonance techniques may better define OPG size. Previous studies have relied on qualitative comparison of tumor size or traditional cross-sectional measures of tumor volume that often fail to account for the diffuse and amorphous shape of OPGs (71). A rigorous volumetric tumor measure may allow tumor volume to be a more robust indicator of vision loss.
Recently, a volumetric method has been developed that partitions the optic nerve, chiasm, and most proximal 1 cm of the optic tract using multisequence MRI to quantify anterior visual pathway (AVP) volume (Fig. 4). This method has been used to help define AVP enlargement to add objective criteria to the diagnosis of OPGs (72). In 38 children with NF1-OPGs, increased AVP and OPG volume using this method was associated with worse visual acuity (73), suggesting that the lack of correlation between tumor size and vision in previous studies may be due to the failure of 1- and 2-dimensional measurements to estimate OPG size. Increased chiasm volume was a sensitive indicator of pRNFL thinning, but volume measures predicted RNFL thickness only in tumors involving both the optic nerve and chiasm, but not in tumors sparing the chiasm. Unfortunately, structures posterior to the proximal optic tract are more difficult to measure because of decreased contrast with neighboring structures and focal areas of signal intensity common in NF1.
FIG. 4.
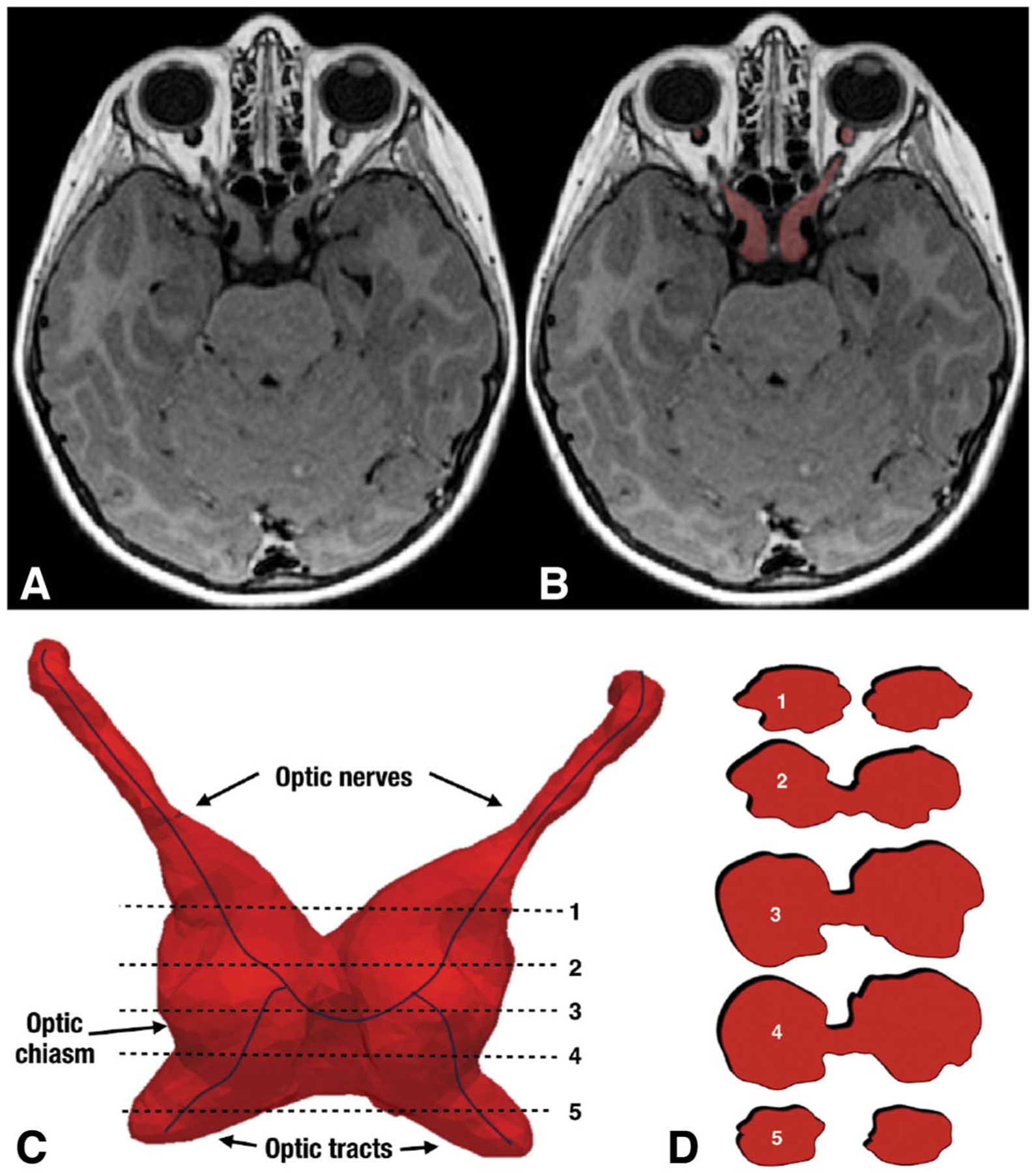
Axial T1 brain MRI (A) with segmentation in red (B) of a patient with bilateral NF1-OPG. Volumetric rendering of the NF1-OPG involving both sides of the anterior visual pathway (C) with 2D slices through the tumor (D). The irregular tumor shape is not amenable to traditional (i.e., 2D) measures. NF1, neurofibromatosis type 1; OPG, optic pathway glioma; 2D, 2 dimensional.
FUTURE DIRECTIONS FOR BIOMARKERS OF VISION
As the pace of development for novel therapeutics accelerates, effective OPG biomarkers of visual loss will be crucial. Clinicians must weigh the risks of conventional chemotherapy against the potential benefit of preventing further vision loss. Recently, orally available biologic agents have shown efficacy in difficult-to-treat NF1-associated plexiform neurofibromas (74). If similar therapies are found to be effective for patients with NF1-OPG, they may offer improved efficacy with fewer complications. Future biomarkers will need to identify a therapeutic window when vision loss is imminent, but preventable, to improve the visual outcome of therapy (75). As more therapeutic options emerge, biomarkers that identify best therapies or recognize therapeutic futility early in therapy may improve therapeutic efficacy.
The development of biomarkers for vision hopefully will lead to a better understanding of the pathophysiology of vision loss in children with OPG. Studies of OCT reveal a threshold of axonal damage past which vision loss is likely. DTI studies demonstrate that anterior damage may propagate to posterior structures as vision loss develops. Understanding the mechanism of vision loss in OPGs may help illuminate the optimal schedule for surveillance as well as when and how treatment can best prevent further deficits. To achieve these goals, potential biomarkers will need to demonstrate not only efficacy but also reliability, accessibility, and cost-effectiveness.
Acknowledgments
Supported in part by the St. Baldrick’s Scholar Award (P.M.K.d.B.) and the National Eye Institute, Bethesda, MD (K23-EY022673, R.A.A.).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. [DOI] [PubMed] [Google Scholar]
- 2.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cnossen MH, Stam EN, Cooiman LC, Simonsz HJ, Stroink H, Oranje AP, Halley DJ, de Goede-Bolder A, Niermeijer MF, de Muinck Keizer-Schrama SM. Endocrinologic disorders and optic pathway gliomas in children with neurofibromatosis type 1. Pediatrics. 1997;100:667–670. [DOI] [PubMed] [Google Scholar]
- 4.Taylor M, Couto-Silva AC, Adan L, Trivin C, Sainte-Rose C, Zerah M, Valteau-Couanet D, Doz F, Chalumeau M, Brauner R. Hypothalamic-pituitary lesions in pediatric patients: endocrine symptoms often precede neuro-ophthalmic presenting symptoms. J Pediatr. 2012;161:855–863. [DOI] [PubMed] [Google Scholar]
- 5.Gan HW, Phipps K, Aquilina K, Gaze MN, Hayward R, Spoudeas HA. Neuroendocrine morbidity after pediatric optic gliomas: a longitudinal analysis of 166 children over 30 years. J Clin Endocrinol Metab. 2015;100:3787–3799. [DOI] [PubMed] [Google Scholar]
- 6.Avery RA, Hardy KK. Vision specific quality of life in children with optic pathway gliomas. J Neurooncol. 2014;116:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Blank PM, Fisher MJ, Lu L, Leisenring WM, Ness KK, Sklar CA, Stovall M, Vukadinovich C, Robison LL, Armstrong GT, Krull KR. Impact of vision loss among survivors of childhood central nervous system astroglial tumors. Cancer. 2016;122:730–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas [review]. J Neuro-ophthalmol. 2011;31:269–278. [DOI] [PubMed] [Google Scholar]
- 10.Shofty B, Ben-Sira L, Freedman S, Yalon M, Dvir R, Weintraub M, Toledano H, Constantini S, Kesler A. Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer. 2011;57:481–485. [DOI] [PubMed] [Google Scholar]
- 11.Kalin-Hajdu E, Decarie JC, Marzouki M, Carret AS, Ospina LH. Visual acuity of children treated with chemotherapy for optic pathway gliomas. Pediatr Blood Cancer. 2014;61:223–227. [DOI] [PubMed] [Google Scholar]
- 12.Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ, Packer RJ, Tabori U, Hoffman RO, Ardern-Holmes SL, Hummel TR, Hargrave DR, Bouffet E, Charrow J, Bilaniuk LT, Balcer LJ, Liu GT. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet A. 2003;122A:95–99. [DOI] [PubMed] [Google Scholar]
- 14.Thiagalingam S, Flaherty M, Billson F, North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology. 2004;111:568–577. [DOI] [PubMed] [Google Scholar]
- 15.Parsa CF, Hoyt CS, Lesser RL, Weinstein JM, Strother CM, Muci-Mendoza R, Ramella M, Manor RS, Fletcher WA, Repka MX, Garrity JA, Ebner RN, Monteiro ML, McFadzean RM, Rubtsova IV, Hoyt WF. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001;119:516–529. [DOI] [PubMed] [Google Scholar]
- 16.Liu GT, Lessell S. Spontaneous visual improvement in chiasmal gliomas. Am J Ophthalmol. 1992;114:193–201. [DOI] [PubMed] [Google Scholar]
- 17.Perilongo G, Moras P, Carollo C, Battistella A, Clementi M, Laverda A, Murgia A. Spontaneous partial regression of low-grade glioma in children with neurofibromatosis-1: a real possibility. J Child Neurol. 1999;14:352–356. [DOI] [PubMed] [Google Scholar]
- 18.Piccirilli M, Lenzi J, Delfinis C, Trasimeni G, Salvati M, Raco A. Spontaneous regression of optic pathways gliomas in three patients with neurofibromatosis type I and critical review of the literature. Childs Nerv Syst. 2006;22:1332–1337. [DOI] [PubMed] [Google Scholar]
- 19.Zuccoli G, Ferrozzi F, Sigorini M, Virdis R, Bassi P, Bellomi M. Early spontaneous regression of a hypothalamic/chiasmatic mass in neurofibromatosis type 1: MR findings. Eur Radiol. 2000;10:1076–1078. [DOI] [PubMed] [Google Scholar]
- 20.Gottschalk S, Tavakolian R, Buske A, Tinschert S, Lehmann R. Spontaneous remission of chiasmatic/hypothalamic masses in neurofibromatosis type 1: report of two cases. Neuroradiology. 1999;41:199–201. [DOI] [PubMed] [Google Scholar]
- 21.Parazzini C, Triulzi F, Bianchini E, Agnetti V, Conti M, Zanolini C, Maninetti MM, Rossi LN, Scotti G. Spontaneous involution of optic pathway lesions in neurofibromatosis type 1: serial contrast MR evaluation. AJNR: Am J Neuroradiol. 1995;16:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 22.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. [DOI] [PubMed] [Google Scholar]
- 23.Avery RA, Bouffet E, Packer RJ, Reginald A. Feasibility and comparison of visual acuity testing methods in children with neurofibromatosis type 1 and/or optic pathway gliomas. Invest Ophthalmol Vis Sci. 2013;54:1034–1038. [DOI] [PubMed] [Google Scholar]
- 24.Avery RA, Ferner RE, Listernick R, Fisher MJ, Gutmann DH, Liu GT. Visual acuity in children with low grade gliomas of the visual pathway: implications for patient care and clinical research. J Neurooncol. 2012;110:1–7. [DOI] [PubMed] [Google Scholar]
- 25.Fisher MJ, Avery RA, Allen JC, Ardern-Holmes SL, Bilaniuk LT, Ferner RE, Gutmann DH, Listernick R, Martin S, Ullrich NJ, Liu GT; REiNS International Collaboration. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;1:S15–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28:262–270. [DOI] [PubMed] [Google Scholar]
- 27.Balcer LJ, Liu GT, Heller G, Bilaniuk L, Volpe NJ, Galetta SL, Molloy PT, Phillips PC, Vaughn S, Maguire MG. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131:442–445. [DOI] [PubMed] [Google Scholar]
- 28.Prada CE, Hufnagel RB, Hummel TR, Lovell AM, Hopkin RJ, Saal HM, Schorry EK. The use of magnetic resonance imaging screening for optic pathway gliomas in children with Neurofibromatosis Type 1. J Pediatr. 2015;167:851–856. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodgshun AJ, Elder JE, Hansford JR, Sullivan MJ. Long-term visual outcome after chemotherapy for optic pathway glioma in children: site and age are strongly predictive. Cancer. 2015;121:4190–4196. [DOI] [PubMed] [Google Scholar]
- 30.Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol. 2014;75:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ, Packer RJ, Tabori U, Hoffman RO, Ardern-Holmes SL, Hummel TR, Hargrave DR, Bouffet E, Charrow J, Bilaniuk LT, Balcer LJ, D’Agostino McGowan L, Liu GT. Gender as a disease modifier in neurofibromatosis type 1 optic pathway glioma. Ann Neurol. 2014;75:799–800. [DOI] [PubMed] [Google Scholar]
- 32.Opocher E, Kremer LC, Da Dalt L, van de Wetering MD, Viscardi E, Caron HN, Perilongo G. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42:1807–1816. [DOI] [PubMed] [Google Scholar]
- 33.Gaudino S, Quaglio F, Schiarelli C, Martucci M, Tartaglione T, Gualano MR, Di Lella GM, Colosimo C. Spontaneous modifications of contrast enhancement in childhood noncerebellar pilocytic astrocytomas. Neuroradiology. 2012;54:989–995. [DOI] [PubMed] [Google Scholar]
- 34.Grill J, Laithier V, Rodriguez D, Raquin M-A, Pierre-Kahn A, Kalifa C. When do children with optic pathway tumours need treatment? An oncological perspective in 106 patients treated in a single centre. Eur J Pediatr. 2000;159:692–696. [DOI] [PubMed] [Google Scholar]
- 35.Campagna M, Opocher E, Viscardi E, Calderone M, Severino SM, Cermakova I, Perilongo G. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer. 2010;55:1083–1088. [DOI] [PubMed] [Google Scholar]
- 36.Kelly JP, Weiss AH. Detection of tumor progression in optic pathway glioma with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin MH, Armstrong GT, Broad JH, Zimmerman R, Bilaniuk LT, Feygin T, Li Y, Liu GT, Fisher MJ. Risk of optic pathway glioma in children with neurofibromatosis type 1 and optic nerve tortuosity or nerve sheath thickening. Br J Ophthalmol. 2016;100:510–514. [DOI] [PubMed] [Google Scholar]
- 38.Ji J, Shimony J, Gao F, McKinstry RC, Gutmann DH. Optic nerve tortuosity in children with neurofibromatosis type 1. Pediatr Radiol. 2013;43:1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habiby R, Silverman B, Listernick R, Charrow J. Precocious puberty in children with neurofibromatosis type 1. J Pediatr. 1995;126:364–367. [DOI] [PubMed] [Google Scholar]
- 40.Josefson JL, Listernick R, Charrow J, Habiby RL. Growth hormone excess in children with optic pathway tumors is a transient phenomenon. Horm Res Paediatr. 2016;86:35–38. [DOI] [PubMed] [Google Scholar]
- 41.Althekair FA, Katowitz WR, Fisher MJ, Belasco JB, Avery RA, Katowitz JA, Liu GT. Debulking optic nerve gliomas for disfiguring proptosis: a globe-sparing approach by lateral orbitotomy alone. 2016: 42nd Annual Meeting North American Neuro-Ophthalmology Society; Tucson, Arizona. [Google Scholar]
- 42.North K, Cochineas C, Tang E, Fagan E. Optic gliomas in neurofibromatosis type 1: role of visual evoked potentials. Pediatr Neurol. 1994;10:117–123. [DOI] [PubMed] [Google Scholar]
- 43.Jabbari B, Maitland CG, Morris LM, Morales J, Gunderson CH. The value of visual evoked potential as a screening test in neurofibromatosis. Arch Neurol. 1985;42:1072–1074. [DOI] [PubMed] [Google Scholar]
- 44.Lund AM, Skovby F. Optic gliomas in children with neurofibromatosis type 1. Eur J Pediatr. 1991;150:835–838. [DOI] [PubMed] [Google Scholar]
- 45.Ng Y, North KN. Visual-evoked potentials in the assessment of optic gliomas. Pediatr Neurol. 2001;24:44–48. [DOI] [PubMed] [Google Scholar]
- 46.Falsini B, Ziccardi L, Lazzareschi I, Ruggiero A, Placentino L, Dickmann A, Liotti L, Piccardi M, Balestrazzi E, Colosimo C, Di Rocco C, Riccardi R. Longitudinal assessment of childhood optic gliomas: relationship between flicker visual evoked potentials and magnetic resonance imaging findings. J Neurooncol. 2008;88:87–96. [DOI] [PubMed] [Google Scholar]
- 47.Kelly JP, Leary S, Khanna P, Weiss AH. Longitudinal measures of visual function, tumor volume, and prediction of visual outcomes after treatment of optic pathway gliomas. Ophthalmology. 2012;119:1231–1237. [DOI] [PubMed] [Google Scholar]
- 48.Danesh-Meyer HV, Carroll SC, Foroozan R, Savino PJ, Fan J, Jiang Y, Vander Hoorn S. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci. 2006;47:4827–4835. [DOI] [PubMed] [Google Scholar]
- 49.Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci. 2008;49:1879–1885. [DOI] [PubMed] [Google Scholar]
- 50.Kardon RH. Role of the macular optical coherence tomography scan in neuro-ophthalmology. J Neuroophthalmol. 2011;31:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng H, Laron M, Schiffman JS, Tang RA, Frishman LJ. The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Invest Ophthalmol Vis Sci. 2007;48:5798–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler. 2008;14:893–905. [DOI] [PubMed] [Google Scholar]
- 53.Talman LS, Bisker ER, Sackel DJ, Long DA Jr, Galetta KM, Ratchford JN, Lile DJ, Farrell SK, Loguidice MJ, Remington G, Conger A, Frohman TC, Jacobs DA, Markowitz CE, Cutter GR, Ying GS, Dai Y, Maguire MG, Galetta SL, Frohman EM, Calabresi PA, Balcer LJ. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frohman E, Costello F, Zivadinov R, Stuve O, Conger A, Winslow H, Trip A, Frohman T, Balcer L. Optical coherence tomography in multiple sclerosis. Lancet Neurol. 2006;5:853–863. [DOI] [PubMed] [Google Scholar]
- 55.Avery RA, Liu GT, Fisher MJ, Quinn GE, Belasco JB, Phillips PC, Maguire MG, Balcer LJ. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151:542–549. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avery RA, Cnaan A, Schuman JS, Trimboli-Heidler C, Chen CL, Packer RJ, Ishikawa H. Longitudinal change of circumpapillary retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2015;160:944–952. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang L, El-Dairi MA, Frempong TA, Burner EL, Bhatti MT, Young TL, Leigh F. Optical coherence tomography in the evaluation of neurofibromatosis type-1 subjects with optic pathway gliomas. J AAPOS 2010;14:511–517. [DOI] [PubMed] [Google Scholar]
- 58.Gu S, Glaug N, Cnaan A, Packer RJ, Avery RA. Ganglion cell layer-inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Invest Ophthalmol Vis Sci. 2014;55:1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avery RA, Cnaan A, Schuman JS, Chen CL, Glaug NC, Packer RJ, Quinn GE, Ishikawa H. Intra- and inter-visit reproducibility of ganglion cell-inner plexiform layer measurements using handheld optical coherence tomography in children with optic pathway gliomas. Am J Ophthalmol. 2014;158:916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avery RA, Hwang EI, Ishikawa H, Acosta MT, Hutcheson KA, Santos D, Zand DJ, Kilburn LB, Rosenbaum KN, Rood BR, Schuman JS, Packer RJ. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol. 2014;132:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Avery RA, Cnaan A, Schuman JS, Chen CL, Glaug NC, Packer RJ, Quinn GE, Ishikawa H. Reproducibility of circumpapillary retinal nerve fiber layer measurements using handheld optical coherence tomography in sedated children. Am J Ophthalmol. 2014;158:780–787. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fard MA, Fakhree S, Eshraghi B. Correlation of optical coherence tomography parameters with clinical and radiological progression in patients with symptomatic optic pathway gliomas. Graefes Arch Clin Exp Ophthalmol. 2013;251:2429–2436. [DOI] [PubMed] [Google Scholar]
- 63.Rajjoub RD, Trimboli-Heidler C, Packer RJ, Avery RA. Reproducibility of retinal nerve fiber layer thickness measures using eye tracking in children with nonglaucomatous optic neuropathy. Am J Ophthalmol. 2015;159:71–77. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Avery RA, Rajjoub RD, Trimboli-Heidler C, Waldman AT. Applications of optical coherence tomography in pediatric clinical neuroscience. Neuropediatrics. 2015;46:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lober RM, Guzman R, Cheshier SH, Fredrick DR, Edwards MS, Yeom KW. Application of diffusion tensor tractography in pediatric optic pathway glioma. J Neurosurg Pediatr. 2012;10:273–280. [DOI] [PubMed] [Google Scholar]
- 66.de Blank PM, Berman JI, Liu GT, Roberts TP, Fisher MJ. Fractional anisotropy of the optic radiations is associated with visual acuity loss in optic pathway gliomas of neurofibromatosis type 1. Neuro Oncol. 2013;15:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hales PW, Smith V, O’Hare P, Mankad K, d’Arco F, Cooper J, Kaur R, Phipps K, Hargrave D, Clark CA. In vivo assessment of tumour invasion of the visual pathway in optic pathway glioma patients using multi-shell diffusion tensor MRI. Platform presentation at the 25th Annual Meeting and Exhibition of the International Society of Magnetic Resonance in Medicine; April 27, 2017; Honolulu, Hawaii. [Google Scholar]
- 68.Yeom KW, Lober RM, Andre JB, Fisher PG, Barnes PD, Edwards MS, Partap S. Prognostic role for diffusion-weighted imaging of pediatric optic pathway glioma. J Neurooncol. 2013;113:479–483. [DOI] [PubMed] [Google Scholar]
- 69.Jost SC, Ackerman JW, Garbow JR, Manwaring LP, Gutmann DH, McKinstry RC. Diffusion-weighted and dynamic contrast-enhanced imaging as markers of clinical behavior in children with optic pathway glioma. Pediatr Radiol. 2008;38:1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Blank P, Fisher MJ, Gittleman H, Barnholtz-Sloan JS, Badve C, Berman JI. Validation of an automated tractography method for the optic radiations as a biomarker of visual acuity in neurofibromatosis-associated optic pathway glioma. Exp Neurol. [published ahead of print June 3, 2017] doi: 10.1016/j.expneurol.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Warren KE, Poussaint TY, Vezina G, Hargrave D, Packer RJ, Goldman S, Wen PY, Pollack IF, Zurakowski D, Kun LE, Prados MD, Rutkowski S, Kieran MW. Challenges with defining response to antitumor agents in pediatric neuro-oncology: a report from the response assessment in pediatric neuro-oncology (RAPNO) working group. Pediatr Blood Cancer. 2013;60:1397–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avery RA, Mansoor A, Idrees R, Biggs E, Alsharid MA, Packer RJ, Linguraru MG. Quantitative MRI criteria for optic pathway enlargement in neurofibromatosis type 1. Neurology. 2016;86:2264–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Avery RA, Mansoor A, Idrees R, Trimboli-Heidler C, Ishikawa H, Packer RJ, Linguraru MG. Optic pathway glioma volume predicts retinal axon degeneration in neurofibromatosis type 1. Neurology. 2016;87:2403–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, Whitcomb P, Martin S, Aschbacher-Smith LE, Rizvi TA, Wu J, Ershler R, Wolters P, Therrien J, Glod J, Belasco JB, Schorry E, Brofferio A, Starosta AJ, Gillespie A, Doyle AL, Ratner N, Widemann BC. Activity of selumetinib in Neurofibromatosis Type 1-related plexiform neurofibromas. N Engl J Med. 2016;375:2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toonen JA, Ma Y, Gutmann DH. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction. Neuro Oncol. 2017;19:808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]


