Abstract
Membrane proteins are widely studied in detergent micelles, a membrane-mimetic system formed byamphiphilic compounds. However, classical detergents have serious limitations in their utility, particularly for unstable proteins such as eukaryotic membrane proteins and membrane protein complexes and thus there is an unmet need for novel amphiphiles with enhanced ability to stabilize membrane proteins. Here, we developed a new class of malonate-derived detergents with four glucosides, designated malonate-derived tetra-glucosides (MTGs) and compared these new detergents with previously reported octyl glucose neopentyl glycol (OGNG) and n-dodecyl-β-D-maltoside (DDM). When tested with two G-protein coupled receptors (GPCRs) and three transporters, a couple of MTGs consistently conferred enhanced stability to all tested proteins compared to DDM and OGNG. As a result of favourable behaviours for a range of membrane proteins, these MTGs have high potential for membrane protein research. This study additionally provides a new detergent design principle regarding the effect of a polar functional group (i.e., ether) on protein stability depending on its position in the detergent scaffold.
Keywords: detergent design, glucoside detergent, detergent micelles, protein structure, protein stabilization
Graphical Abstract
Membrane proteins reside in cell membranes, play essential roles in a variety of cellular activities and thus are major targets for a number of marketed pharmaceutical agents. Atomic resolution protein structure provides a fundamental basis for the understanding of a protein’s mechanism of action in the cellular context and facilitates rational drug design and development.1,2 Thus, tremendous efforts have focused on structure determination of membrane proteins using cutting edge biophysical techniques such as cryo-electron microscopy (cryo-EM), X-ray crystallography, and nuclear magnetic resonance (NMR) spectroscopy.3 However, there is a large difference in the number of membrane proteins of known structure and that of soluble proteins. Despite recent progress in membrane protein structural study, membrane proteins constitute only around 3% of unique protein structures in the protein data bank (PDB) although these bio-macromolecules account for ~30% of the open reading frames (ORFs) in the human genome.4–6 The amphiphilic property of membrane proteins is mainly responsible for the relatively limited progress in structural studies of these proteins. Due to this intrinsic property, these membrane proteins display poor water-solubility and a high propensity to aggregate/denature in aqueous environments. In order to mitigate the issues associated with membrane proteins, diverse membrane mimetic systems have been developed. The traditional yet still the most common system used for membrane protein manipulation are detergent micelles with a hydrophobic core and a hydrophilic exterior.7–10 Small amphiphilic molecules, named detergents or surfactants, interact with phospholipid bilayers and the hydrophobic/ hydrophilic domains of membrane proteins, and are thus capable of extracting and solubilizing membrane proteins from the membranes. Classical detergents, exemplified by dodecyl-β-D-maltoside (DDM) and octyl-β-D-glucoside (OG), have a canonical head-to-tail structure and are widely used for membrane protein structural study. However, many unstable proteins such as eukaryotic membrane proteins and oligomeric complexes, readily denature or aggregate over time even when solubilized in these popular detergents. Micelles formed by a classical detergent are much more dynamic compared to phospholipid bilayers and thus less effective at maintaining membrane protein structures.11,12 In addition, detergent-extracted membrane proteins can lose specific lipid molecules that are important for maintenance of protein function and stability.13,14 Therefore, novel amphiphilic compounds need to be developed for effective downstream analyses of membrane protein structure and function.
Over the past two decades, lipid-based self-assemblies such as bicelles,15 lipidic cubic phase (LCP),16 and nanodiscs (NDs)17 have emerged as useful membrane-mimetic systems. In addition, amphiphilic polymers with a polyacrylamide backbone (amphipols)18 or styrene-maleic acid (SMA) scaffold19 were invented as innovative approaches, along with amphiphilic peptides (e.g., lipopeptide detergents (LPDs),20 β-peptides (BPs),21 peptidisc22 and Salipro23). However, most of these molecules exhibit limited protein extraction properties. Furthermore, only a couple of systems such as bicelles and LCP have proved effective at generating protein crystals suitable for high resolution structure determination. Practicality is one key issue associated with the peptide-based amphiphiles, while the compositional heterogeneity (i.e., polydispersity) and/or sensitivity to solution pH are potential problems associated with polymeric amphiphiles.24 In contrast, small amphiphiles can be used for all the processes of membrane protein research, including protein extraction, purification and crystallization, and are compatible with cutting edge techniques currently used for protein structure determination. Thus, detergent micelles remain currently the optimal system for in vitro analysis of membrane proteins. However, there is substantial scope to improve the stabilization of a wide range of membrane proteins in detergent micelles. Our, and other groups have made substantial efforts to develop novel classes of detergents with enhanced protein stabilization efficacy. Representatives include glyco-tripod amphiphiles (TPAs),25,26 hemifluorinated surfactants (HFSs),27,28 cholate or deoxycholate-based facial amphiphiles (FAs),29,30 neopentyl glycol-based amphiphiles (GNGs/MNGs/NDTs),31–35 mannitol-based or mesitylene-linked glucoside amphiphiles (MNAs and MGAs),36,37 rigid core (norbornane/resorcinarene/terphenyl/1,3,5-triazine)-bearing amphiphiles (NBMs/RGAs/TPMs/TEMs),38–41 rigid hydrophobic group-bearing amphiphiles (e.g., Chobimalt,42 glyco-diosgenin (GDN),43 and penta-phenylene maltoside (PPM)44). Notably, some recent inventions such as pentasaccharide-bearing amphiphiles (PSEs),45 dendronic trimaltosides (DTMs),46 and vitamin E-based glucosides (VEGs)47 showed favourable behaviours for visualization of human β2-adrenergic receptor (β2AR) via EM analysis. Over the past decade, two NG class amphiphiles, MNG-3 and GNG-3 (commercial names, LMNG and OGNG,respectively) have contributed to the determination of more than 40 new membrane protein structures including the β2 adrenergic, acetylcholine and opioid G protein-coupled receptors (GPCRs).48–63 This successful repertoire underlines the contribution and importance of new micellar systems to membrane protein structural determination. The molecular design introduced in this study was inspired by the suitability of OGNG with membrane protein structural study. This glucoside detergent was generally inferior to DDM at stabilizing membrane proteins, yet has contributed to the high-resolution 3D structure determination of several membrane proteins via X-ray crystallography, as exemplified by a sodium-pumping pyrophosphatase, human aquaporin 2 (AQP2), and the N-methyl-D-aspartate (NMDA) receptor.64–66 Thus, we designed malonate-derived tetra-glucoside detergents (MTGs) using the GNG scaffold, with the intention of improving protein stabilization efficacy (Scheme 1). Evaluations of these detergents with model membrane protein systems revealed that a couple of MTGs conferred enhanced stability to all model membrane proteins tested here compared to a gold standard detergent (DDM) and the original GNG (OGNG).
Scheme 1.
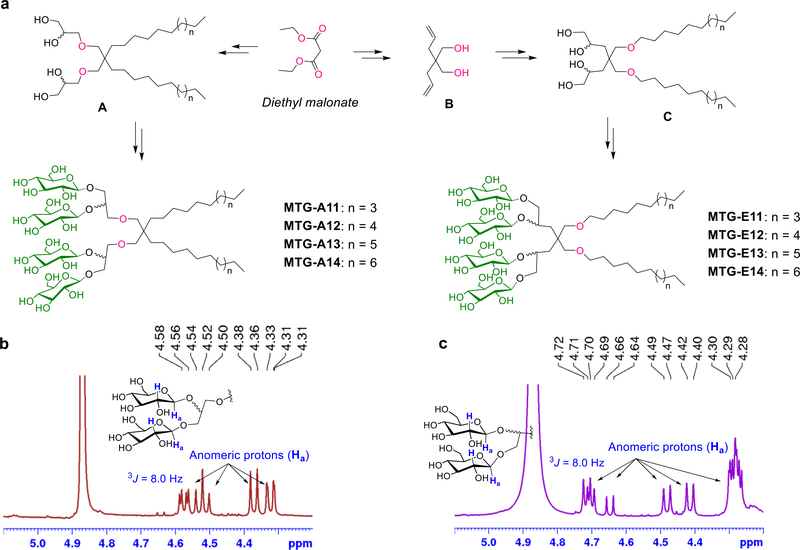
Synthetic scheme (a) and NMR characterization (b,c) of newly prepared malonate-derived tetra-glucoside detergents (MTG-As or MTG-Es). (a) Diethylmalonate was used as starting material for generation of the MTGs. (left) Dialkylated tetra-ols (compounds A) were prepared by a successive operation of dialkylation, reduction, allylation and syn-dihydroxylation. (right) For the preparation of the dialkylated tetra-ols (compounds C), diethylmalonate was first converted into diallylated compounds (B) which were then subjected to similar synthetic steps used for the preparation of compound A. The resulting tetra-ol derivatives containing two alkyl chains (compounds A and C) were stereo-selectively glycosylated using silver triflate (AgOTf) and benzoyl -protected glucosyl bromide as a promoter and glycosyl donor, respectively. Finally, the benzoyl protecting groups were removed by global deprotection to afford the two sets of MTGs. The stereochemistry of the newly formed glycosidic bonds was confirmed by the NMR spectrum of each MTG. As representatives, NMR spectra of (b) MTG-A12 and (c) MTG-E12 focusing on the anomeric region (4.20 ~ 5.10 ppm) are shown with the chemical structures of their head groups. The NMR peaks corresponding to the anomeric protons (Ha) of the MTGs (indicated by the arrows) had coupling constants (3J) of 8.0 Hz with the neighboring protons (H) in both cases, further supporting the β-stereochemistry of the glycosidic bonds.
Results
Detergent structures and physical characterizations
The newly designed amphiphiles have two alkyl chains, as seen in OGNG, but contain four instead of two glucosides in the hydrophilic region (scheme 1). Depending on how the alkyl chains are connected to the central quaternary carbon, the new amphiphiles can be categorized into two sets. One set has the alkyl chains directly attached to the central carbon (MTG-As), while the other set utilizes an ether bond for this connection (MTG-Es). In both sets, four glucoside units were introduced onto the alkyl chains using two 1,2-diol linkers. The diol linkers were connected to the central carbon via an ether linkage (MTG-As) or directly (MTG-Es). The only difference in the chemical structures between the MTG-As and MTG-Es is the relative location of the ether functional group and thus a comparative study of these two sets is ideal to investigate the effect of the relative location of a polar group (i.e., ether) on protein stability. Because of t he presence of four glucosides instead of two, the two alkyl chains of the MTGs could be lengthened compared to the previous GNGs, without compromising water-solubility. The alkyl chain varied from undecyl (C11) to tetradecyl (C14) for the new agents, significantly longer than the previously described GNGs which have alkyl chain ranging from pentyl (C5) to heptyl (C7). This alkyl chain extension is likely beneficial for protein stability because of the enhanced compatibility between the detergent alkyl chain length and the hydrophobic widths of membrane proteins (28~32 Å). In addition, the variation in the alkyl chain length is necessary to find the optimal balance between hydrophilicity and hydrophobicity (i.e., hydrophile-lipophile balance (HLB)), a critical detergent property for effective protein stabilization.67
Both sets of MTGS (MTG-As and MTG-Es) were prepared from diethylmalonate according to a straightforward synthetic protocol. For preparation of the MTG-As, diethylmalonate was converted into dialkylated tetra-ol derivatives (A) via four consecutive reactions: alkylation, reduction, O-allylation and OsO4-based dihydroxylation. In the case of the MTG-Es, 2,2-diallylpropane-1,3-diol (B) was first synthesized by diallylation of diethylmalonate and subsequent reduction of the ester groups with LiAlH4. Next, the alcohol and allyl groups of the compound (B) were connected to alkyl chains and transformed into 1,2-diol groups, respectively, to afford the tetra-ol derivatives (C). Interestingly, the dialkylated tetra-ol derivatives (A and C) were prepared in a single chromatographic separation by combining the initial synthetic steps together. In the following reaction, AgOTf-promoted glycosylation was carried out using the dialkylated tetra-ol compounds (A and C) and perbenzoylated glucosylbromide as glycosyl acceptors and donor, respectively (see Scheme S1†). Multi-gram quantity preparation of these detergent materials was feasible due to the high synthetic yield of each step.
Glycosylation reactions are likely to produce β-selective glycosidic bonds as the benzoyl-protected glucosylbromide was used as a glycosyl donor. β-stereochemistry of the glycosidic bonds was identified from the chemical shifts of anomeric peaks in the 1H NMR spectra of the individual MTGs (Scheme 1b, c & Figure S1†). The presence of multiple peaks in the range of 4.25–4.75 ppm is strong evidence for the formation of the β-selective glycosidic bonds; an α-anomeric proton typically produces an NMR peak around 5.15 ppm. The anomeric peaks observed here showed a rather complex pattern because two kinds of alcohols (primary and secondary) were used to attach the glucose units. The presence of two epimeric carbons resulting from the OsO4-catalyzed dihydroxylation additionally contributes to the complexity of the anomeric peaks. The β-stereochemistry of the glycosidic bonds was further supported by a coupling constant (3J) between the anomeric (C1; Ha) and neighbouring C2 protons (H), estimated to be 8.0 Hz in all the cases. This J value significantly differs from that of an α-anomer (3J = 4.0 Hz).
High water-solubility is a prerequisite for detergent use in biophysical studies of membrane proteins. All MTGs were highly soluble in water (>10 % w/v), indicative of effective micelle formation. We observed no noticeable precipitation in the individual detergent solutions over a one-month incubation at room temperature. Aggregation behaviours of the MTGs were investigated by measuring critical micelle concentrations (CMCs) and hydrodynamic diameters (Dh) of their micelles in aqueous solution. Critical micelle concentrations (CMCs) were estimated by monitoring inclusion of a water-insoluble fluorescent dye68 into detergent micelles, while the hydrodynamic diameters (Dh) of detergent micelles were determined through dynamic light scattering (DLS) measurements. The summarized data for the MTGs along with OGNG, LMNG and DDM are presented in Table 1. All malonate-derived detergents introduced here gave low CMCs ranging from ~12 to ~2 μM, much lower than those of OGNG (~1000 μM) and DDM (170 μM). This result indicates the increased propensity of these agents to self-assemble, which is directly related to micellar stability. Detergent CMCs of the two isomeric MTGs (e.g., MTG-E11 vs. MTG-A11) were comparable. Detergent CMC values were inversely proportional to the alkyl chain length for both sets of MTGs, as expected from hydrophobicity of the detergent alkyl chains. For instance, the CMCs of the MTG-As were reduced from 12 to 2 μM with an alkyl chain length increase from C11 to C14. Micelles formed by the MTGs varied from 6.1 to 7.7 nm in size with the short alkyl-chained MTGs (MTG-A11, MTG-E11 and MTG-E12) forming smaller micelles than DDM (6.0–6.4 vs 6.8 nm), while the long alkyl-chained MTGs (e.g., MTG-A13/A14 or MTG-E14) formed larger micelles than DDM (7.2–7.7 vs 6.8 nm). All MTGs formed smaller micelles than LMNG. For both sets of MTGs the detergent micelle sizes increase with increasing alkyl chain length. Further analysis of the DLS data reveals that all MTGs form highly homogeneous micelles (Figure S2†). When detergent micelle size was investigated over a range of temperatures (15 to 65 °C), little change in micelle size was observed for the tested MTGs or DDM (Figure S3†), indicating the micelles formed by these detergents are thermally stable.
Table 1.
Molecular weights (MWs), critical micelle concentrations (CMCs) of MTGs, OGNG, LMNG and DDM, and hydrodynamic diameter (Dh) (mean±S.D., n = 5) and water-solubility of their micelles.
| Detergent | MWa | CMC (μM) | CMC (wt%) | Dh (nm)b | Solubility (wt%) |
|---|---|---|---|---|---|
| MTG-A11 | 1181.4 | ~ 12 | ~ 0.0014 | 6.4±0.4 | ~ 10 |
| MTG-A12 | 1209.5 | ~ 9 | ~ 0.0011 | 6.8±0.5 | ~ 10 |
| MTG-A13 | 1237.5 | ~ 3.5 | ~ 0.0004 | 7.4±0.4 | ~ 10 |
| MTG-A14 | 1265.6 | ~ 2 | ~ 0.0003 | 7.7±0.2 | ~ 10 |
| MTG-E11 | 1181.4 | ~ 10 | ~ 0.0012 | 6.2±0.2 | ~ 10 |
| MTG-E12 | 1209.5 | ~ 8 | ~ 0.0010 | 6.4±0.4 | ~ 10 |
| MTG-E13 | 1237.5 | ~ 3.5 | ~ 0.0004 | 6.7±0.2 | ~ 10 |
| MTG-E14 | 1265.6 | ~ 2 | ~ 0.0003 | 7.2±0.1 | ~ 10 |
| OGNG | 568.7 | ~ 1000 | ~ 0.057 | 6.1 ±0.0 | ~ 10 |
| LMNGc | 1005.2 | ~ 10 | ~ 0.0010 | 14.4±0.2 | ~ 10 |
| DDM | 510.6 | ~ 170 | ~ 0.0087 | 6.8±0.0 | ~ 10 |
Molecular weight of detergents.
Micelle size determined at 1.0 wt% detergent concentration at room temperature.
Data obtained from ref. 33.
Detergent evaluation for membrane protein stability
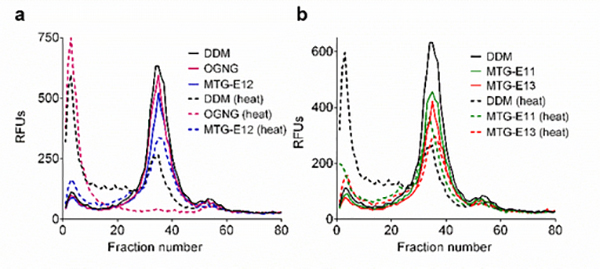
In order to investigate detergent utility as a biochemical tool for membrane protein study, the Arabidopsis thaliana boron transporter 1 (AtBOR1) was first tested with the MTGs.69 AtBOR1 was expressed in Saccharomyces cerevisiae and DDM was used for purification of the transporter. Detergents in DDM-purified AtBOR1 underwent detergent exchange into the individual MTGs or OGNG via sample dilution. The folded state of the transporter was monitored using a thiol-specific fluorescent dye, N-4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl]maleimide (CPM), during a 120-min incubation at 40 °C.70,71 At detergent concentrations of CMC+0.04 wt%, the thermal denaturation profiles showed little difference between the MTG-As and DDM (or OGNG) (Figure S4a†). When detergent concentrations were increased to CMC+0.2 wt%, we found improved detergent efficacy for the alkyl versions of the MTGs; the MTG -As were more effective than DDM and OGNG at maintaining AtBOR1 in a stable state (Figure S4b†). In the case of the ether versions (MTG-Es), the best efficacy was observed for MTG-E14 at CMC+0.04 wt%, while the other MTG-Es (MTG-E11, MTG-E12 and MTG-E13) were only a little better than DDM and OGNG (Figure S5a†). At increased detergent concentrations of CMC+0.2 wt%, MTG-E11 and MTG-E14 were most effective at stabilizing the transporter under the conditions tested (Figure S5b†). As the MTG-Es were generally better than the MTG-As in the CPM assay, we selected these ether versions (MTG-E11/E12/E13/E14) for further analysis with AtBOR1. In order to monitor protein integrity via fluorescence size exclusion chromatography (FSEC), AtBOR1-GFP fusion protein produced in S. cerevisiae membranes was treated with the individual MTG-Es/DDM/OGNG at 1.0 wt%. The stability of the detergent-extracted AtBOR1-GFP was estimated by thermally treating the samples at 46 °C for 10 min. Although most effective in the CPM assay, MTG-E14 failed to extract sufficient amounts of the transporter from the membranes (Table S1; ~10%), and thus was not included in the heated FSEC study. The other MTG-Es all extracted sufficient amounts of the fusion protein, although their solubilisation efficiencies were inferior to DDM (40~60% vs ~85%) (Table S1). When the detergent-extracted AtBOR1-GFP was subjected to FSEC before and after heat treatment, the DDM-solubilized transporter showed a large decrease in the main protein peak (fraction number 35), along with a substantial increase in the aggregation peak (fraction number 2) (Figure 1). The use of OGNG resulted in a complete disappearance of the main peak and appearance of a very large aggregation peak under the same conditions. This result indicates that AtBOR1-GFP in OGNG micelles fully aggregated/denatured over the course of thermal treatment. Following heat treatment, all the tested MTG-Es (MTG-E11, MTG-E12 and MTG-E13) also showed a decrease in the intact protein peak, but the reduction was substantially smaller, particularly in the case of MTG-E11, than that observed for DDM (Figure 1a,b). The combined results of CPM and FSEC assays indicate that the MTGs were moreeffective than DDM and OGNG at stabilizing AtBOR1, with the ether versions superior to the alkyl counterparts.
Figure 1.
Fluorescence size exclusion chromatography (FSEC) profiles of AtBOR1 fused with GFP (AtBOR1-GFP) before and after thermal treatment. Individual MTGs (MTG-E11, MTG-E12 and MTG-E13), DDM and OGNG were used at 1.0 wt% for extraction of the fusion protein. Detergent-extracted AtBOR1-GFP was treated at 46 °C for 10 min before the samples wereloaded onto the SEC column. The data shown is representative of two independent experiments.
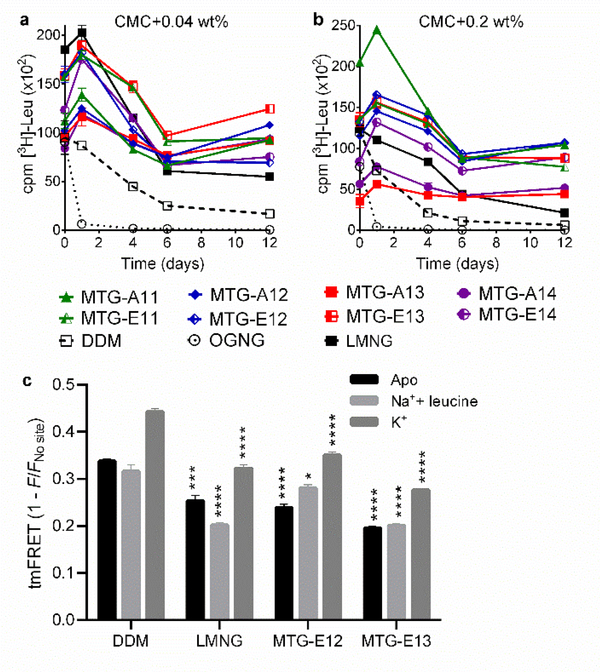
Next, we assessed the MTGs with a bacterial leucine transporter (LeuT) from Aquifex aeolicus.72,73 Protein stability was assessed by measuring substrate binding capability and monitored using a radio-active substrate ([3H]-leucine (Leu)) via scintillation proximity assay (SPA).74 LeuT was first extracted and purified in DDM, which was subjected to detergent exchange from DDM into the individual MTGs, OGNG, LMNG. Using the individual detergents at CMC+0.04 wt%, detergent effect on LeuT stability was monitored over a 12-day incubation period at room temperature. OGNG completely abolished [3H]-Leu binding ability of the transporter within one day, while DDM showed a gradual decrease in Leu binding activity (Figure 2a). All MTGs were superior to DDM at stabilizing the bacterial transporter long-term, with the best performance observed for MTG-E13. At the higher detergent concentrations of CMC+0.2 wt%, LeuT in some MTGs (MTG-A13/A14/E14) had initial activity lower than the transporter in DDM (t = 0 day), but protein activity recovered within a day of incubation. More importantly, the restored substrate binding ability was better retained over time when LeuT was solubilized in the MTGs rather than DDM (Figure 2b). The initial increase in transporter activity may be due to a slow detergent exchange from DDM to the MTG. In comparison with LMNG, a significantly optimized detergent, all MTGs except two long alkyl chained MTG (MTG-A13 and MTG-A14) were better than this novel detergent at stabilizing the transporter. Collectively, the results reveal that most MTGs are superior to DDM/OGNG/LMNG at retaining LeuT stability over time. To assess whether the enhanced protein stability observed for the MTGs is achieved at the expense of protein intrinsic conformational dynamics, we used transition metal ion Förster resonance energy transfer (tmFRET) as a sensitive tool to probe for intramolecular changes in LeuT (Figure 2c).75 We inserted a cysteine (K398C) on the extracellular region of transmembrane helix (TM) 10 on LeuT, and conjugated fluorescein-5-maleimide to this cysteine. A transition metal chelating site (His-X3-His) was introduced on the extracellular loop (EL) 4 (A313H-A317H). We used Ni2+ as the tmFRET acceptor, giving rise to a Förster distance of 12 Å, compatible with assessment of intramolecular distances of two protein domains. Fluorescence emitted by fluorescein is distance-dependently transferred to the chelated Ni2+. Consequently, the tmFRET intensity is dependent on the LeuT conformation induced by the added ligand and the stabilizing detergent. The ligand-induced conformational changes in DDM were in accordance with previous observations.41 When the apo-form of LeuT was incubated in MTG-E12 or MTG-E13 we observed an overall decrease in tmFRET efficiency, suggesting that these MTGs stabilize a more outward-open conformation than DDM, as also observed with LMNG. The preferences of the MTGs and LMNG toward the more outward-open conformation is likely due to the strong binding of detergent molecules to the surface of the transporter. The response to binding of Na+ and leucine was also different from one detergent to another: in DDM this results in a slightly more open outward LeuT compared to the apo-form, while a more inward-open conformation was observed for the transporter in MTG-E12. No apparent effect was observed for protein in MTG-E13. The conformational effect of K+ binding is similar for all detergents tested, inducing a more outward-occluded structure relative to the apo-form. Taken together, the results show that the MTGs stabilize LeuT probably by inducing a more outward-open conformation; however, it is still responsive to the ligands.
Figure 2.
Detergent effects on LeuT stability (a,b) and conformational dynamics (c). LeuT stability was measured using MTGs, DDM, OGNG, and LMNG at two different detergent concentrations (CMC+0.04 wt% (a) or CMC+0.2 wt% (b)). The radio-active substrate ([3H]-leucine (Leu)) was utilized for LeuT stability measurements. Substrate binding ability of the transporter was monitored at regular intervals during the 12-day incubation at room temperature. Error bars: SEM, n = 2–3. (c) Ensemble measurement of ligand-induced changes in intra-domain distances within LeuT in the indicated detergents (DDM, LMNG, MTG-E12 and MTG-E13). tmFRET is performed with detergent concentration of CMC+0.04 wt% in either LeuT unbound (apo) form (black), Na+/leucine-bound (light grey), or K+-bound form (dark grey). The tmFRET efficiency (1-F/Fno site) was measured between donor probe (fluorescein-maleimide) conjugated to an inserted cysteine on TM10 (K398C) and acceptor probe (Ni2+) chelated by an inserted His-X3-His site on EL4 (A313H-A317H) in LeuT. The changes in tmFRET efficiency indicate a change in distance between the two domains of LeuT housing the donor and acceptor probes, respectively. Error bars: SEM, n = 3. *P ≤0.05; **P ≤0.01; ***P ≤0.001; ****P ≤0.0001 relative to tmFRET values for the same condition in DDM.
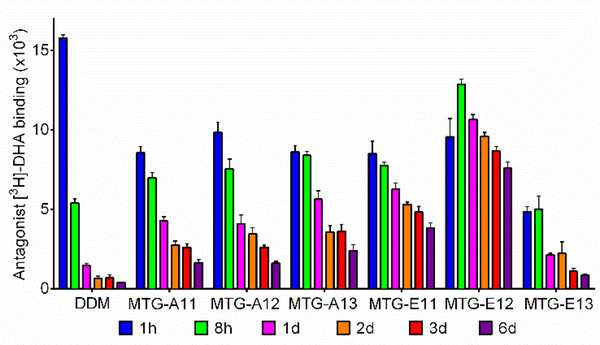
We turned to the human β2 adrenergic receptor (β2AR), a GPCR, to further evaluate the MTGs.76 The receptor purified in DDM was subjected to detergent exchange conducted by a 30-min sample dilution and the final detergent concentrations were CMC+0.2 wt%. A ligand binding assay using a radio-active antagonist ([3H]-dihydroalprenolol (DHA)) was carried out to assess receptor stability.77,78 In a preliminary study where receptor capability of ligand binding was measured right after detergent exchange, OGNG-solubilized receptor showed no ligand binding capability (Figure S6). Intriguingly, the use of the MTGs led to [3H]-DHA-binding capability of the receptor comparable to DDM in all the cases except the two longest alkyl chained detergents (MTG-A14 and MTG-E14). Thus, the six MTGs (MTG-A11/A12/A13 and MTG-E11/E12/E13) were selected to further investigate their effects on receptor stability over time. Receptor activity was monitored at the designated time point over the course of a 6-day incubation at 25 °C (Figure 3). When solubilized in DDM, receptor activity rapidly dropped to give ~ 10% of the initial activity in two days. In contrast, all the selected detergents except MTG-E13 were significantly more effective than DDM at retaining β2AR activity long term, with the best performance detected with MTG-E12.
Figure 3.
Time course ligand binding ability of β2AR solubilized in MTGs, DDM, and OGNG. The radio-active antagonist ([3H]-dihydroalprenolol (DHA)) was utilized for β2AR stability measurements at the detergent concentration of CMC+0.2 wt%. The ligand binding ability of the receptor was monitored at regular intervals during the 6-day incubation at room temperature. Error bars: SEM, n = 3.
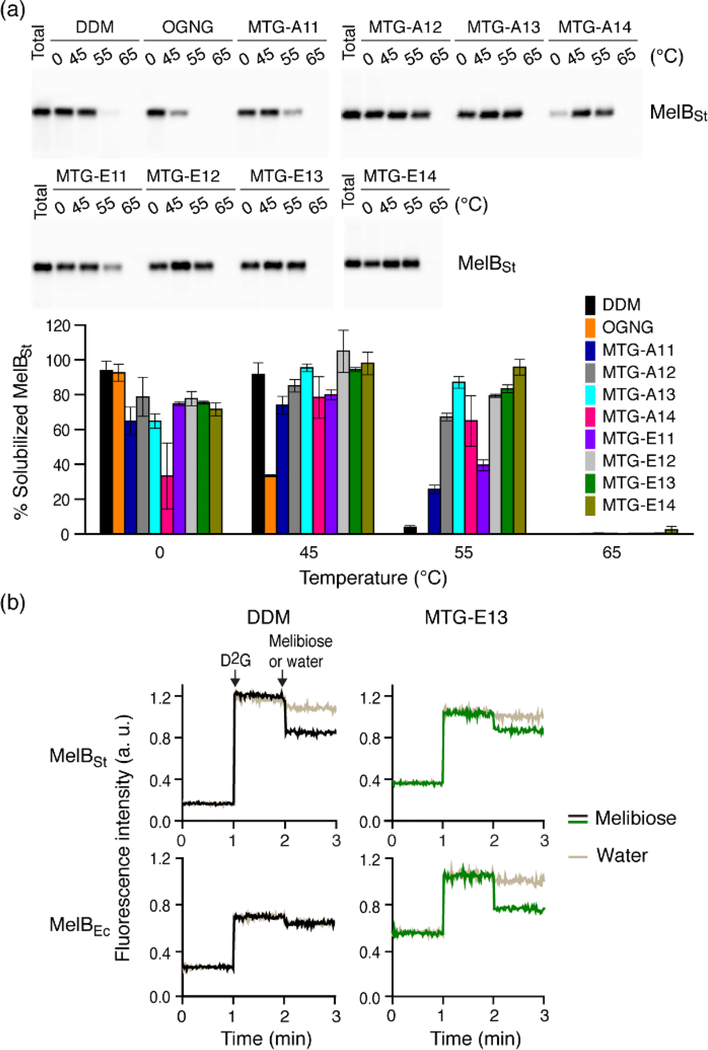
As an additional investigation, we utilized another transporter, melibiose permease from Salmonella typhimurium (MelBSt).79,80 The permease was first produced in E. coli membranes and then extracted on ice by each detergent (MTG/DDM/OGNG) at 1.5 wt% for 90 min, and then the detergent extracts were further thermally treated at a higher temperature (45, 55, or 65 °C) for another 90 min. The amounts of soluble MelBSt were analysed via Western blot and the data is presented as percentages (%) of total transporter originally present in the untreated membranes. The amount of soluble MelBSt detected at 0 °C is used to investigate detergent efficiency for protein extraction. The thermal treatment accelerates protein denaturation/aggregation to varied degrees depending on detergent ability to maintain MelBSt stability. At 0 °C, of the tested detergents, only OGNG yielded an amount of soluble MelBSt comparable to DDM (~100% efficiency), while all new detergents except MTG-A14 yielded 60~80% soluble MelBSt (Figure 4a& Table S2). These solubilization results are more or less consistent with those obtained with AtBOR1. When the detergent extracts were incubated at 45 °C, OGNG gave a markedly decreased amount of soluble MelBSt (~30%), indicating substantial protein aggregation at this temperature, as seen for AtBOR1, LeuT, and β2AR. In contrast, use of each MTG resulted in a higher amount of soluble MelBSt under the same conditions, likely due to enhanced detergent solubility (e.g., MTG-A14). A clear differentiation in detergent efficacy was observed between DDM and the MTGs when the detergents were evaluated at 55 °C. DDM -solubilized MelBSt almost completely disappeared from the solution, while a few MTGs such as MTG-A13, MTG-E12, MTG-E13, and MTG-E14 fully maintained the soluble state of the transporter. The MTG-Es were generally better than the MTG-As for MelBSt stability, consistent with results for AtBOR1. At 65 °C, a trace amount of soluble MelBSt was detected only in the case of MTG-E14. For further verification of MelBSt stability, MTG-E13 was selected for assessment of MelB functionality. For this purpose, we utilized fluorescence response of the transporter over the course of the sequential addition of a fluorescent ligand (i.e., 2’-(N-dansyl)aminoalkyl-1-thio-β-D-galactopyranoside (D2G)) and a non-fluorescent substrate (i.e., melibiose).80–82 Upon addition of D2G, a functional MelBSt would strongly bind to this fluorescent ligand, giving high fluorescence intensity due to the efficient energy transfer from tryptophan to D2G bound to the active site. The subsequent addition of excess melibiose (a non-fluorescent substrate) leads to reduction in a fluorescent signal as a result of a ligand-substrate exchange in the active site. Thus, MelBSt functional state can be examined by monitoring fluorescence intensity with sequential addition of D2G and melibiose. As expected from a mild property of DDM, DDM-solubilized MelBSt showed initial increase and subsequent decrease in the fluorescence signal upon sequential addition of D2G and melibiose (Figure 4b). However, we found a complete loss in fluorescence response of a transporter when less stable MelBEC obtained from E. coli was used for this functional assay. Remarkably, the selected test detergent, MTG-E13, was effective at preserving the functionality of this less stable MelB homologue under the same conditions, indicating that this malonate-based glucoside outperforms DDM with regards to retention of functional MelB.
Figure 4.
(a) Western blot image of detergent-extracted MelBSt. MTGs (MTG-A11/A12/A13/A14 and MTG-E11/E12/E13/E14), DDM and OGNG were used at 1.5 wt% for MelBSt extraction conducted at 0 °C for 90 min. Detergent-extracted samples were further treated at a higher temperature (45, 55, or 65 °C ) for another 90 min. The detergent -extracted samples at 0 °C or thermally treated samples were subjected to ultracentrifugation and then analyzed by Western blot (top panel). The amounts of soluble MelBSt are represented as relative percentages (%) of total MelBSt in the histogram (bottom panel). Error bars, SEM, n = 2. (b) Galactoside binding of MelB in MTG-E13. MelB function was assessed using FRET reversal elicited by a ligand-substrate exchange in the active site. Dansyl-2-galacotside (D2G) and excess melibiose were added to a solution containing DDM/MTG-E13-extracted MelB (MelBSt and MelBEc) at 1-min and 2-min time points, respectively. The resulting responses in fluorescence intensity of the samples were then monitored over the course of the additions. Control data was obtained from addition of water instead of melibiose. Melibiose addition is indicated in black (DDM)/green (MTG-E13), with water addition indicated in gold.
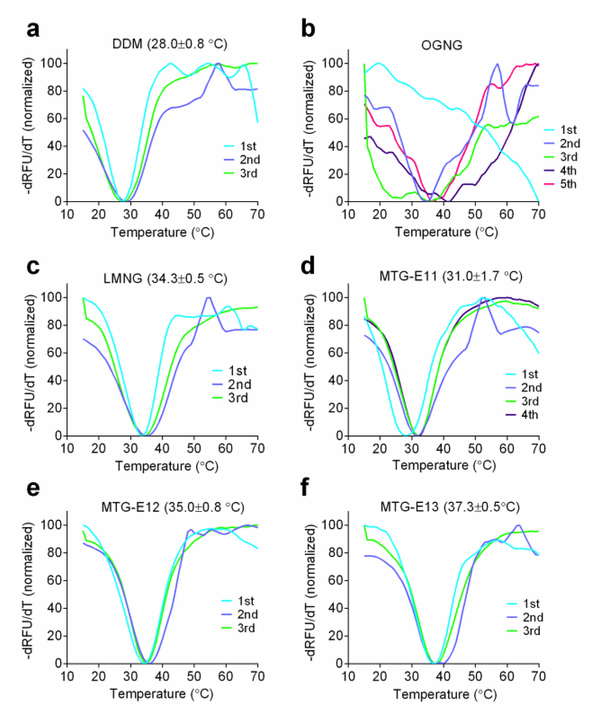
As MTG-E11, MTG-E12 and MTG-E13 displayed the most favorable behaviors with multiple membrane proteins, these MTG-Es were further evaluated with another GPCR, the mouse μ-opioid receptor (MOR).83 Receptor stability was assessed by measuring melting temperature (Tm) using differential scanning fluorimetry analysis. Receptor Tm was estimated by finding the minimum point of negative first order derivative of relative fluorescence units (-dRFU/dT) (Figures 5 and S7). The receptor samples were prepared by diluting DDM-purified receptor into individual detergent-supplemented buffer solutions. The DDM-purified MOR gave a relatively low Tm (28.0 °C), indicating that the receptor is of limited stability in DDM micelles. When OGNG was used in receptor CPM assay, this glucoside detergent showed unusual CPM profiles with a large variation from one experiment to another, strongly suggesting an occurrence of a significant loss in receptor stability during detergent exchange. In contrast, CPM profiles obtained from the tested MTG-Es were highly consistent and receptor Tms were substantially higher than that of DDM. The receptor in MTG-E11 yielded a Tm of 31.0 °C, while MTG-E12 and MTG-E13 produced receptor with Tms of 35.0 and 37.3 °C, respectively. An increase of 9.3 °C in receptor Tm was observed when MTG-E13 was used as a solubilizing agent instead of DDM. This C13 MTG was even more effective at stabilizing this GPCR than LMNG (34.3 °C). This result is a clear demonstration of the enhanced effectiveness of the MTG-Es at stabilizing MOR compared to gold standard conventional detergent or the highly successful novel LMNG.
Figure 5.
Normalized derivative functions of CPM profiles obtained from CPM assay of MOR solubilized in DDM (a), OGNG (b), LMNG (c), MTG-E11 (d), MTG-E12 (e), or MTG-E13 (f). DDM-purified receptor solubilized in the individual detergents thermally denatured in the presence of CPM dye by increasing the temperature from 15 to 70 °C. The value in parenthesis represents average receptor Tm ± SEM (n = 3–5). Tm of OGNG-solubilized MOR could not be obtained due to the inconsistency of the data.
Discussion
The current study showed that the most effective detergent for protein stability varied depending on the individual membrane proteins tested here. AtBOR1 and LeuT were most stable in MTG-E14 and MTG-A12/E13, respectively, while MTG-E12 and MTG-A13/E14 conferred the greatest stability to β2AR and MelB, respectively (Table S3). This protein-specific nature of detergent efficacy can be further illustrated by the behaviour of MTG-E13. This agent was among the best detergents for MelB, LeuT and MOR stability, but was poor when it comes to β2AR stability. Despite the protein-specific nature of detergent efficacy, however, it is noteworthy that MTG-E12 was consistently more effective than DDM at stabilizing all the tested membrane proteins, thereby suggesting that this detergent is widely applicable for protein stabilization. This C12 detergent was particularly effective at stabilizing β2AR, and MelB. MTG-E11 was also consistently better than DDM with the individual membrane proteins tested, but this C11 version was slightly inferior to MTG-E12 for stabilization of MelB and two GPCRs (β2AR and MOR) (Table S3). In the case of MTG-E13, the superior efficacy was observed with all the membrane proteins tested here except β2AR, indicating that this C13 version could be an excellent tool for studying many challenging membrane proteins. Importantly, all the MTGs derived from GNG scaffold were markedly superior to a previous version (i.e., OGNG) at protein stabilization. Furthermore, two MTGs (MTG-E12 and MTG-E13) were even more effective than the significantly optimized LMNG at stabilizing two tested membrane proteins (LeuT and MOR). Thus, the current study exemplifies that a single simple modification of a detergent (i.e., a structural change in the detergent head group from diglucoside to tetraglucoside) greatly enhances detergent efficacy. These detergents feature the presence of a compact head group and sufficiently long alkyl chains compatible with the protein hydrophobic dimensions that are likely responsible for their favourable behaviours. Because of their widely applicable stabilizing effects, MTG-E11, MTG-E12 and MTG-E13 hold significant potential for membrane protein structural study.
The two sets of new agents (MTG-As and MTG-Es) have a minor difference in their chemical structures. The two ether bonds are close to the central quaternary carbon, but these groups were located in the hydrophilic region for the MTG-As, or the lipophilic region for the MTG-Es. As a result, the MTG-As are constitutional isomers of the MTG-E counterparts. Despite the minor differences in their chemical structures, we observed rather large differences in their micelle sizes and efficacies for membrane protein stabilization. The micelles formed by the MTG-As were larger than their isomeric MTG-E counterparts. For example, MTG-A13 formed micelles with a Dh of 7.4 nm, larger than MTG-E13 micelles with a Dh of 6.7 nm. The relatively large micelle sizes of the MTG-As are somewhat unexpected as it seems obvious that the alkyl versions have a larger effective volume of the head group than the ether versions mainly due to the presence of the longer spacer between the carbohydrate units and the central quaternary carbon. This unexpected result means that the effective volume of the tail group is substantially smaller for the ether version (vs. the alkyl counterpart) in a micellar environment. This relatively small volume of the MTG-E tail groups is probably attained by the presence of the ether functional group in the lipophilic region. As a result, the ether versions (i.e., MTG-Es) likely produce more compact and stable packing of the alkyl chains in the micelle interiors than the MTG-As, and this confers greater protein stability in the MTG-Es as seen with AtBOR1 and β2AR. The superior efficacy of the MTG-Es was also found in the detergent evaluations with LeuT and MelB although the detergent efficacy differences were less clear in these cases. Therefore, this study implies that even a very small variation in detergent structure can significantly impact detergent efficacy for membrane protein stabilization.
Conclusions
In summary, we designed and prepared two sets of tetraglucoside-containing detergents (MTG-As and MTG-Es) differing in the relative position of the ether bonds within the detergent architecture. When these agents were evaluated with a set of model membrane proteins including two GPCRs, we identified a few detergents (MTG-E11, MTG-E12 and MTG-E13) that are markedly superior to a gold standard detergent of DDM and the previously reported OGNG at stabilizing almost every protein tested here. Due to universal detergent efficacy for protein stabilization, these malonate-derived glucosides are potential alternatives to classical detergents or other novel detergents for membrane protein study. Furthermore, comparative analysis of the MTG-Es with the MTG-As identified that a small variation in detergent structure has a substantial effect on protein stability, helping rational detergent design and selection for membrane protein research.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF) (2016R1A2B2011257 and 2018R1A6A1A03024231 to P.S.C.). The European Union’s Horizon 2020 research and innovation programme, RAMP-ITN: Rationalising Membrane Protein Crystallisation Innovative Training Network, under the Marie Sklodowska-Curie grant agreement No 722687 (CC) funded this work, also supported by BBSRC grant BB/N016467/1 awarded to BB. This work also supported by the National Institutes of Health (grants R21NS105863 and R01GM122759 to L.G.).
Footnotes
The authors declare no competing financial interest(s).
ASSOCIATED CONTENT
The supporting information is available free of charge via the internet at http://pubs.acs.org, including Figures S1 through S6, Table S1 to S3, supplementary methods on detergent evaluation with membrane proteins, and synthetic protocols and characterizations of the new detergents.
REFERENCES
- (1).Sanders CR and Myers JK (2004) Disease-related misassembly of membrane proteins. Annu. Rev. Biophys. Biomol. Struct, 33, 25–51. [DOI] [PubMed] [Google Scholar]
- (2).Overington JP, Al-Lazikani B and Hopkins AL (2006) How many drug targets are there?. Nat. Rev. Drug Discovery, 5, 993–996. [DOI] [PubMed] [Google Scholar]
- (3).Gautier A (2014) Structure determination of α-helical membrane proteins by solution-state NMR: Emphasis on retinal proteins. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1837, 578–588. [DOI] [PubMed] [Google Scholar]
- (4).Wallin E and Heijne GV (1998)Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci, 7, 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Garavito RM and Ferguson-Miller S (2001) Detergentsas tools in membrane biochemistry. J. Biol. Chem. 276, 32403–32406. [DOI] [PubMed] [Google Scholar]
- (6).Almén MS, Nordström KJ, Fredriksson R and Schiöth HB (2009) Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol, 7, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Helenius A and Simons KAI (1975) Solubilization of membranes by detergents. Biochim. Biophys. Acta, 415, 29–79. [DOI] [PubMed] [Google Scholar]
- (8).Tanford C and Reynolds JA (1976) Characterization of membrane proteins in detergent solutions. Biochim. Biophys. Acta, 457, 133–170. [DOI] [PubMed] [Google Scholar]
- (9).Wallin E and Heijne GV, (1998) Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci, 7, 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Seddon AM, Curnow P and Booth PJ (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1666, 105–117. [DOI] [PubMed] [Google Scholar]
- (11).Bond PJ, Faraldo-Gómez JD, Deol SS and Sansom MSP. (2006) Membrane protein dynamics and detergent interactions within a crystal: A simulation study of OmpA. Proc. Natl. Acad. Sci. U. S. A, 103, 9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Otzen D (2011) Protein–surfactant interactions: A tale of many states. Biochim. Biophys. Acta, 1814, 562–591. [DOI] [PubMed] [Google Scholar]
- (13).Yeagle PL (2014) Non-covalent binding of membrane lipids to membrane proteins. Biochim. Biophys. Acta, 6, 1548–1559. [DOI] [PubMed] [Google Scholar]
- (14).Patrick JW, Boone CD, Liu W, Conover GM, Liu Y, Cong X and Laganowsky A (2018) Allostery revealed within lipid binding events to membrane proteins. Proc. Natl. Acad. Sci. U. S. A, 115, 2976–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ujwal R; and Bowie JU (2011) Crystallizing membrane proteins using lipidic bicelles. Methods, 55, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Martin C (2015) A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes." Acta Crystallographica Section F: Structural Biology Communications, 71, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Denisov IG, and Stephen GS (2016) Nanodiscs for structural and functional studies of membrane proteins. Nat. Struct. Mol. Biol, 23, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Tribet C, Audebert R, and Popot JL (1996). Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl. Acad. Sci. U. S. A, 93, 15047–15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Dörr JM, Scheidelaar S, Koorengevel MC, Dominguez JJ, Schäfer M, van Walree CA, and Killian JA (2016). The styrene–maleic acid copolymer: a versatile tool in membrane research. Eur. Biophys. J. 45, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).McGregor CL, Chen L, Pomroy NC, Hwang P, Go S, Chakrabartty A, and Privé GG (2003). Lipopeptide detergents designed for the structural study of membrane proteins. Nat. Biotechnol. 21, 171–176. [DOI] [PubMed] [Google Scholar]
- (21).Tao H, Lee SC, Moeller A, Roy RS, Siu FY, Zimmermann J, Stevens RC, Potter CS, Carragher B and Zhang Q (2013) Engineered nanostructured ß-sheet peptides protect membrane proteins. Nat. Methods, 10, 759–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Carlson ML, Young JW, Zhao Z, Fabre L, Jun D, Li J, Li J, Dhupar HS, Wason I, Mills AT and Beatty JT (2018) The Peptidisc, a simple method for stabilizing membrane proteins in detergent-free solution Elife, 7, e34085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Frauenfeld J,Löving R, Armache JP,Sonnen AF, Guettou F, Moberg P, Zhu L, Jegerschöld C, Flayhan A, Briggs JA and Garoff H (2016) A saposin-lipoprotein nanoparticle system for membrane proteins. Nature methods, 13, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Popot JL, Althoff T, Bagnard D, Banères JL, Bazzacco P, Billon-Denis E, Catoire LJ, Champeil P, Charvolin D, Cocco MJ and Crémel G (2011) Amphipols from A to Z. Annu. Rev. Biophys 40, 379–408. [DOI] [PubMed] [Google Scholar]
- (25).Chae PS, Wander MJ, Bowling AP, Laible PD and Gellman SH (2008) Glycotripod amphiphiles for solubilization and stabilization of a membrane-protein superassembly: Importance of branching in the hydrophilic portion. ChemBioChem 9, 1706–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Chae PS, Cho KH, Wander MJ, Bae HE, Gellman SH and Laible PD (2014) Hydrophobic variants of ganglio-tripod amphiphiles for membrane protein manipulation. Biochim. Biophys. Acta, 1838, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Frotscher E, Danielczak B, Vargas C, Meister A, Durand G and Keller S (2015) A Fluorinated Detergent for Membrane-Protein Applications. Angew. Chem., Int. Ed. 54, 5069–5073. [DOI] [PubMed] [Google Scholar]
- (28).Abla M, Unger S, Keller S, Bonneté F, Ebel C, Pucci B, Breyton C and Durand G (2015) Micellar and biochemical properties of a propyl-ended fluorinated surfactant designed for membrane–protein study. J. Colloid Interface Sci. 445, 127–136. [DOI] [PubMed] [Google Scholar]
- (29).Chae PS, Gotfryd K, Pacyna J, Miercke LJ, Rasmussen SG, Robbins RA, Rana RR, Loland CJ, Kobilka B, Stroud R, Byrne B, Gether U and Gellman SH (2010) Tandem facial amphiphiles for membrane protein stabilization. J. Am. Chem. Soc, 132, 16750–16752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Lee SC, Bennett BC, Hong WX, Fu Y, Baker KA, Marcoux J, Robinson CV, Ward AB, Halpert JR, Stevens RC and Stout CD (2013) Steroid-based facial amphiphiles for stabilization and crystallization of membrane proteins. Proc. Natl. Acad. Sci. U. S. A. 110, 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chae PS, Rana RR, Gotfryd K, Rasmussen SGF, Kruse AC, Cho KH, Capaldi S, Carlsso E, Kobilka B, Loland CJ, Gether U, Banerjee S, Byrne B, Lee JK and Gellman SH (2013) Glucose-neopentyl glycol (GNG) amphiphiles for membrane protein study. Chem. Commun, 49, 2287–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Chae PS, Rasmussen SGF, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D, Popot JL, Picot D, Fox BG, Guan L, Gether U, Byrne B, Kobilka B and Gellman SH (2010) Maltose—neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat. Methods, 7, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Cho KH, Husri M, Amin A, Gotfryd K, Lee HJ, Go J, Loland CJ, Guan L, Byrne B and Chae PS (2015) Maltose neopentyl glycol-3 (MNG-3) analogues for membrane protein study Analyst, 140, 3157–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bae HE, Du Y, Hariharan P, Mortensen JS, Kumar KK, Ha B, Das M, Lee HS, Loland CJ, Guan, L, Kobilka BK and Chae PS (2019) Asymmetric maltose neopentyl glycol amphiphiles for a membrane protein study: effect of detergent asymmetricity on protein stability. Chem. Sci, 10, 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Sadaf A, Mortensen JS, Capaldi S, Tikhonova E, Hariharan P, Ribeiro O, Loland CJ, Guan L, Byrne B and Chae PS (2016) A class of rigid linker-bearing glucosides for membrane protein structural study. Chem. Sci, 7, 1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hussain H, Du Y, Scull NJ, Mortensen JS, Tarrasch J, Bae HE, Loland CJ, Byrne B, Kobilka BK and Chae PS (2016) Accessible Mannitol-Based Amphiphiles (MNAs) for Membrane Protein Solubilisation and Stabilisation. Chem. —Eur. J, 22, 7068–7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Cho KH, Ribeiro O, Du Y, Tikhonova E, Mortensen JS, Markham K, Hariharan P, Loland CJ, Guan L, Kobilka BK, Byrne B and Chae PS (2016) Mesitylene-Cored Glucoside Amphiphiles (MGAs) for Membrane Protein Studies: Importance of Alkyl Chain Density in Detergent Efficacy. Chem.—Eur. J., 22, 18833–18839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Das M, Du Y, Ribeiro O, Hariharan P, Mortensen JS, Patra D, Skiniotis G, Loland CJ, Guan L, Kobilka BK, Byrne B and Chae PS (2017) Conformationally preorganized diastereomeric norbornane-based maltosides for membrane protein study: Implications of detergent kink for micellar properties. J. Am. Chem. Soc, 139, 3072–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hussain H, Du Y, Tikhonova E, Mortensen JS, Ribeiro O, Santillan C, Das M, Ehsan M, Loland CJ, Guan L, Kobilka BK, Byrne B and Chae PS (2017) Resorcinarene-Based Facial Glycosides: Implication of Detergent Flexibility on Membrane-Protein Stability. Chem.–Eur. J, 23, 6724–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ehsan M, Du Y, Mortensen JS, Hariharan P, Qu Q, Ghani L, Das M, Grethen A, Byrne B, Skiniotis G and Keller S, Loland CJ, Guan L, Kobilka BK and Chae PS. (2019) Self-assembly behaviors and application of terphenyl-cored trimaltosides for membrane protein study: Impact of detergent hydrophobic group geometry on protein stability. Chem.—Eur. J, 25, 11545–11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Ghani L, Munk CF, Zhang X, Katsube S, Du Y, Cecchetti C, Huang W, Bae HE, Saouros S, Ehsan M, Guan L, Liu X, Loland CJ, Kobilka BK, Byrne B, Chae PS (2019) 1,3,5-triazine—cored maltoside amphiphiles for membrane protein extraction and stabilization. J. Am. Chem. Soc. 141, 19677–49687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Howell SC, Mittal R, Huang L, Travis B, Breyer RM and Sanders CR (2010) CHOBIMALT: a cholesterol-based detergent. Biochemistry, Biochemistry, 49, 9572–9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Kruse AC, Manglik A, Cho KH, Nurva S, Gether U, Guan L, Loland CJ, Byrne B, Kobilka BK and Gellman SH (2012) A new class of amphiphiles bearing rigid hydrophobic groups for solubilization and stabilization of membrane proteins. Chem.—Eur. J, 18, 9485–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Ehsan M, Kumar A, Mortensen JS, Du Y, Hariharan P, Kumar KK, Ha B, Byrne B, Guan L, Kobilka BK, Loland CJ and Chae PS (2019) Self-Assembly Behaviors of a Penta-Phenylene Maltoside and Its Application for Membrane Protein Study. Chem. —Asian J, 14, 1926–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Ehsan M, Du Y, Scull NJ, Tikhonova E, Tarrasch J, Mortensen JS, Loland CJ, Skiniotis G, Guan L, Byrne B Kobilka, B. K. and Chae, P. S. (2016) Highly branched pentasaccharide-bearing amphiphiles for membrane protein studies. J. Am. Chem. Soc, 138, 3789–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Sadaf A, Du Y, Santillan C, Mortensen JS, Molist I, Seven AB, Hariharan P, Skiniotis G and Loland CJ, Kobilka BK, Guan L, Byrne B, and Chae PS (2017) Dendronic trimaltoside amphiphiles (DTMs) for membrane protein study. Chem. Sci. 8, 8315–8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Ehsan M, Du Y, Molist I, Seven AB, Hariharan P, Mortensen JS, Ghani L, Loland CJ, Skiniotis G, Guan L and Byrne B, Kobilka BK and Chae PS (2018) Vitamin E-based glycoside amphiphiles for membrane protein structural studies. Org. Biomol. Chem, 16, 2489–2498. [DOI] [PubMed] [Google Scholar]
- (48).Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi H-J, DeVree BT and Sunahara RK (2011) Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature, 469, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Haga K, Kruse AC, Asada H, Yurugi-Kobayashi T, Shiroishi M, Zhang C, and Kobayashi T (2012) Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature, 482, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kruse AC, Ring AM, Manglik A, Hu J, Hu K, Eitel K, and Christopoulos A (2013) Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature, 504, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, and Fujiyoshi Y (2014) Crystal structure of a claudin provides insight into the architecture of tight junctions. Science, 344, 304–307. [DOI] [PubMed] [Google Scholar]
- (52).Dickson VK, Pedi L, and Long SB (2014) Structure and insights into the function of a Ca2+-activated Cl−channel. Nature, 516, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Hauer F, Gerle C, Fischer N, Oshima A, Shinzawa-Itoh K, Shimada S, and Stark H (2015) GraDeR: membrane protein complex preparation for single-particle cryo-EM. Structure, 23, 1769–1775. [DOI] [PubMed] [Google Scholar]
- (54).Yin J, Mobarec JC, Kolb P, and Rosenbaum DM (2015) Crystal structure of the human OX 2 orexin receptor bound to the insomnia drug suvorexant. Nature, 519, 247–250; [DOI] [PubMed] [Google Scholar]; (h) Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, and Xu Q (2015) Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature, 523, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Perez C, Gerber S, Boilevin J, Bucher M, Darbre T, Aebi M, and Locher KP (2015) Structure and mechanism of an active lipid-linked oligosaccharide flippase. Nature, 524, 433–438. [DOI] [PubMed] [Google Scholar]
- (56).Taniguchi R, Kato HE, Font J, Deshpande CN, Wada M, Ito K, and Nureki O (2015) Outward-and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat. Commun, 6, 8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, and Ruda GF (2015) K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science, 347, 1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Paulsen CE, Armache JP, Gao Y, Cheng Y, and Julius D (2015) Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature, 520, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Schmidt HR, Zheng S, Gurpinar E, Koehl A, Manglik A, and Kruse AC (2016) Crystal structure of the human σ 1 receptor. Nature, 532, 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, Thal DM, Furness SG, Christopoulos G, Coudrat T and Danev R (2017) Phase-plate cryo-EM structure of a class B GPCR–G-protein complex. Nature, 546, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).James ZM, Borst AJ, Haitin Y, Frenz B, DiMaio F, Zagotta WN and Veesler D, (2017) CryoEM structure of a prokaryotic cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. U. S. A, 114, 4430–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jörg M, Scammells PJ, May LT, Sexton PM and Christopoulos A (2017) Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell, 168, 867–877. [DOI] [PubMed] [Google Scholar]
- (63).Tanaka Y, Iwaki S and Tsukazaki T (2017) Crystal structure of a plant multidrug and toxic compound extrusion family protein. Structure, 25, 1455–1460. [DOI] [PubMed] [Google Scholar]
- (64).Kellosalo J, Kajander T, Kogan K, Pokharel K and Goldman A (2012) The structure and catalytic cycle of a sodium-pumping pyrophosphatase. Science, 337, 473–476. [DOI] [PubMed] [Google Scholar]
- (65).Frick A, Eriksson UK, de Mattia F, Öberg F, Hedfalk K, Neutze R, Willem J, Deen PM and Törnroth-Horsefield S (2014) X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. U. S. A, 111, 6305–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Karakas EandFurukawa H(2014)Crystalstructureofa heterotetrameric NMDA receptor ion channel. Science, 344, 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Chae PS, Kruse AC, Gotfryd K, Rana RR, Cho KH, Rasmussen SG, Bae HE, Chandra R, Gether U, Guan L, Kobilka BK, Loland CJ, Byrne B and Gellman SH (2013) Novel tripod amphiphiles for membrane protein analysis. Chem.—Eur. J, 19, 15645–15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Chattopadhyay A and London E (1984) Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal. Biochem. 139, 408–412. [DOI] [PubMed] [Google Scholar]
- (69).Thurtle-Schmidt BH and Stroud RM (2016) Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers. Proc. Natl. Acad. Sci. U. S. A, 113, 10542–10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Alexandrov AI, Mileni M, Chien EY, Hanson MA and Stevens RC (2008) Microscale fluorescent thermal stability assay for membrane proteins. Structure, 16, 351–359. [DOI] [PubMed] [Google Scholar]
- (71).Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P and Stevens RC (2008) A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure, 16, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Deckert G, Warren PV, Gaasterland T, Young WG, Lenox AL, Graham DE, Overbeek R, Snead MA, Keller M, Aujay M, Huber R, Feldman RA, Short JM, Olsen GJ and Swanson RV (1998) The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature, 392, 353–358. [DOI] [PubMed] [Google Scholar]
- (73).Yamashita A, Singh SK, Kawate T, Jin Y and Gouaux E (2005) Crystal structure of a bacterial homologue of Na+/Cl− dependent neurotransmitter transporters. Nature, 437, 215–223. [DOI] [PubMed] [Google Scholar]
- (74).Quick M and Javitch JA (2007) Monitoring the function of membrane transport proteins in detergent-solubilized form. Proc. Natl. Acad. Sci. U. S. A, 104, 3603–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Billesbølle CB, Mortensen JS, Sohail A, Schmidt SG, Shi L, Sitte HH, Gether U and Loland CJ (2006) Transition metal ion FRET uncovers K+ regulation of a neurotransmietter/sodium symporter. Nat. Commun. 7, 12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC and Kobilka BK (2007) GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science, 318, 1266–1273. [DOI] [PubMed] [Google Scholar]
- (77).Yao X, Parnot C, Deupi X, Ratnala VR, Swaminath G, Farrens D and Kobilka B (2006) Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat. Chem. Biol. 2, 417–422. [DOI] [PubMed] [Google Scholar]
- (78).Swaminath G, Steenhuis J, Kobilka B and Lee TW (2002) Allosteric modulation of β2-adrenergic receptor by Zn2+. Molecular pharmacology, Mol. Pharmacol 61, 65–72. [DOI] [PubMed] [Google Scholar]
- (79).Guan L, Nurva S and Ankeshwarapu SP (2011) Mechanism of melibiose/cation symport of the melibiose permease of Salmonella typhimurium. J. Biol. Chem, 286, 6367–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Ethayathulla AS, Yousef MS, Amin A, Leblanc G, Kaback HR and Guan L (2014) Structure-based mechanism for Na+/melibiose symport by MelB. Nat. Commun, 5, 3009–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Cordat E, Mus-Veteau I and Leblanc G (1998) Structural Studies of the Melibiose Permease of Escherichia coli by Fluorescence ResonanceEnergyTransferII. IDENTIFICATION OF THE TRYPTOPHAN RESIDUES ACTING AS ENERGY DONORS. J. Biol. Chem, 273, 33198–33202. [DOI] [PubMed] [Google Scholar]
- (82).Amin A, Hariharan P, Chae PS and Guan L (2015) Effect of detergents on galactoside binding by Melibiose permeases. Biochemistry, 54, 5849–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK and Granier S (2012) Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature, 485, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.