Abstract
Introduction:
The general assumption is that blood glucose (BG) and interstitial fluid glucose (IntFG) are practically the same. We aimed to determine whether the typical patient with type 2 diabetes can use IntFG to estimate BG.
Description:
The study was conducted on an 83-year-old white male with type 2 diabetes. One hundred pairs of IntFG and BG observations mg/dL (n = 50 simultaneous; n = 50 with 15-minute lag) were made over a 10-day period. We used paired t tests, correlation coefficients, and linear regression to predict relationships between IntFG and BG.
Results:
There were significant (P < .0001) mean differences between IntFG and BG (simultaneous: 53.8 mg/dL; 15-minute time lag: 46.4 mg/dL). There were significant (P < .0001) positive correlations between IntFG and BG (simultaneous: r = 0.641; 15-minute time lag: r = 0.712). Linear regression revealed that increased IntFG was significantly (P < .0001) associated with declines in mean predicted BG.
Conclusion:
The typical type 2 diabetes patient cannot use IntFG level to estimate BG.
Keywords: diabetes, measurement, patient education, patient feedback, quantitative methods
Introduction
Self-monitoring of blood glucose (BG) is a critical element in diabetes management (1). In fact, self-monitoring of BG represents one of the most important advancements in diabetes management since the advent of insulin in 1920 (2).
It has been the practice for decades to base therapeutic decisions for patients with diabetes on glucose measurements using fingertip capillary blood samples. Knowledge gained from clinical studies on the impact of metabolic control on diabetes-related complications is based on such measurements (3). However, although it has so far eluded us, noninvasive continuous blood glucose monitoring (CGM) is thought to be the ideal method for that purpose.
Recent introduction of minimally invasive means for continuous glucose monitoring yield measures of glucose in interstitial fluid (IntFG) (4). Despite some controversy, the general assumption is that glucose levels in blood and interstitial fluid are practically the same and that the information provided can be used by type 2 diabetes patients interchangeably (5).
Currently, Abbott Labs FreeStyle Libre CGM device measures interstitial fluid glucose (IntFG; mg/dL) levels through a small sensor that can be applied to the back of the upper arm. The FreeStyle Libre CGM system provides real-time glucose readings for up to 10 days (4). Consumers are told in FreeStyle Libre CGM marketing information that IntFG is a good way to track BG, and that BG, based on finger capillary blood samples, is no longer needed.
The relationship between IntFG and BG levels is well known to medical experts as regard both the difference—and the reason for the difference—between actual values observed and the time lapse between them (IntFG lags BG by approximately 15 minutes) (6,7). However, this information is not common knowledge to the typical type 2 diabetes patient (the focus of this study) who is told in marketing information that his or her BG levels can readily be tracked by observing interstitial tissue levels, as shown in their device readout.
The purpose of this single-subject case study was to determine whether a typical type 2 diabetes patient can actually estimate serum glucose level from IntFG level. The first part of this study examined the relationship between temporal-contiguous samples of IntFG and finger-capillary BG readings in mg/dL, obtained ad libitum—exactly as would the typical user. The second part of this study aimed to determine whether IntFG would reliably represent BG level after a time lapse of 15 minutes, as reported in previous studies (6,7).
Methods
Subject
The study was conducted on an 83-year-old white male, diagnosed with type 2 diabetes and chronic heart disease.
Apparatus
An Abbott FreeStyle Libre continuous glucose monitoring device and a OneTouch UltraMini glucometer sampling fingertip capillary blood were used to collect IntFG and BG measures. No attempt was made to calibrate the devices, as the measures obtained were paired comparisons rather than absolute measures. The report of the manufacturers as to accuracy was accepted prima facie.
Procedures
For procedure 1, 50 simultaneous IntFG- (mg/dL) and BG- (mg/dL) level observations were made ad libitum over a 10-day period. During that time the subject followed a treatment regimen consisting of 75 mg. Precose (oral α-glucosidase inhibitor for use in the management of type 2 diabetes mellitus) and 0.5 mg. Repaglinide (oral BG-lowering drug of the glinide class) 3 times per day, taken at meals.
For procedure 2, using the same apparatus, and following the same procedure, over a 10-day period, 50 separate observations of BG level were made likewise ad libitum, each followed 15 minutes later by IntFG level.
For both procedures, the patient was told to collect 50 pairs of observations. We chose 50 pairs because in conventional parametric statistics, t is approximately normally distributed when n > 30. The patient was also instructed not to follow any temporal pattern but to collect ad libitum. First, this was done in order to avoid any BG cycle highs and lows repetition observations and so generate the greatest possible diversity (variability) of observations; second, ad libitum collection likely mirrors what a typical user might do, consistent with the aim of the study.
Statistical Analysis
For both procedures, we used paired t tests to detect significant differences in mean IntFG and BG levels (mg/dL). The paired sample t test is a statistical test used to ascertain whether the mean difference between 2 sets of observations is zero. In a paired sample t test, each subject is measured twice, resulting in pairs of observations (8). We further used the Pearson product–moment correlation coefficient (denoted by r) to measure the strength of the linear association between IntFG and BG levels (mg/dL). Pearson’s r can range from −1 (perfect negative linear relationship) to +1 (perfect positive linear relationship) (9). Finally, we used simple linear regression to predict the effect of a single predictor (ie, IntFG mg/dL) on a single-continuous outcome (ie, BG mg/dL). Simple linear regression models the relationship between 2 variables by fitting a linear equation to observed data (10). The regression equation with 1 dependent and 1 independent variable is written formulaically as: y = β0 + β1 × x, where y = predicted BG mg/dL, β0 = constant, β1 = regression coefficient, and x = IntFG mg/dL (10). All analyses were performed with SAS 9.3 (SAS Corp, Cary, North Carolina), were 2-tailed, and conducted with α = .05 significance level.
Results
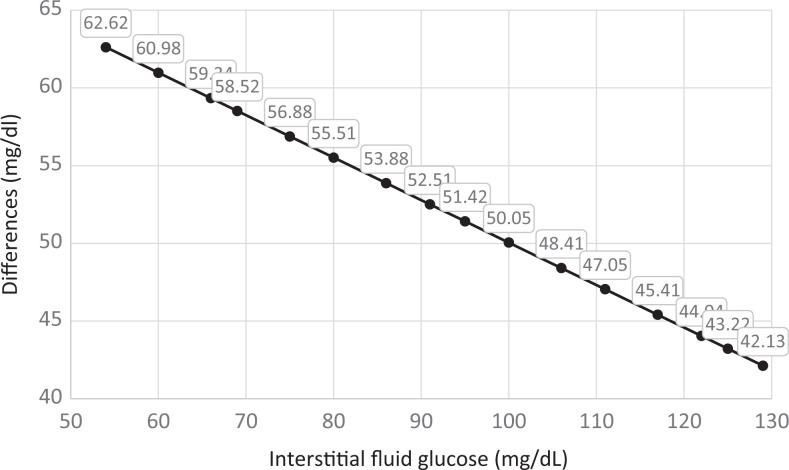
When IntFG and BG were measured simultaneously (Procedure 1), there was a mean difference of 53.8 mg/dL (standard deviation [SD] = 17.4 mg/dL) between IntFG and BG (t49 = 21.79; P < .0001). The correlation between these 2 samples was significant and positive (r = 0.641; P < .0001). In using linear regression to predict the effect of IntFG on BG measured simultaneously, we generated the following predictive model: y (estimated BG mg/dL) = 77.36616 + 0.72684 (IntFG mg/dL). Table 1 displays the mean differences between predicted BG and observed IntFG measured simultaneously, for a range of values. As shown in Figure 1 that difference regularly declines as IntFG rises. For instance, the difference decreases from 62.6 mg/dL when IntFG is 54 mg/dL, to 42.1 mg/dL when IntFG is 129 mg/dL, a difference of 20.5 mg/dL (Figure 1). Supplementary Appendix I provides the complete set of 50 simultaneous IntFG- (mg/dL) and BG- (mg/dL) level observations.
Table 1.
Differences Between Simultaneous Interstitial Fluid Glucose and Predicted Blood Glucose for a Range of Values.
| Observed IntFG | Observed BG | Predicted BGa | Difference Between Predicted BG and Observed IntFG |
|---|---|---|---|
| 54 | 128 | 116.62 | 62.62 |
| 60 | 130 | 120.98 | 60.98 |
| 66 | 138 | 125.34 | 59.34 |
| 69 | 127 | 127.52 | 58.52 |
| 75 | 129 | 131.88 | 56.88 |
| 80 | 131 | 135.51 | 55.51 |
| 86 | 136 | 139.88 | 53.88 |
| 91 | 143 | 143.51 | 52.51 |
| 95 | 154 | 146.42 | 51.42 |
| 100 | 165 | 150.05 | 50.05 |
| 106 | 147 | 154.41 | 48.41 |
| 111 | 179 | 158.05 | 47.05 |
| 117 | 149 | 162.41 | 45.41 |
| 122 | 168 | 166.04 | 44.04 |
| 125 | 222 | 168.22 | 43.22 |
| 129 | 190 | 171.13 | 42.13 |
Abbreviations: BG, blood glucose (mg/dL); IntFG, interstitial fluid glucose (mg/dL).
aBG = 77.36616 + 0.72684(IntFG).
Figure 1.
Differences between predicted blood glucose and observed interstitial fluid glucose (measured simultaneously).
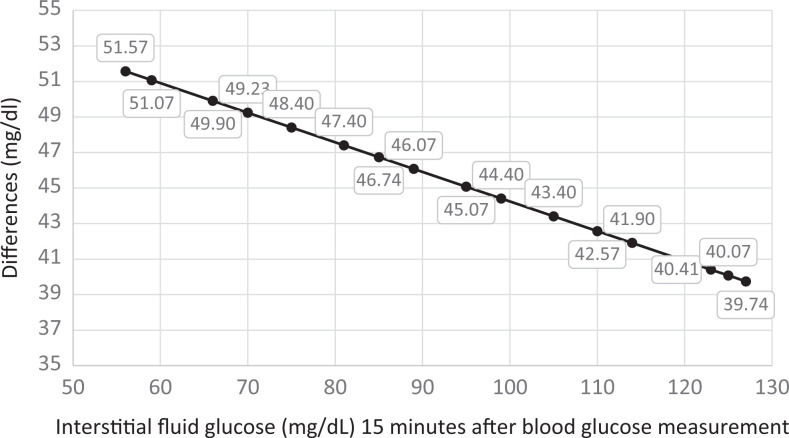
When IntFG mg/dL was measured 15 minutes after BG mg/dL (Procedure 2), there was a mean difference of 46.4 mg/dL (SD = 14.7 mg/dL) between IntFG and BG (t49 = 22.25; P < .0001). The correlation between these 2 samples was significant and positive (r = 0.712; P < .0001). In using linear regression to predict the effect of IntFG on BG 15 minutes earlier, we generated the following predictive model: y (estimated BG mg/dL 15-minutes earlier) = 60.89927 + 0.83342 (IntFG mg/dL). Table 2 displays the differences between predicted BG mg/dL 15 minutes earlier and observed IntFG mg/dL, for a range of values. As shown in Figure 2 the difference regularly declines as IntFG rises. For instance, the difference between IntFG and predicted BG 15 minutes earlier decreases from 51.5 mg/dL when IntFG is 56 mg/dL, to 39.7 mg/dL when IntFG is 127 mg/dL, a difference of 11.8 mg/dL (Figure 1). Supplementary Appendix II provides the complete set of 50 observations of BG level, each followed 15 minutes later by IntFG level observations.
Table 2.
Differences Between Observed Interstitial Fluid Glucose and Predicted Blood Glucose 15-Minutes Earlier for a Range of Values.
| Observed IntFG | Observed BG | Predicted BGa | Difference Between Predicted BG and Observed IntFG |
|---|---|---|---|
| 56 | 158 | 107.57 | 51.57 |
| 59 | 146 | 110.07 | 51.07 |
| 66 | 158 | 115.90 | 49.90 |
| 70 | 128 | 119.23 | 49.23 |
| 75 | 153 | 123.40 | 48.40 |
| 81 | 121 | 128.40 | 47.40 |
| 85 | 176 | 131.74 | 46.74 |
| 89 | 152 | 135.07 | 46.07 |
| 95 | 161 | 140.07 | 45.07 |
| 99 | 126 | 143.40 | 44.40 |
| 105 | 106 | 148.40 | 43.40 |
| 110 | 161 | 152.57 | 42.57 |
| 114 | 114 | 155.90 | 41.90 |
| 123 | 169 | 163.41 | 40.41 |
| 125 | 189 | 165.07 | 40.07 |
| 127 | 147 | 166.74 | 39.74 |
Abbreviations: BG, blood glucose (mg/dL); IntFG, interstitial fluid glucose (mg/dL).
aY(predicted) = 60.89927 + 0.83342 (IntFG).
Figure 2.
Differences between predicted blood glucose and observed interstitial fluid glucose measured 15 minutes after blood glucose measurement.
Discussion
In light of these findings, estimating BG level from any value of IntFG by simply correcting BG by adding, the mean difference (53.8 or 46.4) as a constant is not valid because the difference between them is in fact not constant over the BG-level span. However, if these data hold up on replication, it may be possible to provide the user with a detailed table that shows average estimated BG mg/dL from observed IntFG mg/dL taking into account the progressive change in the difference in IntFG and BG as IntFG rises. However, the reliability of that estimate varies with the level of IntFG due to non-homoscedasticity.
Since there is approximately a 15-minute time lag between IntFG and BG, the second part of this study aimed to determine the reliability of the estimate of BG 15 minutes before observing IntFG. In other words, given IntFG can one reliably determine what the corresponding serum BG level was 15 minutes earlier?
Curiously, using observed IntFG level to predict BG level 15 minutes earlier is more reliable as the difference between IntFG mg/dL and BG mg/dL 15 minutes earlier is smaller and does not change appreciably over the BG spectrum. This “backward estimation” measure may be of use to type 2 diabetes patients for whom, unlike type 1 diabetes patients, that time difference may not be as crucial.
We conclude that the typical type 2 diabetes patient may find it difficult to use their IntFG measures to reliably estimate their time-congruous BG levels. At best, the correlation between IntFG and BG being positive, the typical type 2 diabetes patient can at least use changes in IntFG to get a sense of changes in BG.
In light of these findings, we are reminded of a study by Cobelli et al (2016) which used simulations to examine the relationship between interstitial fluid glucose and blood serum glucose. Consistent with our findings, the authors found that IntFG is not simply a shifted-in-time version of blood serum glucose but exhibits a more complex pattern (11). These findings suggest, however, that while IntFG is not exactly equivalent to BG, it could prove a useful index of trend.
Limitations
This study of type 2 diabetes with an N of one has important limitations. First, results may not be generalizable to other patients with type 2 diabetes—in particular, those taking different treatment regiments or whose glucose measurements are not done around meal time. Second, since type 1 diabetes, and commonly insulin therapy, creates its own unique problems, results are not generalizable to type 1 diabetes patients. Finally, since this study involved a single 83-year-old male, our results need to be confirmed by studies with larger samples sizes.
Lessons Learned
To effectively “track” BG by means of IntFG, a user would need to be provided with a detailed chart showing average estimated BG, predicted by observed IntFG, to help guide treatment decisions.
Supplemental Material
Supplemental Material, Appendix_I_R1_JPX for Can Type 2 Diabetes Sufferers Actually Estimate Serum Glucose Level From Interstitial Fluid Glucose Level: A Diabetes Patient’s Experience by Dennis A Fried and Robert Fried in Journal of Patient Experience
Author Biographies
Dennis A Fried is assistant professor, Department of Epidemiology, Rutgers School of Public Health, Newark, NJ; Health Science Specialist, US Department of Veterans Affairs, War-Related Illness & Injury Study Center, East Orange, NJ.
Robert Fried is emeritus professor, Doctoral Faculty in Behavioral Neuroscience, City University of New York (CUNY). Emeritus Member, American Physiological Society (APS — Cardiovascular and Respiration Divisions) & FASEB.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Dennis A Fried, PhD, MPH, MBA  https://orcid.org/0000-0003-3517-0758
https://orcid.org/0000-0003-3517-0758
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Fried R, Carlton RM. Type 2 Diabetes: Cardiovascular and Related Complications and Evidence-Based Complementary Treatments. Boca Raton, FL: CRC Press/Taylor & Francis Group; 2018. [Google Scholar]
- 2. Cengiz E, Tamborlane WV. A tale of two compartments: Interstitial versus blood glucose monitoring. Diabetes technology & therapeutics. 2009;11(S1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried R, Carlton RM. Type 2 Diabetes—Cardiovascular and Related Complications and Evidence-Based Complementary Treatments. Boca Raton, FL: CRC Press/Div Taylor & Francis Group; 2018. [Google Scholar]
- 4. Chen C, Zhao XL, Li ZH, Zhu ZG, Qian SH, Flewitt AJ. Current and emerging technology for continuous glucose monitoring. Sensors (Basel). 2017;17:182 doi:10.3390/s17010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegmund T, Heinemann L, Kolassa R, Thomas A. Discrepancies between blood glucose and interstitial glucose—technological artifacts or physiology: implications for selection of the appropriate therapeutic target. J Diabetes Sci Technol. 2017:11:766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossetti P, Bondia J, Vehí J, Fanelli CG. Review estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors (Basel). 2010;10:10936–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aussedat B, Dupire-Angel M, Gifford R, Klein JC, Wilson GS, Reach G. Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metab. 2000;278:E716–28. [DOI] [PubMed] [Google Scholar]
- 8. Kleinbaum D, Kupper L, Nizam A, Rosenberg E. Applied Regression Analysis and Other Multivariable Methods. Toronto: Nelson Education; 2013. [Google Scholar]
- 9. Trapp RG, Dawson B. Basic & Clinical Biostatistics. Norwalk, CT: Appleton & Lange; 1990. [Google Scholar]
- 10. Montgomery DC, Peck EA, Vining GG. Introduction to Linear Regression Analysis (Vol. 821). Hoboken: John Wiley & Sons; 2012. [Google Scholar]
- 11. Cobelli C, Schiavon M, Dalla Man C, Basu A, Basu R. Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror image of blood glucose: insight from an in silico study. Diabetes Technol Ther. 2016;18:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Appendix_I_R1_JPX for Can Type 2 Diabetes Sufferers Actually Estimate Serum Glucose Level From Interstitial Fluid Glucose Level: A Diabetes Patient’s Experience by Dennis A Fried and Robert Fried in Journal of Patient Experience