INTRODUCTION
One to two percent of non–small-cell lung cancers (NSCLCs) harbor ROS1 gene rearrangements.1-3 ROS1 gene rearrangement leads to constitutive activation receptor tyrosine kinase that activates downstream mitogen-activated protein kinase, phosphoinositide-3 kinase, signal transducer and activator of transcription 3, and other pathways leading to oncogenesis.4
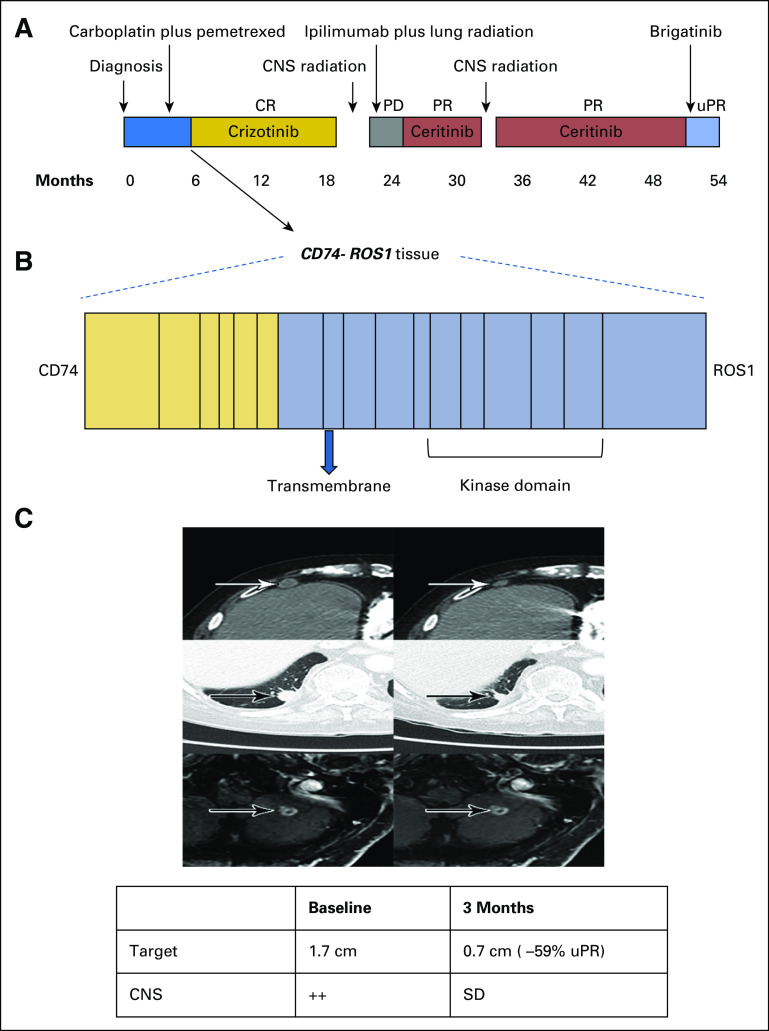
Because ROS1 shares 49% amino acid sequence homology with anaplastic lymphoma kinase (ALK) in the kinase domain, ROS1 rearranged (ROS1-positive) NSCLC tends to be sensitive to ALK inhibitors.5 Crizotinib, a mesenchymal epithelial transition factor (MET)/ALK/ROS1 tyrosine kinase inhibitor (TKI), is the only US Food and Drug Administration–approved drug for ROS1-positive NSCLC.6 Ceritinib and brigatinib, second-generation ALK inhibitors, have demonstrated activity in crizotinib-naïve ROS1-positive NSCLC but lacked activity in patients who were crizotinib resistant in anecdotal cases.7,8 However, the efficacy of both ceritinib and brigatinib in patients who are crizotinib resistant remains unclear. We previously reported the preliminary systemic and intracranial activity of ceritinib in a crizotinib-refractory patient.9 Herein, we report the activity of brigatinib in the same patient (Fig 1A).
FIG 1.
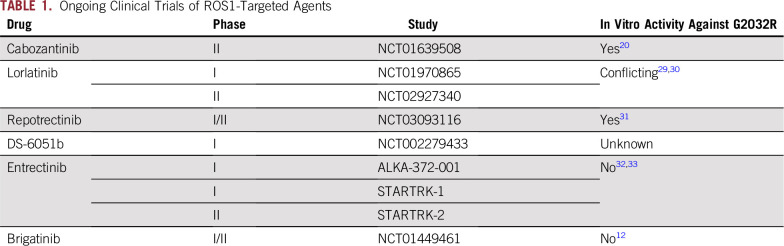
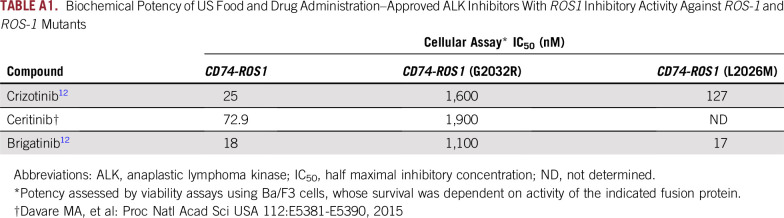
Clinical activity of brigatinib in crizotinib- and ceritinib-resistant ROS1-positive non–small-cell lung cancer. (A) The various treatments the patient received for metastatic ROS1-positive non–small-cell lung cancer, along with the duration of and best response to each treatment. (B) Fusion event: 5′-CD74(x1-6 NM_004355)-3′-ROS1(x33-43 NM_002944) breakpoints CD74 intron 6, ROS1 intron 32. (C) Computed tomography and magnetic resonance images of the patient’s right anterior diaphragmatic lymph node, right lower lobe nodule, and left cerebellar metastases before and at the indicated times after he initiated treatment with brigatinib. A radiologic unconfirmed partial response by Response Evaluation Criteria in Solid Tumors 1.1 was achieved after 3 months in the target lesion (right lower lobe nodule), with concurrent response in the right anterior diaphragmatic lymph node and stable cerebellar metastases. CR, complete response; PD, progressive disease; PR, partial response; uPR, unconfirmed partial response; SD, stable disease.
CASE REPORT
A 77-year-old white man with a prior history of smoking presented with shortness of breath. Chest radiograph revealed a right lower lobe (RLL) nodule. Computed tomography (CT) scan of the chest confirmed a 1.5-cm irregular nodular opacity within the RLL. Biopsy indicated a cytokeratin 7– and thyroid transcription factor-1–positive adenocarcinoma. He underwent RLL lobectomy, which confirmed T2aN1M0, stage IIA NSCLC. Hot spot molecular testing was negative for EGFR, KRAS, and BRAF mutations, and fluorescence in situ hybridization was negative for ALK gene rearrangement. He received adjuvant carboplatin and pemetrexed. Fludeoxyglucose positron emission tomography–CT scan after completing four cycles of chemotherapy showed increasing pleural nodularity. Comprehensive next-generation sequencing of tumor tissue obtained revealed a CD74-ROS1 rearrangement (Fig 1B). Crizotinib 250 mg orally twice daily was initiated. After two cycles of crizotinib, positron emission tomography–CT imaging showed resolution of metastatic disease. He remained disease free for 13 months until a follow-up CT scan of the chest showed relapse, with two RLL nodules. He received stereotactic ablative radiotherapy to two RLL nodules (50 Gy in four fractions). Chest CT scan performed 1 month after stereotactic ablative radiation showed treatment response in one of the two RLL nodules but multiple new pleural nodules. Magnetic resonance imaging (MRI) of the brain also demonstrated new bilateral cerebellar enhancing lesions. Each of the two cerebellar lesions was treated with gamma knife radiosurgery (20 Gy to 50% isodose), and the patient was enrolled in an anti–cytotoxic T-cell lymphocyte-4 antibody ipilimumab and radiation trial (ClinicalTrials.gov identifier: NCT02239900). As per trial protocol, he received external beam radiotherapy to the RLL lesion (60 Gy in 10 fractions) with concurrent ipilimumab. There was disease progression noted in mediastinal lymph nodes and pleural metastasis while on the aforementioned trial. In addition, he developed autoimmune hypophysitis and was off therapy for 3 months.10 Restaging confirmed progression in multiple sites. He was then enrolled in the modular phase II basket SIGNATURE (ClinicalTrials.gov identifier: NCT02186821) trial with ceritinib for ROS1-aberrant cancers at 750 mg orally daily.11 Restaging scans after two cycles and once again after four cycles confirmed a partial response (PR; 56% decrease) per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. In addition, MRI showed reduction in brain metastasis. He continued to have PR while receiving ceritinib for 8 months, until it had to be held for grade 3 elevation in AST and ALT. During this treatment hiatus, he developed new brain metastases and had to be taken off the trial. He received whole-brain radiation therapy (30 Gy in 10 fractions). After multidisciplinary consensus, given his prior response to ceritinib, he restarted ceritinib at 600 mg orally daily off-label. Ceritinib continued to clinically benefit the patient, demonstrating both systemic and CNS activity for another 17 months. Eventually, he experienced progression in brain metastases, mediastinal lymph nodes, and RLL lesions. Plasma cell-free DNA (cfDNA) testing (Guardant panel, Guardant Health, Redwood, CA) showed NOTCH S2435S, TP53 G245A, and P190T, as well as FBXW7 G477S mutations but no CD74-ROS1 fusions or other ROS1 pathway aberrations. Given the preclinical activity of brigatinib in ROS1 fusion–positive cancers,12 he was administered brigatinib 90 mg orally once daily 12 days after discontinuing ceritinib. Four days after starting brigatinib, it was held because of grade 2 fatigue (National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03). Brigatinib was restarted 3 weeks later at 30 mg orally once daily and escalated to 90 mg over the course of 3 days. He continued to receive brigatinib 90 mg daily for 2 more months, with grade 1 fatigue and no other adverse effects. Restaging MRI after two cycles of brigatinib showed stable brain metastases and no new lesions. CT scan of the chest also showed 59% decrease in target lesions by RECIST 1.1 criteria (unconfirmed PR), with decrease in RLL lesions and resolution of mediastinal lymphadenopathy (Fig 1C). Unfortunately, 1 month later he experienced a fall, leading to hospitalization. MRI at this juncture continued to show stable brain metastases with no new lesions. However, owing to a significant decline in performance status (Eastern Cooperative Oncology Group score, 4) due to other comorbidities and prolonged hospitalization, he was transitioned to hospice care.
DISCUSSION
To our knowledge, this is the first report of crizotinib-resistant ROS1-positive NSCLC responding for an extended period of time (25 months), to ceritinib9 and subsequently to brigatinib. Mechanisms of resistance to crizotinib in ROS1-positive NSCLC include mutations involving the ROS1 kinase domain and activation of bypass signaling pathways.13-15 The most common of the ROS1 resistance mutations is the G2032R solvent front mutation that causes steric interference to crizotinib binding.15,16 The G2032R mutation occurs in close to 50% of crizotinib-resistant NSCLC.15 In vitro studies have demonstrated that both ceritinib and brigatinib are unable to overcome the common G2032R resistance mutation.12,17 Various other mutations in the ROS1 kinase domain confer resistance to crizotinib.18-21 Of these, the L2026M gatekeeper mutation maintains sensitivity to ceritinib and brigatinib.12,20 Brigatinib is a TKI that has preclinical activity against ROS1.12 In an in vitro kinase inhibitory screen of more than 300 kinases, ALK (half maximal inhibitory concentration[IC50]; 0.6 nM) was the only kinase inhibited with an IC50 less than 1 nM. Excluding ALK variants, 10 kinases (3% of those assayed) were inhibited by brigatinib with an IC50 within 10-fold of ALK (ie, less than 6 nM), including ROS1 (IC50, 1.9 nM). Brigatinib potently inhibited viability of Ba/F3 cells expressing FIG-, CD74-, SDC4-, or EZR-ROS1 fusions (IC50 from 16 nM to 41 nM) with potency similar to that of crizotinib (IC50, 17 to 52 nM). Introduction of a mutation at the gatekeeper residue (L2026M) of a CD74-ROS1 fusion had no effect on the potency of brigatinib, whereas crizotinib potency was reduced five-fold.12 Biochemical potency of crizotinib, ceritinib, and brigatinib, all of which our patient received, is listed in Appendix Table A1. One explanation is the presence of L2026M or another mutation that conferred resistance to crizotinib but remained sensitive to ceritinib and brigatinib. Another possibility is the re-emergence of the CD74-ROS1 fusion during ipilimumab plus radiation and the subsequent 3-month treatment hiatus because of lack of selection pressure in the absence of crizotinib, rendering the tumor sensitive to ceritinib. However, the lapse between ceritinib and brigatinib therapy was only 12 days, making such a phenomenon less likely.
One of the major limitations of this study is the lack of on- and post-progression biopsies, which are critical to understanding response and/or resistance mechanisms. These were not performed because the patient declined biopsy, considering advanced age, comorbidities, clinical urgency of treatment at progression, and the risk of pneumothorax. Instead, we obtained cfDNA testing (Guardant panel) at disease progression, which was nondiagnostic, either because of ROS1 cfDNA suppression by ongoing ceritinib treatment or limited sensitivity of the cfDNA platform in detecting the ROS1 fusion. Evolving cfDNA platforms will improve our ability to detect these fusions.22,23
Apart from ROS1 resistance mutations, poor CNS penetration of crizotinib is a frequent cause of CNS progression and failure of therapy.24,25 In a phase II study of ceritinib in ROS1-positive NSCLC, 25% and 63% of patients had intracranial response and intracranial disease control, respectively.7 Brigatinib demonstrated superior CNS activity in both preclinical and clinical studies of ALK-rearranged NSCLC.12,26 In an exploratory analysis of the phase I/II ALTA (ClinicalTrials.gov identifier: NCT02094573) trial, brigatinib intracranial response rate was 53%.26 Intracranial response rate was similar in both patients without prior irradiation and those who had progressed after radiation. Although CNS activity of brigatinib in ROS1-positive NSCLC is not documented, it is likely superior to crizotinib and possibly even ceritinib, given the findings in ALK-rearranged NSCLC. The CNS activity of both ceritinib and brigatinib in our patient is noteworthy. Ceritinib induced a response in previously irradiated CNS lesions, and brigatinib was able to maintain intracranial disease control in twice-irradiated CNS metastases. Our patient was refractory to ipilimumab and radiation but responded promptly to subsequent ROS1-directed therapy, once again highlighting the lack of benefit of second-line immune checkpoint monotherapy in patients with certain subsets of oncogene-driven NSCLC.27,28
Several ROS1-directed TKIs are being studied in clinical trials (Table 1). While outcomes of these trials are awaited, ceritinib and brigatinib may still have a role in the treatment of crizotinib-resistant ROS1-positive NSCLC. This report further emphasizes the need for efficacious ROS1 inhibitors with activity against resistance mutations and better CNS penetrance.
TABLE 1.
Ongoing Clinical Trials of ROS1-Targeted Agents
Appendix
TABLE A1.
Biochemical Potency of US Food and Drug Administration–Approved ALK Inhibitors With ROS1 Inhibitory Activity Against ROS-1 and ROS-1 Mutants
Footnotes
Supported in part by the National Institutes of Health/National Cancer Institute under award number P30CA016672 and by the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy Grant No. 1U01 CA180964.
AUTHOR CONTRIBUTIONS
Conception and design: Vivek Subbiah
Financial support: Vivek Subbiah
Administrative support: Funda Meric-Bernstam, Vivek Subbiah
Provision of study material or patients: David S. Hong, Vivek Subbiah
Collection and assembly of data: Aparna Hegde, David S. Hong, Siraj M. Ali, Vivek Subbiah
Data analysis and interpretation: Aparna Hegde, Amini Behrang, Siraj M. Ali, Luke Juckett, Funda Meric-Bernstam, Vivek Subbiah
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
David S. Hong
Stock and Other Ownership Interests: MolecularMatch, Oncoresponse, Presagia
Honoraria: Adaptimmune, Baxter, Merrimack Pharmaceuticals, Bayer
Consulting or Advisory Role: Alpha Insights, Axiom, Adaptimmune, Baxter, Bayer, Genentech, GLG Pharma, Group H, Guidepoint Global, Infinity, Janssen Pharmaceuticals Merrimack, Medscape, Numab, Pfizer, Seattle Genetics, Takeda, Trieza Therapeutics
Research Funding: AbbVie,Adaptimmune, Amgen, Astra-Zeneca, Bayer, BMS, Daiichi-Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa, Lilly, LOXO, Merck, MedImmune, Mirati, MiRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Pfizer, Seattle Genetics, Takeda
Travel, Accommodations, Expenses: Loxo Oncology, Mirna Therapeutics
Siraj M. Ali
Employment: Foundation Medicine
Leadership: Incysus
Stock and Other Ownership Interests: Exelixis, Blueprint Medicines, Agios, Genocea Biosciences
Patents, Royalties, Other Intellectual Property: Patents via Foundation Medicine; patents via Seres Health on microbiome interventions stuff in non-neoplastic disease (I)
Luke Juckett
Employment: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine
Funda Meric-Bernstam
Honoraria: Sumitomo Group, Dialectica
Consulting or Advisory Role: Genentech, Inflection Biosciences, Pieris Pharmaceuticals, ClearLight Diagnostics, Darwin Health, Samsung Bioepis, Spectrum Pharmaceuticals, Aduro Biotech, OrigiMed, Xencor, Debiopharm Group
Research Funding: Novartis, AstraZeneca, Taiho Pharmaceutical, Genentech, Calithera Biosciences, Debiopharm Group, Bayer, Aileron Therapeutics, PUMA Biotechnology, CytomX Therapeutics, Jounce Therapeutics, Zymeworks, Curis, Pfizer, eFFECTOR Therapeutics, AbbVie, Boehringer Ingelheim (I), Guardant Health (Inst)
Vivek Subbiah
Consulting or Advisory Role: MedImmune
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), MultiVir (Inst), Blueprint Medicines (Inst), Loxo Oncology (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), InhibRx (Inst), Exelixis (Inst)
Travel, Accommodations, Expenses: PharmaMar, Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bergethon K, Shaw AT, Ou S-HI, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai W, Li X, Su C, et al. ROS1 fusions in Chinese patients with non-small-cell lung cancer. Ann Oncol. 2013;24:1822–1827. doi: 10.1093/annonc/mdt071. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida A, Kohno T, Tsuta K, et al. ROS1-rearranged lung cancer: A clinicopathologic and molecular study of 15 surgical cases. Am J Surg Pathol. 2013;37:554–562. doi: 10.1097/PAS.0b013e3182758fe6. [DOI] [PubMed] [Google Scholar]
- 4.Chin LP, Soo RA, Soong R, et al. Targeting ROS1 with anaplastic lymphoma kinase inhibitors: A promising therapeutic strategy for a newly defined molecular subset of non-small-cell lung cancer. J Thorac Oncol. 2012;7:1625–1630. doi: 10.1097/JTO.0b013e31826baf83. [DOI] [PubMed] [Google Scholar]
- 5.Ou S-HI, Tan J, Yen Y, et al. ROS1 as a ‘druggable’ receptor tyrosine kinase: Lessons learned from inhibiting the ALK pathway. Expert Rev Anticancer Ther. 2012;12:447–456. doi: 10.1586/era.12.17. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Ou S-HI, Bang Y-J, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim SM, Kim HR, Lee J-S, et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35:2613–2618. doi: 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]
- 8.Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: A single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:1683–1696. doi: 10.1016/S1470-2045(16)30392-8. [DOI] [PubMed] [Google Scholar]
- 9.Subbiah V, Hong DS, Meric-Bernstam F. Clinical activity of ceritinib in ROS1-rearranged non-small cell lung cancer: Bench to bedside report. Proc Natl Acad Sci USA. 2016;113:E1419–E1420. doi: 10.1073/pnas.1522052113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ileana-Dumbrava E, Subbiah V. Autoimmune hypophysitis. Lancet Oncol. 2018;19:e123. doi: 10.1016/S1470-2045(17)30577-6. [DOI] [PubMed] [Google Scholar]
- 11.Kang BP, Slosberg E, Snodgrass S, et al. The signature program: Bringing the protocol to the patient. Clin Pharmacol Ther. 2015;98:124–126. doi: 10.1002/cpt.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Anjum R, Squillace R, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22:5527–5538. doi: 10.1158/1078-0432.CCR-16-0569. [DOI] [PubMed] [Google Scholar]
- 13.Davies KD, Mahale S, Astling DP, et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS One. 2013;8:e82236. doi: 10.1371/journal.pone.0082236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dziadziuszko R, Le AT, Wrona A, et al. An activating KIT mutation induces crizotinib resistance in ROS1-positive lung cancer. J Thorac Oncol. 2016;11:1273–1281. doi: 10.1016/j.jtho.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gainor JF, Tseng D, Yoda S, et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol. doi: 10.1200/PO.17.00063. doi: 10.1200/PO.17.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368:2395–2401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong CR, Bahcall M, Capelletti M, et al. Identification of existing drugs that effectively target NTRK1 and ROS1 rearrangements in lung cancer. Clin Cancer Res. 2017;23:204–213. doi: 10.1158/1078-0432.CCR-15-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davare MA, Saborowski A, Eide CA, et al. Foretinib is a potent inhibitor of oncogenic ROS1 fusion proteins. Proc Natl Acad Sci USA. 2013;110:19519–19524. doi: 10.1073/pnas.1319583110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drilon A, Somwar R, Wagner JP, et al. A novel crizotinib-resistant solvent-front mutation responsive to cabozantinib therapy in a patient with ROS1-rearranged lung cancer. Clin Cancer Res. 2016;22:2351–2358. doi: 10.1158/1078-0432.CCR-15-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res. 2015;21:166–174. doi: 10.1158/1078-0432.CCR-14-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song A, Kim TM, Kim D-W, et al. Molecular changes associated with acquired resistance to crizotinib in ROS1-rearranged non-small cell lung cancer. Clin Cancer Res. 2015;21:2379–2387. doi: 10.1158/1078-0432.CCR-14-1350. [DOI] [PubMed] [Google Scholar]
- 22.Schrock AB, Pavlick D, Klempner SJ, et al. Hybrid capture-based genomic profiling of circulating tumor DNA from patients with advanced cancers of the gastrointestinal tract or anus. Clin Cancer Res. 2018;24:1881–1890. doi: 10.1158/1078-0432.CCR-17-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark TA, Chung JH, Kennedy M, et al. Analytical validation of a hybrid capture-based next-generation sequencing clinical assay for genomic profiling of cell-free circulating tumor DNA. J Mol Diagn. 2018;20:686–702. doi: 10.1016/j.jmoldx.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 25.Patil T, Smith DE, Bunn PA, et al. The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol. 2018;13:1717–1726. doi: 10.1016/j.jtho.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camidge DR, Kim D-W, Tiseo M, et al. Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non–small-cell lung cancer and brain metastases in two clinical trials. J Clin Oncol. 2018;36:2693–2701. doi: 10.1200/JCO.2017.77.5841. [DOI] [PubMed] [Google Scholar]
- 27.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer: A meta-analysis. J Thorac Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Zou HY, Li Q, Engstrom LD, et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci USA. 2015;112:3493–3498. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Facchinetti F, Loriot Y, Kuo MS, et al. Crizotinib-resistant ROS1 mutations reveal a predictive kinase inhibitor sensitivity model for ROS1- and ALK-rearranged lung cancers. Clin Cancer Res. 2016;22:5983–5991. doi: 10.1158/1078-0432.CCR-16-0917. [DOI] [PubMed] [Google Scholar]
- 31.Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov. 2018;8:1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]
- 32.Ardini E, Menichincheri M, Banfi P, et al. Entrectinib, a pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther. 2016;15:628–639. doi: 10.1158/1535-7163.MCT-15-0758. [DOI] [PubMed] [Google Scholar]
- 33.Chong CR, Bahcall M, Capelletti M, et al. Identification of existing drugs that effectively target NTRK1 and ROS1 rearrangements in lung cancer. Clin Cancer Res. 2017;23:204–213. doi: 10.1158/1078-0432.CCR-15-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]