Abstract
This article comments on:
Dixon LE, Pasquariello M, Boden SA. 2020. TEOSINTE BRANCHED1 regulates height and stem internode length in bread wheat. Journal of Experimental Botany 71, 4742–4750.
Keywords: Domestication genes, plant architecture, plant height, TCP transcription factors
Dixon et al. (2020) report a novel role for the TB1 transcription factor gene in wheat, controlling plant height. This gene and its orthologues have long been known to affect branching in a number of crops and plant species. Its involvement in the determination of plant height opens up new avenues for the modification of this key trait, which affects multiple agronomic aspects of annual crops, from emergence to harvest index.
Plant height: key for modern agriculture
Plant height, the distance in centimetres from the ground to the tip of the spike (in wheat, for instance), is a deceptively simple trait with big agronomic repercussions. In the Poaceae family, it is affected by a number of signalling pathways, mainly (but not limited to) gibberellins (GAs), brassinosteroids, and strigolactones. This trait was at the core of the transformation of cereal cultivation spurred by the Green Revolution. Semi-dwarf varieties were an essential part of the genetic–technological package combining new lodging-resistant, high harvest index varieties (a high proportion of assimilates going to the grain), with the application of higher fertilizer rates. The widely known ‘reduced height’ alleles Rht-B1 and Rht-D1 were the protagonists of this story. They were the result of a ‘silver bullet’ approach, which reaped more profits than downsides. Briefly, germplasm was surveyed, and natural mutants with large effects on plant height were found and bred into adapted genetic backgrounds all over the world. This kind of approach still yields good results, as the study by Dixon et al. (2020) reveals. They report a novel effect of TEOSINTE BRANCHED1 (TB1) on wheat height. However, the authors were not taking a shot in the dark. They targeted a domestication gene originally identified in maize, with known effects on plant architecture and fertility in a large number of species, including wheat, as demonstrated in a previous article by the same group (Dixon et al., 2018). Exploring the function of domestication genes across species seems a sensible thing to do. These genes show different outcomes due to alternative modes of regulation, in many cases time and space dependent, rather than to simple differences in protein function (Dong et al., 2019a). They are usually placed high up in gene hierarchy and, therefore, affect different pathways and, ultimately, a variety of phenotypic outputs. Domestication genes are the low-hanging fruits of crop genetics and, apparently, some are still waiting to be fully harvested.
Genes that modulate plant height
The catalogue of major genes affecting plant height in wheat, without causing substantial deleterious agronomic effects, is not large. Even the genes broadly used in wheat breeding still present minor issues, as summarized in Dixon et al. (2020) and previous reports. Essentially, Rht-B1 and Rht-D1 present suboptimal seedling emergence, reduced biomass, and lowered fertility at temperatures above 24 °C. Given this last effect, it is not surprising that their presence in southern European cultivars is scarce (Würschum et al., 2017). Other genes, such as Rht18/24, have been successfully used in breeding since well before their identification as quantitative trait loci (QTLs) (Würschum et al 2017). These genes, and the recently discovered Rht25 (Mo et al., 2018), all act in the GA signalling pathway. Rht8 (Gasperini et al., 2012) is the exception to this rule, as it responds to brassinosteroids, expanding the options for flexible tuning of plant height to breeding targets.
The discovery of new plant height genes is challenging, particularly for loss-of-function alleles, because forward genetics in wheat is complicated by the buffering effect of the homoeologue genes (Adamski et al., 2020). The rich knowledge on Poaceae genes affecting plant height could be leveraged for wheat breeding, through either the search of natural variants, the induction of new alleles through gene editing, or the introduction of genes from wild relatives, barley, or rice. In fact, wheat breeding has exploited mainly the GA signalling pathway so far, while brassinosteroid effects are well known mostly in barley (Dockter and Hansson, 2015), with the mentioned exception of Rht8, and strigolactones in rice (Liu et al., 2018).
The arrival of TB1 is a welcome addition to the catalogue of wheat plant height genes, giving more leeway to breeders worldwide. Recently, Dixon et al. (2018) identified natural variation for TB1 in bread wheat associated with changes in inflorescence architecture (see Box 1). In the present work, the same group goes one step further, indicating that TB1 also regulates plant height, with the bonus of not affecting coleoptile length, and hence not compromising plant emergence. The TB1 height-reducing effect, as described by Dixon et al. (2020), depends on gene dosage. The authors found a highly branched (hb) wheat line, characterized by reduced tillering and the formation of multiple paired spikelets in the inflorescence. The hb line is tetrasomic for chromosome 4D, containing two copies of TB-D1, and had higher expression of TB1 in stems, which limited elongation. The role of TB-D1 was indisputably validated through a transgenic approach. Additionally, the authors provide convincing confirmation of the involvement of another homoeologue, TB-B1, in plant height, via the comparison of two naturally occurring alleles. TB-A1 was found to be weakly expressed and thus not considered. Natural alleles of wheat TB1 genes are shown in Box 1, together with other TILLING alleles predicted to be deleterious in Ensembl Plants (Howe et al., 2020), which could be interesting research materials to confirm the phenotypes observed by Dixon et al. (2020).
Box 1. Natural and TILLING mutants of wheat TB1 homoeologues.
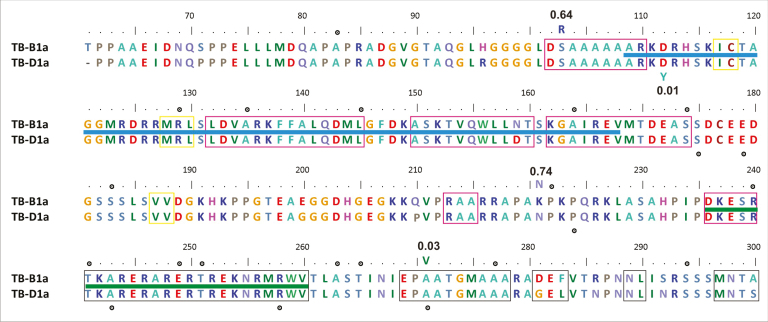
Partial sequence alignment of the TB-B1a and TB-D1a alleles. Residue numbers correspond to TB-B1a. Natural variants found by Dixon et al. (2018, 2020) are indicated next to the SIFT score (bold) computed at Ensembl Plants. Two of them (D112Y and A271V) have scores <0.05 and are thus expected to be deleterious. Large dots mark other deleterious substitutions found in TILLING lines of wheat cultivars Kronos (tetraploid) and Cadenza (hexaploid), which can be browsed at Ensembl Plants (TraesCS4B02G042700.1 and TraesCS4D02G040100.1) and ordered at SEEDSTOR. These and other resources for wheat are reviewed at Adamski et al. (2020). Secondary elements (yellow strands and pink helices), as well as the DNA-binding TCP domain (blue) and the R motif (green), are overlaid to give structural context to the mutants. For instance, in Arabidopsis thaliana, Davière et al. (2014) observed that DELLA proteins can interact with the TCP domain (just for class I TCP transcription factors), and mutants in that region affect plant height.
TB1: ‘a finger in every pie’
It is surprising to realize the number of studies finding new roles for this gene in a large variety of species. The original TB1 was discovered in maize, as the gene underpinning the shift from axillary branching to apical dominance that transformed teosinte into cultivated maize. Numerous orthologues found in other species such as Arabidopsis, barley, rice, or wheat, among many others, share its core functions of negatively regulating axillary bud outgrowth, and modulating inflorescence architecture (Doebley et al., 1997; Takeda et al., 2003; Aguilar-Martínez et al., 2007; Ramsay et al., 2011). TB1 belongs to the Teosinte branched1/Cycloidea/Proliferating cell factor (TCP) class II gene family, a group of phylogenetically related, plant-specific transcription factors that share a non-canonical basic helix–loop–helix motif, the TCP domain (Nicolas and Cubas, 2016). Class II TCP genes are tightly regulated at multiple levels and prevent plant growth and proliferation. The function of the TB1 gene is generally conserved across species, although cases which have several copies are common, and some may have adopted specialized functions (Box 2). Loss-of-function mutations of the gene are associated with increased branching or tillering. Increased dosage or overexpression results in reduced lateral branching, fewer tillers, and reduced culm length. Actually, TB1 involvement in plant height had already been hinted at in wheat, and reported in rice (Choi et al., 2012), as well as in maize (Studer et al., 2017).
Box 2. Number of TB1 orthologues across vascular plants.
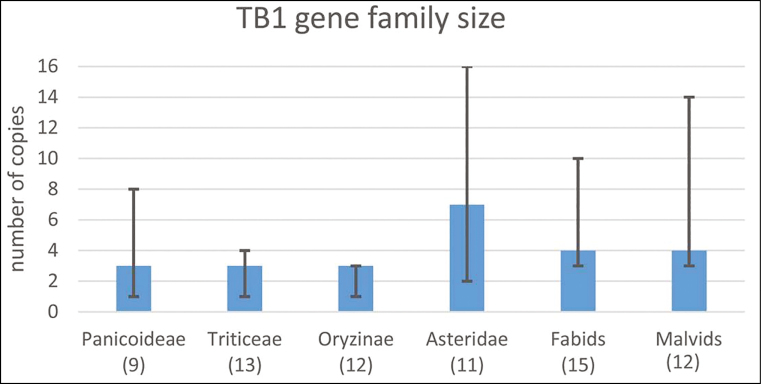
Dixon et al. (2018) discovered that the number of TB1 genes in wheat is key to observe phenotypes. The bars are estimated TB1 gene family sizes for several clades computed from a phylogenetic tree in Ensembl Plants. Note that subgenomes of polyploids were analysed on their own. The number of genomes in each clade is in parentheses. The maximum and minimum copies observed in each clade are also indicated, revealing that this gene family has systematically grown in some clades (Asteridae) or in individual species such as maize (8), soybean (10), or sunflower (16). There is evidence that some of these copies have neofunctionalized (Lyu et al., 2020).
The mechanism of growth repression by TB1 in grasses, or its orthologue gene BRC1 in dicotyledonous species, is still not well known today. TB1-like genes seem to integrate signals from phytohormones (strigolactone, auxin, and cytokinins) and light stimuli (Nicolas and Cubas, 2016; Studer et al., 2017) as part of the shade avoidance syndrome. Regulation of TB1/BRC1 presents similarities and differences between monocots and dicots (Barbier et al., 2019). In the latter group, BRC1 is inhibited by increased sucrose availability and, in rice, it is targeted by IPA1, a functional transcription activator, with profound effects on rice architecture.
As a transcription factor gene, TB1 targets other domestication loci in maize, including teosinte glume architecture1 (tga1) and prol1.1/grassy tillers1 (gt1), as well as its own promoter. It is also involved in regulating biosynthesis and downstream signalling of GAs, abscisic acid, and jasmonic acid (Dong et al., 2019b). TB1/BRC1 pathways are not fully elucidated in any species, but Dong et al. (2019a), in an extensive review, define TB1 as ‘a master regulator operating in a large regulatory hierarchy that targets other domestication loci, all with wide phenotypic effects’. This view is confirmed in many studies which recurrently report that this gene and its orthologues are central integrators in multiple pathways. Among them, evidence in Arabidopsis and wheat demonstrates that TB1 interacts with FLOWERING LOCUS T1 (FT1), a gene well known to all crop scientists, which integrates signals from several flowering time pathways to induce the transition to reproductive growth. TB1 modulates FT1 activity in the axillary buds to prevent premature floral transition (Niwa et al., 2013), reducing FT1-dependent activation of spikelet meristem identity genes (Dixon et al., 2018), which affects spike fertility, hence yield potential. Given the dosage-dependent effect of TB1 and its involvement in a wide diversity of physiological mechanisms, we hypothesize that the number of orthologues of this gene in plant species would provide some hints on the diversity of roles they might have adopted across vascular plants. Box 2 summarizes the number of orthologous copies of TB1 on a variety of plant clades represented in Ensembl Plants.
Further outlook
Future research efforts should aim at elucidating the interactions between genes affecting plant height in cereals. In this respect, the study of their gene regulatory networks, as proposed by Lavarenne et al. (2018), could shed light on their relationships, and point to potential synergies or redundancies that may guide their use, and even find other possible genes of interest. Additionally, wheat breeding would benefit from further research on pleiotropic effects of TB1, on genotype-by-environment interactions (are TB1 effects dependent on temperature or water stress?), and genotype-by-management interactions (such as the response of TB1 to widely applied growth regulators). The dynamics of expression of TB1 underpin the multiple effects observed for this gene and its orthologues. For example, there is no report of TB1 involvement in plant height in barley, but this is not surprising since its expression is detected only in inflorescences, and not in leaves or stems (Rapazote-Flores et al., 2019). Also, TB-A1 expression is almost absent in wheat stems (Dixon et al., 2018). These results indicate that tissue-specific TB1 regulation could be a sensible target for breeding in cereals.
Acknowledgements
The authors receive strategic funding from the Government of Aragón, research group A08-17R, the Spanish Ministry of Science and Innovation [AGL2016-80967-R], the UK Biosciences and Biotechnology Research Council [BB/P016855/1], the National Sciences Foundation [1127112], and the European Molecular Biology Laboratory.
References
- Adamski NM, Borrill P, Brinton J, et al. 2020. A roadmap for gene functional characterisation in crops with large genomes: lessons from polyploid wheat. eLife 9, e55646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. 2007. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. The Plant Cell 19, 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA. 2019. An update on the signals controlling shoot branching. Trends in Plant Science 24, 220–236. [DOI] [PubMed] [Google Scholar]
- Choi M-S, Woo M-O, Koh E-B, Lee J, Ham T-H, Seo HS, Koh H-J. 2012. Teosinte Branched 1 modulates tillering in rice plants. Plant Cell Reports 31, 57–65. [DOI] [PubMed] [Google Scholar]
- Davière J-M, Wild M, Regnault T, Baumberger N, Eisler H, Genschik P, Achard P. 2014. Class I TCP–DELLA interactions in inflorescence shoot apex determine plant height. Current Biology 24, 1923–1928. [DOI] [PubMed] [Google Scholar]
- Dixon LE, Greenwood JR, Bencivenga S, Zhang P, Cockram J, Mellers G, Ramm K, Cavanagh C, Swain SM, Boden SA. 2018. TEOSINTE BRANCHED1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum). The Plant Cell 30, 563–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LE, Pasquariello M, Boden SA. 2020. TEOSINTE BRANCHED1 regulates height and stem internode length in bread wheat (Triticum aestivum). Journal of Experimental Botany 71, 4742–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockter C, Hansson M. 2015. Improving barley culm robustness for secured crop yield in a changing climate. Journal of Experimental Botany 66, 3499–3509. [DOI] [PubMed] [Google Scholar]
- Doebley J, Stec A, Hubbard L. 1997. The evolution of apical dominance in maize. Nature 386, 485–488. [DOI] [PubMed] [Google Scholar]
- Dong Z, Alexander M, Chuck G. 2019a Understanding grass domestication through maize mutants. Trends in Genetics 35, 118–128. [DOI] [PubMed] [Google Scholar]
- Dong Z, Xiao Y, Govindarajulu R, Feil R, Siddoway ML, Nielsen T, Lunn JE, Hawkins J, Whipple C, Chuck G. 2019b The regulatory landscape of a core maize domestication module controlling bud dormancy and growth repression. Nature Communications 10, 3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini D, Greenland A, Hedden P, Dreos R, Harwood W, Griffiths S. 2012. Genetic and physiological analysis of Rht8 in bread wheat: an alternative source of semi-dwarfism with a reduced sensitivity to brassinosteroids. Journal of Experimental Botany 63, 4419–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KL, Contreras-Moreira B, De Silva N, et al. 2020. Ensembl Genomes 2020—enabling non-vertebrate genomic research. Nucleic Acids Research 48, D689–D695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavarenne J, Guyomarc’h S, Sallaud C, Gantet P, Lucas M. 2018. The spring of systems biology-driven breeding. Trends in Plant Science 23, 706–720. [DOI] [PubMed] [Google Scholar]
- Liu F, Wang P, Zhang X, Li X, Yan X, Fu D, Wu G. 2018. The genetic and molecular basis of crop height based on a rice model. Planta 247, 1–26. [DOI] [PubMed] [Google Scholar]
- Lyu J, Huang L, Zhang S, et al. 2020. Neo-functionalization of a Teosinte branched 1 homologue mediates adaptations of upland rice. Nature Communications 11, 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y, Vanzetti LS, Hale I, Spagnolo EJ, Guidobaldi F, Al-Oboudi J, Odle N, Pearce S, Helguera M, Dubcovsky J. 2018. Identification and characterization of Rht25, a locus on chromosome arm 6AS affecting wheat plant height, heading time, and spike development. Theoretical and Applied Genetics 131, 2021–2035. [DOI] [PubMed] [Google Scholar]
- Nicolas M, Cubas P. 2016. TCP factors: new kids on the signaling block. Current Opinion in Plant Biology 33, 33–41. [DOI] [PubMed] [Google Scholar]
- Niwa M, Daimon Y, Kurotani K, et al. 2013. BRANCHED1 interacts with FLOWERING LOCUS T to repress the floral transition of the axillary meristems in Arabidopsis. The Plant Cell 25, 1228–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay L, Comadran J, Druka A, Marshall DF, Thomas WT, Macaulay M, MacKenzie K, Simpson C, Fuller J, Bonar N. 2011. INTERMEDIUM-C, a modifier of lateral spikelet fertility in barley, is an ortholog of the maize domestication gene TEOSINTE BRANCHED 1. Nature Genetics 43, 169–172. [DOI] [PubMed] [Google Scholar]
- Rapazote-Flores P, Bayer M, Milne L, et al. 2019. BaRTv1.0: an improved barley reference transcript dataset to determine accurate changes in the barley transcriptome using RNA-seq. BMC Genomics 20, 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer AJ, Wang H, Doebley JF. 2017. Selection during maize domestication targeted a gene network controlling plant and inflorescence architecture. Genetics 207, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Suwa Y, Suzuki M, Kitano H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M, Ueguchi C. 2003. The OsTB1 gene negatively regulates lateral branching in rice. The Plant Journal 33, 513–520. [DOI] [PubMed] [Google Scholar]
- Würschum T, Langer SM, Longin CFH, Tucker MR, Leiser WL. 2017. A modern Green Revolution gene for reduced height in wheat. The Plant Journal 92, 892–903. [DOI] [PubMed] [Google Scholar]