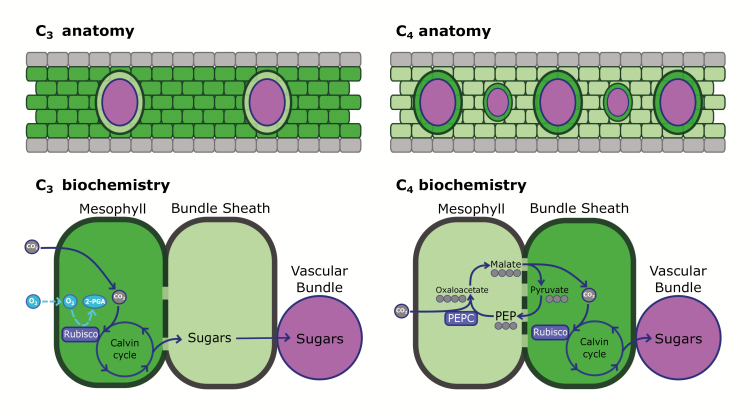
We review possible physiological, anatomical, and evolutionary limitations that may explain the rarity of C4 photosynthesis in trees and discuss how the C4 trees in Euphorbia are exceptions to this.
Keywords: C4 photosynthesis, Chamaesyce, disjunct veins, Euphorbia, Euphorbiaceae, phloem loading, symplastic, trees, quantum yield
Abstract
Since C4 photosynthesis was first discovered >50 years ago, researchers have sought to understand how this complex trait evolved from the ancestral C3 photosynthetic machinery on >60 occasions. Despite its repeated emergence across the plant kingdom, C4 photosynthesis is notably rare in trees, with true C4 trees only existing in Euphorbia. Here we consider aspects of the C4 trait that could limit but not preclude the evolution of a C4 tree, including reduced quantum yield, increased energetic demand, reduced adaptive plasticity, evolutionary constraints, and a new theory that the passive symplastic phloem loading mechanism observed in trees, combined with difficulties in maintaining sugar and water transport over a long pathlength, could make C4 photosynthesis largely incompatible with the tree lifeform. We conclude that the transition to a tree habit within C4 lineages as well as the emergence of C4 photosynthesis within pre-existing trees would both face a series of challenges that together explain the global rarity of C4 photosynthesis in trees. The C4 trees in Euphorbia are therefore exceptional in how they have circumvented every potential barrier to the rare C4 tree lifeform.
Introduction
C4 photosynthesis arises from anatomical and biochemical modifications to the ancestral C3 photosynthetic machinery that serve to concentrate CO2 around Rubisco (Box 1). This CO2-concentrating mechanism (CCM) acts to elevate CO2 assimilation, while functionally increasing the apparent specificity of Rubisco for CO2, over O2, thus minimizing oxygenation and resultant photorespiration (Tcherkez et al., 2006). It follows that the evolution of C4 photosynthesis is favoured by environmental conditions that would promote high rates of photorespiration in C3 species, namely low ambient CO2 concentrations, warmth, bright light, aridity, and salinity (Chollet and Ogren, 1975; Ehleringer et al., 1991; Sage et al., 2018). Since C4 photosynthesis was first observed >50 years ago (Kortschak et al., 1965; Hatch and Slack, 1966), numerous studies have attempted to elucidate exactly which modifications are typically required to assemble the components of C4 physiology (Box 2). To travel the path of C4 evolution, an ancestral C3 progenitor arrived in an environment selective for a C4 benefit (Ehleringer et al., 1997; Sage, 2001a; Sage et al., 2018), and, starting from an initial set of genetic and anatomical pre-adaptations (Monson, 2003; Christin et al., 2013, 2015; Griffiths et al., 2013), evolved developmental and genetic modifications (Stata et al., 2016; Moreno-Villena et al., 2018; Dunning et al., 2019a; Lundgren et al., 2019), navigated energetic constraints (Bellasio and Lundgren, 2016), and underwent progressive optimization (Rondeau et al., 2005; Christin et al., 2009)—or, in some cases, potentially ‘cheated’ this lengthy final step via horizontal gene transfer (Christin et al., 2012a, b; Dunning et al., 2019b). Given that some version of this path has been repeatedly travelled nearly 70 times by diverse plant lineages spanning a wide range of lifeforms and ecological niches (Sage et al., 2011a), it seems unusual that only a single group of true trees (i.e. defined here as tall, perennial, woody lifeforms with secondary growth) has evolved C4 leaves (Pearcy and Troughton, 1975; Table 1). These exceptional trees are members of Euphorbia, a global genus of Euphorbiaceae spanning the semideserts of East Africa to the rainforests of the Pacific Islands, and encompassing growth forms from herbs to xerophytic stem succulents to trees of up to 30 m in height (Horn et al., 2012).
Box 1. C4 photosynthesis arises from both anatomical and biochemical modifications to the ancestral C3 photosynthetic system.
There are several anatomical and biochemical differences that arise during the transition from the C3 photosynthetic system to the C4 CO2-concentrating mechanism (CCM). In C3 plants, the majority of chloroplasts (and associated Rubisco) are localized to the mesophyll, which is largely exposed to ambient CO2 and O2 concentrations. The featureless nature of CO2 and O2 makes them enzymatically hard to distinguish, such that Rubisco has catalytic affinity for both molecules, and will catalyse the carboxylation and oxygenation of RuBP. Because the oxygen availability to Rubisco is high in C3 plants, oxygenation and subsequently photorespiration occur at high rates, especially in high-temperature and low-CO2 conditions. Some C3 plants have evolved higher specificity of Rubisco for CO2 over O2; however, this comes at the cost of slower catalytic turnover (Tcherkez et al., 2006).
The C4 CCM allows plants to avoid this specificity–efficiency trade-off by increasing CO2 concentrations at the Rubisco active site, leading to an apparent increase in the specificity of Rubisco for CO2. In C4 plants, CO2 is biochemically shuttled from the mesophyll into the bundle sheath via the carbonic anhydrase–phosphoenolpyruvate carboxylase (PEPC) system, which builds up CO2 concentrations around Rubisco within bundle sheath cells, drastically reducing the incidence of oxygenation and increasing net carbon assimilation.
To facilitate the C4 cycle, C4 plants have higher bundle sheath to mesophyll area ratios (Hattersley, 1984), often via increased density of vascular bundles (Lundgren et al., 2019). In addition, the connectivity of the mesophyll and bundle sheath cells is enhanced in C4 plants by an increased density of plasmodesmata at the cell interface, which allows for the increased flux of metabolites that is required for a functional C4 cycle (Danila et al., 2016).
Grey, leaf epidermis; dark green, cells with many chloroplasts; light green, cells with fewer chloroplasts; purple circles/ovals, vascular tissue; solid purple lines, photosynthesis; dashed light blue lines, oxygenation; dashed dark green line, mesophyll–bundle sheath interface.
Box 2. Key developments in understanding C4 evolution.
There are numerous evolutionary steps on the path from C3 to C4 (Stata et al., 2019). To understand this complex evolutionary progression, it is most useful to examine it in study systems that contain individuals across the entire spectrum of photosynthetic types from C3 to C4. While there are many fully C3 and C4Euphorbia species, there are only two known C3–C4 intermediates, and this limits the use of Euphorbia as a model for understanding C4 evolution. However, study of the eudicot genus Flaveria has been crucial in understanding the evolutionary transitions from C3–C4 to C4 in particular (Monson and Moore, 1989), while studies of the grass Alloteropsis semialata provide unparalleled insight into C4 evolution owing to its extreme intraspecific photosynthetic diversity, which reduces the confounding effects of long divergence times in phylogenetic analyses and species comparisons (Lundgren et al., 2016; Dunning et al., 2019a). Key developments in these systems have started to pick apart the anatomical and biochemical components required to construct a functional C4 photosynthetic system. The remarkable convergence of the C4 trait across the plant kingdom means that the findings from Flaveria and A. semialata can be applied to other distantly related C4 lineages, such as Euphorbia, despite differences in life history, to understand which stages on the path to C4 might conflict with the tree habit.
High expression of key genes is important in the establishment of a weak C 4 cycle. Moreno-Villena et al. (2018) demonstrated that highly expressed genes in grasses, and also possibly in Flaveria, were preferentially co-opted for C4 photosynthesis regardless of tissue specificity. The importance of high expression levels of C4 cycle genes was further shown by the observed increases in expression of the key C4 genes phosphenolpyruvate carboxykinase (PEPCK) and phosphenolpyruvate carboxylase (PEPC) across the C3 to C3–C4 transition (Dunning et al., 2019a). These genes underwent duplication and a resultant dosage-dependent increase concurrently with their co-option for C4 photosynthesis in Alloteropsis semialata (Bianconi et al., 2018).
C 4 anatomy can evolve via a single developmental change: an increase in vein density. Lundgren et al. (2019) demonstrated that an increase in vein density driven by proliferation of minor veins is sufficient, given necessary anatomical pre-conditions, to produce a functional C4 leaf anatomy and create an evolutionary entry to more complex C4 syndromes in A. semialata. Christin et al. (2013) present the necessary anatomical pre-conditions (or ‘enablers’) in grasses as a high (>15%) proportion of bundle sheath tissue (combination of a short distance between bundle sheaths and large bundle sheath cells), which, when combined with environmental changes, facilitated the emergence of the C4 trait.
A reduction in chloroplast numbers and increased chloroplast size is associated with changes in C 4 metabolic activity. Stata et al. (2016) showed that across C3, C3–C4, and C4 species in the genus Flaveria, chloroplast number and coverage of the mesophyll cell periphery increase with increased strength of C4 metabolism, while increased C4 cycle strength was associated with increased chloroplast size. The reduced chloroplast volume at the mesophyll cell periphery and associated increased cytosolic exposure to the atmosphere could enhance diffusion of CO2 to PEPC, thus facilitating the incorporation of CO2 into the C4 metabolic cycle.
Lateral gene transfer is an important force in C 4 evolution, spreading functional genes among grasses. A total of 59 genes in the genome of A. semialata were laterally acquired from other grasses, including those known to be involved in C4 photosynthesis (Dunning et al., 2019b; Olofsson et al., 2019).
While these anatomical and biochemical modifications that assemble the C4 trait were selected for by increased fitness in hot, dry environments, they have been retained by the entire Hawaiian Euphorbia across multiple transitions from open habitats into the moist forest understorey following the colonization of the Hawaiian Islands, and multiple transitions to the tree habit (Yang et al., 2018). Considering these recent developments in our understanding of C4 evolution, alongside the ecology and biogeography of the Hawaiian radiation, is key to understanding the overall evolutionary progression of the lineage.
Table 1.
Where are C4 trees found?
| Species | Varieties | Form | Geography | Environment | Reference |
|---|---|---|---|---|---|
| Euphorbiaceae | |||||
| Euphorbia olowaluana | Tree (up to 9 m) | Open and subalpine forest | Dry | a, b, d, e, f | |
| E. herbstii* | Tree (3–8 m) | Forest | Mesic to wet | a, c, d, e, f | |
| E. remyi | kauaiensis | Small tree (2–3 m) | Forestb,d,e | Wet b,d,e | a, b, d, e, f |
| E. rockii | rockii b,e grandifolia b,e | Shrub to tree (1–8 m) | Open ridge to forest | Mesic to wet | a, b, d, e, f |
| E. celastroides | lorifolia | Shrub to tree (1–9 m) | Open forest | Dry | a, b, d, e, f |
| E. atrococca | Shrub to small tree (up to 3 m) | Forest | Dry to mesic | a, b, d, e, f | |
| Chenopodiaceae (tribe Salsoleae sensu stricto) | |||||
| Haloxylon persicum** | Large shrub to tree (up to 8 m) | Desert | Dry | g, h, i | |
| H. ammodendron** | Large shrub to tree (up to 8 m) | Desert | Dry | g, h, i |
a Pearcy and Troughton, 1975; bKoutnik, 1987; cRobichaux and Pearcy, 1980; dSporck, 2011 (p70); eYang et al., 2018; fYang, 2012; gSage, 2001a, b; hSage, 2016; iPyankov et al., 1999.
*formerly E. forbesii; **these species have C4 photosynthetic stems, C3 leaf-like cotyledons, and no true leaves, and become arborescent with age.
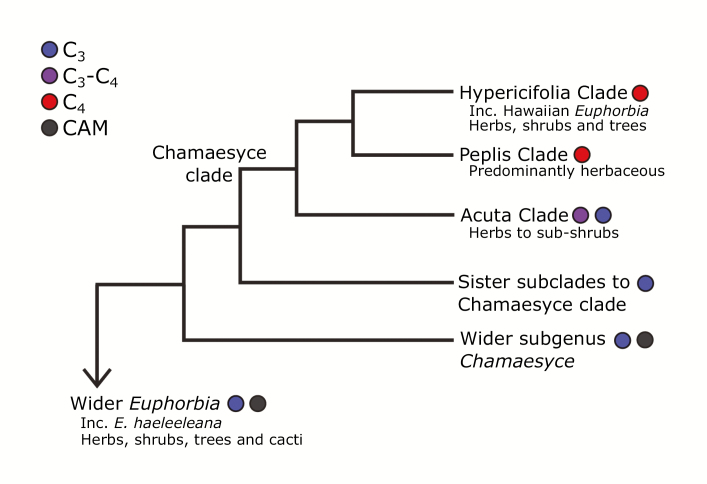
Euphorbia is the only known genus to contain plants using crassulacean acid metabolism (CAM), C3, C3–C4, and C4 photosynthetic types (Yang and Berry, 2011). The C4 lineage in Euphorbia, the largest single C4 lineage among the eudicots, is found within subgenus Chamaesyce, a subclade of Euphorbia that includes C3 and C4 species, as well as C3–C4 evolutionary intermediates (Sage et al., 1999a, 2011b; Yang and Berry, 2011; Box 3). Subgenus Chamaesyce underwent a radiation on the Hawaiian Islands, resulting in 27 taxa that all use C4 photosynthesis, as indicated by δ 13C values spanning –14.5‰ to –12.0 ‰ (Pearcy and Troughton, 1975; Sporck, 2011). The Hawaiian species in Chamaesyce (hereafter referred to as Hawaiian Euphorbia, still recognizing that a separate colonization event led to the origin of a C3 Hawaiian tree not in Chamaesyce, Euphorbia haeleeleana) include a variety of growth forms, from sub-shrub to tree, which exist across a diverse range of environments, from bright and arid habitats to mesic and wet forest understoreys (Table 1). Though there are some relatively shade-tolerant C4 monocots (e.g. Lundgren et al., 2015), shade-tolerant C4 eudicots are rare (Sage et al., 1999b) and thus the ubiquity of C4 photosynthesis across the diverse habitats of Hawaiian Euphorbia is surprising (Pearcy and Troughton, 1975; Sage, 2016). Given that the entire Hawaiian radiation uses C4 photosynthesis, it is likely that the progenitor for this radiation was also a C4 species that arrived on the Hawaiian Islands. This idea is supported by recent phylogenetic work that suggests that the closest relatives of Hawaiian Euphorbia are in fact C4 herbs from the southern USA, Mexico, and/or the Caribbean, and that the woody state evolved after arrival on the Hawaiian Islands (Yang and Berry, 2011; Yang et al., 2018; Box 3).
Box 3. Phylogenetic relationships in Euphorbia and closest relatives of Hawaiian taxa.
C4 photosynthesis is present in all species of the core Chamaesyce, which includes both the Hypercifolia and Peplis clades. A phylogeny of the Chamaesyce clade (Euphorbia subg. Chamaesyce sect. Anisophyllum) identifies four close relatives and possible progenitors of Hawaiian Euphorbia: Euphorbia stictospora, E. velleriflora, E. mendezii, and E. leucantha (Yang and Berry, 2011). These species are members of the Hypercifolia clade and are herbaceous annuals commonly found in the southern USA, northern Mexico, and/or the Caribbean. Euphorbia haeleeleana, a woody C3 species that is part of Euphorbia subg. Euphorbia, represents a separate colonization of the Hawaiian Islands. The closest relatives of this taxa are the Australian succulents E. plumerioides and E. sarcostemmoides, also from subg. Euphorbia (Zimmermann et al., 2010).
There has been periodic interest in Hawaiian Euphorbia, and the wider rarity of C4 trees (e.g. Pearcy and Troughton, 1975; Ehleringer, 1978; Pearcy, 1983; Sporck, 2011; Sage, 2014; Sage and Sultmanis, 2016; Yang et al., 2018); however, there has been little definitive progress towards understanding why C4 trees are indeed so rare. Given that C4 trees do exist, there cannot be any fundamental incompatibility between C4 photosynthesis and the tree habit, or any physiological explanation for the reduced competitive ability of a C4 tree versus a C3 tree that is true under all conditions. Therefore, in explaining the rarity of C4 trees, all the factors that could limit, but not preclude, the evolution of the two syndromes in the same species must be considered, whether that evolution is via adaptation of a C4 progenitor to an environment inhabited by trees, as is likely to be the case for Hawaiian Euphorbia, or via an existing tree traversing the adaptive landscape from C3 to C3–C4 to C4 (Yang and Berry, 2011). This review will consider the key steps on the path to C4 photosynthesis, where these steps might conflict with the tree lifeform, and argue that Hawaiian Euphorbia present a unique opportunity to study the evolution of the C4 trait in trees as a target for future research.
C4 trees may perform poorly under a closed canopy
Rates of photorespiration increase in warm environments, making the C4 pathway—which largely avoids photorespiration—superior to C3 photosynthesis in plants with similar lifeforms (Ehleringer, 1978). As such, C4 grasses frequently dominate in areas with warm climates where trees cannot grow, for example due to high levels of disturbance, while C3 forests—and thus canopies—establish in warm areas where conditions are such that trees thrive. The theory that follows is that C4 trees have failed to become widespread forest species due to their poor performance under canopies, where conditions are cool, shady, and often enriched in CO2 (Sage et al., 1999a). However, this theory may not hold true given insights into the physiological performance of Euphorbia species in the Hawaiian forest understorey (Robichaux and Pearcy, 1980; Pearcy et al., 1985) and, more recently, C4 photosynthesis under low light canopy conditions (Bellasio and Griffiths, 2014; Sage, 2014).
Reduced quantum yield as a limitation
Ehleringer (1978) proposed an early hypothesis that the quantum yield of photosynthesis, defined as the rate of photosynthesis relative to that of photon absorption, is important in determining the distribution of C4 species, especially grasses. Maximum quantum yield is inherently lower in C4 plants than in C3 plants due to the greater energy requirements of the C4 system, though the quantum yield of C3 plants declines with increasing temperature while that of C4 plants remains constant (Long, 1999; Monson, 1999). If below-canopy temperatures are sufficiently cool that the additional energy requirement of the C4 system is greater than the light energy lost to photorespiration in an energetically inexpensive C3 plant, then the quantum yield of the C3 plant would be greater than that of a theoretical C4 tree, and that tree would probably be outcompeted. Indeed, for Atriplex species native to grassland, desert, and coastal strand habitats, the temperature at which the quantum yields of C3 and C4 species are equal is 30 °C, at least at atmospheric CO2 (at that time 325 ppm) and O2 concentrations (Ehleringer and Björkman, 1977). Quantum yield also varies with CO2 concentration, life history, and C4 biochemical subtype. The lower quantum yield of eudicots compared with monocots may contribute to the relative scarcity of shade-tolerant C4 eudicot herbs compared with forest-shade grasses (Monson, 1999). Similarly, the higher quantum yields of plants using the NADP-ME biochemical subtype of C4 photosynthesis (such as Hawaiian Euphorbia) compared with those using the NAD-ME subtype (Ehleringer and Pearcy, 1983; Pearcy and Ehleringer, 1984) may partially explain the shade tolerance of the understorey C4 tree Euphorbia herbstii (formerly E. forbesii), whose quantum yield equals that of an equivalent C3 tree at a leaf temperature of 22–23 °C (approximately the same value as the mean midday leaf temperature at the site where these plants were collected) (Robichaux and Pearcy, 1980).
Direct comparison between the quantum yields of C3 and C4 species, however, does not adequately address the question of whether or not quantum yield could limit the evolution of a C4 tree: the quantum yields of intermediate species on the path from C3 to C4 must be considered. In the incipient C3–C4 phases of C4 evolution in Flaveria, the poor integration of the C3 and C4 cycles causes futile cycling in the C4 assimilation of CO2 and thus reduced quantum yields (Monson et al., 1986; Stata et al., 2019). Inefficient transfer of CO2 from the C4 to the C3 cycle may create an ‘adaptive trough’, expressed through reduced quantum yields in C3–C4 species, compared with fully coupled C3 or C4 taxa, which could act as a barrier to the evolution of C3–C4 traits in species native to shady habitats (Monson, 1989). Thus, the limitations of the C3–C4 intermediate state could make it difficult for the C4 pathway to evolve in a tree under a forest canopy. However, this limitation does not apply where the transition to the forest understorey occurred subsequently to the evolution of the full C4 trait, as was likely to have been the case for Hawaiian Euphorbia (Yang and Berry, 2011).
Poor ability to utilize sunflecks as a limitation
Sage et al. (1999a) proposed that C4 plants may be maladapted to shady understorey environments due to their inefficient utilization of sunflecks, which represent the primary source of light available under the canopy. However, sunfleck use does not seem to be a limiting factor in Euphorbia, as the C4 tree E. herbstii is as efficient in utilizing sunflecks as a comparative C3 tree (Pearcy et al., 1985). Similarly, study of the C4 grass maize shows that, while it responds more slowly to short sunflecks, it otherwise has a similar sunfleck use efficiency to C3 crop species (Krall and Pearcy, 1993), suggesting that C4 plants may not be inherently limited by poor sunfleck exploitation.
Inability to meet the increased energy demands of C4 in the shade
Under low light, C4 plants suffer increased ‘bundle sheath leakiness’—the rate of diffusion of CO2 out of the bundle sheath relative to that of phosphoenolpyruyvate (PEP) carboxylation (Kromdijk et al., 2008). This is driven by a slow down in the C4 carboxylation process due to a reduction in ATP availability under low light. Thus, in the low-light forest interior, the higher leakiness would limit carbon gain and lead to poorer performance of a theoretical C4 tree. However, maize plants grown under diffuse light can acclimate and thereby reduce leakiness, possibly by allocating proportionally more energy to C3 cycle activity to reduce CO2 overcycling, optimizing scarce ATP resources, and then trapping a greater proportion of CO2 in the bundle sheath (Bellasio and Griffiths, 2014).
Disjunct veins, which shade-tolerant Hawaiian Euphorbia species possess (Herbst, 1971), may be another mechanism adapted to tolerate shade. This modification to leaf anatomy allows these species to have a low density of functional veins (i.e. those that are connected to the vascular network) to reduce leaf costs, as is typical of shade species (Sporck, 2011), while establishing islands of bundle sheath tissue to increase the relative bundle sheath tissue area and maintain the close proximity of mesophyll and bundle sheath cells required for a functional C4 system (Box 1). Therefore, it seems that optimization of C4 physiology in combination with modifications to leaf anatomy should allow C4 plants to thrive under the canopy, including C4 trees such as E. herbstii and E. rockii (Table 1). However, not all C4 lineages may be equally primed to adopt these modifications.
Evolutionary factors shaped the pathway to C4 trees
There are several key historical factors and evolutionary ‘opportunities’ that have played a role in shaping the evolution of the C4 trait, and subsequently the tree habit, in Hawaiian Euphorbia.
Evolution of the C4 trait in Euphorbia
First, it is important to consider the age of the eudicot C4 lineages and the timing of C4 evolution relative to historical climatic changes. C4 eudicots are not overall younger than C4 monocots, and all lineages of C4 plants evolved in the low CO2 atmosphere that has shaped plant evolution over the last 30 million years (Christin et al., 2011). This low CO2 atmosphere was probably a key evolutionary opportunity for Euphorbia and is associated with the evolution of at least 17 independent CCMs, which are mostly CAM but also include the C4 lineage (subsect. Hypericifoliae) of Euphorbia subg. Chamaesyce (Horn et al., 2014; Sage, 2016).
Secondly, relatively short generation times in Euphorbia, owing to rapid flowering and high levels of reproductive output per plant, favour the comparatively fast evolution of traits, probably including those associated with C4 photosynthesis, as well as the rapid accumulation of duplicated and neofunctionalized genes as a resource for C4 evolution (Monson, 2003; Emms et al., 2016; Bianconi et al., 2018; Box 2). However, this rapid generation time probably offers less potential evolutionary benefit than lateral gene transfer, which is increasingly recognized as an important force in shaping C4 evolution in the monocots, but has not been documented in C4 eudicots (Christin et al., 2012a, b; Dunning et al., 2019b; Olofsson et al., 2019; Box 2).
Thirdly, lifeform may have also played a role in the evolutionary potential of these plants. The ancestor of the C4 lineage in Euphorbia was likely to have been herbaceous, while all 16–21 independent origins of CAM in Euphorbia occurred in woody ancestors (Horn et al., 2014). While this is only a single example, it suggests that the woody ancestor of Euphorbia may have needed to undergo a transition to the herbaceous lifeform prior to the evolution to C4 photosynthesis, and perhaps the evolution of C4 is less favourable than that of CAM in a woody species. Furthermore, the herbaceous lifeform of the ancestor of Hawaiian Euphorbia facilitated its dispersal to the Hawaiian Islands (Yang and Berry, 2011). Indeed, much of the Hawaiian woody flora evolved from herbaceous ancestors, which had greater dispersal ability to reach the remote Hawaiian Islands (Carlquist, 1970; Panero et al., 1999; Eggens et al., 2007; Dunbar-Co et al., 2008). Before European contact and the influx of invasive species, Hawaii had an environment with available niches along with topographic heterogeneity providing barriers to gene flow (Givnish et al., 2009). Thus, C4 trees or their progenitors (none of which are able to dominate the forest canopy) did not need to invade established C3 communities or areas of high disturbance that typically occlude the tree lifeform, so providing an evolutionary opportunity for a C4 ancestor to transition to the tree habit.
Finally, Euphorbia have a remarkably high degree of variation in morphological characteristics, level of adaptive plasticity, and species richness compared with other plant lineages of similar age (Horn et al., 2012). In particular, there are several characteristics of the ancestral Euphorbia that may be synergistically associated with the origin of the C4 lineage, and thus may have facilitated C4 evolution. The combination of plagiotropic branches and a distichous leaf arrangement maximize the leaf area exposed to sunlight. The co-evolution of C4 photosynthesis with these growth traits would maximize photosynthetic rates and minimize photorespiration rates in high-light, high-temperature environments. In addition, the high adaptive plasticity of Euphorbia may have facilitated the evolution of further adaptations in progenitors of the C4 trees, for example the development of shade-tolerant leaves, circumventing constraints that the C4 state places on phenotypic plasticity (Herbst, 1971; Robichaux and Pearcy, 1980; Sage and McKown, 2006; Horn et al., 2012; Lundgren et al., 2014). It is also worth noting that adaptive plasticity in Hawaiian Euphorbia specifically may have been furthered by their allopolyploid origin resulting in increased heterozygosity (Yang and Berry, 2011).
Evolution of the tree habit in C4Euphorbia
There are many factors that drive, and constrain, the evolution of the tree habit. These include, but are not limited to, protection from animal herbivory, improved dispersal, avoiding (self-)shading, and maintaining water balance. It is unclear what has driven the evolution of trees in Chamaesyce, as Hawaiian Euphorbia tree taxa do not appear to perform better than closely related shrub taxa by measure of abundance.
In terms of evolutionary constraints, in order to diversify to a forest understorey niche, such as that of E. herbstii, a C4 tree must acquire some shade tolerance. However, shade tolerance does not universally constrain the evolution of the tree habit: C4 shrubs can displace grasses in high-light scrubland, as is observed for the C4 shrubs Atriplex confertifolia and A. canescens (Sage and Sultmanis, 2016), and the C4 tree Euphorbia olowaluana is a pioneer species on newly formed Hawaiian lava fields, occurring sparsely in high-light conditions (see fig. 1H in Yang and Berry, 2011). Therefore, the ability to develop shade-tolerant leaves alone does not dictate whether or not a C4 plant can evolve the tree habit, and there may be other factors acting to constrain the evolution of trees in existing C4 lineages, such as the age of these lineages (Sage and Sultmanis, 2016).
The evolution of the tree habit in a herbaceous C4 ancestor requires sufficient evolutionary time following the appearance of the C4 trait. C4 trees, and C4 shrubs that become arborescent with age, are found in two of the oldest C4 eudicot lineages: Euphorbia (19.3 Mya) and tribe Salsoleae sensu stricto (Chenopodiaceae, 23.4 Mya), respectively (Table 1; Sage and Sultmanis, 2016). However, in the case of Hawaiian Euphorbia, 19.3 My is much longer than the time required for the transition from herbaceous to tree lifeform: the initial colonization event of the Hawaiian Islands was ~5 Mya and the true tree species themselves are ~1 My old (Yang et al., 2018). Therefore, it may be more accurate to say that it is not evolutionary time, but the rate of evolution that can act to constrain the transition to a tree lifeform in a C4 lineage. Many eudicot families that have C4 lineages also have trees, but it may be that the C4 state limits the rate at which the tree lifeform can be acquired within C4 lineages by reducing adaptive plasticity (Sage and McKown, 2006; Bellasio and Lundgren, 2016). The aforementioned high adaptive plasticity of Hawaiian Euphorbia, or the fact that the lineage had woody ancestors that had previously undergone a transition to the herbaceous state, may have favoured a comparatively rapid evolution of the tree lifeform in this C4 lineage (Horn et al., 2014). Interestingly, the ancestor of the C4 lineage in Salsoleae may have been a shrub or sub-shrub (Schüssler et al., 2017), so while the transition to true tree has not been completed and thus is slower than that in Euphorbia, they may have been advantaged in this transition by acquiring C4 photosynthesis in an already woody or semi-woody ancestor.
Passive symplastic phloem loading and/or hydraulic limitation negate the benefits of C4 anatomy in trees
Tree height, and indeed the tree growth form, is limited by difficulties in sustaining water and sugar transport over the long pathlength (Ryan and Asao, 2014; Liesche et al., 2017; Savage et al., 2017). We propose that three elements of sugar and water transport design can contribute to limitations on the evolution of C4 trees.
First, trees tend to exhibit high numbers of plasmodesmatal connections between mesophyll cells and minor vein cells that are devoted to phloem loading, a phenotype that is frequently associated with a passive symplastic loading mechanism (Davidson et al., 2011). The persistently strong sugar sinks of trees, which have meristems and storage sites in their trunks and roots, and also high rates of photosynthesis and thus photosynthetic export from leaves, may actually select for passive symplastic phloem loading as it is less energetically demanding than active mechanisms (Turgeon, 2010). C4 species, on the other hand, have a high density of plasmodesmatal contacts between mesophyll and bundle sheath cells to allow for the flux of intermediate metabolites (Sowiński et al., 2008; Danila et al., 2016). In a C4 passive phloem loader, the export of photosynthate would require a large number of plasmodesmatal contacts between bundle sheath and phloem cells. However, this would form a complete plasmodesmatal route from mesophyll to phloem, with C4 intermediates moving from mesophyll to bundle sheath (and back), while sugar loading proceeds from the bundle sheath into the phloem. Due to the passive nature of this process and lack of compartmentalization, it would be difficult to regulate the flux of C4 metabolites; that is, prevent leakage of C4 intermediates into the phloem. Avoiding leakage of C4 sugars may place a limit on phloem loading via plasmodesmata and thus on passive loading.
Secondly, the combination of C4 intermediate diffusion with direct plasmodesmatal pathways for sugar transport from the mesophyll to the bundle sheath and into the phloem might be very difficult to sustain, given that sugar movement would be against the transpiration stream. Theoretical analysis has shown that transpiration-induced bulk flow from veins to stomata and passive sugar loading into the phloem by diffusion can co-exist (Rockwell et al., 2018), but these analyses assumed very low plasmodesmatal fluxes between the bundle sheath and mesophyll, as is typical of C3 species (i.e. without the extensive plasmodesmatal contact typical of C4 species). More work is needed to determine if transpirational counterflow might present an obstacle to a C4 passive loader.
Thirdly, taller trees tend to have upper canopy leaves with both lower leaf water potentials and a greater heterogeneity in cell water potentials, due to the tension associated with gravity, greater resistance pathlengths, and exposure of canopies to strong fluctuations in light and temperature (Zwieniecki et al., 2004; Burgess and Dawson, 2007). Plasmodesmata may lose transport capacity when strong pressure differences are generated between adjacent cells or tissues (Oparka and Prior, 1992). Thus, the C4 pathway, depending on plasmodesmatal transport, may not be feasible at very negative leaf water potentials and/or given the large heterogeneity of water status within the leaf, and so its evolution may be precluded in trees, particularly tall canopy-forming species unlike Hawaiian Euphorbia (Table 1).
Even if these three limitations could be overcome, the increased photosynthetic efficiency of the C4 system combined with the reduced ratio of source tissue to phloem requires an increase in phloem loading efficiency to avoid accumulation of photosynthate in the leaves. In C4 grasses, this is achieved by two mechanisms: first, by up-regulation of active transporters for bundle sheath sugar export, such as maize SWEET-13, a transporter that was duplicated and retained during C4 evolution (Emms et al., 2016); and secondly, by increasing plasmodesmatal density at the interface of the bundle sheath and the vascular parenchyma to increase passive photosynthate transport in plants grown under high-light conditions (Sowiński et al., 2007). Such adjustments would probably not be possible or effective in a C4 tree, in the first instance owing to the absence of an active loading mechanism, and in the second instance due to the aforementioned limitations on plasmodesmatal transport. Inability to increase the maximum rate of phloem loading and associated accumulation of leaf non-structural carbohydrates would result in a downward adjustment of photosynthetic capacity that could partially or fully negate the potential benefits of the C4 system in a tree (Paul and Foyer, 2001; Paul and Pellny, 2003).
How the trees of Hawaiian Euphorbia have circumvented these limitations is unknown, and little is known of their phloem loading mechanism. Notably, the anatomical diversity of Hawaiian Euphorbia is exceptional, from their striking disjunct veins, which vary strongly across species, to their variation in growth form, leaf surfaces, and leaf cross-sectional anatomy (Herbst, 1971; Koutnik, 1987; Horn et al., 2012). More work is needed to discover how phloem loading relates to this diversity, and if a specialized anatomy evolved that mitigates limitations on C4 tree evolution dictated by phloem processes.
Conclusions
Previous commentary on the rarity of C4 trees has pointed towards hypotheses of physiology and life history, and found that there is no single explanation that is satisfactory, with Hawaiian Euphorbia (and possibly Salsoleae sensu stricto, Table 1) acting as exceptions to every argument. Each hypothesis seems to have a caveat, whereby if a species is exceptionally shade tolerant, is markedly efficient at sunfleck use, utilizes a particular C4 biochemical subtype, is notably morphologically diverse, evolved in an especially low competition environment, or has circumvented difficulties in phloem loading, then it may be the exception to the rule. Only by examining all of the interconnected aspects of a complex trait such as C4 photosynthesis can we begin to understand why a few unique trees have travelled the pathway to C4, whereas other would-be C4 trees cannot complete the journey. New directions into understanding the rarity of C4 trees also merit investigation, including comparative genomic approaches and investigations into any role that the characteristic latex and laticifers of Euphorbia plants may play in overcoming limitations imposed by passive symplastic phloem loading and hydraulic constraints. These Hawaiian Euphorbia represent a crucial resource in advancing our understanding in these areas. However, they are becoming increasingly threatened in their native habitat: 10 taxa are state and federally listed as endangered and several others are observed to be rare (MJS-K, unpublished data). The narrow endemism of these species and lack of appropriate, protected habitats for conservation mean they are vulnerable to fires, invasive species, human activity, and climate change.
With Hawaiian Euphorbia most probably arising from a herbaceous-to-woody transition in a C4 ancestor (Yang and Berry, 2011), it is still unclear whether an existing tree could evolve the C4 trait, and there are currently no known C3–C4 intermediate tree species to indicate that this is a possibility. Indeed, the low quantum yield of these C3–C4 intermediates could mean that, at least under an existing canopy, the transition to C4 would require the traversal of an adaptive trough (Monson et al., 1986; Stata et al., 2019). Additionally, the occurrence of passive symplastic phloem loading could also act as a barrier to C4 evolution in existing trees. While these considerations may not apply to the evolution of a tree habit in a C4 ancestor, this alternative evolutionary path is still constrained by adaptive plasticity (Sage and McKown, 2006; Bellasio and Lundgren, 2016). Thus, both routes to the evolution of a C4 tree are potentially tortuous, which may together explain the global rarity of C4 tree species.
Acknowledgements
SNRY is funded by a Natural Environment Research Council Envision Doctoral Training Partnership studentship. MRL is funded by a Leverhulme Early Career Fellowship (ECF-2018-302).
References
- Bellasio C, Griffiths H. 2014. Acclimation to low light by C4 maize: implications for bundle sheath leakiness. Plant, Cell & Environment 37, 1046–1058. [DOI] [PubMed] [Google Scholar]
- Bellasio C, Lundgren MR. 2016. Anatomical constraints to C4 evolution: light harvesting capacity in the bundle sheath. New Phytologist 212, 485–496. [DOI] [PubMed] [Google Scholar]
- Bianconi ME, Dunning LT, Moreno-Villena JJ, Osborne CP, Christin PA. 2018. Gene duplication and dosage effects during the early emergence of C4 photosynthesis in the grass genus Alloteropsis. Journal of Experimental Botany 69, 1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SS, Dawson TE. 2007. Predicting the limits to tree height using statistical regressions of leaf traits. New Phytologist 174, 626–636. [DOI] [PubMed] [Google Scholar]
- Carlquist S. 1970. Hawaii: a natural history. New York: Natural History Press. [Google Scholar]
- Chollet R, Ogren WL. 1975. Regulation of photorespiration in C3 and C4 species. The Botanical Review 41, 137–179. [Google Scholar]
- Christin PA, Arakaki M, Osborne CP, Edwards EJ. 2015. Genetic enablers underlying the clustered evolutionary origins of C4 photosynthesis in angiosperms. Molecular Biology and Evolution 32, 846–858. [DOI] [PubMed] [Google Scholar]
- Christin PA, Edwards EJ, Besnard G, Boxall SF, Gregory R, Kellogg EA, Hartwell J, Osborne CP. 2012a Adaptive evolution of C4 photosynthesis through recurrent lateral gene transfer. Current Biology 22, 445–449. [DOI] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proceedings of the National Academy of Sciences, USA 110, 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Sage RF, Arakaki M, Edwards EJ. 2011. C4 eudicots are not younger than C4 monocots. Journal of Experimental Botany 62, 3171–3181. [DOI] [PubMed] [Google Scholar]
- Christin PA, Petitpierre B, Salamin N, Büchi L, Besnard G. 2009. Evolution of C4 phosphoenolpyruvate carboxykinase in grasses, from genotype to phenotype. Molecular Biology and Evolution 26, 357–365. [DOI] [PubMed] [Google Scholar]
- Christin PA, Wallace MJ, Clayton H, Edwards EJ, Furbank RT, Hattersley PW, Sage RF, Macfarlane TD, Ludwig M. 2012b Multiple photosynthetic transitions, polyploidy, and lateral gene transfer in the grass subtribe Neurachninae. Journal of Experimental Botany 63, 6297–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danila FR, Quick WP, White RG, Furbank RT, von Caemmerer S. 2016. The metabolite pathway between bundle sheath and mesophyll: quantification of plasmodesmata in leaves of C3 and C4 monocots. The Plant Cell 28, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A, Keller F, Turgeon R. 2011. Phloem loading, plant growth form, and climate. Protoplasma 248, 153–163. [DOI] [PubMed] [Google Scholar]
- Dunbar-Co S, Wieczorek AM, Morden CW. 2008. Molecular phylogeny and adaptive radiation of the endemic Hawaiian Plantago species (Plantaginaceae). American Journal of Botany 95, 1177–1188. [DOI] [PubMed] [Google Scholar]
- Dunning LT, Moreno-Villena JJ, Lundgren MR, et al. 2019. a Key changes in gene expression identified for different stages of C4 evolution in Alloteropsis semialata. Journal of Experimental Botany 70, 3255–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning LT, Olofsson JK, Parisod C, et al. 2019. b Lateral transfers of large DNA fragments spread functional genes among grasses. Proceedings of the National Academy of Sciences, USA 116, 4416–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggens F, Popp M, Nepokroeff M, Wagner WL, Oxelman B. 2007. The origin and number of introductions of the Hawaiian endemic Silene species (Caryophyllaceae). American Journal of Botany 94, 210–218. [DOI] [PubMed] [Google Scholar]
- Ehleringer J, Björkman O. 1977. Quantum yields for CO2 uptake in C3 and C4 plants: dependence on temperature, CO2, and O2 concentration. Plant Physiology 59, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J, Pearcy RW. 1983. Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiology 73, 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR. 1978. Implications of quantum yield differences on the distributions of C3 and C4 grasses. Oecologia 31, 255–267. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112, 285–299. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Sage RF, Flanagan LB, Pearcy RW. 1991. Climate change and the evolution of C4 photosynthesis. Trends in Ecology and Evolution 6, 95–99. [DOI] [PubMed] [Google Scholar]
- Emms DM, Covshoff S, Hibberd JM, Kelly S. 2016. Independent and parallel evolution of new genes by gene duplication in two origins of C4 photosynthesis provides new insight into the mechanism of phloem loading in C4 species. Molecular Biology and Evolution 33, 1796–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish TJ, Millam KC, Mast AR, Paterson TB, Theim TJ, Hipp AL, Henss JM, Smith JF, Wood KR, Sytsma KJ. 2009. Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae). Proceedings of the Royal Society B: Biological Sciences 276, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H, Weller G, Toy LF, Dennis RJ. 2013. You’re so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant, Cell & Environment 36, 249–261. [DOI] [PubMed] [Google Scholar]
- Hatch MD, Slack CR. 1966. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. The Biochemical Journal 101, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley PW. 1984. Characterization of C4 type leaf anatomy in grasses (Poaceae). mesophyll:bundle sheath area ratios. Annals of Botany 53, 163–180. [Google Scholar]
- Herbst D. 1971. Disjunct foliar veins in hawaiian Euphorbias. Science 171, 1247–1248. [DOI] [PubMed] [Google Scholar]
- Horn JW, van Ee BW, Morawetz JJ, Riina R, Steinmann VW, Berry PE, Wurdack KJ. 2012. Phylogenetics and the evolution of major structural characters in the giant genus Euphorbia L. (Euphorbiaceae). Molecular Phylogenetics and Evolution 63, 305–326. [DOI] [PubMed] [Google Scholar]
- Horn JW, Xi Z, Riina R, Peirson JA, Yang Y, Dorsey BL, Berry PE, Davis CC, Wurdack KJ. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68, 3485–3504. [DOI] [PubMed] [Google Scholar]
- Kortschak HP, Hartt CE, Burr GO. 1965. Carbon dioxide fixation in sugarcane leaves. Plant Physiology 40, 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutnik DL. 1987. A taxonomic revision of the Hawaiian species of the genus Chamaesyce (Euphorbiaceae). Allertonia 4, 331–388. [Google Scholar]
- Krall JP, Pearcy RW. 1993. Concurrent measurements of oxygen and carbon dioxide exchange during lightflecks in maize (Zea mays L.). Plant Physiology 103, 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Schepers HE, Albanito F, Fitton N, Carroll F, Jones MB, Finnan J, Lanigan GJ, Griffiths H. 2008. Bundle sheath leakiness and light limitation during C4 leaf and canopy CO2 uptake. Plant Physiology 148, 2144–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesche J, Pace MR, Xu Q, Li Y, Chen S. 2017. Height-related scaling of phloem anatomy and the evolution of sieve element end wall types in woody plants. New Phytologist 214, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP. 1999. Environmental responses. In: Sage RF, Monson RK, eds. C4 plant biology. Academic Press, 215–249. [Google Scholar]
- Lundgren MR, Besnard G, Ripley BS, et al. 2015. Photosynthetic innovation broadens the niche within a single species. Ecology Letters 18, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Christin PA, Escobar EG, Ripley BS, Besnard G, Long CM, Hattersley PW, Ellis RP, Leegood RC, Osborne CP. 2016. Evolutionary implications of C3–C4 intermediates in the grass Alloteropsis semialata. Plant, Cell & Environment 39, 1874–1885. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Dunning LT, Olofsson JK, et al. 2019. C4 anatomy can evolve via a single developmental change. Ecology Letters 22, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren MR, Osborne CP, Christin PA. 2014. Deconstructing Kranz anatomy to understand C4 evolution. Journal of Experimental Botany 65, 3357–3369. [DOI] [PubMed] [Google Scholar]
- Monson RK. 1989. On the evolutionary pathways resulting in C4 photosynthesis and crassulacean acid metabolism. Advances in Ecological Research 19, 57–110. [Google Scholar]
- Monson RK. 1999. The origins of C4 genes and evolutionary pattern in the C4 metabolic phenotype. In: Sage RF, Monson RK, eds. C4 plant biology. Academic Press, 377–410. [Google Scholar]
- Monson RK. 2003. Gene duplication, neofunctionalization, and the evolution of C4 photosynthesis. International Journal of Plant Sciences 164(3 Suppl), S43–S54. [Google Scholar]
- Monson RK, Moore BD. 1989. On the significance of C3–C4 intermediate photosynthesis to the evolution of C4 photosynthesis. Plant, Cell & Environment 12, 689–699. [Google Scholar]
- Monson RK, Moore BD, Ku MS, Edwards GE. 1986. Co-function of C3- and C4-photosynthetic pathways in C3, C4 and C3–C4 intermediate Flaveria species. Planta 168, 493–502. [DOI] [PubMed] [Google Scholar]
- Moreno-Villena JJ, Dunning LT, Osborne CP, Christin PA. 2018. Highly expressed genes are preferentially co-opted for C4 photosynthesis. Molecular Biology and Evolution 35, 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Prior DAM. 1992. Direct evidence for pressure-generated closure of plasmodesmata. The Plant Journal 2, 741–750. [Google Scholar]
- Olofsson JK, Dunning LT, Lundgren MR, et al. 2019. Population-specific selection on standing variation generated by lateral gene transfers in a grass. Current Biology 29, 3921–3927. [DOI] [PubMed] [Google Scholar]
- Panero JL, Francisco-Ortega J, Jansen RK, Santos-Guerra A. 1999. Molecular evidence for multiple origins of woodiness and a New World biogeographic connection of the Macaronesian island endemic Pericallis (Asteraceae: Senecioneae). Proceedings of the National Academy of Sciences, USA 96, 13886–13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH. 2001. Sink regulation of photosynthesis. Journal of Experimental Botany 52, 1383–1400. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Pellny TK. 2003. Carbon metabolite feedback regulation of leaf photosynthesis and development. Journal of Experimental Botany 54, 539–547. [DOI] [PubMed] [Google Scholar]
- Pearcy RW. 1983. The light environment and growth of C3 and C4 tree species in the understory of a Hawaiian forest. Oecologia 58, 19–25. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Ehleringer JR. 1984. Comparative ecophysiology of C3 and C4 plants. Plant, Cell & Environment 7, 1–13. [Google Scholar]
- Pearcy RW, Osteryoung K, Calkin HW. 1985. Photosynthetic responses to dynamic light environments by hawaiian trees: time course of CO2 uptake and carbon gain during sunflecks. Plant Physiology 79, 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy RW,, Troughton J. 1975. C4 photosynthesis in tree form Euphorbia species from Hawaiian rainforest sites. Plant Physiology 55, 1054–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyankov VI, Black CC, Artyusheva EG, Voznesenskaya EV, Ku MSB, Edwards GE. 1999. Features of photosynthesis in Haloxylon species of Chenopodiaceae that are dominant plants in Central Asian Deserts. Plant & Cell Physiology 40, 125–134. [Google Scholar]
- Robichaux RH, Pearcy RW. 1980. Photosynthetic responses of C3 and C4 species from cool shaded habitats in Hawaii. Oecologia 47, 106–109. [DOI] [PubMed] [Google Scholar]
- Rockwell FE, Gersony JT, Holbrook NM. 2018. Where does Münch flow begin? Sucrose transport in the pre-phloem path. Current Opinion in Plant Biology 43, 101–107. [DOI] [PubMed] [Google Scholar]
- Rondeau P, Rouch C, Besnard G. 2005. NADP-malate dehydrogenase gene evolution in Andropogoneae (Poaceae): gene duplication followed by sub-functionalization. Annals of Botany 96, 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MG, Asao S. 2014. Phloem transport in trees. Tree Physiology 34, 1–4. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2001a Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Biology 3, 202–213. [Google Scholar]
- Sage RF. 2001b C4 plants. In: Levin SA, ed. Encyclopedia of biodiversity. Elsevier, 575–598. [Google Scholar]
- Sage RF. 2014. Stopping the leaks: new insights into C4 photosynthesis at low light. Plant, Cell & Environment 37, 1037–1041. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2016. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. Journal of Experimental Botany 67, 4039–4056. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin PA, Edwards EJ. 2011a The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage RF, Li M, Monson RK. 1999a The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 plant biology. Academic Press, 551–584. [Google Scholar]
- Sage RF, McKown AD. 2006. Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? Journal of Experimental Botany 57, 303–317. [DOI] [PubMed] [Google Scholar]
- Sage RF, Monson RK, Ehleringer JR, Adachi S, Pearcy RW. 2018. Some like it hot: the physiological ecology of C4 plant evolution. Oecologia 187, 941–966. [DOI] [PubMed] [Google Scholar]
- Sage TL, Sage RF, Vogan PJ, Rahman B, Johnson DC, Oakley JC, Heckel MA. 2011b The occurrence of C2 photosynthesis in Euphorbia subgenus Chamaesyce (Euphorbiaceae). Journal of Experimental Botany 62, 3183–3195. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sultmanis S. 2016. Why are there no C4 forests? Journal of Plant Physiology 203, 55–68. [DOI] [PubMed] [Google Scholar]
- Sage RF, Wedin DA, Li M. 1999b The biogeography of C4 photosynthesis: patterns and controlling factors. In: Sage RF, Monson RK, eds. C4 plant biology. Academic Press, 313–373. [Google Scholar]
- Savage JA, Beecher SD, Clerx L, Gersony JT, Knoblauch J, Losada JM, Jensen KH, Knoblauch M, Holbrook NM. 2017. Maintenance of carbohydrate transport in tall trees. Nature Plants 3, 965–972. [DOI] [PubMed] [Google Scholar]
- Schüssler C, Freitag H, Koteyeva N, Schmidt D, Edwards G, Voznesenskaya E, Kadereit G. 2017. Molecular phylogeny and forms of photosynthesis in tribe Salsoleae (Chenopodiaceae). Journal of Experimental Botany 68, 207–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowiński P, Bilska A, Barańska K, Fronk J, Kobus P. 2007. Plasmodesmata density in vascular bundles in leaves of C4 grasses grown at different light conditions in respect to photosynthesis and photosynthate export efficiency. Environmental and Experimental Botany 61, 74–84. [Google Scholar]
- Sowiński P, Szczepanik J, Minchin PE. 2008. On the mechanism of C4 photosynthesis intermediate exchange between Kranz mesophyll and bundle sheath cells in grasses. Journal of Experimental Botany 59, 1137–1147. [DOI] [PubMed] [Google Scholar]
- Sporck MJ. 2011. T he Hawaiian C4 Euphorbia adaptive radiation: an ecophysiological approach to understanding leaf trait diversification. PhD thesis, University of Hawaii, Manoa. [Google Scholar]
- Stata M, Sage TL, Hoffmann N, Covshoff S, Ka-Shu Wong G, Sage RF. 2016. Mesophyll chloroplast investment in C3, C4 and C2 species of the genus Flaveria. Plant & Cell Physiology 57, 904–918. [DOI] [PubMed] [Google Scholar]
- Stata M, Sage TL, Sage RF. 2019. Mind the gap: the evolutionary engagement of the C4 metabolic cycle in support of net carbon assimilation. Current Opinion in Plant Biology 49, 27–34. [DOI] [PubMed] [Google Scholar]
- Tcherkez GG, Farquhar GD, Andrews TJ. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences, USA 103, 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R. 2010. The role of phloem loading reconsidered. Plant Physiology 152, 1817–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. 2012. Phylogenetics and evolution of Euphorbia Subgenus Chamaesyce. PhD thesis, University of Michigan, Ann Arbor. [Google Scholar]
- Yang Y, Berry PE. 2011. Phylogenetics of the Chamaesyce clade (Euphorbia, Euphorbiaceae): reticulate evolution and long-distance dispersal in a prominent C4 lineage. American Journal of Botany 98, 1486–1503. [DOI] [PubMed] [Google Scholar]
- Yang Y, Morden CW, Sporck-Koehler MJ, Sack L, Wagner WL, Berry PE. 2018. Repeated range expansion and niche shift in a volcanic hotspot archipelago: radiation of C4 Hawaiian Euphorbia subgenus Chamaesyce (Euphorbiaceae). Ecology and Evolution 8, 8523–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann NFA, Ritz CM, Hellwig FH. 2010. Further support for the phylogenetic relationships within Euphorbia L. (Euphorbiaceae) from nrITS and trnL-trnF IGS sequence data. Plant Systematics and Evolution 286, 39–58. [Google Scholar]
- Zwieniecki MA, Boyce CK, Holbrook NM. 2004. Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant, Cell and Environment 27, 357–365. [Google Scholar]