We have identified a 31.7 Mbp interval on the short arm of wheat chromosome 4D that is involved in increased Type II susceptibility to Fusarium head blight.
Keywords: Aneuploid, barley, deletion, Fusarium, scab, susceptibility, wheat
Abstract
Fusarium head blight (FHB) causes significant grain yield and quality reductions in wheat and barley. Most wheat varieties are incapable of preventing FHB spread through the rachis, but disease is typically limited to individually infected spikelets in barley. We point-inoculated wheat lines possessing barley chromosome introgressions to test whether FHB resistance could be observed in a wheat genetic background. The most striking differential was between 4H(4D) substitution and 4H addition lines. The 4H addition line was similarly susceptible to the wheat parent, but the 4H(4D) substitution line was highly resistant, which suggests that there is an FHB susceptibility factor on wheat chromosome 4D. Point inoculation of Chinese Spring 4D ditelosomic lines demonstrated that removing 4DS results in high FHB resistance. We genotyped four Chinese Spring 4DS terminal deletion lines to better characterize the deletions in each line. FHB phenotyping indicated that lines del4DS-2 and del4DS-4, containing smaller deletions, were susceptible and had retained the susceptibility factor. Lines del4DS-3 and del4DS-1 contain larger deletions and were both significantly more resistant, and hence had presumably lost the susceptibility factor. Combining the genotyping and phenotyping results allowed us to refine the susceptibility factor to a 31.7 Mbp interval on 4DS.
Introduction
Fusarium head blight (FHB) is an economically important fungal disease of various cereal crop species, in particular wheat (Triticum aestivum) and barley (Hordeum vulgare). In wheat, the primary symptom is the premature bleaching of spikelets that progressively spreads through the head. Infected spikelets produce shrivelled and chalky grain, which can have a significant negative impact on yield. Furthermore, mycotoxins accumulate in infected grain, which are harmful to humans and animal consumers. The most important mycotoxin is deoxynivalenol (DON) which acts as a virulence factor in wheat by promoting the spread of the fungus (Bai et al., 2002; Langevin et al., 2004). Fusarium graminearum and F. culmorum are the most prevalent species responsible for FHB. Both species are capable of producing large quantities of DON (Scherm et al., 2013) and hence tend to be the most aggressive pathogens of wheat.
Resistance to initial infection (Type I) and to the spread of infection through the rachis (Type II) were first proposed by Schroeder and Christensen (1963) and remain the two most widely considered forms of resistance. Numerous small-effect Type II and fewer Type I FHB quantitative trait loci (QTLs) have been reported and are reviewed by Buerstmayr et al. (2009) and more recently by Buerstmayr et al. (2019). In addition to these two main types of FHB resistance, there is resistance to kernel infection (Type III), host tolerance to FHB and/or DON (Type IV), and resistance to the accumulation of DON (Type V) (Boutigny et al., 2008; Gunupuru et al., 2017). Single amino acid changes to the DON target, ribosomal protein L3 (RPL3), have been demonstrated to improve tolerance to DON in yeast, and hence this is a possible target to improve type IV resistance (Mitterbauer et al., 2004; Lucyshyn et al., 2007). Type V resistance is commonly considered to be a component of Type II resistance, as it typically limits disease spread (Gunupuru et al., 2017), and can be subdivided into Class 1, processes that chemically modify DON to a less toxic form; and Class 2, processes that prevent the accumulation of DON and other trichothecene mycotoxins (Boutigny et al., 2008). The most widely reported form of host detoxification of DON is by UDP-glucosyltransferase (UGT) proteins, which glucosylate DON to the less toxic DON-3-O-glucoside (D3G) (Poppenberger et al., 2003). More recent studies have identified other pathways capable of detoxifying DON. For example, bacterial aldo-keto reductases were demonstrated to be involved in epimerizing DON to 3-epi-DON (Hassan et al., 2017; He et al., 2017).
Wheat and barley differ noticeably in Type II resistance. Wheat typically possesses some degree of Type II susceptibility whilst, in contrast, barley is generally highly resistant to fungal spread through the rachis (Langevin et al., 2004). Furthermore, whilst DON has been shown to function as a virulence factor in wheat (Langevin et al., 2004), DON does not appear to possess such a role during infection of barley heads (Maier et al., 2006).
The reasons for this marked difference in Type II susceptibility of wheat and barley are not well understood. Defined genetic stocks of wheat containing all or part of barley chromosomes offer an insight into which barley chromosomes contribute most strongly to Type II FHB resistance and whether this resistance can be expressed, and potentially utilized, in a wheat genetic background. Herein, we report on a series of experiments to establish whether this difference in FHB susceptibility is because barley carries genes conferring resistance, wheat carries genes conferring susceptibility, or whether it is a combination of both factors. Following this, we investigated the location of a major effect identified on wheat chromosome 4D that appears to significantly compromise resistance to FHB spread through the rachis (Type II resistance).
To date, there have been few reports of FHB susceptibility factors. Garvin et al. (2015) identified a spontaneous deletion of a portion of the long arm of 3D, which appeared to be responsible for increased FHB resistance, suggesting that the deleted region carries an FHB susceptibility factor in the cultivar Apogee. Ma et al. (2006) point-inoculated the existing ditelosomic (DT) lines of Chinese Spring that each lack individual chromosome arms. They found that the loss of individual chromosome arms can improve, as well as compromise, FHB resistance (Ma et al., 2006). Their data suggested that some chromosome arms, especially 7AS, 3BL, 7BS, and 4DS, are likely to contain FHB susceptibility factors (Ma et al., 2006). Although the gene(s) underlying Fhb1, the most widely deployed FHB resistance QTL, remains controversial, there is evidence that Fhb1 may be considered a disrupted susceptibility factor (Su et al., 2018, 2019). Plant hormones play an important role in responding to disease. Host response to FHB infection is particularly sensitive to disrupting phytohormone production or perception. Plants insensitive to ethylene and brassinosteroid signalling exhibit increased FHB resistance, which suggests that the fungus is exploiting such physiological processes (Chen et al., 2009; Goddard et al., 2014). There is significant potential in identifying and characterizing susceptibility factors, with the aim of eliminating them from elite cultivars to enhance resistance to FHB and other economically important diseases.
Materials and methods
Plant material
Wheat–barley addition, substitution, and translocation lines were developed at the Hungarian Academy of Sciences, Agricultural Institute, Centre for Agricultural Research, Hungary (Table 1). An independent set of wheat–barley addition lines, of the wheat variety Chinese Spring and the barley donor variety Betzes, were generated by Islam et al. (1981) and obtained from the Genetic Resources Unit at the John Innes Centre, Norwich, UK.
Table 1.
Wheat–barley addition, substitution, translocation, and centric fusion lines used in FHB experiments
| Line abbreviation | Description | Reference |
|---|---|---|
| Mv9kr1 | Martonvasari9 kr1 | Molnar-Lang et al. (1996) |
| 1HS add | Mv9kr1–Igri 1HS disomic addition | Szakacs and Molnar-Lang (2007) |
| 2H add | Mv9kr1–Igri 2H disomic addition | Szakacs and Molnar-Lang (2007) |
| 3H add | Mv9kr1–Igri 3H disomic addition | Szakacs and Molnar-Lang (2007) |
| 4H add | Mv9kr1–Igri 4H disomic addition | Szakacs and Molnar-Lang (2007) |
| 6HS add | Mv9kr1–Igri 6HS disomic addition | Szakacs and Molnar-Lang (2010) |
| 7H add | Mv9kr1–Igri 7H disomic addition | Szakacs and Molnar-Lang (2010) |
| 2D-1H trans | 2DS.2DL-1HS translocation | Nagy et al. (2002) |
| 3HS.3BL centric | 3HS.3BL centric fusion | Nagy et al. (2002) |
| 4H(4D) sub | 4H(4D) wheat–barley substitution | Molnar et al. (2007) |
| 6B-4H trans | 6BS.6BL–4HL translocation | Nagy et al. (2002) |
| 7D-5H trans | 5HS-7DS.7DL wheat–barley translocation | Kruppa et al. (2013) |
The primary wheat parent was Martonvasari 9 kr1 (Mv9kr1) for all lines, and the barley donor parents were Igri or Betzes. Associated references contain detailed descriptions of line generation and composition.
Chinese Spring and its 4D DT lines were acquired from the Germplasm Resource Unit, John Innes Centre, Norwich, UK. The lines DT(4DL) and DT(4DS) lack 4DS and 4DL, respectively. Four homozygous Chinese Spring terminal deletion (CSTD) lines of 4DS, described by Endo and Gill (1996), were obtained from Kansas State University, USA. The lines acquired were 4532 L1 [fraction length (FL)= 0.53], 4532 L2 (FL=0.82), 4532 L3 (FL=0.67), and 4532 L4 (FL=0.77), henceforth referred to as del4DS-1, del4DS-2, del4DS-3, and del4DS-4, respectively.
Marker development and genotyping
Homoeologue non-specific markers were designed to simultaneously amplify fragments of homoeologous genes on 4A, 4B, and 4D. Sequence information of 4D genes and corresponding homoeologous genes was obtained from Ensembl Plants (http://plants.ensembl.org/Triticum_aestivum/Info/Index). Gene names and the physical positions reported correspond to the IWGSC RefSeq v1.1 wheat genome assembly (IWGSC, 2018). Sequence insertions and deletions (indels) between homoeologous gene sequences were exploited to enable distinction of the three resulting PCR products. Forward primers were M13-tailed to enable incorporation of a fluorescent adaptor to PCR products, as described by Schuelke (2000). A total of 37 markers designed as such were used to characterize the deletions in four 4DS CSTD lines (Table 2).
Table 2.
Homoeologue non-specific markers used to genotype four Chinese Spring 4DS terminal deletion lines
| Marker | Forward primer | Reverse primer | Fragment A; B; D (bp) | 4D gene target |
|---|---|---|---|---|
| BH0001 | tgtaaaacgacggccagtTCCTCCAATAAGAAGGTATGTC | TGGCACTGCCCTTATAGCAA | 356; 330; 228 | TraesCS4D02G001400 |
| BH0002 | tgtaaaacgacggccagtTGTCGTTGTTCCAGTTAAAG | TCAGGCGCATCAGACATTTG | 205; 172; 163 | TraesCS4D02G009200 |
| BH0013 | tgtaaaacgacggccagtGGGGAATTGTCCAAAGCGT | TGCAAGAGATGTTGGGATTTT | 211; 155; 207 | TraesCS4D02G014500 |
| BH0003 | tgtaaaacgacggccagtCTCCACTTTATCATTTGAAGACA | ACAAAACCTTTCACATGGCC | 452; 264; 491 | TraesCS4D02G017300 |
| BH0004.2 | tgtaaaacgacggccagtGTGTTCCCATTGTCGCCG | TAGTCCGCCTCCTTGCTCCT | 168; 152; 194 | TraesCS4D02G035700 |
| BH0025.2 | tgtaaaacgacggccagtACAATCCCGAGGTTGCCAGA | CGAAGAGGAGGGCATACATA | 275; 359; 378 | TraesCS4D02G039400 |
| BH0005.2 | tgtaaaacgacggccagtTGGTGCTTCATTATCCTTCTGAT | TGGTGTCCAGAGTAAACTCGATA | 443; 448; 319 | TraesCS4D02G040700 |
| BH0020 | tgtaaaacgacggccagtCGACCTCCTCTCAGCTTTTAG | ATGAGGATACACGGTGCTGC | 304; 193; 220 | TraesCS4D02G045500 |
| BH0029 | tgtaaaacgacggccagtGAGCAGATCTTCAACGTACG | ATCACAAAGGGATGGACCTG | 183; 196; 159 | TraesCS4D02G050300 |
| BH0024 | tgtaaaacgacggccagtAAAGTAAAATCCTCTTCCCTGAG | GCTAAACTTGCTGTCAGACAAG | 274; 298; 389 | TraesCS4D02G051400 |
| BH0006.2 | tgtaaaacgacggccagtGGCCAAGGTGCGTAATCCA | CGCGAGCTGAACACAAGC | 265; 121; 313 | TraesCS4D02G052300 |
| BH0022 | tgtaaaacgacggccagtAGTATTAGGCAATGTGTTCCACT | TGAGAAGGTTCCAAGAACCAAC | 288; 459; 260 | TraesCS4D02G057100 |
| BH0021 | tgtaaaacgacggccagtTCATTCAACATGCAGATCTAGGC | GACAAACTTCAATGGCATAAGC | 123; 155; 130 | TraesCS4D02G065300 |
| BH0014 | tgtaaaacgacggccagtCCATTGCATTCCTTCACTTGT | CGTCGTCCCATACTTCACAAA | 110; 113; 107 | TraesCS4D02G066900 |
| BH0026 | tgtaaaacgacggccagtCGATACACCAGTTAATTGAAATATG | CTAGGAGTTCCTTCATGGACATT | 289; 471; 318 | TraesCS4D02G073200 |
| BH0015.2 | tgtaaaacgacggccagtCACAACTTGTGCAGGTATAACC | GGAAAGTCAAGACAGGCACAA | 198; 346; 426 | TraesCS4D02G074200 |
| BH0008 | tgtaaaacgacggccagtGTATCGACGAAGCCGCAGTT | TTCCGGAGCGTCCTACGACAA | 309; 190; 199 | TraesCS4D02G074500 |
| BH0040 | tgtaaaacgacggccagtGCGCAGTGAGACAAAACTC | AAGTAGAAGAGCAGCGCCAT | 442; 448; 451 | TraesCS4D02G075300 |
| BH0041 | tgtaaaacgacggccagtAACAAATCCATGTGACCCC | CTACAAGGACGCGTGGTTAT | 299; 338; 302 | TraesCS4D02G076000 |
| BH0042 | tgtaaaacgacggccagtCGGACAACATTTCAGGATTTC | ACCGGAACAAGGCTGCAC | 379; 135; 125 | TraesCS4D02G077600 |
| BH0027.3 | tgtaaaacgacggccagtGGTAACATTCCTTTGGTATACTCGG | TGTGCTAAGATCTACAACATC | 303; 350; 266 | TraesCS4D02G078900 |
| BH0032 | tgtaaaacgacggccagtTTGTGGCCTGCTTACATTGC | TGATCTGCAGGTGTTGGC | 317; 305; 300 | TraesCS4D02G079900 |
| BH0033 | tgtaaaacgacggccagtTGCCCGTGTTTTATGCACTG | GGTAAGTAAAATGGGAAGAAAGC | 201; 167; 185 | TraesCS4D02G081000 |
| BH0034 | tgtaaaacgacggccagtCTGCCGTATCTCCAACTC | ATGAGCGCCATCAGGAAC | 209; 297; 217 | TraesCS4D02G082500 |
| BH0035 | tgtaaaacgacggccagtACGCGGACCCGAATTCAAA | TCCTTGGGCATAGAGGAAG | 190; 167; 162 | TraesCS4D02G083100 |
| BH0036 | tgtaaaacgacggccagtATGTTAGCCGTCCTTTGTTTC | TGGCTGACAGCTATACTTCTAGT | 246; 255; 223 | TraesCS4D02G084000 |
| BH0037 | tgtaaaacgacggccagtGACGGACAATTCTTATGATTGTG | TATGTCCTGCCCCTTCTCCAT | 191; 187; 166 | TraesCS4D02G085100 |
| BH0038 | tgtaaaacgacggccagtATCTGCGTCCAGGTGAGC | TCAGCTAAGACAACTGGCAC | 359; 341; 318 | TraesCS4D02G085900 |
| BH0009.3 | tgtaaaacgacggccagtTAGAGGGAGCAGGGATGACAT | TCTCCGTCTGGTTCATTCGT | 106; 103; 111 | TraesCS4D02G087200 |
| BH0010.2 | tgtaaaacgacggccagtACGTGGTCTTCAAATCTGGC | CTGCAATATAAGGTGGCAAATC | 189; 155; 159 | TraesCS4D02G098400 |
| BH0017 | tgtaaaacgacggccagtCAGATTGTACGAACATCTTCTGC | AGCAGAACAAAATCTCATGG | 252; 246; 263 | TraesCS4D02G105100 |
| BH0018 | tgtaaaacgacggccagtGTGAGCAGAGCACCCTCC | CTGCACCACCACAGAAAAGA | 226; 195; 214 | TraesCS4D02G107300 |
| BH0011 | tgtaaaacgacggccagtATGCTCGTCTTCATCGAGGTAA | ATGCATTGCAGACACATCAAG | 128; 160; 135 | TraesCS4D02G114700 |
| BH0012.2 | tgtaaaacgacggccagtGGTCCTTCATGAAGCTTGTTC | GGCAAATAAGAGAGTTGCATAGG | 275; 289; 280 | TraesCS4D02G117800 |
| BH0030 | tgtaaaacgacggccagtGGCAATGTGATCCTGCAGTTC | GCCCAAAGAAATAGCAAGGGAAA | 145; 174; 189 | TraesCS4D02G126600 |
| BH0057 | tgtaaaacgacggccagtGCACATCCTGCTGTACCA | CTCCTTGGGAATCTTAATGCA | 464; 356; 322 | TraesCS4D02G147800 |
| BH0058 | tgtaaaacgacggccagtCCATTTAGATTCATGGCGAT | AGGCATATTGCAAACCCAAC | 190; 315; 179 | TraesCS4D02G149800 |
Primer sequences, fragment sizes (corresponding to the 4A, 4B, and 4D homoeologous gene targets), and the 4D gene target of markers used to characterize the deletion sizes present in four Chinese Spring 4DS terminal deletion lines. The lower case sequence in the forward primer indicates the M13 tail. All markers were amplified at 58 °C annealing temperature.
DNA was extracted from freeze-dried leaf tissue as described by Pallotta et al. (2003). PCRs were prepared using HotStarTaq Mastermix (Qiagen) following the manufacturer’s instructions and amplified using the following steps: 95 °C 15 min; 35 cycles of 95 °C 1 min, 58 °C 1 min, 72 °C 1 min; and 72 °C 10 min. PCR products were separated using an ABI 3730xl DNA analyser (Applied Biosystems) and resolved using Peak Scanner 2 software (Applied Biosystems). Up to five markers were multiplexed following PCR to increase assay efficiency.
Primers were designed to specifically amplify within a 5H barley UGT (HORVU5Hr1G047150), whilst avoiding amplification of wheat orthologues (primer sequences: GATGAGGTTTGAGATTTGCGGA, CACGAGCACAACAGATGAATTCA). PCRs were prepared using Taq Mastermix (Qiagen) and amplified using the following PCR settings: 94 °C 3 min; 35 cycles of 94 °C 30 s, 58 °C 30 s, 72 °C 1 min; and 72 °C 10 min. PCR products were separated on a 0.8% (w/v) agarose gel.
FHB evaluation and statistical analysis
Highly virulent DON-producing isolates of F. graminearum (UK1) or F. culmorum (Fu42) were used in disease experiments. Production of inoculum was carried out as described previously in Gosman et al. (2005). Wheat heads were inoculated at mid-anthesis. The conidial suspension, adjusted to 1×106 spores ml−1, was injected into a spikelet approximately central on the wheat head. The spread of disease symptoms was scored at 2–4 d intervals between 7 and 21 days post-inoculation (dpi). Polytunnel experiments were organized in a randomized complete block design with four replicates each containing four or five plants per line. For the glasshouse experiment, at least 16 plants per lines were randomized and individual inoculated heads were considered as replicates.
Disease data were analysed using a linear mixed model (REML) in Genstat software (v18.1) to assess the variation attributable to line (fixed), inoculation date (fixed), the interaction between line and inoculation date (fixed), and replicate (random), where factors were significant in the model. Data from which residuals were not normally distributed or where residuals did not appear independent of fitted values were log10 transformed, which was sufficient in correcting for these assumptions. Predicted mean and SE values were calculated for lines included in the REML. Pairwise comparisons were made between the wild-type wheat parent/genetic background and the other genotypes tested in each experiment using Fisher’s protected least significant difference. All predicted values generated from transformed data were back-transformed to the original scale for presentation.
DON evaluation and statistical analysis
DON was purified to >98% at IFA-Tulln, as described by Altpeter and Posselt (1994). DON application was carried out on wheat spikes at mid-anthesis, following a protocol modified from Lemmens et al. (2005). Two adjacent spikelets opposite to each other on the wheat head and approximately central on the head were cut with scissors approximately central on the spikelet. At 1–2 h after cutting, 10 µl of DON solution [10 mg ml–1 amended with 0.01% (v/v) Tween-20] was applied to the two outer florets of each cut spikelet, between the palea and lemma. To increase the humidity at the site of DON application, treated wheat heads were bagged. At 48 h post-application, the DON application was repeated, and heads were bagged again. Hence, each treated wheat head received a total application of 0.8 mg of DON. After a further 48 h, crossing bags were removed from the DON-treated heads. The severity of bleaching for each treated wheat head was scored, out of 10, daily between 5 d and 9 d post-application (from the first application). A score of zero was given when no evidence of DON damage was present and a score of 10 was recorded when the spike was completely bleached above the point of DON application. Scores between 1 and 9 were used to record the progressive yellowing and bleaching of the DON-treated wheat heads, which occurred relatively uniformly above the point of DON application in the case of Chinese Spring (Supplementary Fig. S1 at JXB online). After the experiment, DON-treated and untreated heads from each plant were harvested. From each plant with a DON-treated head, a comparable untreated head (with similar spikelet number and head length) was selected for grain weight analysis. Grain number and grain weight data were collected from DON-treated and comparable untreated heads from each plant, to observe any difference in the effect of DON on grain filling.
DON bleaching data and associated grain data were analysed using a REML. In all cases of both DON bleaching data and grain data, transformation of data was not necessary, as residuals appeared to be normally distributed and independent of fitted values based on visual checking. For bleaching data, line was included as a fixed term and replicate as a random term in the model. For DON grain data, the REML model was constructed using line, treatment (DON-treated or untreated heads), and the interaction between line and treatment as fixed terms, and replicate as a random term.
Results
Effect of barley chromosome additions, substitutions, translocations, and centric fusions on Type II FHB susceptibility in the winter wheat variety Martonvasari 9 (Mv9kr1)
FHB point inoculation experiments of the wheat–barley material were conducted twice and are described as experiment 1 (Fig. 1a) and experiment 2 (Fig. 1b) henceforth. The experiments showed very similar results for most of the lines tested. FHB symptoms were always restricted in both barley varieties, Igri and Betzes, and did not spread from the inoculated spikelet. For this reason, Igri and Betzes were only included as control lines in experiment 1 (Fig. 1a). The primary wheat parent, Mv9kr1, was susceptible to the spread of the fungus in both repeats of the experiment.
Fig. 1.
FHB disease above the inoculation point in wheat–barley addition, substitution, translocation, and centric fusion lines from (a) polytunnel experiment 1, including barley parents Igri and Betzes as controls, and (b) polytunnel experiment 2. Predicted means were generated using a linear mixed model. Error bars are ±SE. *P<0.05; **P< 0.01; ***P<0.001 compared with Mv9kr1.
The addition of barley chromosomes 2H (2H add) and 6HS (6HS add) appeared to have no effect on FHB resistance in either experiment. Disease symptoms in these lines were not statistically significantly different from those of Mv9kr1. The 6BS.6BL-4HL translocation (6B-4H trans) was significantly more susceptible than Mv9kr1 (P <0.001 in both experiments). Whilst the 3HS.3BL centric fusion line (3HS.3BL centric) was more highly susceptible in experiment 1 (P <0.001), the line showed similar disease to Mv9kr1 in experiment 2 (P=0.566). The addition of chromosomes 1HS (1HS add) and 7H (7H add), in addition to the 5HS-7DS.7DL wheat–barley translocation (5H-7D trans) and the 2DS.2DL-1HS translocation line (2D-1H trans), all showed highly significant increases in FHB resistance compared with Mv9kr1 (P <0.001 in both experiments for all lines). The 3H addition (3H add) was inconsistent between the two experiments. In experiment 1, the 3H addition was significantly more susceptible to FHB than Mv9kr1 (P=0.004) whilst, in experiment 2, it was significantly more resistant (P<0.001).
A particularly strong resistant phenotype was seen with the 4H(4D) substitution, in which disease was almost entirely restricted to the inoculated spikelet in both experiments (P<0.001 in both instances). In contrast to this, the addition of barley 4H (4H add) showed similar disease levels to Mv9kr1 in experiment 1 (P=0.841, Fig. 1a) and exhibited only a small increase in resistance in experiment 2 (P=0.021, Fig. 1a).
Effect of barley chromosome additions, substitutions, translocations, and centric fusions on Type II FHB susceptibility in the spring wheat variety Chinese Spring
An FHB point inoculation experiment was performed on wheat–barley addition lines of the varieties Chinese Spring and Betzes, respectively (Fig. 2). These lines include addition lines of 5HS and 5HL, which were absent in the lines generated in the Mv9kr1 wheat background. As previously observed, Betzes showed almost no disease spread from the inoculation point. Chinese Spring, on the other hand, showed evidence of disease spread. FHB symptoms in the majority of addition lines were not significantly different from those in Chinese Spring.
Fig 2.
FHB disease, as a percentage of total number of bleached spikelets, from data combined from 13 dpi and 14 dpi. Predicted means were generated using a linear mixed model. Error bars are ±SE. *P=0.05–0.01; ***P<0.001 compared with Chinese Spring.
The 5HL addition line exhibited significantly increased FHB resistance when compared with Chinese Spring (P<0.001), although the line was still significantly more susceptible than Betzes (P=0.042). The 5HS addition line was also statistically significantly more resistant compared with Chinese Spring (P=0.039). A marker targeting the barley UGT gene, HORVU5Hr1G047150, confirmed that this gene was present in Betzes and the 5HL addition line, but was absent in the 5HS addition line (Supplementary Fig. S2). Consistent with the previous experiments, the 4HL and 4HS addition lines both showed similar FHB susceptibility to Chinese Spring.
Type II FHB susceptibility and DON susceptibility in Chinese Spring 4D ditelosomic lines
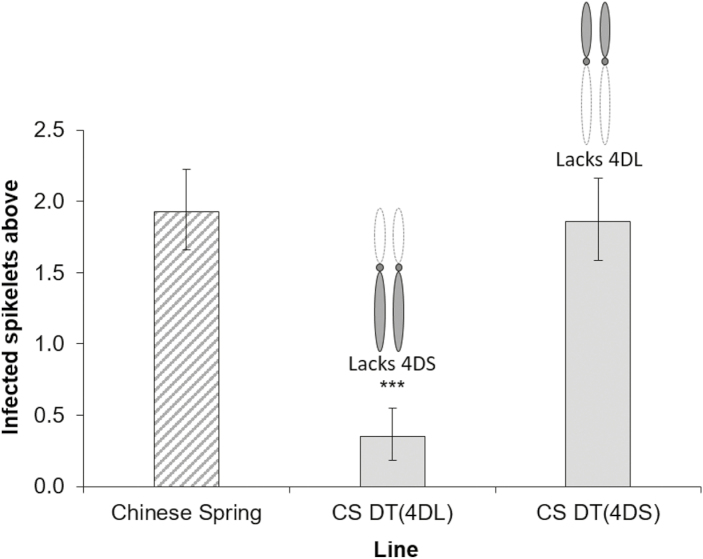
The contrast in the effect of adding 4H or substituting 4D with 4H indicated that the presence of 4D may be responsible for a significant proportion of the susceptibility of both Mv9kr1 and Chinese Spring. To test this possibility, Chinese Spring and two DT lines: DT(4DL) and DT(4DS), missing 4DS and 4DL, respectively, were tested in three independent FHB point inoculation experiments. Data are presented here from a 2013 experiment conducted in a glasshouse, but the results were replicated in a 2013 experiment under controlled conditions and in a polytunnel experiment conducted in 2016. Chinese Spring and DT(4DS), missing 4DL, showed very similar disease symptoms to each other (Fig. 3). In contrast to this, DT(4DL), missing 4DS, was highly resistant to the spread of infection when compared with wild-type Chinese Spring (P<0.001).
Fig. 3.
FHB disease at 17 dpi in euploid Chinese Spring and 4D ditelosomic lines DT(4DL) and DT(4DS), missing 4DS and 4DL, respectively. Diagrams of 4D are included above ditelosomic lines to illustrate their genetic state. Error bars are ±SE. ***P<0.001 compared with Chinese Spring.
DON is widely believed to contribute towards Type II susceptibility by promoting the spread of FHB. Hence, it is possible that the susceptibility factor may be responding to DON and not the fungus itself. To confirm whether DON is involved, we applied purified DON to wheat heads of Chinese Spring and two DT lines; DT(4DL) and DT(4DS). Chinese Spring was moderately susceptible to DON, with an average bleaching score of 3.39 (Fig. 4). DT(4DS), lacking 4DL, was not significantly different from Chinese Spring (mean=2.88; P=0.222) (Fig. 4). On the other hand, DT(4DL), lacking 4DS, was significantly more susceptible to DON-induced bleaching (mean=7.64; P<0.001) (Fig. 4).
Fig. 4.
DON application experiment to heads of Chinese Spring and ditelosomic lines DT(4DL) and DT(4DS), lacking 4DS and 4DL, respectively. (a) Average DON bleaching scores at 7 d post-application. Predicted means were generated using a linear mixed model. Error bars are ±SE. P<0.001 compared with Chinese Spring. (b) DON-treated and untreated mean grain weights (mg) above the application point, or the comparable point in untreated heads, dissected after the experiment. Predicted means were generated using a linear mixed model. Error bars are ±SE. (c) Photograph showing three representative examples of untreated and DON-treated grain taken from above the DON application point, or the comparable point in untreated heads.
Grain was harvested and dissected from DON-treated and untreated heads to assess any difference in grain weight. These data mirrored the bleaching data. Chinese Spring and DT(4DS) showed similar reductions in grain weight when comparing DON-treated and untreated heads (percentage reductions of 50.0% and 48.9%, respectively) (Fig. 4). Average grain weight in untreated heads of DT(4DL) was smaller than in untreated Chinese Spring. However, grain of DON-treated DT(4DL) heads had a proportionally greater reduction in grain weight compared with untreated heads (72.3%) (Fig. 4). The difference is evident when visually comparing treated and untreated grain from the three lines; treated grain from DT(4DL) are visibly smaller than those of Chinese Spring and DT(4DS) (Fig. 4).
These data suggest that DON is not implicated in the function of the susceptibility factor. However, there does appear to be an independent DON resistance factor also on 4DS.
Precise characterization of deletion sizes in Chinese Spring 4DS terminal deletion lines
Experiments using 4D DT lines strongly suggest that the FHB susceptibility attributed to chromosome 4D is isolated to the short arm (4DS). Genotyping was performed on four Chinese Spring lines with terminal deletions on 4DS to verify the deletions present and more precisely position the deletion breakpoint in each line relative to the physical map. Markers were designed that can reliably detect genes on 4D and their homoeologues on 4A and 4B. The ability to detect and distinguish all three homoeologues provides two internal positive controls for each marker when identifying deletions of any particular homoeologue. Up to five markers, tagged using different fluorophores (NED, FAM, PET, or VIC), were multiplexed into a single sample for efficiency, using markers designed to produce PCR product sizes sufficiently different for each gene target and its respective homoeologues when resolved using capillary electrophoresis (Supplementary Fig. S3).
Genotyping was successful in identifying genes and their respective physical positions, flanking the deletion breakpoint in all four 4DS CSTD lines (Table 3). A marker (BH0001) targeting the gene TraesCS4D02G001400 at the extreme distal end of 4DS confirmed that all four lines were true terminal deletions. The terminal deletion in del4DS-2 extends to between 50.6 Mbp and 51.6 Mbp. Line del4DS-4 is deleted up to between 53.9 Mbp and 54.8 Mbp. The deletion in del4DS-3 ends between 83.3 Mbp and 85.6 Mbp. The deletion breakpoint in the CSTD line containing the largest terminal deletion, del4DS-1, ends between 111.1 Mbp and 140.9 Mbp.
Table 3.
Flanking genes and markers of deletion breakpoints in four Chinese Spring 4DS terminal deletion lines
| Line | Left flank gene | Left flank marker | Right flank gene | Right flank marker | Breakpoint interval (kb) |
|---|---|---|---|---|---|
| del4DS-2 | TraesCS4D02G076000 | BH0041 | TraesCS4D02G077600 | BH0042 | 976 |
| del4DS-4 | TraesCS4D02G079900 | BH0032 | TraesCS4D02G081000 | BH0033 | 949 |
| del4DS-3 | TraesCS4D02G105100 | BH0017 | TraesCS4D02G107300 | BH0018 | 2313 |
| del4DS-1 | TraesCS4D02G126600 | BH0030 | TraesCS4D02G147800 | BH0057 | 29776 |
The breakpoint interval is the size of the interval between two adjacent markers where the marker signal was retrieved, indicating the end of the deletion.
Type II FHB susceptibility and DON susceptibility in Chinese Spring terminal deletion lines of 4DS
Euploid Chinese Spring and the four genotyped 4DS CSTD lines (del4DS-2, del4DS-4, del4DS-3, and del4DS-1, in ascending order of terminal deletion size) were point-inoculated in a polytunnel experiment in 2017 (Fig. 5). Chinese Spring showed moderate levels of disease in this experiment, with mean disease above the inoculation point of 1.84 bleached spikelets at 13 dpi. Lines del4DS-2 (P=0.796) and del4DS-4 (P=0.278) showed similar disease levels to that of euploid Chinese Spring (Fig. 5). Lines del4DS-3 and del4DS-1 both had significantly reduced disease with respect to euploid Chinese Spring (P<0.001 for both lines) (Fig. 5).
Fig. 5.
(a) FHB disease above the inoculation point at 13 dpi, following point inoculation of euploid Chinese Spring and four terminal deletion bins: del4DS-2, del4DS-4, del4DS-3, and del4DS-1. Error bars are ±SE. ***P<0.001 compared with Chinese Spring. (b) Representative FHB disease symptoms in the Chinese Spring terminal deletion lines del4DS-4 and del4DS-3 at 16 dpi. (c) Diagrams of 4DS in euploid Chinese Spring and four 4DS terminal deletion lines, as characterized by genotyping with 35 markers spanning 4DS. The spotted interval indicates the breakpoint interval; the distance between two markers where the 4D signal was retrieved. The bottom diagram indicates the interval on 4DS inferred to contain an FHB susceptibility factor (diagonal stripes), following point inoculation of the Chinese Spring terminal deletion lines. Values in bold indicate the physical position in Mbp.
This information was used to infer that the susceptibility factor was present in the two deletion lines carrying the smaller deletions (del4DS-2 and del4DS-4) but was lost in the two lines containing the larger deletions (del4DS-3 and del4DS-1). Hence, the FHB susceptibility factor appears to reside between the deletion breakpoints of del4DS-4 and del4DS-3—a 31.73 Mbp interval (Fig. 5).
The 4DS CSTD lines were also subjected to DON application to refine the position of the DON resistance factor also believed to be on 4DS (Fig. 6). Chinese Spring showed similar levels of bleaching to the previous experiment conducted on DT lines (mean=3.91). The CSTD line containing the smallest terminal deletion, del4DS-2, was significantly more susceptible to DON-induced bleaching than Chinese Spring (mean=4.66, P=0.019). However, del4DS-4 (mean=5.73, P<0.001) del4DS-3 (mean=5.83, P<0.001), and del4DS-1 (mean=6.32, P<0.001) all showed statistically significantly higher susceptibility to DON bleaching symptoms than Chinese Spring (Fig. 6).
Fig. 6.
DON application experiment to heads of Chinese Spring and four terminal deletion lines. (a) Average DON bleaching scores at 8 d post-application. Predicted means were generated using a linear mixed model. Error bars are ±SE. P<0.001 compared with Chinese Spring. (b) DON-treated and untreated mean grain weights (mg) above the application point, or a comparable point in untreated heads, dissected after the experiment. Predicted means were generated using a linear mixed model. Error bars are ±SE.
Whilst all 4DS CSTD lines were significantly more susceptible to DON-induced bleaching than Chinese Spring, a greater differential was observed between del4DS-2 and del4DS-4 than was seen between Chinese Spring and del4DS-2. Grain were dissected from Chinese Spring, del4DS-2, and del4DS-4 to understand whether this result was also reflected in the grain data. Chinese Spring showed a 60.1% reduction in grain weight when comparing DON-treated and untreated grain. The 4DS CSTD lines, del4DS-2 and 4DS-4, showed similarly larger grain weight reductions as Chinese Spring (73.6% and 72.2%, respectively).
Discussion
Previous studies have shown that barley is able to detoxify DON through glucosylation by the UGT HvUGT13248 (Schweiger et al., 2010). This gene has been transgenically expressed in Arabidopsis where it was demonstrated to increase DON resistance (Shin et al., 2012). Furthermore, expression of HvUGT13248 in wheat, under the maize ubiquitin promoter, increased FHB resistance, and transformants were demonstrated to more efficiently convert DON to the less toxic D3G (Li et al., 2015). However, Xing et al. (2018) demonstrated that overexpression of a wheat UGT also increased FHB resistance and reduced the DON concentration in grain. How HvUGT13248 performs in wheat under its native barley promoter has not yet been demonstrated and hence the increase in resistance attributed to HvUGT13248 in wheat may be due to overexpression. HvUGT13248 is encoded by the gene HORVU5Hr1G047150 which is present near the centromere on chromosome 5H (Ensembl Plants). If the breakpoints in the wheat–barley 5HS and 5HL DT addition lines are not centromeric, this may explain the findings relating to the high level of resistance conferred by addition of both 5HS and 5HL. To confirm this, we designed primers specific to the barley copy of the UGT that does not amplify from the orthologous wheat copies in the wheat–barley additions. This assay confirmed that HvUGT13248 was present in the 5HL addition line and was absent in the 5HS addition line. Hence, it is likely that an independent source of FHB resistance is present on 5HS.
In this study, we also found that additions of barley chromosome 7H (7H add) and the short arm of chromosome 1H (1HS add), or the translocation of 1H to 2D (2D-1H trans), significantly increased Type II FHB resistance in the winter wheat variety Mv9kr1. Despite enhanced FHB resistance from the 7H addition to Mv9kr1, the addition of neither 7HS nor 7HL had an effect in the Asian spring wheat cultivar Chinese Spring. 1H addition lines were not available in the Chinese Spring–Betzes addition set, so this could not be compared between populations. These findings suggest that barley contains genes conferring Type II resistance that are lacking in one or both wheat varieties. The addition of barley chromosomes 5H and perhaps 1H and 7H is likely to offer the best opportunity of enhancing FHB resistance, when considering the use of wheat–barley introgressions.
We confirmed that the presence of the short arm of chromosome 4D was increasing Type II FHB susceptibility in three independent experiments. The loss of 4DS [line DT(4DL)] resulted in a high level of FHB resistance, whilst the loss of 4DL [line DT(4DS)] resulted in little change compared with Chinese Spring. Ma et al. (2006) phenotyped Chinese Spring DT lines for FHB susceptibility and they also reported an increase in FHB resistance in the line missing 4DS. Together, these studies strongly suggest that there is an FHB susceptibility factor in both winter (Mv9kr1) and spring (Chinese Spring) wheat genetic backgrounds. We applied purified DON to the 4D DT lines to test whether or not the susceptibility factor is being influenced by DON. However, the loss of 4DS resulted in increased DON susceptibility, assessed both by scoring DON-induced bleaching and by comparing grain weights. This would indicate that there is an independent DON resistance factor present on 4DS and that the susceptibility factor is increasing susceptibility to the fungus.
Endo and Gill (1996) developed a set of terminal deletion lines in Chinese Spring. The lines have deletions from the ends of each chromosome arm, varying in size. These stocks are a valuable resource for physically mapping genes to a defined interval of a chromosome arm. The lines were characterized using C-banding and the deletion size was breported as an FL value, effectively the proportion of the chromosome arm estimated to have been retained. C-banding is unlikely to be capable of reliably detecting more complex deletions, such as interstitial deletions or chromosome substitutions. Since their development, the Chinese Spring terminal deletion stocks have not been more precisely characterized using more recent advancements in genotyping. We have genotyped four lines containing terminal deletions of 4DS, using a total of 37 novel homoeologue non-specific markers spanning the chromosome arm. These markers take advantage of the hexaploid nature of wheat to create a robust genotyping assay for the detection of deletions on 4DS, and homoeologous regions on 4BS and 4AL. A similar assay was used by Chia et al. (2017) to verify deletions across homoeologous regions, but this study expands on this technique, using a much higher density of markers to characterize deletion size. Homoeologous genes are simultaneously amplified with a single pair of primers but are distinguishable due to differences in the size of PCR products corresponding to the A, B, and D genome copies. The signals from the retained homoeologues act as internal controls for a deletion in any homoeologue; in this case, the 4D copy. This technique verified that all four 4DS CSTD lines were indeed true terminal deletions and that the size of the deletions was consistent with the FL values calculated by Endo and Gill (1996). For the lines del4DS-2 and del4DS-4, the physical position of the deletion endpoint was determined to intervals <1 Mbp. The interval containing the deletion endpoint in del4DS-3 has been refined to ~2.3 Mbp. The breakpoint in the largest deletion, del4DS-1, was less precisely characterized (29.8 Mbp) because FHB susceptibility was associated with the interval between the deletion breakpoints in lines del4DS-4 and del4DS-3.
We performed FHB disease experiments on the four genotyped 4DS CSTD lines. This clearly demonstrated that the lines with the two smaller deletions (del4DS-2 and del4DS-4) retained the susceptibility factor and showed a similar phenotype to euploid Chinese Spring, while the two lines containing the larger deletions (del4DS-3 and del4DS-1) showed significantly increased FHB resistance and hence the susceptibility factor has presumably been lost. As del4DS-4 exhibited wild-type FHB susceptibility but del4DS-3 was comparatively more resistant, the cause must be situated between the deletion breakpoints of these two lines, restricting the susceptibility factor to a 31.7 Mbp interval containing 266 high confidence genes (Supplementary Table S1; IWGSC RefSeq v1.1). The positive effect from deleting the susceptibility factor appears to be restricted to 4D and hence it is possible that the gene responsible is 4D specific and does not possess homoeologues. BLAST searches of each 4D gene in the interval, followed by validation in Ensembl Plants, identified nine genes that appear to lack homoeologues and hence are 4D specific (Supplementary Table S1). Alternatively, the 4D homoeologue may be preferentially expressed or may have diverged in function compared with the 4A and 4B copies.
It may be considered surprising that an FHB susceptibility factor with such a powerful effect has not been detected before now. However, we hypothesize that the FHB susceptibility factor is highly conserved among wheat cultivars. The susceptibility factor exists both in the Hungarian winter wheat cultivar Martonvasari 9 and in the Asian spring wheat variety Chinese Spring. Preliminary experiments of a γ-irradiated Paragon line containing a deletion of the entire 31.7 Mbp FHB susceptibility interval indicates that this line possesses potent Type II resistance and hence confirms that the susceptibility factor is also present in the UK spring cultivar Paragon (data not shown). If there was sufficient allelic variation at the locus, the effect of the susceptibility factor is likely to have been detected as an FHB QTL in existing mapping populations. In the absence of such reports, we predict that the FHB susceptibility factor is fixed in both spring and winter wheats.
Reduced height-D1 (Rht-D1) is located on the short arm of chromosome 4D. The mutant alleles Rht-D1b and homoeologous Rht-B1b (on chromosome 4B) result in reduced gibberellin sensitivity, causing decreased stem elongation and hence reduced plant height. These semi-dwarfing alleles were widely deployed during the Green Revolution and resulted in greatly increased crop yields (Hedden, 2003). However, Rht-D1b and Rht-B1b have also been implicated in increased susceptibility to FHB (Srinivasachary et al., 2009; Saville et al., 2012; Buerstmayr and Buerstmayr, 2016). Both Chinese Spring and Paragon possess the wild-type allele Rht-D1a (TraesCS4D02G040400) (P. Nicholson, personal communication). Rht-D1 is at 18.78 Mbp on chromosome 4D, meaning that Rht-D1 is distal to the FHB susceptibility interval identified in this study (EMBL-EBI, 2019). Rht-D1 will have been deleted in the smallest terminal deletion line (del4DS-2), which did not show altered FHB symptoms. Furthermore, no obvious sign of altered plant height was observed in any of the Chinese Spring 4D DT or terminal deletion lines (data not shown). For these reasons, Rht-D1 is unlikely to be responsible for the altered FHB susceptibility observed in the 4DS deletion lines.
We also used 4D CSTD lines in a DON application experiment. All four lines showed increased susceptibility to DON-induced bleaching compared with Chinese Spring. The effect on grain weight was less pronounced compared with the 4D DT lines. However, both del4DS-2 and del4DS-4 showed greater reductions in grain weight compared with Chinese Spring. These data suggest that DON resistance is associated with the region distal to 51.6 Mbp on 4DS in Chinese Spring. Further experiments are necessary to confirm whether the DON resistance is present in additional wheat varieties.
Genetic resistance to fungal diseases is critical to the protection of food crops such as wheat. The search for and incorporation of resistance factors is common practice in cereal breeding. However, identifying novel sources of resistance to FHB is challenging and time consuming. FHB resistance is quantitative, highly polygenic, and often environmentally labile. Few large-effect FHB QTLs have been identified. Attempts to clone the gene underlying the best-known source of FHB resistance, the Fhb1 QTL, have been inconsistent and controversial (Ma et al., 2017; Rawat et al., 2017; Steiner et al., 2017; Su et al., 2017). Su et al. (2018) identified that the presence of a deletion at the 5' end of a histidine-rich calcium-binding gene (TaHRC) within the Fhb1 locus was sufficient in identifying varieties carrying Fhb1. Su et al. (2019) have since reported that Fhb1 possesses enhanced resistance due to the loss of function of TaHRC and that the wild-type allele is hence functioning as a susceptibility factor. Li et al. (2019) also identified that a deletion of the TaHRC gene was responsible for Fhb1 resistance. However, in conflict with the findings of Su et al. (2019), their data suggest that this is due to a gain of function resulting from a different start codon positioned upstream to the original (Li et al., 2019). Our data on the 3HS-3BL centric fusion line do not suggest that 3BS contains a susceptibility factor, as the line was either wild-type-like or more highly susceptible to the spread of FHB. This concurs with Ma et al. (2006), who reported that the Chinese Spring DT line missing 3BS [DT(3BL)] was more susceptible to FHB, which is not compatible with the hypothesis that FHB resistance results from Fhb1 being a loss-of-function susceptibility factor. It remains possible that more than one gene is responsible for FHB resistance conferred by Fhb1.
There has been relatively little research into susceptibility factors in cereals and how they may be used in plant breeding. The barley mildew resistance locus o (Mlo) is one of the earliest and best characterized examples of how disruption of a susceptibility factor could be exploited to improve disease resistance; in this case, to powdery mildew caused by the biotrophic fungus Blumeria graminis f. sp. hordei (Jorgensen, 1992). This topic is covered in review articles by Acevedo-Garcia et al. (2014) and Kusch and Panstruga (2017).
R genes, usually nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes, are typically used by plants to detect and respond to attack by biotrophic fungi. However, necrotrophic pathogens have evolved methods of exploiting such plant defences to aid infection. Parastagonospora nodorum and Pyrenophora tritici-repentis are necrotrophic pathogens of wheat that utilize this strategy. Susceptibility to these diseases operates in an inverse gene-for-gene interaction, in which a fungal necrotrophic effector is detected by a corresponding host sensitivity gene product (usually an NBS-LRR), triggering a hypersensitive response that results in necrosis that benefits the fungus (Faris et al., 2010). Absence of either the necrotrophic effector or host sensitivity gene makes the interaction impossible and host resistance is maintained. There have been few reports of how NBS-LRRs are involved in interactions with Fusarium spp. However, Zhang et al. (2019) found that the expression of an LRR gene appeared to increase susceptibility to F. graminearum in soybean (Glycine max).
Fusarium graminearum leads a hemibiotrophic lifestyle whereby the hyphal front remains surrounded by living tissue but cell death is triggered soon after colonization (Brown et al., 2010). Phytohormones play important roles in defence and there is considerable evidence to suggest that F. graminearum modifies phytohormone signalling for its own benefit. Disruption of ethylene signalling in wheat (Chen et al., 2009) and brassinosteroid signalling in barley and Brachypodium distachyon (Goddard et al., 2014) resulted in enhanced resistance to FHB infection, suggesting that the fungus is exploiting phytohormone signalling in order to aid infection. Expression of 9-lipoxygenases are also manipulated by F. graminearum in bread wheat and Arabidopsis thaliana and hence operate as susceptibility factors (Nalam et al., 2015).
In the present study, we provide compelling evidence for FHB susceptibility associated with a 31.7 Mbp interval on the short arm of chromosome 4D. We have demonstrated that the removal of the susceptibility interval is sufficient to significantly improve Type II FHB resistance. A population possessing smaller deletions is required to further refine the position of the FHB susceptibility factor. We intend to utilize a γ-irradiated population of the UK spring wheat variety Paragon (Shaw et al., 2013; Wheat Genetic Improvement Network, 2019) to improve the resolution for the physical mapping of the FHB susceptibility factor. Once this has been achieved, we will use the Cadenza TILLING population to validate the effect of mutations in individual gene candidates (Uauy et al., 2009).
Supplementary data
Table S1. Genes present in the defined FHB susceptibility interval, including summarized functional annotations.
Fig. S1. DON-treated wheat heads showing representative bleaching for the assigned bleaching scores (/10).
Fig. S2. Agarose gel image following PCR of Chinese Spring–Betzes 5HS and 5HL addition lines using primers targeting the barley UDP-glucosyltransferase gene HORVU5Hr1G047150.
Fig. S3. Example outputs of five multiplexed homoeologue non-specific markers used to genotype 4DS CSTD lines.
Acknowledgements
The authors would like to thank the Biotechnology and Biological Sciences Research Council (BBSRC) for funding through the Designing Future Wheat institute strategic programme (BB/P016855/1) and the John Innes Foundation for funding this work. BH would like to thank BBSRC (BB/M016919/1) and RAGT Seeds for supporting his PhD studentship.
Glossary
Abbreviations
- CSTD
Chinese Spring terminal deletion
- dpi
days post-inoculation
- DON
deoxynivalenol
- D3G
DON-3-O-glucoside
- DT
ditelosomic
- FHB
Fusarium head blight
- FL
fraction length
- QTL
quantitative trait locus
- UGT
UDP-glucosyltransferase
References
- Acevedo-Garcia J, Kusch S, Panstruga R. 2014. Magical mystery tour: MLO proteins in plant immunity and beyond. New Phytologist 204, 273–281. [DOI] [PubMed] [Google Scholar]
- Altpeter F, Posselt UK. 1994. Production of high quantities of 3-acetyldeoxynivalenol and deoxynivalenol. Applied Microbiology and Biotechnology 41, 384–387. [Google Scholar]
- Bai GH, Desjardins AE, Plattner RD. 2002. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 153, 91–98. [DOI] [PubMed] [Google Scholar]
- Boutigny AL, Richard-Forget F, Barreau C. 2008. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. European Journal of Plant Pathology 121, 411–423. [Google Scholar]
- Brown NA, Urban M, van de Meene AM, Hammond-Kosack KE. 2010. The infection biology of Fusarium graminearum: defining the pathways of spikelet to spikelet colonisation in wheat ears. Fungal Biology 114, 555–571. [DOI] [PubMed] [Google Scholar]
- Buerstmayr H, Ban T, Anderson JA. 2009. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breeding 128, 1–26. [Google Scholar]
- Buerstmayr M, Buerstmayr H. 2016. The semidwarfing alleles Rht-D1b and Rht-B1b show marked differences in their associations with anther-retention in wheat heads and with fusarium head blight susceptibility. Phytopathology 106, 1544–1552. [DOI] [PubMed] [Google Scholar]
- Buerstmayr M, Steiner B, Buerstmayr H. 2019. Breeding for fusarium head blight resistance in wheat—progress and challenges. Plant Breeding 26, doi: 10.1111/pbr.12797. [Google Scholar]
- Chen X, Steed A, Travella S, Keller B, Nicholson P. 2009. Fusarium graminearum exploits ethylene signalling to colonize dicotyledonous and monocotyledonous plants. New Phytologist 182, 975–983. [DOI] [PubMed] [Google Scholar]
- Chia T, Adamski NM, Saccomanno B, Greenland A, Nash A, Uauy C, Trafford K. 2017. Transfer of a starch phenotype from wild wheat to bread wheat by deletion of a locus controlling B-type starch granule content. Journal of Experimental Botany 68, 5497–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMBL-EBI 2019. Ensembl Plants release 43. https://plants.ensembl.org [Google Scholar]
- Endo TR, Gill BS. 1996. The deletion stocks of common wheat. Journal of Heredity 87, 295–307. [Google Scholar]
- Faris JD, Zhang Z, Lu H, et al. 2010. A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proceedings of the National Academy of Sciences, USA 107, 13544–13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin DF, Porter H, Blankenheim ZJ, Chao S, Dill-Macky R. 2015. A spontaneous segmental deletion from chromosome arm 3DL enhances Fusarium head blight resistance in wheat. Genome 58, 479–488. [DOI] [PubMed] [Google Scholar]
- Goddard R, Peraldi A, Ridout C, Nicholson P. 2014. Enhanced disease resistance caused by BRI1 mutation is conserved between Brachypodium distachyon and barley (Hordeum vulgare). Molecular Plant-Microbe Interactions 27, 1095–1106. [DOI] [PubMed] [Google Scholar]
- Gosman N, Chandler E, Thomsett M, Draeger R, Nicholson P. 2005. Analysis of the relationship between parameters of resistance to Fusarium head blight and in vitro tolerance to deoxynivalenol of the winter wheat cultivar WEK0609 (R). European Journal of Plant Pathology 111, 57–66. [Google Scholar]
- Gunupuru LR, Perochon A, Doohan FM. 2017. Deoxynivalenol resistance as a component of FHB resistance. Tropical Plant Pathology 42, 175–183. [Google Scholar]
- Hassan YI, He JW, Perilla N, Tang K, Karlovsky P, Zhou T. 2017. The enzymatic epimerization of deoxynivalenol by Devosia mutans proceeds through the formation of 3-keto-DON intermediate. Scientific Reports 7, 6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WJ, Zhang L, Yi SY, Tang XL, Yuan QS, Guo MW, Wu AB, Qu B, Li HP, Liao YC. 2017. An aldo-keto reductase is responsible for Fusarium toxin-degrading activity in a soil Sphingomonas strain. Scientific Reports 7, 9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P. 2003. The genes of the Green Revolution. Trends in Genetics 19, 5–9. [DOI] [PubMed] [Google Scholar]
- Islam AKMR, Shepherd KW, Sparrow DHB. 1981. Isolation and characterization of euplasmic wheat–barley chromosome addition lines. Heredity 46, 161. [Google Scholar]
- IWGSC 2018. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361, earr7191. [DOI] [PubMed] [Google Scholar]
- Jorgensen JH. 1992. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 63, 141–152. [Google Scholar]
- Kruppa K, Sepsi A, Szakács É, Röder MS, Molnár-Láng M. 2013. Characterization of a 5HS-7DS.7DL wheat–barley translocation line and physical mapping of the 7D chromosome using SSR markers. Journal of Applied Genetics 54, 251–258. [DOI] [PubMed] [Google Scholar]
- Kusch S, Panstruga R. 2017. mlo-based resistance: an apparently universal ‘weapon’ to defeat powdery mildew disease. Molecular Plant-Microbe Interactions 30, 179–189. [DOI] [PubMed] [Google Scholar]
- Langevin F, Eudes F, Comeau A. 2004. Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereal species. European Journal of Plant Pathology 110, 735–746. [Google Scholar]
- Lemmens M, Scholz U, Berthiller F, et al. 2005. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Molecular Plant-Microbe Interactions 18, 1318–1324. [DOI] [PubMed] [Google Scholar]
- Li X, Shin S, Heinen S, Dill-Macky R, Berthiller F, Nersesian N, Clemente T, McCormick S, Muehlbauer GJ. 2015. Transgenic wheat expressing a barley UDP-glucosyltransferase detoxifies deoxynivalenol and provides high levels of resistance to Fusarium graminearum. Molecular Plant-Microbe Interactions 28, 1237–1246. [DOI] [PubMed] [Google Scholar]
- Li G, Zhou J, Jia H, et al. 2019. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nature Genetics 51, 1106–1112. [DOI] [PubMed] [Google Scholar]
- Lucyshyn D, Busch BL, Abolmaali S, Steiner B, Chandler E, Sanjarian F, Mousavi A, Nicholson P, Buerstmayr H, Adam G. 2007. Cloning and characterization of the ribosomal protein L3 (RPL3) gene family from Triticum aestivum. Molecular Genetics and Genomics 277, 507–517. [DOI] [PubMed] [Google Scholar]
- Ma HX, Bai GH, Gill BS, Hart LP. 2006. Deletion of a chromosome arm altered wheat resistance to fusarium head blight and deoxynivalenol accumulation in Chinese spring. Plant Disease 90, 1545–1549. [DOI] [PubMed] [Google Scholar]
- Ma Z, Li G, Zhou J, et al. 2017. Map-based cloning of Fhb1 revealed unique mutation of a well-conserved gene resulting in resistance to wheat Fusarium head blight. In: Buerstmayr H, Lang-Mladek C, Steiner B, Michel S, Buerstmayr M, Lemmens M, Vollmann J, Grausgruber H, eds. 13th International Wheat Genetics Symposium. Tulln, Austria, BOKU-University of Natural Resources and Life Sciences, Vienna, 68. [Google Scholar]
- Maier FJ, Miedaner T, Hadeler B, Felk A, Salomon S, Lemmens M, Kassner H, Schäfer W. 2006. Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Molecular Plant Pathology 7, 449–461. [DOI] [PubMed] [Google Scholar]
- Mitterbauer R, Poppenberger B, Raditschnig A, Lucyshyn D, Lemmens M, Glössl J, Adam G. 2004. Toxin-dependent utilization of engineered ribosomal protein L3 limits trichothecene resistance in transgenic plants. Plant Biotechnology Journal 2, 329–340. [DOI] [PubMed] [Google Scholar]
- Molnar I, Linc G, Dulai S, Nagy ED, Molnar-Lang M. 2007. Ability of chromosome 4H to compensate for 4D in response to drought stress in a newly developed and identified wheat–barley 4H(4D) disomic substitution line. Plant Breeding 126, 369–374. [Google Scholar]
- Molnar-Lang M, Linc G, Sutka J. 1996. Transfer of the recessive crossability allele kr1 from Chinese Spring into the winter wheat variety Martonvasari 9. Euphytica 90, 301–305. [Google Scholar]
- Nagy ED, Molnár-Láng M, Linc G, Láng L. 2002. Identification of wheat–barley translocations by sequential GISH and two-colour FISH in combination with the use of genetically mapped barley SSR markers. Genome 45, 1238–1247. [DOI] [PubMed] [Google Scholar]
- Nalam VJ, Alam S, Keereetaweep J, Venables B, Burdan D, Lee H, Trick HN, Sarowar S, Makandar R, Shah J. 2015. Facilitation of Fusarium graminearum Infection by 9-lipoxygenases in Arabidopsis and wheat. Molecular Plant-Microbe Interactions 28, 1142–1152. [DOI] [PubMed] [Google Scholar]
- Pallotta M, Warner P, Fox RL, Kuchel H, Jefferies SJ, Langridge P. 2003. Marker assisted wheat breeding in the southern region of Australia. In: Pogna N, McIntosh R, eds. Proceedings of the 10th international wheat genetics symposium Vol. 2 Paestum, Italy: Istituto Sperimentale per la Cerealicoltura, 789–791. [Google Scholar]
- Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glössl J, Luschnig C, Adam G. 2003. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. Journal of Biological Chemistry 278, 47905–47914. [DOI] [PubMed] [Google Scholar]
- Rawat N, Pumphrey MO, Liu S, Zhang X, Tiwari VK, Trick HN, Akhunov E, Anderson JA, Gill BS. 2017. Pore-forming toxin-like gene provides resistance against Fusarium head blight in wheat. In: Buerstmayr H, Lang-Mladek C, Steiner B, Michel S, Buerstmayr M, Lemmens M, Vollmann J, Grausgruber H, eds. 13th International Wheat Genetics Symposium. Tulln, Austria: BOKU-University of Natural Resources and Life Sciences, Vienna, 67. [Google Scholar]
- Saville RJ, Gosman N, Burt CJ, Makepeace J, Steed A, Corbitt M, Chandler E, Brown JK, Boulton MI, Nicholson P. 2012. The ‘Green Revolution’ dwarfing genes play a role in disease resistance in Triticum aestivum and Hordeum vulgare. Journal of Experimental Botany 63, 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherm B, Balmas V, Spanu F, Pani G, Delogu G, Pasquali M, Migheli Q. 2013. Fusarium culmorum: causal agent of foot and root rot and head blight on wheat. Molecular Plant Pathology 14, 323–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder HW, Christensen JJ. 1963. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 53, 831–838. [Google Scholar]
- Schuelke M. 2000. An economic method for the fluorescent labeling of PCR fragments. Nature Biotechnology 18, 233–234. [DOI] [PubMed] [Google Scholar]
- Schweiger W, Boddu J, Shin S, Poppenberger B, Berthiller F, Lemmens M, Muehlbauer GJ, Adam G. 2010. Validation of a candidate deoxynivalenol-inactivating UDP-glucosyltransferase from barley by heterologous expression in yeast. Molecular Plant-Microbe Interactions 23, 977–986. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Turner AS, Herry L, Griffiths S, Laurie DA. 2013. Mutant alleles of Photoperiod-1 in wheat (Triticum aestivum L.) that confer a late flowering phenotype in long days. PLoS One 8, e79459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Torres-Acosta JA, Heinen SJ, McCormick S, Lemmens M, Paris MP, Berthiller F, Adam G, Muehlbauer GJ. 2012. Transgenic Arabidopsis thaliana expressing a barley UDP-glucosyltransferase exhibit resistance to the mycotoxin deoxynivalenol. Journal of Experimental Botany 63, 4731–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasachary Gosman N, Steed A, Hollins TW, Bayles R, Jennings P, Nicholson P. 2009. Semi-dwarfing Rht-B1 and Rht-D1 loci of wheat differ significantly in their influence on resistance to Fusarium head blight. Theoretical and Applied Genetics 118, 695–702. [DOI] [PubMed] [Google Scholar]
- Steiner B, Zimmerl S, Polzer R, Mühl S, Lemmens M, Adam G, Till B, Schweiger W, Buerstmayr H. 2017. Functional identification of the wheat gene enhancing mycotoxin detoxification of the major Fusarium resistance QTL Fhb1. In: Buerstmayr H, Lang-Mladek C, Steiner B, Michel S, Buerstmayr M, Lemmens M, Vollmann J, Grausgruber H, eds. 13th International Wheat Genetics Symposium. Tulln, Austria: BOKU-University of Natural Resources and Life Sciences, Vienna, 70. [Google Scholar]
- Su Z, Bernardo A, Li C, Lu P, Cai S, Bai G. 2017. TaHRC is the key gene underlying Fhb1 resistance to Fusarium head blight in wheat. In: Buerstmayr H, Lang-Mladek C, Steiner B, Michel S, Buerstmayr M, Lemmens M, Vollmann J, Grausgruber H, eds. 13th International Wheat Genetics Symposium. Tulln, Austria: BOKU-University of Natural Resources and Life Sciences, Vienna, 69. [Google Scholar]
- Su Z, Bernardo A, Tian B, et al. 2019. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nature Genetics 51, 1099–1105. [DOI] [PubMed] [Google Scholar]
- Su Z, Jin S, Zhang D, Bai G. 2018. Development and validation of diagnostic markers for Fhb1 region, a major QTL for Fusarium head blight resistance in wheat. Theoretical and Applied Genetics 131, 2371–2380. [DOI] [PubMed] [Google Scholar]
- Szakács E, Molnár-Láng M. 2007. Development and molecular cytogenetic identification of new winter wheat-winter barley (‘Martonvásári 9 kr1’–‘Igri’) disomic addition lines. Genome 50, 43–50. [DOI] [PubMed] [Google Scholar]
- Szakács E, Molnár-Láng M. 2010. Identification of new winter wheat–winter barley addition lines (6HS and 7H) using fluorescence in situ hybridization and the stability of the whole ‘Martonvásári 9 kr1’–‘Igri’ addition set. Genome 53, 35–44.m [DOI] [PubMed] [Google Scholar]
- Uauy C, Paraiso F, Colasuonno P, Tran RK, Tsai H, Berardi S, Comai L, Dubcovsky J. 2009. A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biology 9, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat Genetic Improvement Network 2019. http://www.wgin.org.uk/ Wheat Genetic Improvement Network.
- Xing LP, Gao L, Chen QG, Pei HY, Di ZC, Xiao J, Wang HY, Ma LL, Chen PD, Cao AZ, Wang XE. 2018. Over-expressing a UDP-glucosyltransferase gene (Ta-UGT (3)) enhances Fusarium Head Blight resistance of wheat. Plant Growth Regulation 84, 561–571. [Google Scholar]
- Zhang C, Zhao X, Qu Y, Teng W, Qiu L, Zheng H, Wang Z, Han Y, Li W. 2019. Loci and candidate genes in soybean that confer resistance to Fusarium graminearum. Theoretical and Applied Genetics 132, 431–441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.