Two phospholipase C2s in Nicotiana benthamiana, PLC2-1 and PLC2-2, complement each other during disease resistance responses and play important roles the induction of PAMP-triggered immunity.
Keywords: Jasmonic acid, Nicotiana benthamiana, pathogen-associated molecular pattern-triggered immunity, phosphatidylinositol-phospholipase C2, Ralstonia solanacearum, virus-induced gene silencing
Abstract
Phospholipid signaling plays an important role in plant immune responses against phytopathogenic bacteria in Nicotiana benthamiana. Here, we isolated two phospholipase C2 (PLC2) orthologs in the N. benthamiana genome, designated as PLC2-1 and 2-2. Both NbPLC2-1 and NbPLC2-2 were expressed in most tissues and were induced by infiltration with bacteria and flg22. NbPLC2-1 and NbPLC2-2 (NbPLC2s) double-silenced plants showed a moderately reduced growth phenotype. The induction of the hypersensitive response was not affected, but bacterial growth and the appearance of bacterial wilt were accelerated in NbPLC2s-silenced plants when they were challenged with a virulent strain of Ralstonia solanacearum that was compatible with N. benthamiana. NbPLC2s-silenced plants showed reduced expression levels of NbPR-4, a marker gene for jasmonic acid signaling, and decreased jasmonic acid and jasmonoyl-L-isoleucine contents after inoculation with R. solanacearum. The induction of pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) marker genes was reduced in NbPLC2s-silenced plants after infiltration with R. solanacearum or Pseudomonas fluorescens. Accordingly, the resistance induced by flg22 was compromised in NbPLC2s-silenced plants. In addition, the expression of flg22-induced PTI marker genes, the oxidative burst, stomatal closure, and callose deposition were all reduced in the silenced plants. Thus, NbPLC2s might have important roles in pre- and post-invasive defenses, namely in the induction of PTI.
Introduction
Plants combat invading pathogens by employing a two-layered innate immune system (Thom ma et al., 2011). The first layer is triggered upon perception of conserved molecular structures termed pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors localized to the plasma membrane, and this is designated as PAMP-triggered immunity (PTI). Well-studied examples of PTI are Arabidopsis FLS2 and EFR, which recognize the bacterial flagellar component flg22 and the elongation factor thermo unstable (EF-Tu), respectively. Adapted pathogens have evolved a number of virulence mechanisms to suppress PTI by acquiring effector proteins (Bigeard et al., 2015). As a counter-measure, plants have evolved the second layer of the innate immune system to directly or indirectly recognize effector proteins by their cognate resistance proteins, resulting in the initiation of effector-triggered immunity (ETI) (Gassmann and Bhattacharjee, 2012). PTI and ETI share signaling components that have distinct activation dynamics and amplitudes (Tsuda and Katagiri, 2010). Generally, PTI is characterized by broad-spectrum, transient, and relatively mild immune responses without an associated programmed cell death hypersensitive response (HR) (Segonzac et al., 2011; Bigeard et al., 2015). In contrast, ETI is characterized by specific, sustainable, and robust immune responses with HR (Jones and Dangl, 2006).
During PTI and ETI, plants trigger activation of diverse signaling cascades, such as generation of reactive oxygen species (ROS), spikes in cellular Ca2+, activation of MAP kinase, production of phytohormones, and transcriptional reprogramming. The most characterized Ca2+ spike results from an influx from the apoplast and endoplasmic reticulum that causes a rapid increase in the cytosolic concentration (Blume et al., 2000; Lecourieux et al., 2005). Plant cyclic nucleotide-gated ion channels provide a pathway for conductance of Ca2+ across the plasma membrane and thus facilitate the elevation of the cytosolic concentration. In Arabidopsis, cyclic nucleotide-gated ion channel 2 plays a pivotal role in allowing entry of Ca2+ into cells in response to pathogen signals (Ma and Berkowitz, 2011). The elevated cytosolic Ca2+ reportedly activates downstream intracellular signaling components. ROS generation is another event in immune signaling, and is mainly mediated by a respiratory-burst oxidase homologue (Rboh). ROS act not only as direct antimicrobial agents that cross-link components for cell-wall strengthening, but also as second messengers during immune signaling (Torres, 2010). In Nicotiana benthamiana, NbRbohA and NbRbohB are required for ROS generation to occur after treatment with hyphal cell wall components and the INF1 elicitin from Phytophthora infestans (Yoshioka et al., 2003). The rapid activation of MAP kinase cascades is another important event in the downstream signal transduction during induction of PTI and ETI. In N. tabacum (tobacco) and N. benthamiana, protein kinases induced by salicylic acid (SA) and wounding are also activated rapidly after elicitation (Seo et al., 1995; Lebrun-Garcia et al., 1998; Zhang and Klessig, 1998; Dahan et al., 2009).
Phospholipid-based signaling cascades are important for signal transduction in plant immune responses. Phospholipid turnover is mainly composed of cascades associated with diacylglycerol kinase and phospholipase C (PLC) and phospholipase D (PLD). Treatment with SA significantly increases the generation of phosphatidic acid (PA) by the activation of PLD (Rodas-Junco et al., 2015). PLD is involved in defense signaling in non-host resistance against powdery mildew, and PLDδ may be the main participating isoform in Arabidopsis (Pinosa et al., 2013). The silencing of diacylglycerol kinase cluster III abolishes PA production and strongly inhibits the ROS burst in tobacco in response to the elicitin cryptogein (Cacas et al., 2017). We have previously identified the SEC14 gene in N. benthamiana. Suppression of PLC and PLD activities, and production of diacylglycerol (DAG) and PA were observed in NbSEC14-silenced plants, resulting in compromised disease resistance against phytopathogenic bacteria through the jasmonic acid (JA)-dependent pathway (Kiba et al., 2012, 2014, 2016). Thus, phospholipid turnover may play an important role in the induction of immune responses in N. benthamiana.
PLCs are an important group of lipid-hydrolysing enzymes in both plants and animals. In plants, PLCs can be subdivided into the well-studied phosphatidylinositol-specific PLCs (PI-PLCs) and the recently identified phosphatidylcholine-PLCs (PC-PLCs). PI-PLCs act upon a specific substrate, PI (4,5) P2, at the glycerophosphate ester linkages of membrane phospholipids and lead to the generation of secondary messengers, such as DAG and inositol 1,4,5-trisphosphate (IP3) (Singh et al. 2015). A total of seven PLCs have been identified in tomato (Abd-El-Haliem et al., 2016). We have searched the recently completed N. benthamiana genome sequence (https://solgenomics.net/) and identified 12 PLCs, and our objective here was to clarify their roles in plant immunity. We isolated two PLC2 orthologs, NbPLC2-1 and NbPLC2-2 (NbPLC2s), and determined the effects of silencing both on the induction of PTI and ETI in N. benthamiana by using several PTI-inducers and effectors, and by using Pseudomonas syringae pv. Tabaci and Ralstonia solanacearum. In the light of our results, we discuss the regulatory roles of NbPLC2s in immune responses in N. benthamiana.
Materials and methods
Biological and chemical materials
The flg22 peptide was obtained from the Funakoshi Co. Ltd. (Tokyo, Japan), and Nicotiana benthamiana was grown from seeds under a 16/8-h photoperiod in a growth room as described previously (Maimbo et al., 2007, 2010). Pseudomonas fluorescens 55 (an effective PTI inducer in N. benthamiana), P. syringae pv. tabaci 6605, the virulent compatible Ralstonia solanacearum strain OE1-1 (RsOE1-1), the avirulent R. solanacearum strain 8107, and hrpY-deficient RsOE1-1 that lacks the ability to deliver effectors into plant cells were cultured in peptone yeast-extract medium containing appropriate antibiotics as described previously (Chakravarthy et al., 2010; Maimbo et al., 2010; Ito et al., 2014a, 2014b). Based on previous reports, the bacterial population of P. fluorescens 55 was adjusted to 107 (Chakravarthy et al., 2010), P. syringae pv. tabaci 6605 to 104 (Ito et al., 2014a, 2014b), RsOE1-1 and Rs8107 to 108 (Maimbo et al., 2010), and hrpY-deficient RsOE1-1 to 108 (Kiba et al., 2018). Agrobacterium tumefaciens was cultured in YEB medium (Maimbo et al., 2010). The bacterial populations were determined by plating bacterial suspensions on R. solanacearum selective Hara-Ono medium plates at specified time-points (Maimbo et al., 2010). The bacterial suspensions and the flg22 solution were infiltrated using syringes as described previously (Maimbo et al., 2007, 2010; Nguyen et al., 2010). Control plants were infiltrated with water.
RNA isolation and cDNA synthesis
Total RNAs were isolated from the stamens, gynoecium, petals, leaves, stems, petioles, and roots of 2-month-old N. benthamiana plants using NucleoSpin RNA Plant Kits (Macherey-Nagel), and qRT-PCR was completed according to the method described by Maimbo et al. (2007). A 1-µg sample of total RNA was used as the template for reverse-transcription using a ReverTra Ace® qPCR RT Kit (Toyobo Co., Ltd.).
Sequence analysis was performed using the M4 and RV primers (Supplementary Table S1 at JXB online) with the reagents for the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems) and a 3100 Avant Automated Sequencer (Applied Biosystems) according to the manufacturer’s instructions. The sequence analysis was carried out using DNASIS (version 3.6; Hitachi) and the the BLAST network service from the NCBI (Altschul et al., 1990).
Virus-induced gene silencing
cDNA fragments for NbPLC2-1, NbPLC2-2, and the combined NbPLC2-1 and NbPLC2-2 sequences (NbPLC2s) were amplified using the primers listed in Supplementary Table S1 using N. benthamiana cDNA as the template. These cDNA fragments were independently subcloned into the TA cloning site of the pMD20 vector (TaKaRa Bio.) to create pMD-NbPLC2-1, pMD-NbPLC2-2, and pMD-NbPLC2s. These plasmids were digested with SalI (TaKaRa Bio.) and ligated into SalI-digested pPVX201 (Maimbo et al. 2007). The plasmids used for the virus-induced gene silencing (VIGS) experiments are listed in Supplementary Table S2. VIGS was conducted with pPVX201 containing the NbPLC2-1, NbPLC2-2, and combined NbPLC2s sequences. The cDNA fragments were amplified with the primers listed in Supplementary Table S1. The pPVX201 plasmid lacking any insert was used as a control, as previously described (Kiba et al., 2012). These binary plasmids were transformed into A. tumefaciens strain GV3101 and inoculated into leaves of 4-week-old N. benthamiana as described previously (Nakano et al., 2013). At 4 weeks after the initial inoculation, bacteria and flg22 were inoculated into a leaf located 3–4 leaves above the Agrobacterium-inoculated one as a challenge inoculation. The silencing efficiency was assessed by quantitative real-time PCR (qRT-PCR) assays (Supplementary Fig. S3).
Bacterial population and disease index
Bacterial suspensions (108 CFU ml–1) of RsOE1-1 and hrpY-deficient R. solanacearum were inoculated into N. benthamiana leaves. The bacterial populations after 24 h and 48 h were determined by plating on Hara-Ono plates. Plants inoculated with rsOE1-1 were labeled and inspected daily for wilting symptoms for 14 d. For each plant, the disease index on a scale of 0–4 was calculated as described previously (Maimbo et al., 2010).
Quantitative real-time PCR
Gene expression analysis was carried out using qRT-PCR and the ΔΔCT method as described by Maimbo et al. (2007). Briefly, the qRT-PCR was carried out in 20 µl of reaction mixture, containing 1 µl of cDNA template, 10 pM of the respective primers (Supplementary Table S1) and THUNDERBIRD qPCR MIX (Toyobo Co.), on an Applied Biosystems 7300 real-time PCR instrument. The cycling parameters were the same for all the primers: an initial 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 1 min. Melting-curve runs were performed at the end of each PCR reaction to verify the specificity of the primers by the presence of a single amplification product. The relative quantification of gene expression was performed according to the manufacturer’s instructions using the comparative cycle threshold [Ct] method for the calculation of the Qty value. All values were normalized to the expression of the actin gene, which was used as an internal standard in each cDNA stock.
Estimation of cell death
Cell death was measured by ion conductivity (Nakano et al., 2013) using a Twin Cord B-173 conductivity meter (HORIBA, Kyoto, Japan).
Phytohormone analysis
Phytohormone contents were determined using a method described previously by Kiba et al. (2012). Extracted samples were measured on a triple-quadrupole LC-MS/MS 6410 (Agilent Technologies) equipped with a Zorbax SB-C18 column (2.1 mm id×50 mm, 1.8 µm; Agilent Technologies). The amounts of hormones were calculated from the ratios of the endogenous hormone peaks and the known amounts of internal standards, and expressed in relation to the fresh weight of the samples used for extraction.
Callose deposition assays
Callose deposition was detected using a modified version of a staining method using aniline blue (Ton and Mauch-Mani, 2004). Briefly, leaves of N. benthamiana were fixed and de-stained in 95% ethanol. The leaves were then washed with 0.07 M phosphate buffer (pH 9.0) and incubated for 1–2 h in 0.07 M phosphate buffer containing 0.01% aniline blue (Sigma). Samples were observed using a BX-51 epifluorescence microscope with a UV filter (Olympus). Callose deposition was quantified based on the number of deposits detected in digital photographs using the Photoshop Elements 7 software (Adobe Systems).
PTI assays based on cell death
PTI assays based on cell death were conducted as described previously by Chakravarthy et al. (2010), with P. fluorescens 55 (1×109 CFU ml−1) and P. syringae pv. tabaci 66455 (1×104 CFU ml−1) as the inducer and challenger, respectively. The PTI was induced by infiltrating the leaves with the inducer, then 24 h later the challenger was applied to a partially overlapping area. Images were taken 5 d after the challenge inoculations.
ROS measurements
ROS measurements were performed as described by Kobayashi et al. (2007). Leaves of N. benthamiana were infiltrated with 0.5 mM L-012 (Wako Pure Chemical Industries Ltd, Osaka, Japan) in 10 mM MOPS-KOH (pH 7.4) using a needleless syringe. Chemiluminescence was monitored continuously using a photon image processor equipped with a sensitive CCD camera (ARGUS-50 or Aquacosmos 2.5; Hamamatsu Photonics). Photons were integrally incorporated for 5 min after the treatment.
Epidermal strip bioassays
Epidermal strip bioassays were carried out as described by Chen et al. (2004) with slight modifications. Leaves of N. benthamiana were incubated in MES buffer (10 mM MES-Tris, pH 6.0, containing 30 mM KCl and 0.1 mM CaCl2) for 90 min under light to open the stomata. The strips were then transferred to MES buffer in the absence or presence of 100 nM flg22 for 3 h and images of stomatal apertures were captured with an Olympus BX43 microscope. Measurements of 50 randomly selected stomata were taken. Each assay was repeated three times.
Statistical analysis
Significant differences between means were determined using Student’s t-tests (two-sided).
Results
Identification of phosphatidylinositol phospholipase C2 from Nicotiana benthamiana
Based on PLC sequences from tomato (Solanum lycopersicum cv. Microtom), we searched for orthologs in N. benthamiana using the Sol Genomics Network (https://solgenomics.net/) and identified a total of 12. A phylogenic analysis of the amino acid sequences divided the NbPLCs into seven classes (Supplementary Fig. S1A). Two of them (Niben101Scf02221g00009 and Niben101Scf00318g03011) were classified into the same clade as SlPLC2; however, AtPLC2 belonged to a different clade. The deduced amino acid sequences of the full-length cDNAs of the PLC2 orthologs contained the PI-PLC-X, PI-PLC-Y, and PI3K-C2 domains, suggesting that they were phosphatidylinositol-specific PLCs (PI-PLCs; Supplementary Fig. S1B, C). We designated them as NbPLC2-1 (Niben101Scf02221g00009) and NbPLC2-2 (Niben101Scf00318g03011).
Expression patterns of NbPLC2-1 and NbPLC2-2
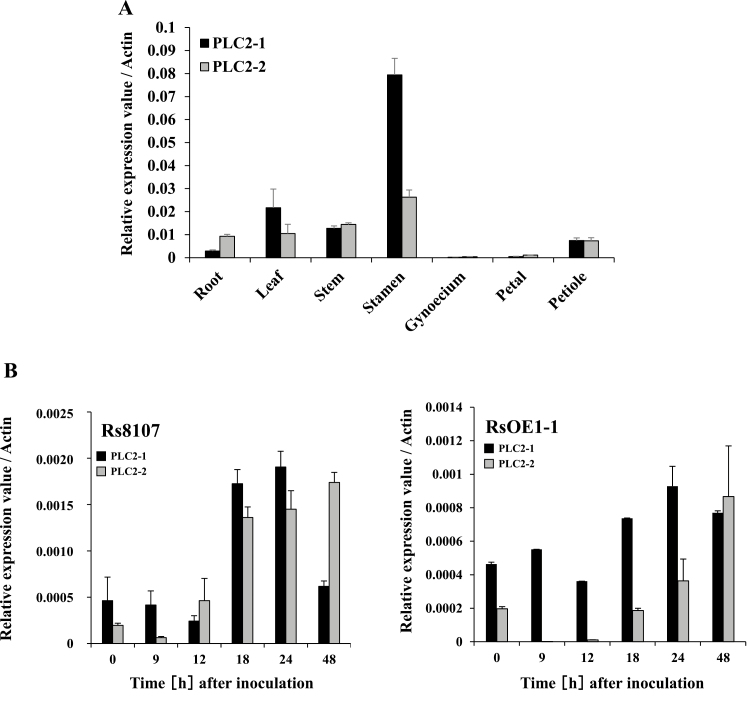
Total RNAs were isolated from various organs of the plants, and qRT-PCR showed that NbPLC2-1 and NbPLC2-2 were expressed in all of them (Fig. 1A). The highest level of expression of NbPLC2-1 was observed in the stamens, followed by the leaves, stems, petioles, roots, petals, and gynoecium. The highest level of expression of NbPLC2-2 was also observed in the stamens, followed by the stems, leaves and roots equally, petioles, petals, and gynoecium.
Fig. 1.
Expression patterns of NbPLC2s in Nicotiana benthamiana. (A) Relative expression of NbPLC2-1 and NbPLC2-2 in different tissues. (B) Relative expression of NbPLC2-1 and NbPLC2-2 in leaves of plants inoculated with avirulent Ralstonia solanacearum 8107 (Rs8107) or with virulent compatible R. solanacearum OE1-1 (RsOE1-1). Expression levels were determined by qRT-PCR and are relative to the Actin housekeeping gene. Data are means (±SD) of n=3 replicates.
To determine the expression profiles of NbPLC2s in response to inoculation with R. solanacearum, we used the virulent compatible strain OE1-1 (RsOE1-1) and the avirulent strain 8107 (Rs8107), and total RNAs were isolated from leaves at between 0–48 h after inoculation. Strong induction of both NbPLC2-1 and NbPLC2-2 was observed in leaves inoculated with Rs8107 (Fig. 1B), with the expression of NbPLC2-1 showing a peak at 24 h whilst the highest expression of NbPLC2-2 was observed at 48 h. The expression levels of NbPLC2-1 and NbPLC2-2 were lower in leaves inoculated with RsOE1-1 and increases in expression were not evident until 18–24 h after inoculation.
Effects of silencing of NbPLC2s on resistance against Ralstonia solanacearum
To examine the roles of NbPLC2s in plant immunity, we carried out virus-induced gene silencing (VIGS). We created constructs for silencing both PLC2s (PLC2s-VIGS), and individually for NbPLC2-1 (PLC2-1-VIGS) and NbPLC2-2 (PLC2-2-VIGS) (Supplementary Fig. S2). We checked the specific suppression of NbPLC2-1 and NbPLC2-2 using qRT-PCR, which confirmed that the expression levels of both genes were reduced in plants inoculated with Agrobacterium carrying the NbPLC2s-VIGS construct and that the expression levels of the individual genes were reduced in the individual VIGS constructs (Supplementary Fig. S3A). It was notable that the expression of NbPLC2-2 was increased in PLC2-1-VIGS plants and the expression of NbPLC2-1 was increased in PLC2-2-VIGS plants. In terms of phenotype, compared with water-inoculated controls, PLC2s-VIGS plants showed a significant reduction in growth at 3 weeks after inoculation with the construct whilst no differences were observed in the PLC2-1-VIGS and PLC2-2-VIGS plants (Supplementary Fig. S3B, C).
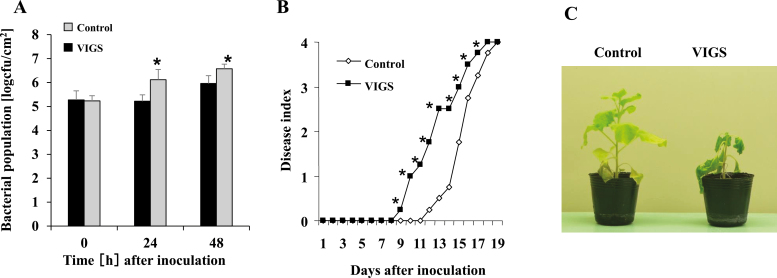
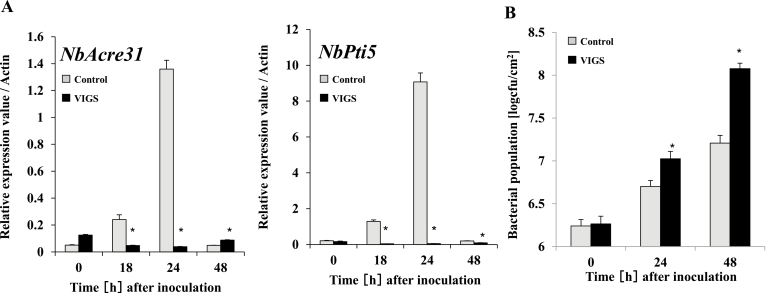
We then examined the roles of NbPLC2s using a N. benthamiana–R. solanacearum interaction model. We first inoculated PLC2s-VIGS, PLC2-1-VIGS, and PLC2-2-VIGS plants with the RsOE1-1 strain, a compatible pathogen that causes characteristic wilt symptoms in N. benthamiana. At 24 h after inoculation with RsOE1-1, the bacterial population was ~10-fold greater in PLC2s-VIGS plants compared with the control (Fig. 2A). Bacterial wilt was first observed in control plants at 12 d, and the plants were completely wilted at 18 d (Fig. 2B). In PLC2s-VIGS plants, accelerated wilting was observed, with symptoms first being visible at 9 d, and the plants were completely wilted by 17 d after inoculation (Fig. 2B, C). In contrast, no significant changes in disease development or bacterial populations were observed in the PLC2-1-VIGS and PLC2-2-VIGS plants (Supplementary Fig. S4). The results therefore indicated that NbPLC2s might play important roles in induced defense against R. solanacearum OE1-1, and that NbPLC2-1 and NbPLC2-2 might cooperatively regulate the responses. Another possibility was that activity of just one of the two PLC2s was sufficient to mediate the defense responses. Therefore, we used PLC2s-VIGS plants for further functional analysis of NbPLC2s in plant immunity.
Fig. 2.
Responses of NbPLC2s-silenced Nicotiana benthamiana plants to virulent compatible strain of Ralstonia solanacearum. Leaves of plants at 8 weeks old were infiltrated with R. solanacearum OE1-1. Plants were silenced using VIGS. (A) Bacterial populations of R. solanacearum following inoculation. Data are means (±SD) of n=5 replicates. (B) Disease development of bacterial wilt according to a disease index scale of 0–4. Data are means of n=10 plants; for clarity, the error bars are not shown. (C) Images of control and VIGS plants at 12 d after inoculation. Significant differences between control and VIGS plants were determined using Student’s t-test: *P<0.05.
Next, we inoculated plants with Rs8107, which is an incompatible pathogen that induces a hypersensitive response (HR) in N. benthamiana. HR lesions developed in both the control and PLC2s-VIGS plants 24 h after inoculation (Supplementary Fig. S5), and the magnitude of induced cell death and its timing were also similar, suggesting that the HR-mediated immune responses might have been independent of NbPLC2. Furthermore, silencing of the NbPLC2s had no effect on the induction of HR by Agrobacterium-mediated transient expression of the R. solanacearum effectors AvrA and PopP1 (Poueymiro et al., 2009; Supplementary Fig. S5), and hence we concluded that NbPLC2 was not essential for the induction of effector-triggered immunity (ETI).
Silencing of NbPLC2s reduces jasmonic acid-dependent defenses against Ralstonia solanacearum
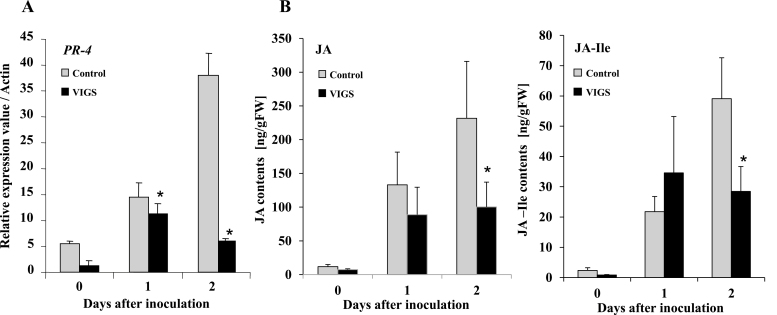
Phospholipid turnover plays an important role in defense responses against RsOE1-1 through JA signaling (Kiba et al., 2012, Nakano et al., 2013), and therefore we examined the effects of silencing NbPLC2s on JA signaling. Total RNA was extracted from leaves of control and PLC2s-VIGS plants at 0–2 d after inoculation with RsOE1-1. The expression level of PR-4, a marker gene for the JA signaling pathway, increased dramatically in control plants at both 1 d and 2 d after inoculation (Fig. 3A). In contrast, the increase in expression in PLC2s-VIGS plants at 1 d was significantly lower than in the control, and it had decreased at 2 d. Similar patterns were observed for the contents of JA and JA-L-isoleucine (Fig. 3B): whilst no significant differences were observed compared with the controls at 1 d after inoculation, the contents were both significantly reduced at 2 d in the PLC2s-VIGS plants. These results therefore suggested that NbPLC2s might be involved in JA-mediated immune responses against R. solanacearum.
Fig. 3.
Suppression of jasmonic acid-dependent defense responses in NbPLC2s-silenced Nicotiana benthamiana plants inoculated with a virulent compatible strain of Ralstonia solanacearum. Leaves of plants at 8 weeks old were infiltrated with R. solanacearum OE1-1. Plants were silenced using VIGS. (A) Expression of NbPR-4 (a marker gene for jasmonic acid signaling) was determined relative to that in the control and values were normalized against Actin. Data are means (±SD) of n=3 replicates. (B) Total contents of jasmonic acid (JA) and jasmonoyl-L-isoleucine (JA-Ile) were determined using LC-MS/MS. Data are means (±SD) of n=5 replicates. Significant differences between control and VIGS plants were determined using Student’s t-test: *P<0.05.
Stimulation of expression of NbPLC2s by PAMP-triggered immunity inducers
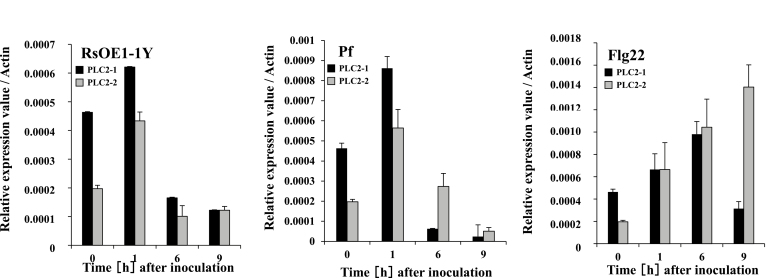
The silencing of NbPLC2s essentially had no effect on the induction of HR by the incompatible Rs8107 strain and by type III effectors from R. solanacearum. However, PLC2s-VIGS plants showed a reduced-resistance phenotype against the compatible RsOE1-1 strain. Jones and Dangl (2006) defined resistance activated by virulent pathogens on susceptible hosts as basal disease resistance. Accordingly, it can be described as PAMP-triggered immunity (PTI) plus weak ETI minus effector-triggered susceptibility. Basal disease resistance is then mainly covered by PTI (Jones and Dangle, 2006). We therefore examined the correlation between NbPLC2s and PTI by first determining their expression patterns in the presence of PTI inducers. We used a type III secretion system (hrpY)-deficient mutant of RsOE1-1 that lacks the ability to deliver effectors into plant cells and induces PTI in N. benthamiana, and we also used Pseudomonas fluorescens and the bacterial flagellar component flg22, which are effective PTI inducers in N. benthamiana (Chakravarthy et al., 2010). Strong inductions of NbPLC2-1 and NbPLC2-2 were observed 1 h after inoculation with the hrp-deficient mutant and 1 h after inoculation with P. fluorescens (Fig. 4). Inoculation with flg22 induced increasing expression of NbPLC2-1 from 1–6 h, after which it declined, whilst expression of NbPLC2-2 increased from 1–9 h after treatment. NbPLC2s thus appeared to be expressed after the perception of PAMPs.
Fig. 4.
Induction of NbPLC2-1 and NbPLC2-2 in Nicotiana benthamiana by pathogen-associated molecular pattern (PAMP)-triggered immunity inducers. Plants were inoculated with either a hrpY-deficient mutant of virulent compatible Ralstonia solanacearum OE1-1 (RsOE1-1Y), Pseudomonas fluorescens (Pf), or 100nM flg22 peptide (Flg22). Expression levels of NbPLC2-1 and NbPLC2-2 were determined by qRT-PCR and are relative to the control, with values normalized against Actin. Data are means (±SD) of n=3 replicates.
Silencing of NbPLC2 reduces PAMP-triggered immune responses to Ralstonia solanacearum
Because the expression levels of NbPLC2-1 and NbPLC2-2 were induced by the hrp-deficient mutant of RsOE1-1, by P. fluorescens, and by flg22, we hypothesized that NbPLC2s may be involved in the initiation of PTI. To assess the effects of silencing of NbPLC2s on the induction of PTI, we examined the expression levels of the PTI marker genes NbAcre31 and NbPti5 after inoculation with RsOE1-1. In control plants, increased expression of NbAcre31 was observed between 18–24 h after inoculation and increased expression of NbPti5 was observed between 24–48 h after inoculation (Fig. 5). In agreement with our hypothesis, the expression levels of both genes were significantly reduced in PLC2s-VIGS plants relative to the controls following inoculation.
Fig. 5.
Suppression of pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) in Nicotiana benthamiana NbPLC2s-silenced plants inoculated with a virulent compatible strain of Ralstonia solanacearum. Leaves of plants at 8 weeks old were infiltrated with R. solanacearum OE1-1. Plants were silenced using VIGS. Expression of the PTI marker genes NbAcre31 and NbPti5 were determined by qRT-PCR and are relative to the control, with values normalized against Actin. Data are means (±SD) of n= 3 replicates. Significant differences between control and VIGS plants were determined using Student’s t-test: *P<0.05.
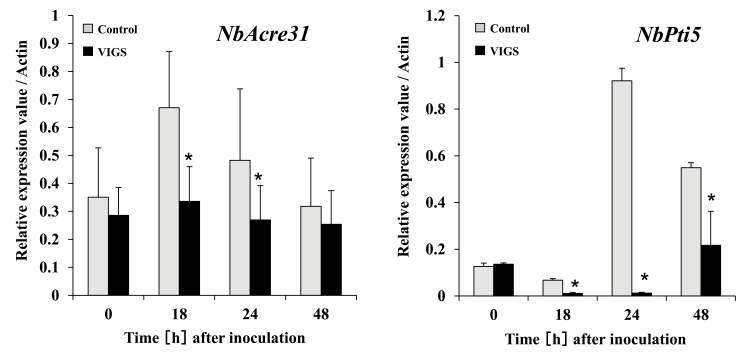
We then inoculated plants with the hrp-deficient mutant of RsOE1-1 and observed increased expression of NbAcre31 and NbPti5 after 18–24 h in the controls, whereas expression in the PLC2s-VIGS plants was significantly lower at these time-points (Fig. 6A). To further confirm their role in PTI, we determined the effects of NbPLC2-silencing on the growth of the hrp-deficient mutant. Bacterial growth increased following inoculation in both control and PLC2s-VIGS plants, but the rate was higher in the latter with the result that populations were ~10-fold greater after 48 h in the silenced plants (Fig. 6B). These results further supported the involvement of NbPLC2s in the induction of PTI.
Fig. 6.
Effects of silencing NbPLC2s in Nicotiana benthamiana on pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) in response to a hrpY-deficient mutant of Ralstonia solanacearum. Leaves of plants at 8 weeks d old were infiltrated with the R. solanacearum mutant. Plants were silenced using VIGS. (A) Expression of the PTI marker genes NbAcre31 and NbPti5 were determined by qRT-PCR and are relative to the control, with values normalized against Actin. (B) Bacterial populations of the R. solanacearum mutant following inoculation. Data are means (±SD) of n=5 replicates. Significant differences between control and VIGS plants were determined using Student’s t-test: *P<0.05.
Silencing of NbPLC2s reduces PAMP-triggered immune responses to Pseudomonas fluorescens
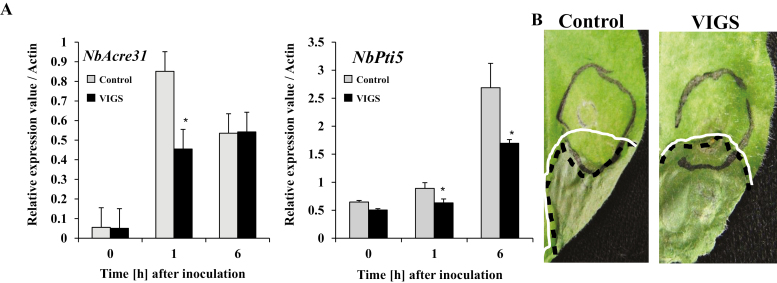
Pseudomonas fluorescens is an effective PTI inducer in N. benthamiana (Chakravarthy et al., 2010), and so we used it to inoculate control and NbPLC2-silenced plants to induce responses. Expression of NbAcre31 increased after 1 h following inoculation in both control and PLC2s-VIGS plants, but it was significantly higher in the control (Fig. 7A). The levels were then the same after 6 h. For NbPti5, expression increased at 1 h and 6 h after inoculation and at both time-points it was significantly higher in the controls than in the silenced plants.
Fig. 7.
Effect of NbPLC2s-silencing in Nicotiana benthamiana on pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) in response to Pseudomonas fluorescens (an effective PTI inducer). Leaves of plants at 8 weeks old were infiltrated with P. fluorescens. Plants were silenced using VIGS. (A) Expression of the PTI marker genes NbAcre31 and NbPti5 were determined by qRT-PCR and are relative to the control, with values normalized against Actin. Data are means (±SD) of n=3 replicates. Significant differences between control and VIGS plants were determined using Student’s t-test: *P<0.05. (B) A cell death-based assay for PTI. Pseudomonas syringae pv. tabaci was used as the death-inducible challenger, and P. fluorescens was infiltrated into the leaves to induce PTI (area within grey circle). At 7 h after P. fluorescens inoculation, the same leaves were challenged with P. syringae pv. tabaci (area within the white line). The area within the black dotted line indicates the necrotic lesions caused by P. syringae pv. tabaci. The images were taken 5 d after inoculation with P. syringae pv. tabaci.
An assay based on cell death using P. fluorescens has been reported to be an effective tool for examining PTI responses (Oh and Collmer, 2005). We used P. fluorescens as a PTI inducer and P. syringae pv. tabaci as a challenger. In the control and PLC2s-VIGS plants, infection with P. syringae pv. tabaci resulted in necrotic lesions in leaf regions in which P. fluorescens was not infiltrated (Fig. 7B). In control plants, necrotic lesions were suppressed in the region in which P. fluorescens and P. syringae pv. tabaci overlapped, indicating that PTI was effectively induced. In contrast, necrotic lesions were observed in the overlapping area of PLC2s-VIGS plants inoculated with both bacteria. This suggested that the silencing of the NbPLC2s impaired the PTI response by P. fluorescens.
Silencing of NbPLC2s reduces PAMP-triggered immune responses to flg22
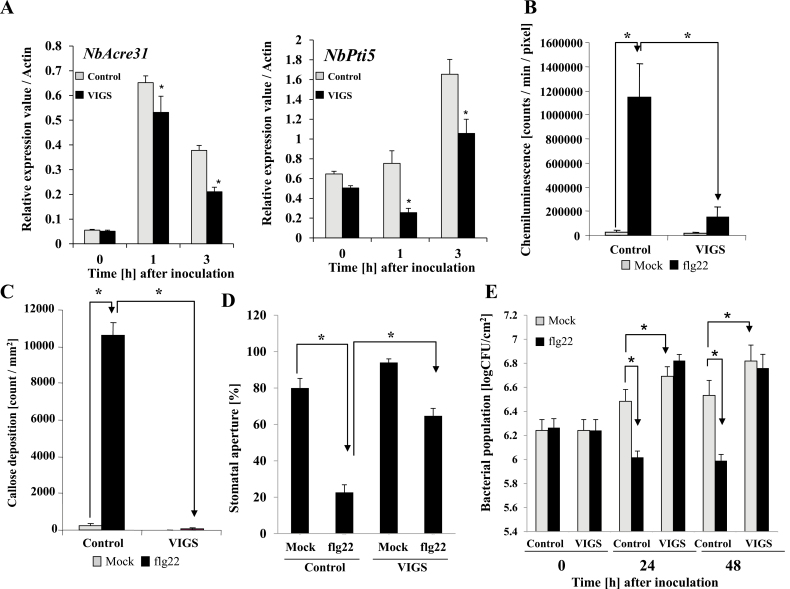
In both control and PLC2s-VIGS plants, a strong increase in expression of NbAcre31 was observed 1 h after treatment with the flg22 elicitor, and expression remained elevated at 3 h (Fig. 8A); however, at both time-points the expression in the silenced plants was significantly lower than in the controls. For NbPti5, the highest levels of expression were observed after 3 h, but again at both time-points the expression in the silenced plants was significantly lower than in the controls.
Fig. 8.
Effects of NbPLC2s-silencing in Nicotiana benthamiana on pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) induced by flg22. Flg22 (100 nM) was used as the PTI-inducer and infiltrated into leaves of 8-week -old plants. Plants were silenced using VIGS. (A) Expression of the PTI marker genes NbAcre31 and NbPti5 were determined by qRT-PCR and are relative to the control, with values normalized against Actin. Data are means (±SD) of n=3 replicates. (B) Levels of reactive oxygen species determined 30 min after the flg22 treatment. Chemiluminescence intensities mediated by L-012 were quantified using a photon image processor. Photons were integrally incorporated for 5 min after the flg22 treatment. Data are means (±SD) of n=4 replicates. (C) Callose deposition at 24 h after flg22 treatment, as determined by staining with aniline blue. Fluorescent deposits were visualized using fluorescence microscopy. Data are means (±SD) of n=5 replicates. (D) Stomatal apertures in the leaves at 3 h after treatment with flg22. Data are means (±SD) of n=50 replicates from three independent experiments. (E) Flg22 was infiltrated into the leaves of control and NbPLC2s-silenced plants. After 24 h, a virulent compatible strain of Ralstonia solanacearum, OE1-1, was inoculated as a challenger into the flg22-infiltrated area, and the bacterial populations were determined at subsequent time-points. Data are means (±SD) of n=5 replicates. Significant differences between means were determined using Student’s t-test: *P<0.05.
ROS generation is another hallmark of a PTI response, and we found that it was dramatically induced 30 min after the inoculation with flg22 in the control plants (Fig. 8B). Whilst an increase in ROS was also observed in PLC2s-VIGS plants, the response was much less than in the control plants.
Callose deposition occurs during PTI responses to counteract pathogen invasions. In control plants, we found a large increase in callose deposition 24 h after the inoculation with flg22 while PLC2s-VIGS plants showed no response (Fig. 8C, Supplementary Fig. S6A).
Guard cells exhibit innate immune responses to pathogens and PAMP compounds, such as flg22, which induce stomatal closure (Zhang et al., 2009). At 3 h following treatment with flg22, the size of the stomatal apertures was clearly reduced in both control and PLC2s-VIGS plants, suggesting induced closure (Fig 8D, Supplementary Fig. S6B). However, the induced closure was significantly less in PLC2s-VIGS plants relative to the control.
We then treated leaves with flg22, and 24 h later inoculated the treated areas with R. solanacearum strain OE1-1. The bacterial population increased at 24 h and 48 h after inoculation with RsOE1-1 in control plants, and populations were greater in PLC2s-VIGS plants than in the controls (Fig. 8E; see also Fig. 2A). The proliferation of bacteria was suppressed by pre-treatment with flg22 in the control plants, indicating that it effectively induced PTI against RsOE1-1. In contrast, no suppression of bacterial growth by flg22 was observed in the PLC2s-VIGS plants.
Discussion
Phospholipase Cs (PLCs) represent an important group of lipid-hydrolysing enzymes in both plants and animals (Pokotylo et al., 2014). In plants, phosphatidylinositol-specific (PI-)PLCs act on a specific substrate, PI (4,5) P2, at glycerophosphate ester linkages of membrane phospholipids and lead to the generation of secondary messengers, such as DAG and IP3. The phosphorylated products of DAG and IP3, namely PA, diacylglycerol pyrophosphate, and hexakisphosphate may function as second messengers in plants (van Leeuwen et al., 2007, Xue et al., 2007). Plant PI-PLCs have been implicated in a number of cellular processes and signal transduction events during differentiation and development. In Arabidopsis, nine PI-PLCs (PLC1–9) have been identified (Tasma et al., 2008). More recently, PLCs have been detected in the tomato genome and classified into seven groups (Abd-El-Haliem et al., 2016). In our current study, we found 12 PLC orthologs in N. benthamiana (Supplementary Fig. S1). Based on a phylogenic analysis of the amino acid sequences, we designated two PLC orthologs, Niben101Scf02221g00009 and Niben101Scf00318g03011, as NbPLC2-1 and NbPLC2-2, respectively. Intriguingly, AtPLC2 belonged to a different clade from NbPLC2-1, NbPLC2-2, and SlPLC2 (Supplementary Fig. S1A; Abd-El-Haliem et al., 2016). In addition, 15 amino acids were lacking in the central regions of NbPLC2-1, NbPLC2-2, and SlPLC2 (Supplementary Fig. S1B). Hence, the structures of PLC2s from the Solanaceae were different from those of the Brassicaceae, and these differences might be correlated with functional differences.
PLC1 and PLC3 are expressed in Arabidopsis pollen and have significant roles in pollen-tube growth (Dowd et al., 2006, Helling et al., 2006). PI-PLC has also been found to play a significant role during the process of asymmetric cell division that generates stomatal complexes in maize (Apostolakos et al., 2008). In petunia, the catalytically inactive form of PLC1 competes with the native form, and this results in an alteration of the Ca2+ gradient and reorganization of the cytoskeleton, leading to delocalized growth and swollen tips in pollen tubes (Dowd et al., 2006). Among the nine Arabidopsis PLCs, only PLC2 is expressed dominantly and ubiquitously in most tissues (Hirayama et al., 1997; Li et al., 2015) and it is the primary phospholipase in phosphoinositide metabolism (Kanehara et al., 2015). Disruption of PLC2 can lead to sterility because the development of both male and female gametophytes is severely perturbed in homozygous plc2 Arabidopsis mutants (Li et al., 2015; Di Fino et al., 2017). PLC2 also functions in auxin‐modulated root development in Arabidopsis (Chen et al., 2019). Thus, plant PI-PLCs may act as important regulators of various signaling pathways in different processes of growth and development. In our study, expression of NbPLC2-1 and NbPLC2-2 was observed in the stamen, gynoecium, petals, leaves, stems, and roots (Fig. 1A). NbPLC2s-silenced plants displayed phenotypes with moderately retarded growth relative to controls, with ~40% reduced height (Supplementary Fig. S3B, C). However, there were no phenotypic changes in plants with NbPLC2-1 or NbPLC2-2 silenced individually. Intriguingly, the expression level of NbPLC2-2 was significantly increased in NbPLC2-1-silenced plants, whilst the expression of NbPLC2-1 was significantly increased in NbPLC2-2-sielnced plants. Taken together, our results indicate that NbPLC2s appear to be involved in growth and development in N. benthamiana plants, at least in the stages following the initiation of VIGS in our experiments. In addition, NbPLC2-1 and NbPLC2-2 may mutually complement each other at the transcriptional level during these developmental stages.
Several PLCs participate in abiotic stress responses. The overexpression of maize and tobacco PLCs confers higher drought and salt tolerance levels in transgenic plants (Wang et al., 2008, Tripathy et al., 2012). In Arabidopsis, PI-PLCs have been implicated in the accumulation of the osmolyte proline that leads to adaptive responses following ionic hyperosmotic stress (Parre et al., 2007). A PLC-mediated signal transduction pathway is also induced during cold stress in plants (Ruelland et al., 2002). PLCs may also have significant roles in responses to heat stress. For example, PI-PLC proteins accumulate in pea plants after heat-stress treatments (Liu et al., 2006; Ruelland and Zachowski, 2010). Arabidopsis PLC9 has been implicated in heat-stress responses, and the atplc9 mutant exhibits a highly thermosensitive phenotype. Accumulation of HSP18.2 and HSP25.3 is reduced in atplc9 and enhanced in AtPLC9-overexpressing lines after exposure to heat stress (Zheng et al., 2012). An important role for AtPLC3 in thermo-tolerance has been established in Arabidopsis through a reduction in the heat-induced levels of Ca2+ in atplc3 plants (Gao et al., 2014). In rice, PLC1-mediated Ca2+ signaling is essential for controlling the accumulation of Na+ that leads to salt tolerance (Li et al., 2017). In contrast, AtPLC4 negatively regulates the salt tolerance of Arabidopsis seedlings through Ca2+ regulatory processes (Xia et al., 2017). PLC2 is involved in stress responses related to the endoplasmic reticulum in Arabidopsis (Kanehara et al., 2015). It remains to be determined whether the NbPLC2s isolated in our study may also participate in abiotic stress responses.
As well as abiotic stress responses, PLCs are also involved in responses to biotic stress, including plant immunity (Canonne et al., 2011). The PI-PLC family is required for HR-mediated defense responses and induction of effector-triggered immunity (ETI). Phytoalexin and ROS production, together with the HR, are reduced by the PI-PLC inhibitor U73122 in riboflavin- and Cf-4/Avr4-elicited tobacco cells (de Jong et al., 2004; Wang et al., 2013). Silencing of SlPLC6 results in a reduction of HR and increased colonization of Avr4-carriyng Cladosporium fulvum in Cf-4 containing tomato plants (Vossen et al., 2010). Avr4-induced HR is also reduced in the resistance Cf-4 carrying tomato after SlPLC4-silencing. In addition, the heterologous expression of SlPLC4 results in accelerated Avr4/Cf-4-induced HR in N. benthamiana (Abd-El-Haliem et al., 2016). Treatment of tomato suspension cells with HR-inducible fungal xylanase leads to a rapid increase in nitric oxide, which is responsible for PI-PLC activity and consequent defense responses (Raho et al., 2011). The production of nitric oxide and induction of HR are required to increase the expression levels of SlPLC5 in the xylanase-treated cells. Thus, numerous examples show that induction of HR and ETI are dependent on the functions of PI-PLC family members in tomato, and possibly in other plants. SlPLC2 is also required for HR, as shown by the expression of hsr203J being suppressed in SlPLC2-silenced plants (Gonorazky et al., 2014). Our results showed that the NbPLC2s were also required for ROS production (Fig. 8B), similar to SlPLC2. In contrast, the silencing of the NbPLC2s did not affect ETI, including induction of HR (Supplementary Fig. S5A, B). Thus, the NbPLC2s did not appear to be required for ETI responses.
SlPLC2 is also required for the xylanase-induced expression of defense-related genes. Reduced expression of the SA-dependent PR-1a gene is observed in SlPLC2-silenced plants, indicating it has a role in SA signaling (Gonorazky et al., 2014). SlPLC2 is also required for plant susceptibility against Botrytis cinerea through the competitive suppression of JA-dependent defenses caused by up-regulation of the SA-signaling pathway (Gonorazky et al., 2016). In contrast, silencing of NbPLC2s affected JA-related defenses (Fig. 3), and hence they may have a role in JA-dependent defense responses in N. benthamiana.
In addition to HR-mediated defenses and ETI, PLCs play important roles in defenses without HR, including induction of PTI. Both the flagellin-triggered response and the internalization of the corresponding receptor, FLS2, in Arabidopsis are suppressed by the inhibition of PLC activity (Abd-El-Haliem et al., 2016). PLC2-silenced Arabidopsis plants are susceptible to a spray treatment of the type-III secretion system-deficient bacterial strain P. syringae pv. tomato DC3000 (hrcC–) but not to the wild-type P. syringae pv. tomato DC3000 (D’Ambrosio et al., 2017). In contrast, PLC2 does not affect bacterial growth when the bacteria are syringe-infiltrated into the apoplast. In response to flg22, PLC2-silenced Arabidopsis show reduced stomatal closures. Thus, PLC2s may control stomatal pre-invasive, but not post-invasive, immunity. In our present study, expression of NbPLC2-1 and NbPLC2-2 were induced by PTI inducers, such as hrp-deficient R. solanacearum, P. fluorescens, and flg22 (Fig. 4); however, intriguingly, the induction patterns were different. Therefore, we speculate that NbPLC2-1 and NbPLC2-2 might have different roles during the immune responses. Silencing the NbPLC2s negatively affected the expression of PTI reporter genes when control and NbPLC2-silenced plants were infiltrated with wild-type R. solanacearum, hrp-deficient R. solanacearum, P. fluorescens, or flg22 (Figs 3–7). The suppression of PTI induction was observed using a cell death-based assay with P. fluorescens and P. syringae pv. tabaci. Flg22-induced callose deposition and hence disease resistance were also compromised in NbPLC2s-silenced plants (Fig. 8C, Supplementary Fig. S6A). These results collectively support a significant role for PLC2 in PTI responses. In addition, flg22-induced stomatal closure was reduced by silencing of the NbPLC2s (Fig. 8D, Supplementary Fig. S6B). Furthermore, enhanced bacterial growth occurred in NbPLC2s-silenced plants when either R. solanacearum or a hrpY mutant were infiltrated into the apoplast area. These results suggest that NbPLC2s control not only stomatal pre-invasive, but also post-invasive, immunity in N. benthamiana.
In summary, we have demonstrated that NbPLC2s contribute to the induction of pre- and post-invasive PTI responses in N. benthamiana. While undergoing PTI induction, NbPLC2s may be activating JA and JA-mediated immune responses, leading to the suppression of bacterial infections. These results provide novel insights into the roles of PLC2 and the PLC family in the regulation of plant immunity. Further studies are necessary to clarify the complex mechanisms by which the NbPLC2 protein is engaged in PTI responses, and to characterize the phospholipid turnover involved in the PTI-signaling cascade.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Characterization of the phosphatidylinositol-specific phospholipase C in Nicotiana benthamiana.
Fig. S2. Nucleotide sequences of NbPLC2-1 and NbPLC2-2.
Fig. S3. Phenotypes of NbPLC2s-, NbPLC2-1-, and NbPLC2-2-silenced plants.
Fig. S4. Responses of NbPLC2s-, NbPLC2-1-, and NbPLC2-2-silenced plants to compatible Ralstonia solanacearum.
Fig. S5. Effects of NbPLC2s-silencing on the induction of the hypersensitive response by effectors from incompatible Ralstonia solanacearum.
Fig. S6. Callose deposition and stomatal closure in NbPLC2s-silenced plants.
Table S1. Primers used in this study.
Table S2. Plasmids used in this study.
Acknowlegements
The authors thank Dr David C. Baulcombe of the Sainsbury Laboratory, John Innes Centre, UK, and G. Martin of Cornell University, USA, for providing the PVX vector and Pseudomonas fluorescens 55, respectively. The authors also thank Dr Y. Ichinose for kindly providing Pseudomonas syringae pv. tabaci 6605. This work was supported by a Cabinet Office Grants-in-Aid, the Advanced Next-Generation Greenhouse Horticulture by the Internet of Plants (IoP), Japan. This research was also supported by the Ministry of Education, Culture, Sports, Science and Technology as part of the Joint Research Program implemented at the Institute of Plant Science and Resources, Okayama University, Japan. LC-MS/MS instrumentation was supported by the Japan Advanced Plant Science Network. AK is also grateful for financial support from a Grants-in-Aid for Scientific Research (24580066) from the Ministry of Education, Science, Sports, and Culture, Japan, the Asahi Glass Foundation, the Agricultural Chemical Research Foundation, and the Sapporo Bioscience Foundation. We thank Dr Lesley Benyon from the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Glossary
Abbreviations
- HR
hypersensitive response
- hrp
hypersensitive response and pathogenicity
- PAMPs
pathogen-associated molecular patterns
- PLC
phospholipase C
- PTI
PAMPtriggered immunity
- Rs
Ralstonia solanacearum
- VIGS
virus-induced gene silencing
Author contributions
AK, KO, and YH designed the research; AK, MH, HN, IG, and MN performed the research; AK, IG, and TS analysed the data and wrote the paper.
References
- Abd-El-Haliem AM, Vossen JH, van Zeijl A, Dezhsetan S, Testerink C, Seidl MF, Beck M, Strutt J, Robatzek Joosten MHAJ. 2016. Biochemical characterization of the tomato phosphatidylinositol-specific phospholipase C (PI-PLC) family and its role in plant immunity. Biochimica et Biophysica Acta 1861, 1365–1378. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Apostolakos P, Panteris E, Galatis B. 2008. The involvement of phospholipases C and D in the asymmetric division of subsidiary cell mother cells of Zea mays. Cell Motility and the Cytoskeleton 65, 863–875. [DOI] [PubMed] [Google Scholar]
- Bigeard J, Colcombet J, Hirt H. 2015. Signaling mechanisms in pattern-triggered immunity (PTI). Molecular Plant 8, 521–539. [DOI] [PubMed] [Google Scholar]
- Blume B, Nürnberger T, Nass N, Scheel D. 2000. Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. The Plant Cell 12, 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacas JL, Gerbeau-Pissot P, Fromentin J, Cantrel C, Thomas D, Jeannette E, Kalachova T, Mongrand S, Simon-Plas F, Ruelland E. 2017. Diacylglycerol kinases activate tobacco NADPH oxidase-dependent oxidative burst in response to cryptogein. Plant, Cell & Environment 40, 585–598. [DOI] [PubMed] [Google Scholar]
- Canonne J, Froidure-Nicolas S, Rivas S. 2011. Phospholipases in action during plant defense signaling. Plant Signaling & Behavior 6, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy S, Velásquez AC, Ekengren SK, Collmer A, Martin GB. 2010. Identification of Nicotiana benthamiana genes involved in pathogen-associated molecular pattern-triggered immunity. Molecular Plant-Microbe Interactions 23, 715–726. [DOI] [PubMed] [Google Scholar]
- Chen YL, Huang R, Xiao YM, Lü P, Chen J, Wang XC. 2004. Extracellular calmodulin-induced stomatal closure is mediated by heterotrimeric G protein and H2O2. Plant Physiology 136, 4096–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li L, Xu B, Zhao S, Lu P, He Y, Ye T, Feng YQ, Wu Y. 2019. Phosphatidylinositol-specific phospholipase C2 functions in auxin-modulated root development. Plant, Cell & Environment 42, 1441–1457. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio JM, Couto D, Fabro G, Scuffi D, Lamattina L, Munnik T, Andersson MX, Álvarez ME, Zipfel C, Laxalt AM. 2017. Phospholipase C2 affects MAMP-triggered immunity by modulating ROS production. Plant Physiology 175, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan J, Pichereaux C, Rossignol M, Blanc S, Wendehenne D, Pugin A, Bourque S. 2009. Activation of a nuclear-localized SIPK in tobacco cells challenged by cryptogein, an elicitor of plant defence reactions. The Biochemical Journal 418, 191–200. [DOI] [PubMed] [Google Scholar]
- de Jong CF, Laxalt AM, Bargmann BO, de Wit PJ, Joosten MH, Munnik T. 2004. Phosphatidic acid accumulation is an early response in the Cf-4/Avr4 interaction. The Plant Journal 39, 1–12. [DOI] [PubMed] [Google Scholar]
- Di Fino LM, D’Ambrosio JM, Tejos R, van Wijk R, Lamattina L, Munnik T, Pagnussat GC, Laxalt AM. 2017. Arabidopsis phosphatidylinositol-phospholipase C2 (PLC2) is required for female gametogenesis and embryo development. Planta 245, 717–728. [DOI] [PubMed] [Google Scholar]
- Dowd PE, Coursol S, Skirpan AL, Kao TH, Gilroy S. 2006. Petunia phospholipase C1 is involved in pollen tube growth. The Plant Cell 18, 1438–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Liu YL, Li B, Zhou RG, Sun DY, Zheng SZ. 2014. Arabidopsis thaliana phosphoinositide-specific phospholipase C isoform 3 (AtPLC3) and AtPLC9 have an additive effect on thermotolerance. Plant & Cell Physiology 55, 1873–1883. [DOI] [PubMed] [Google Scholar]
- Gassmann W, Bhattacharjee S. 2012. Effector-triggered immunity signaling: from gene-for-gene pathways to protein–protein interaction networks. Molecular Plant-Microbe Interactions 25, 862–868. [DOI] [PubMed] [Google Scholar]
- Gonorazky G, Guzzo MC, Abd-El-Haliem AM, Joosten MH, Laxalt AM. 2016. Silencing of the tomato phosphatidylinositol-phospholipase C2 (SlPLC2) reduces plant susceptibility to Botrytis cinerea. Molecular Plant Pathology 17, 1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonorazky G, Ramirez L, Abd-El-Haliem A, Vossen JH, Lamattina L, ten Have A, Joosten MH, Laxalt AM. 2014. The tomato phosphatidylinositol-phospholipase C2 (SlPLC2) is required for defense gene induction by the fungal elicitor xylanase. Journal of Plant Physiology 171, 959–965. [DOI] [PubMed] [Google Scholar]
- Helling D, Possart A, Cottier S, Klahre U, Kost B. 2006. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. The Plant Cell 18, 3519–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T, Mitsukawa N, Shibata D, Shinozaki K. 1997. AtPLC2, a gene encoding phosphoinositide-specific phospholipase C, is constitutively expressed in vegetative and floral tissues in Arabidopsis thaliana. Plant Molecular Biology 34, 175–180. [DOI] [PubMed] [Google Scholar]
- Ito M, Takahashi H, Sawasaki T, Ohnishi K, Hikichi Y, Kiba A. 2014a Novel type of adenylyl cyclase participates in tabtoxinine-β-lactam-induced cell death and occurrence of wildfire disease in Nicotiana benthamiana. Plant Signaling & Behavior 9, e27420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yamamoto Y, Kim CS, Ohnishi K, Hikich Y, Kiba A. 2014b Heat shock protein 70 is required for tabtoxinine-β-lactam-induced cell death in Nicotiana benthamiana. Journal of Plant Physiology 171, 173–178. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kanehara K, Yu CY, Cho Y, Cheong WF, Torta F, Shui G, Wenk MR, Nakamura Y. 2015. Arabidopsis AtPLC2 is a primary phosphoinositide-specific phospholipase C in phosphoinositide metabolism and the endoplasmic reticulum stress response. PLoS Genetics 11, e1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba A, Galis I, Hojo Y, Ohnishi K, Yoshioka H, Hikichi Y. 2014. SEC14 phospholipid transfer protein is involved in lipid signaling-mediated plant immune responses in Nicotiana benthamiana. PLoS ONE 9, e98150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba A, Imanaka Y, Nakano M, Galis I, Hojo Y, Shinya T, Ohnishi K, Hikichi Y. 2016. Silencing of Nicotiana benthamiana SEC14 phospholipid transfer protein reduced jasmonic acid dependent defense against Pseudomonas syringae. Plant Biotechnology 33, 111–115. [Google Scholar]
- Kiba A, Nakano M, Ohnishi K, Hikichi Y. 2018. The SEC14 phospholipid transfer protein regulates pathogen-associated molecular pattern-triggered immunity in Nicotiana benthamiana. Plant Physiology and Biochemistry 125, 212–218. [DOI] [PubMed] [Google Scholar]
- Kiba A, Nakano M, Vincent-Pope P, Takahashi H, Sawasaki T, Endo Y, Ohnishi K, Yoshioka H, Hikichi Y. 2012. A novel Sec14 phospholipid transfer protein from Nicotiana benthamiana is up-regulated in response to Ralstonia solanacearum infection, pathogen associated molecular patterns and effector molecules and involved in plant immunity. Journal of Plant Physiology 169, 1017–1022. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. 2007. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. The Plant Cell 19, 1065–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Garcia A, Ouaked F, Chiltz A, Pugin A. 1998. Activation of MAPK homologues by elicitors in tobacco cells. The Plant Journal 15, 773–781. [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Lamotte O, Bourque S, Wendehenne D, Mazars C, Ranjeva R, Pugin A. 2005. Proteinaceous and oligosaccharidic elicitors induce different calcium signatures in the nucleus of tobacco cells. Cell Calcium 38, 527–538. [DOI] [PubMed] [Google Scholar]
- Li L, He Y, Wang Y, Zhao S, Chen X, Ye T, Wu Y, Wu Y. 2015. Arabidopsis PLC2 is involved in auxin-modulated reproductive development. The Plant Journal 84, 504–515. [DOI] [PubMed] [Google Scholar]
- Li L, Wang W, Yan P, Jing W, Zhang C, Kudla J, Zhang W. 2017. A phosphoinositide-specific phospholipase C pathway elicits stress-induced Ca2+ signals and confers salt tolerance to rice. New Phytologist 214, 1172–1187. [DOI] [PubMed] [Google Scholar]
- Liu HT, Huang WD, Pan QH, Weng FH, Zhan JC, Liu Y, Wan SB, Liu YY. 2006. Contributions of PIP2-specific-phospholipase C and free salicylic acid to heat acclimation-induced thermotolerance in pea leaves. Journal of Plant Physiology 163, 405–416. [DOI] [PubMed] [Google Scholar]
- Ma W, Berkowitz GA. 2011. Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytologist 190, 566–572. [DOI] [PubMed] [Google Scholar]
- Maimbo M, Ohnishi K, Hikichi Y, Yoshioka H, Kiba A. 2007. Induction of a small heat shock protein and its functional roles in Nicotiana plants in the defense response against Ralstonia solanacearum. Plant Physiology 145, 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimbo M, Ohnishi K, Hikichi Y, Yoshioka H, Kiba A. 2010. S-glycoprotein-like protein regulates defense responses in Nicotiana plants against Ralstonia solanacearum. Plant Physiology 152, 2023–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Nishihara M, Yoshioka H, Takahashi H, Sawasaki T, Ohnishi K, Hikichi Y, Kiba A. 2013. Suppression of DS1 phosphatidic acid phosphatase confirms resistance to Ralstonia solanacearum in Nicotiana benthamiana. PLoS ONE 8, e75124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HP, Chakravarthy S, Velásquez AC, McLane HL, Zeng L, Nakayashiki H, Park DH, Collmer A, Martin GB. 2010. Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana. Molecular Plant-Microbe Interactions 23, 991–999. [DOI] [PubMed] [Google Scholar]
- Oh HS, Collmer A. 2005. Basal resistance against bacteria in Nicotiana benthamiana leaves is accompanied by reduced vascular staining and suppressed by multiple Pseudomonas syringae type III secretion system effector proteins. The Plant Journal 44, 348–359. [DOI] [PubMed] [Google Scholar]
- Parre E, Ghars MA, Leprince AS, Thiery L, Lefebvre D, Bordenave M, Richard L, Mazars C, Abdelly C, Savouré A. 2007. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiology 144, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinosa F, Buhot N, Kwaaitaal M, Fahlberg P, Thordal-Christensen H, Ellerström M, Andersson MX. 2013. Arabidopsis phospholipase Dδ is involved in basal defense and nonhost resistance to powdery mildew fungi. Plant Physiology 163, 896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokotylo I, Kolesnikov Y, Kravets V, Zachowski A, Ruelland E. 2014. Plant phosphoinositide-dependent phospholipases C: variations around a canonical theme. Biochimie 96, 144–157. [DOI] [PubMed] [Google Scholar]
- Poueymiro M, Cunnac S, Barberis P, Deslandes L, Peeters N, Cazale-Noel AC, Boucher C, Genin S. 2009. Two type III secretion system effectors from Ralstonia solanacearum GMI1000 determine host-range specificity on tobacco. Molecular Plant-Microbe Interactions 22, 538–550. [DOI] [PubMed] [Google Scholar]
- Raho N, Ramirez L, Lanteri ML, Gonorazky G, Lamattina L, ten Have A, Laxalt AM. 2011. Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. Journal of Plant Physiology 168, 534–539. [DOI] [PubMed] [Google Scholar]
- Rodas-Junco BA, Muñoz-Sánchez JA, Vázquez-Flota F, Hernández-Sotomayor SM. 2015. Salicylic-acid elicited phospholipase D responses in Capsicum chinense cell cultures. Plant Physiology and Biochemistry 90, 32–37. [DOI] [PubMed] [Google Scholar]
- Ruelland E, Cantrel C, Gawer M, Kader JC, Zachowski A. 2002. Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiology 130, 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelland E, Zachowski A. 2010. How plants sense temperature. Environmental and Experimental Botany 69, 225e–232.. [Google Scholar]
- Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen PJ. 2011. Hierarchy and roles of PAMP-induced responses in Nicotiana benthamiana. Plant Physiology 156, 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. 1995. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science 270, 1988–1992. [DOI] [PubMed] [Google Scholar]
- Singh A, Bhatnagar N, Pandey A, Pandey GK. 2015. Plant phospholipase C family: regulation and functional role in lipid signaling. Cell Calcium 58, 139–146. [DOI] [PubMed] [Google Scholar]
- Tasma IM, Brendel V, Whitham SA, Bhattacharyya MK. 2008. Expression and evolution of the phosphoinositide‐specific phospholipase C gene family in Arabidopsis thaliana. Plant Physiology and Biochemistry 46, 627–637. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Nürnberger T, Joosten MH. 2011. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. The Plant Cell 23, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Mauch-Mani B. 2004. β-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming of callose. The Plant Journal 38, 119–130. [DOI] [PubMed] [Google Scholar]
- Torres MA. 2010. ROS in biotic interactions. Physiologia Plantarum 138, 414–429. [DOI] [PubMed] [Google Scholar]
- Tripathy MK, Tyagi W, Goswami M, Kaul T, Singla-Pareek LS, Deswal R, Reddy MK, Sopory SK. 2012. Characterization and functional validation of tobacco PLC delta for abiotic stress tolerance. Plant Molecular Bioliology Reporter 30, 488–497. [Google Scholar]
- Tsuda K, Katagiri F. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Current Opinion in Plant Biology 13, 459–465. [DOI] [PubMed] [Google Scholar]
- van Leeuwen W, Vermeer JE, Gadella TW Jr, Munnik T. 2007. Visualization of phosphatidylinositol 4, 5-bisphosphate in the plasma membrane of suspension-cultured tobacco BY-2 cells and whole Arabidopsis seedlings. The Plant Journal 52, 1014–1026. [DOI] [PubMed] [Google Scholar]
- Vossen JH, Abd-El-Haliem A, Fradin EF, et al. 2010. Identification of tomato phosphatidylinositol-specific phospholipase-C (PI-PLC) family members and the role of PLC4 and PLC6 in HR and disease resistance. The Plant Journal 62, 224–239. [DOI] [PubMed] [Google Scholar]
- Wang CR, Yang AF, Yue GD, Gao Q, Yin HY, Zhang JR. 2008. Enhanced expression of phospholipase C 1 (ZmPLC1) improves drought tolerance in transgenic maize. Planta 227, 1127–1140. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhu X, Liu J, Chu X, Jiao J, Liang Y. 2013. Involvement of phospholipases C and D in the defence responses of riboflavin-treated tobacco cells. Protoplasma 250, 441–449. [DOI] [PubMed] [Google Scholar]
- Xia K, Wang B, Zhang J, Li Y, Yang H, Ren D. 2017. Arabidopsis phosphoinositide-specific phospholipase C 4 negatively regulates seedling salt tolerance. Plant, Cell & Environment 40, 1317–1331. [DOI] [PubMed] [Google Scholar]
- Xue H, Chen X, Li G. 2007. Involvement of phospholipid signaling in plant growth and hormone effects. Current Opinion in Plant Biology 10, 483–489. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JD, Doke N. 2003. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. The Plant Cell 15, 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Fang Q, Zhang Z, Wang Y, Zheng X. 2009. The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hypersensitive response in Nicotiana benthamiana. Journal of Experimental Botany 60, 3109–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. 1998. The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proceedings of the National Academy of Sciences, USA 95, 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SZ, Liu YL, Li B, Shang ZL, Zhou RG, Sun DY. 2012. Phosphoinositide-specific phospholipase C9 is involved in the thermotolerance of Arabidopsis. The Plant Journal 69, 689–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.