Review of grapevine drought responses and the search for drought-tolerant varieties that can increase the sustainability of viticulture in the context of climate change.
Keywords: Agriculture, climate change, fruit ripening, viticulture, water deficit, wine
Abstract
Water availability is arguably the most important environmental factor limiting crop growth and productivity. Erratic precipitation patterns and increased temperatures resulting from climate change will likely make drought events more frequent in many regions, increasing the demand on freshwater resources and creating major challenges for agriculture. Addressing these challenges through increased irrigation is not always a sustainable solution so there is a growing need to identify and/or breed drought-tolerant crop varieties in order to maintain sustainability in the context of climate change. Grapevine (Vitis vinifera), a major fruit crop of economic importance, has emerged as a model perennial fruit crop for the study of drought tolerance. This review synthesizes the most recent results on grapevine drought responses, the impact of water deficit on fruit yield and composition, and the identification of drought-tolerant varieties. Given the existing gaps in our knowledge of the mechanisms underlying grapevine drought responses, we aim to answer the following question: how can we move towards a more integrative definition of grapevine drought tolerance?
Introduction
Grapevine (Vitis vinifera) is one of the most economically important fruit crops worldwide (Alston and Sambucci, 2019), and its use in wine production has played an important cultural role in many parts of the world. Grapevines are now cultivated in more than 90 countries for wine, distilled liquors, juice, table grapes, and raisin production (FAO-OIV, 2016). Because of its global economic importance, the climatic diversity of the producing regions, and the large number of studies (from genomics to production practices), grapevine has emerged as a model perennial fruit crop species.
Almost all wine regions in the world are located in temperate zones and many have a Mediterranean climate characterized by warm and dry summers. In these regions grapevines are regularly exposed to periods of drought unless irrigation is applied, and currently much of the world’s winegrape production is not irrigated. For instance, as of 2016, fewer than 10% of vineyards in Europe were irrigated (Costa et al., 2016). However, this percentage appears to be increasing for a variety of reasons including climate change and the relaxation of irrigation restrictions in many traditionally rainfed regions. For example, in Spain the irrigated vineyard area had increased from ~2% in the 1950s to almost 27% in 2015 (Ayuda et al., 2020), and looking at the most recent data from Spain in 2018, that percentage has grown further to ~30% (data obtained from the ‘Anuario de Estadística’, Spanish Ministry of Agriculture). Water deficits impair vine growth and decrease yield, but can improve grape and wine quality unless they are severe (Chaves et al., 2010). In regions where irrigation is used, much attention is placed on developing deficit irrigation strategies (i.e. application of irrigation at levels below what would be required to sustain 100% evapotranspiration) with the goal of producing high quality grapes, particularly for red wines, while minimizing yield losses. The relative importance of yield versus quality varies with the type of grapes being produced and the producer’s goals.
Studies indicate that climate change will exacerbate drought events in many traditional wine growing regions, which will likely increase the need for irrigation (Intergovernmental Panel on Climate Change, 2014). Many argue that irrigation is not a sustainable solution, and although this is likely true for water-scarce regions, putting concrete numbers on this assertion is extremely difficult (Hannah et al., 2013). Quantitative studies that predict the increase in freshwater resource consumption by expanding vineyard irrigation are needed in many regions.
Because of the lingering questions surrounding the sustainability of irrigation, there has been a strong focus on the need to understand the differences in drought tolerance between existing grapevine varieties (Medrano et al., 2018). The logic is that a detailed mechanistic understanding of the differences in drought tolerance between existing genotypes would (i) increase sustainability through informing the optimum choice of plant material for a given climate-production context and (ii) identify the key traits responsible for these differences in order to better design and target phenotyping approaches for the development of new drought-tolerant varieties. It is important to keep in mind that grapevines are perennial crops that typically produce for many decades. Thus, drought tolerance from the point of view of viticulture must comprise both the ability to maintain productivity within the current season and equally the ability to avoid negative carry-over effects, and perhaps drought-induced mortality, across many seasons (Gambetta, 2016).
In order to characterize the differences in drought tolerance between grapevine genotypes, many studies have focused on key agronomic indicators such as yield and grape composition (i.e. fruit quality), while others have focused on finer scale physiology such as stomatal regulation and carbon assimilation. Despite extensive study, how different varieties respond to drought in terms of water use, growth, fruit quality, and yield remains an open and critical question for managing water in vineyards. Yield is impaired under drought conditions but a recent meta-analysis suggests this decrease may be variety specific (discussed below; Mirás-Avalos and Intrigliolo, 2017). Recent work suggests that all genotypes regulate vine water use (i.e. stomatal conductance) in order to protect against more severe damage such as petiole or leaf cavitation and leaf shedding (Hochberg et al., 2017c; Dayer et al., 2020). However, it is unclear to what extent differences in the regulation of vine water use between varieties result from innate genotypic differences, as was traditionally thought, or environmental factors (Hochberg et al., 2018). Furthermore, grapevine appears to almost always operate within a ‘safe’ margin of water potentials in which stem cavitation is extremely rare (Charrier et al., 2018), but we still do not know the exact mortality thresholds for grape. Thus, numerous gaps remain in our understanding of what really constitutes a drought-adapted grapevine variety, making it difficult to robustly address future climate challenges. Given the existing gaps in our knowledge, this review focuses on the following question: How can we move towards a more integrative definition of grapevine drought tolerance? Accordingly, we synthesize the current state-of-the-art regarding grapevine drought stress physiology, including the impact on leaves and fruit composition, and use this background to discuss an integrative approach to define and characterize drought-adapted varieties.
Grapevine drought responses
Stomatal regulation of vine water use
According to the cohesion–tension theory (reviewed by Tyree and Zimmermann, 2002), water flows from the soil to the atmosphere through the plant xylem network under tension (i.e. under a negative pressure), pulled by the more negative water potentials (Ψ) in the leaf tissues where water is transpired into the atmosphere through stomata (Fig. 1). By regulating its stomatal and hydraulic conductance the plant can determine its Ψ anywhere between the soil water potential and the atmospheric water potential. In grapevines, Ψ usually ranges between −0.3 and −2.0 MPa (e.g. Charrier et al., 2018). Because the water flows in a metastable state (i.e. under tension), when the tension exceeds certain limits, cavitation occurs, leading to hydraulic failure and the risk of mortality (Tyree and Sperry, 1989).
Fig. 1.
Cryo-scanning electron microscopy images of the underside of a grapevine (Vitis rupestris) leaf at various magnifications. Islands of stomata (A) are visible in between leaf vascular traces, and there is ample aperture diversity (B) of stomata in more closed (C) or open (D) states. Scale bars are shown in each panel.
Stomata are key players in a plant’s response to drought. During drought stomata close to reduce transpiration, avoid critical Ψ, and conserve water (Darwin, 1898). Without this reduction in transpiration, the fast flow could lead to large pressure drops between the soil and leaf (i.e. increasingly negative Ψ). Irrigated vineyards typically function within a safe range of water potentials (Fig. 2, Ψ stem>−1.5 MPa) that do not lead to cavitation or turgor loss. Even non-irrigated vineyards seldom surpass these values (Charrier et al., 2018). When vineyard managers utilize deficit irrigation strategies, most commonly in association with premium red winegrape production, they normally target levels of water deficit (Ψ stem −1.2 to −1.4 MPa) that are certainly great enough to decrease stomatal conductance (gs), transpiration, photosynthesis, and ultimately fruit yield (Fig. 2). More severe water deficit (Ψ stem<−1.6 MPa) might induce turgor loss and xylem cavitation that could lead to leaf shedding and even vine mortality.
Fig. 2.
The sequence and thresholds of grapevine responses to water deficit. Irrigated vineyards typically function within a safe range of water potentials (green transpiration zone, Ψ stem>−1.5 MPa). In this zone grapevines can experience decreased transpiration (E), stomatal conductance (gs), hydraulic conductance (K), photosynthesis, and fruit yield (purple). ABA concentration ([ABA]) does not increase until after stomata are mostly closed. As water deficit increases vines can acclimate through numerous processes. One is osmotic adjustment where their leaf turgor loss point (Ψ TLP, blue) can become more negative allowing the vine to operate across a wider range of Ψ stem. If Ψ stem becomes increasingly negative (~Ψ stem<−1.6 MPa) grapevines can experience cavitation producing embolisms in the water-conducting xylem vessels (quantified as the percentage loss of conductivity, PLC). Grapevines exhibit ‘vulnerability segmentation’ in which leaves (orange) are more vulnerable to embolism than perennial tissues (e.g. trunks and stems; red). Embolism in leaves acts as a hydraulic fuse protecting perennial organs from experiencing levels of water stress that would lead to embolism. Extensive embolism can lead to vine mortality (grey).
Different grapevine genotypes exhibit a continuum of stomatal sensitivities to water deficit (Lavoie-Lamoureux et al., 2017; Charrier et al., 2018; Levin et al., 2019; Dayer et al., 2020) where some varieties tend to close their stomata earlier than others (discussed below). Regardless of these differences all vines close their stomata in response to water deficit within a relatively narrow range of water potentials when compared with the breadth of plant taxa in general (e.g. compare Lavoie-Lamoureux et al., 2017; Martin-StPaul et al., 2017). Understanding the underlying mechanisms that drive the regulation of stomata under drought allows the identification of targets that could be leveraged in the breeding of more drought-tolerant varieties and/or rootstocks.
The mechanisms contributing to stomatal regulation
One of the reasons why the elucidation of the mechanisms driving stomatal regulation remains so challenging is that they are numerous and interdependent, involving biochemical and hydraulic signals among others (Buckley, 2019). The most prominent biochemical player studied is the plant hormone abscisic acid (ABA), which has become synonymous with drought stress, although ABA functions in numerous plant processes. ABA directly affects gs at the guard cell level, and the molecular mechanisms driving these direct effects are well studied. ABA binds to the PYR/PYL/RCAR ABA receptors that interact with, and remove the inhibitory action of, the 2C protein phosphatases (PP2Cs) (Nakashima and Yamaguchi-Shinozaki, 2013). Other molecular messengers eventually activate ion efflux transporters that decrease guard cell turgor and close the stomata (Boneh et al., 2012; Munemasa et al., 2015). Changes in Ψ leaf and/or hydraulic conductance (K) also affect guard cell turgor and gs. Changes in K likely involve the regulation of aquaporins, cell membrane proteins that facilitate water and small molecule transport through plasma membranes (e.g. Gambetta et al., 2017; Maurel and Prado, 2017). Aquaporins comprise a large gene family whose regulation is complex, and they are expressed in all grapevine tissues (Wong et al., 2018). As in other plant species, aquaporins have been linked to grapevine stomatal regulation and to differences in this regulation between varieties (Vandeleur et al., 2009; Vitali et al., 2016; Coupel-Ledru et al., 2017).
The relative contribution of these biochemical and hydraulic mechanisms is still unknown and likely dependent on genotype (McAdam and Brodribb, 2015; Coupel-Ledru et al., 2017; Peccoux et al., 2018) as well as environment (Lovisolo et al., 2016; Hochberg et al., 2017b). When the entire breadth of plant taxa is considered, we observe a spectrum of relative contributions of these two modes (hydraulic and biochemical) from gymnosperms that utilize a purely hydraulic mode to angiosperms whose regulation is largely ABA-dependent (McAdam and Brodribb, 2015). In grapevine, studies suggest that both hydraulics and ABA play a role in stomatal regulation although the relative contribution of these two modes remains unclear (Tombesi et al., 2015; Peccoux et al., 2018). Studies in grape have found that ABA increases only when gs falls to very low levels (≤50 mmol m−2 s−1), suggesting that early stomatal closure is not ABA driven (Fig. 2 ; Pou et al., 2008; Hochberg et al., 2013b; Tombesi et al., 2015; Degu et al., 2019).
The hydraulic and biochemical modes of stomatal regulation are interdependent, making a strict division between the two extremely challenging both theoretically and experimentally. A good example of this conundrum is illustrated by the links between ABA and K. Changes in Ψ are modulated in part through changes in K and, in turn, K is modulated by ABA, creating an interdependence that has yet to be disentangled. This interdependence appears to be present in most plant organs. For example, leaf hydraulic conductance (Kleaf) is modulated by ABA (Shatil-Cohen et al., 2011; Pantin et al., 2013). In grape specifically, genotypes appear to differ in the sensitivity of Kleaf to exogenous ABA treatments (Coupel-Ledru et al., 2017). This implies that when ABA concentrations increase in leaves under water deficit there are effects on both gs and K, both of which when integrated would affect Ψ leaf, and thus ABA, setting up a circular loop. Furthermore, cell shrinkage was shown to increase ABA biosynthesis (McAdam and Brodribb, 2016), suggesting the reduction in Ψ leaf would reinforce the ABA effect on the stomata. The same situation is present in roots, where root hydraulic conductance also appears to be modulated by ABA (reviewed in Gambetta et al., 2017). The challenge of this complexity is not restricted to the examples highlighted above and researchers have suggested a number of other mechanisms including changes in guard cell ABA sensitivity as a function of Ψ leaf (distinct from changes in ABA concentration) and other potential biochemical signals like pH (Chaves et al., 2010; Geilfus, 2017) and reactive oxygen species (Cruz de Carvalho, 2008; Miller et al., 2010).
Decreases in photosynthesis
During drought, photosynthesis is limited by both stomatal closure and impairment of the photosynthetic machinery (i.e. metabolic factors). The relative contributions of these two mechanism are not always clear and an in-depth discussion of these factors is outside the scope of the current review (see the review by Flexas et al., 2004). In grape, the photosynthetic machinery appears to be very tolerant to mild, and even medium, levels of water deficit (Flexas et al., 1998; Chaves et al., 2010; Dayer et al., 2019). For example, Flexas et al. (2002) demonstrated that gs needed to be reduced by 60–70% before changes in electron transport rate and non-photochemical quenching of chlorophyll fluorescence were observed. Intrinsic water use efficiency (WUEi) increases under mild drought, and then later decreases under more severe or long-term drought because of the damage or inhibition of photosynthesis (Bota et al., 2016). It seems that non-stomatal limitations become dominant only when grapevine gs falls below 50 mmol m−2 s−1 (Flexas and Medrano, 2002).
Under long-term drought, mesophyll conductance decreases, negatively affecting CO2 diffusion and limiting photosynthesis (Ouyang et al., 2017). In grape, decreases in mesophyll conductance appear to be coincident with an impairment of the photosynthetic machinery (Flexas et al., 2002), although the exact magnitude and timing of the decrease is likely variety specific and related to leaf anatomical structure (Tomás et al., 2014). Research in other species has demonstrated that modulation of mesophyll conductance results in part from the regulation of leaf aquaporin expression and/or activity (Maurel and Prado, 2017) and the same is also likely true in grape (Flexas et al., 2010).
Drought exacerbates photoinhibition (i.e. a transient decrease of the photochemical efficiency of PSII as a result of excess photosynthetic photon flux density), and different varieties have developed various strategies to cope with this (Guan et al., 2004). Beis and Patakas (2012) in a study of Greek Vitis vinifera varieties found that the drought-tolerant Sabatiano removed excess absorbed light by thermal dissipation (non-photochemical quenching) and protected cells via rapid activation of the antioxidant defense system. In contrast, Mavrodafni relied on the activation of photorespiration, which is an alternative process to dissipate excess light energy and thus prevent damage to the photosynthetic apparatus. Hochberg et al. (2013a) observed a link between photosynthetic efficiency and photorespiration rate and stomatal regulation when comparing Cabernet Sauvignon and Syrah. Cabernet Sauvignon displayed tighter stomatal control and higher photosynthesis and photorespiration rate, which may help ameliorate the photoinhibition caused by the stomatal closure.
Leaf osmotic adjustment
The concentration of solutes in the symplast enables leaves to maintain turgor pressure under negative Ψ (Hsiao et al., 1976). For this reason, the Ψ at which leaf cells lose turgor (i.e. turgor loss point; Ψ TLP) has been used as a leaf drought tolerance trait across species. Leaf turgor loss is correlated with stomatal closure (Brodribb et al., 2003) and the decline in Kleaf (Scoffoni et al., 2011; Trifiló et al., 2016) so that leaves with a more negative Ψ TLP are able to maintain stomatal and hydraulic conductance and growth under drier conditions (Bartlett et al., 2012). The same relationships between Ψ TLP and stomatal closure have been found in grapevines (Fig. 2; Hochberg et al., 2017a; Dayer et al., 2020).The Ψ TLP is a plastic trait and most plant species decrease the osmotic potential under drought conditions by accumulating osmotically active solutes in leaf cells (i.e. osmotic adjustment). This allows them to maintain positive cell turgor despite decreasing Ψ (Bartlett et al., 2014; Maréchaux et al., 2017).
Previous research has shown that grapevines osmotically adjust in response to drought conditions (Downton and Millhouse, 1983; During, 1984; Rodrigues et al., 1993; Schultz and Matthews, 1993; Patakas and Noitsakis, 1999; Patakas et al., 2002; Martorell et al., 2015; Hochberg et al., 2017a). However, it is important to mention that different species accumulate different osmolytes (Sofo et al., 2008; Warren et al., 2012) and little information is available regarding the compounds involved in the osmoregulation of grapes. Nitrogen metabolism, particularly amino acids, is responsive to drought stress in grapevine and might have a role in the osmotic adjustment (Hochberg et al., 2013b). Proline has been suggested as a prominent osmolyte because of its accumulation under stress, but it seems that its contribution to the osmotic potential is marginal. Patakas et al. (2002) and more recently Degu et al. (2019) suggested that calcium accumulation could have a pivotal role in the osmotic adjustment of grape leaves together with other inorganic ions such as potassium. Interestingly, some authors found an increase in calcium oxalate crystals in leaves of grapevines under drought, suggesting that these structures in the mesophyll could either play a functional role in water stress and calcium regulation, or represent an unintended result of the increased calcium accumulation (Liu et al., 2015; Doupis et al., 2016).
Hydraulic vulnerability segmentation
Severe water deficits can increase the tension in the water column to levels at which embolisms form within the xylem vessel rendering the xylem conduit in which they occur permanently or temporarily dysfunctional (Fig. 3). Widespread embolism (hydraulic failure) can lead to leaf shedding and vine mortality. Grapevines, however, exhibit a special trait referred to as hydraulic vulnerability segmentation, which protects the perennial tissues (e.g. trunks and canes) from experiencing levels of water stress that would lead to embolism (Tyree and Ewers, 1991). Plants exhibit hydraulic vulnerability segmentation when distal organs, such as leaves, are more vulnerable to embolism than the perennial organs. As Ψ becomes more and more negative, petioles and leaves experience embolism first, severing the water column connecting leaves to the perennial organs. This acts as a ‘hydraulic fuse’ protecting the perennial organs from more negative Ψ (Tyree and Ewers, 1991). Many species exhibit vulnerability segmentation (Tsuda and Tyree, 2000; Pivovaroff et al., 2014; Peguero-Pina et al., 2015; Johnson et al., 2016), conferring the ability to conserve their vital organs in the face of severe drought.
Fig. 3.
Non-invasive visualization of embolisms in a 1-year-old grapevine cane using high-resolution computed tomography. Functional xylem vessels appear bright white (visualized via the contrasting agent iohexol), but some vessels have become embolized (black xylem vessels, examples shown with red arrowheads) and do not conduct water.
In grapevine, studies using destructive methods to measure hydraulic conductivity have suggested the presence of vulnerability segmentation (Schultz, 2003; Lovisolo et al., 2008; Choat et al., 2010; Tombesi et al., 2014; Shelden et al., 2017). Recent studies using non-destructive in vivo visualization via magnetic resonance imaging and synchrotron-based microcomputed tomography revealed the same vulnerability segmentation (Hochberg et al., 2016a, 2017c; Charrier et al., 2016). When synthesized, studies in grapevine demonstrate that petioles and leaves are significantly more vulnerable than stems (Fig. 2). Further, the hydraulic vulnerability of basal leaves is greater than that of the apical leaves, which enables grapevines to preferentially shed the older and less photosynthetically efficient basal leaves (Hochberg et al., 2017c). The vulnerability segmentation strategy suggests that under severe drought grapevines have a ‘wait it out’ strategy, in which the vine is designed to abandon the current year’s canopy to preserve the perennial structure until the following season.
The mechanisms that bring about vulnerability segmentation in grape are not understood. Generally, larger xylem vessels are more susceptible to embolism than smaller vessels due to higher inter-vessel pit surface areas and more pits (Wheeler et al., 2005). However, the xylem vessel diameter of the petioles was smaller than that of stems (Hochberg et al., 2016a), which implies that petioles would be more resistant to embolism than the stem solely based on this criterion. Hochberg et al. (2016a) proposed that the larger proportion of primary xylem than secondary xylem in petioles may, in part, explain this discrepancy as primary xylem is more embolism sensitive. Differences in pit pore properties, xylem vessel arrangement, along with other factors may also contribute to the segmentation (Holbrook and Zwieniecki, 2005). More research is needed to identify the source of the observed vulnerability differences.
Extreme drought
High levels of embolism in perennial organs, referred to as ‘hydraulic failure’, can lead to plant mortality (McDowell et al., 2008). Although their vulnerability segmentation protects against high levels of embolism in stems and trunks, grapevines can still die from drought. Extreme water deficits (Fig. 2, Ψ stem<−2 MPa) generally result in the loss of much or all of the canopy and crop in the current season. The following season the vine may have the potential to regrow if some buds have survived the drought episode although the exact desiccation thresholds of bud tissues are unknown. In potted-plant experiments even when vines are stressed to levels that result in nearly complete defoliation and 100% loss of conductivity due to embolism in stems, a large percentage of vines still regrow the following season (Tombesi et al., 2018; G. Charrier and G. A. Gambetta, unpublished data). The ability of vines to recover from and/or repair extensive embolism over winter may involve their ability to refill embolized xylem vessels in the stem (Knipfer et al., 2015a; Nardini et al., 2017). However, leaves and petioles do not appear to recover from embolism. Embolized petioles were not refilled even when submerged overnight in water (Hochberg et al., 2016b) and embolized leaves were shed even after irrigation was administered and stress was relieved (Hochberg et al., 2017c).
Assuming a vine does recover over winter from an extreme drought event there are still many questions regarding the extent to which the vine may be compromised for future events and how future fruit yields could be impacted in subsequent seasons. One concern is cavitation fatigue, in which previous cavitation events lead to an increase in vulnerability to cavitation (Hochberg et al., 2017a). It is reasonable to assume that vines that have undergone a drought event that resulted in severe levels of embolism may be more vulnerable to future events. With respect to season-to-season carry-over effects on fruit yield, even less extreme water deficits can sometimes lead to decreased yields in the following season (Buttrose, 1974; Williams and Matthews, 1990; Dayer et al., 2013), but to date few studies have investigated the carry-over effects of more severe drought events (Tombesi et al., 2018).
Defining drought tolerance in grapevine
The hydraulic variability between grapevine cultivars has been well known for several decades and has been the subject of dozens of studies (e.g. Schultz, 2003; Rogiers et al., 2012). Different cultivars have been shown to have different sensitivities to deficit irrigation in terms of changes in fruit yield and composition (e.g. Hochberg et al., 2015a; Martorell et al., 2015). It is critical to understand the physiological source of this variability in order to improve predictions of varietal behavior in response to climate and to optimize irrigation strategies. Unfortunately, there are numerous hydraulic traits that determine a vine’s response to water availability: xylem architecture (Hochberg et al., 2015b), aquaporin regulation (Vandeleur et al., 2009), ABA dynamics (Soar et al., 2006), osmotic adjustment (Martorell et al., 2015), root characteristics (Alsina et al., 2011), and stomatal regulation (Schultz, 2003) are all linked to hydraulic variability between cultivars. Even if we were to explore all of these traits in a single cultivar (which to date has never been done), transformation of the resulting physiological traits matrix into a hydraulic strategy exceeds our current physiological models. Therefore, to simplify cultivars’ hydraulic classification, researchers turned to a phenomenological scale (Tardieu and Simonneau, 1998) ranking species from isohydric to anisohydric.
The iso/anisohydric terminology was introduced into grapevine hydraulics by Schultz (2003), who used this terminology to distinguish between Grenache and Syrah based on seasonal water potential (Ψ) homeostasis. Later, the terms gained additional meanings, describing daily Ψ homeostasis (Soar et al., 2006) or the Ψ leading to stomatal closure (Lavoie-Lamoureux et al., 2017). The classification became popular since it was relatively easy to measure based on a few physiological traits (i.e. gs and Ψ) and could presumably indicate the variety’s performance under drought or the underlying physiological traits (e.g. hydraulic vulnerability (Schultz, 2003), aquaporin expression (Vandeleur et al., 2009), and transciptomic responses (Dal Santo et al., 2016). However, it can be argued that the iso/anisohydric paradigm has not provided much information about cultivars’ response to water stress. A case in point, after 17 years, with over 80 grapevine studies that have used the terminology, it is still unclear which of the two (iso/anisohydric) represents cultivars that are better adapted to drought.
Recently, Hochberg et al. (2018) pointed out that the use of the iso/anisohydric terminology should be abandoned for two reasons: (i) the different definitions (seasonal Ψ homeostasis, daily Ψ homeostasis, and stomatal regulation) are not necessarily in agreement with one another, creating confusion as to the actual meaning of the terms; and (ii) the environmental effects are at least as significant as the genotypic effect, and thus we cannot predict a cultivar’s hydraulic behavior without accounting for the environment (Hochberg et al., 2018; Villalobos-González et al., 2019). It is important to explicitly state that the call to abandon the terminology is not tantamount to a denial of the hydraulic variability between grape cultivars. It only stresses that we should seek other approaches in order to more accurately describe, and better predict, varietal responses to water deficit.
The environmental effect on varietal responses could be approached through large cultivar comparisons in common garden experiments (e.g. Gowdy et al., 2018) or phenotyping facilities (e.g. Coupel-Ledru et al., 2014; Charrier et al., 2018; Dayer et al. 2020). In addition to the effects that directly originate from environmental factors (soil water potential, soil hydraulic conductivity, and vapor pressure deficit), the exposure to different environments will also shape the vine’s hydraulic properties (i.e. hydraulic plasticity; Lovisolo et al., 2010). Turgor loss point, stomatal regulation, and hydraulic architecture were all shown to have high environmental plasticity (Lovisolo et al., 2010; Martorell et al., 2015; Hochberg et al., 2015b, 2017a; Munitz et al., 2018), meaning that cultivar comparisons under different conditions, and at different developmental stages, might not always lead to the same hydraulic behavior. This may be one reason for the variable behaviors that have been observed in the same variety between different studies (e.g. Chaves et al., 2010; Charrier et al., 2018). Common garden experiments or phenotyping facilities should reveal the hydraulic differences that originate from genotypic variability and provide the hydraulic base that could be later expanded into plasticity studies.
To move beyond the iso/anisohydric terminology we propose to describe a specific variety’s drought tolerance with the following four core physiological traits that when integrated describe a vine’s response to water stress.
(i) Maximal transpiration rate (Emax), a good indicator for the soil reservoir depletion rate. A rapidly transpiring vine is more likely to experience stress in between water supplies (either rain or irrigation). Measurements should be made on healthy well-watered vines and always referenced to the potential evapotranspiration (Penman, 1948). Emax could be estimated indirectly through a combination of leaf area and E measurements for specific leaves, but those are likely to overestimate the actual Emax due to the large variability in E between leaves (Escalona et al., 2016). Alternatively, Emax could be evaluated from lysimeter measurements. In field scale lysimeters Emax ranged between 4 and 60 litres day−1, depending on leaf area and potential evapotranspiration (Williams and Ayars, 2005). However, due to the heavy cost of field scale lysimeters, large cultivar comparisons of Emax are more feasible in small pots (Coupel-Ledru et al., 2014).
(ii) Stomatal regulation, one of the most widely studied traits impacting drought tolerance. It is expressed as the gs–Ψ leaf curve and indicates the maximal photosynthetic capacity (Medrano et al., 2003; Brodribb et al., 2020) as well as the critical Ψ leaf that plants are trying to avoid (Meinzer et al., 2009; Choat et al., 2018). The latter could be used to calculate the soil water reservoir that is available to the vine (in combination with root volume and the soil water retention curve). Of the four core traits, stomatal regulation is by far the most widely studied, probably due to the relative simplicity of the Ψ leaf and gs measurements. However, curve fitting and its extrapolation to predict Ψ crit are not trivial, especially when attempting to determine the Ψ leaf that leads to stomatal closure. Since the curve tends to g s=0, the error when calculating the Ψ leaf that leads to 1% gs (compared with the maximal gs) might be in the magnitude of several MPa. Therefore, it is recommended to select values from the center of the curve (Ψ leaf that leads to 10–25% of the maximal gs) that could serve as proxies for stomatal closure (Lavoie-Lamoureux et al., 2017). In a meta-analysis of 40 studies, Lavoie-Lamoureux et al. (2017) showed that at −1.2 MPa, gs varied between 45 and 338 mmol m−2 s−1, reflecting the large variability of this trait. Interestingly, these authors found that studies that used porometers measured on average two times higher gs compared with studies that used infra-red gas analysers (Lavoie-Lamoureux et al., 2017). It is clear that measuring the actual gs is not as trivial as portrayed by commercial producers of gas exchange devices, and that in addition to using best practices such as making comparisons using the same instrument within studies, substantial variation should be expected between studies.
(iii) Turgor loss point (Ψ TLP), a well-defined physiological trait that is relatively easy to measure (Bartlett et al., 2012; Petruzzellis et al., 2019). Ψ TLP is a good predictor of stomatal closure (Martorell et al., 2015) and the onset of molecular responses to stress (McAdam and Brodribb, 2018). The latter is possibly a result of the activation of membrane proteins when turgor loss leads to membrane shrinkage. It is important to reiterate that this trait is very plastic and can be shifted by up to 1 MPa along the season or in response to water deficit (Alsina et al., 2007; Martorell et al., 2015). The extent of Ψ TLP plasticity is likely to be a good indicator for the capacity of a cultivar to osmotically adjust to withstand low water potentials.
(iv) Root volume, one of the most basic and enigmatic physiological traits. When combined with the soil retention curve, the rooting volume could be used to determine the soil water reservoir that is available to the vine. Alsina et al. (2011) found that drought-adapted rootstocks tend to have deeper roots. Currently, reliable methods to estimate rooting volume are being developed, such as 3D soil tomography (Zhu et al., 2014) and water isotope quantification (Savi et al., 2018), which may provide insight into this trait in the future.
By considering all of these core traits together, one could more holistically evaluate a vine’s drought tolerance. Here we introduce the idea of a vine’s ‘stress distance’. The stress distance would be a quantitative value with the units of ‘time’ that would represent the amount of time that a particular vine could go without water, under a given set of environmental conditions, until it reached the critical water potential threshold, or Ψ crit. The stress distance can be described quantitatively as:
where Ψ 0 is the initial water potential and Ψ crit is evaluated from the gs–Ψ leaf relationship or as Ψ TLP.
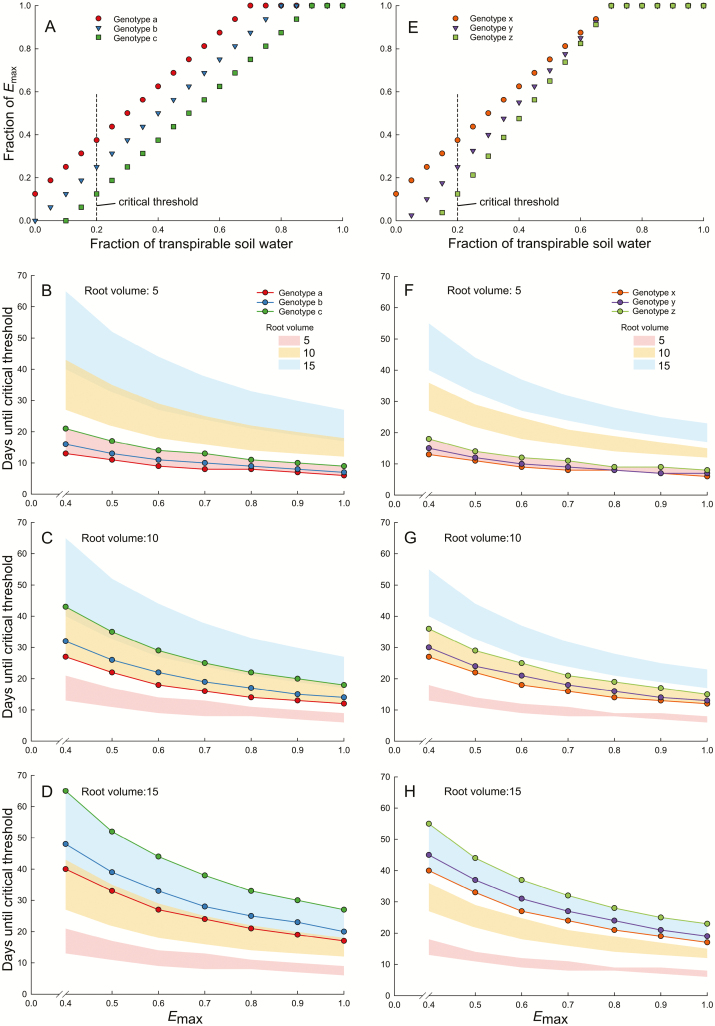
This is an oversimplification, but a useful one, and is not intended to supersede more rigorous attempts at modeling vine water use (Lebon et al., 2003; Peccoux et al., 2018; Zhu et al., 2018, 2019). Using this framework in a simplified thought experiment gives insight into the relative importance of the various traits. Figure 4 illustrates how the stress distance would change when varying stomatal regulation, root volume, and Emax under generic scenarios (Ψ crit does not vary in this example). High Emax decreases the relative impacts of varying stomatal regulation and root volume. The importance of Emax is reinforced by the two examples of varied stomatal control, in which changing the point at which E begins to be regulated (Fig. 4A–D) has a greater impact on increasing the stress distance than changing the slope of the regulation (Fig. 4E–H). The idea of a variable Emax is nuanced and could reflect innate differences between genotypes when Emax (or gmax) is considered on a per unit leaf area basis (Levin et al., 2019; Dayer et al., 2020), and/or could reflect differences in canopy size, architecture, and/or planting density (Lebon et al., 2003; van Leeuwen et al., 2019). Stomatal regulation, on the other hand, becomes increasingly important as the stress distance increases, through decreased Emax and/or increased root volume (Fig. 4).
Fig. 4.
Comparing the relative importance of stomatal regulation, root volume, and maximum transpiration (Emax) in determining the amount of time until a critical threshold (here defined as 20% of transpirable soil water) is reached. We created six generic genotypes with different stomatal control behaviors, three (A–D) where only the break point varies (i.e. when stomata regulation starts), and three (E–H) where only the slope varies (i.e. the speed at which stomata close). We calculated the days until the critical threshold would be reached (i.e. the stress distance) varying the root volume with generic values of 5 (B, F), 10 (C, G), 15 (D, H), and Emax. The shading in the panels is provided for reference and represents the range of values across the three genotypes for a given root volume.
The stress distance thought experiment provides several interesting conclusions regarding what constitutes drought tolerance in grapevine. The first is to highlight the importance of Emax. This reinforces traditional viticultural practices for dry climates, where low planting densities (theoretically increasing the rooting volume) and training systems favoring smaller canopies (theoretically decreasing Emax) have traditionally been used. It also suggests that within a single season, reducing canopy size (i.e. pruning) could provide an excellent emergency strategy for vineyards that face extreme drought in the absence of irrigation infrastructure. The stress distance thought experiment also speaks to the impact of using genotypes with a tighter stomatal regulation, and suggests that this strategy will be much more effective if used together with strategies that slow the dry-down, by decreasing maximum water use and/or increasing water availability to the vine. Slowing the dry-down has the added advantage that it provides more time for the vine to acclimate (e.g. decrease Ψ TLP, Hochberg et al., 2017a).
Berry drought responses: hydraulics, yield, and metabolism
Berries hydraulics
Red winegrape production is peculiar in that growers often hope for, or purposefully apply, water deficits. Water deficits increase red winegrape quality through numerous mechanisms that include increasing the accumulation of quality-related metabolites (detailed below) and decreasing berry size leading to an increased ratio of berry skin (where many quality related metabolites accumulate, e.g. phenolics) to flesh. Berry fresh weight largely results from the accumulation of water, and to a lesser extent sugar, so how berries are hydraulically integrated with the parent vine is critical in controlling growth, ripening, and the response to water deficit.
Berry water relations are markedly different before and after the onset of ripening (veraison). Before veraison berries are hydraulically connected to the vine, and although they have very few, or no, functional stomata they exhibit high rates of cuticular transpiration (Blanke and Leyhe, 1987; Zhang and Keller, 2015). Pre-veraison berries are also sensitive to changes in the vine’s Ψ and can experience drought-induced shriveling (Fig. 5; Hardie and Considine, 1976; Greenspan et al., 1994). After veraison the hydraulic connection between berries and the vine changes; transpiration decreases due to a reduction of the cuticular conductance—likely resulting from changes in cuticular wax composition (Rogiers et al., 2004; Dimopoulos et al., 2020). The pathway of water transport into the berry changes from the xylem to the phloem in concert with the sharp increase in sugar transport and accumulation (Greenspan et al., 1994; Greer and Rogiers, 2009). Additionally, the hydraulic conductance of the berry and pedicel decreases (Tyerman et al., 2008; Choat et al., 2009; Knipfer et al., 2015b). In contrast with pre-veraison berries, post-veraison berries are buffered from changes in the vine’s Ψ and largely insensitive to drought-induced shriveling (Fig. 5; Hardie and Considine, 1976; Greenspan et al., 1994; Keller et al., 2015).
Fig. 5.
Examples of the hydraulic buffering that occurs at veraison in grapevine. When a vine is subjected to water deficit, pre-veraison berries (i.e. green berries) are sensitive to drought-induced shriveling, and berries that are either undergoing veraison (A, B, reddish-green berries) or post-veraison (C, purple berries) are insensitive. Vines shown are under a very severe water deficit (Ψ stem<−1.5 MPa). Photos courtesy of Markus Keller.
The mechanisms that result in this hydraulic buffering of the berry are still unknown. Although studies have measured a decrease in the hydraulic conductance of the berry and pedicel, the magnitude of this decrease does not appear great enough to account for the buffering (see discussions of Choat et al., 2009; Knipfer et al., 2015b), and other studies have clearly demonstrated that the xylem pathway remains functional after veraison (Bondada et al., 2005; Keller et al., 2006; Chatelet et al., 2008). It is even likely that excess water imported via the phloem could be recycled into the parent plant through the xylem (Tilbrook and Tyerman, 2009; Choat et al., 2009; Keller et al., 2015; Zhang and Keller, 2017). One possibility is that the decreases in hydraulic conductance have been underestimated because the viscosity of the xylem sap has not been taken into account. Grape berries accumulate an incredibly high amount of sugars (among the highest of all fruit) and studies have suggested that sugars are almost equally distributed between the berry apoplast and symplast (Wada et al., 2008, 2009). When the viscosity is taken into account the decreases in hydraulic conductance could be between ~25% (at 10 °Bx) and ~100% (at 20 °Bx) greater. Another possibility is that sugar accumulation alone could alter berry water relations in a way that creates a buffered system. The exact details are difficult to imagine, especially since turgor and elasticity of post-veraison berries are very low (Thomas et al., 2006; Wada et al., 2008, 2009; Castellarin et al., 2016).
Berry size and yield
Water deficits reduce berry size and yield, and studies have shown that the decreases are linearly related to decreases in Ψ. Grimes and,Williams (1990) reported a 41% decrease in yield per MPa decrease in Ψ and a more recent work by the same group found a 66% decrease in berry weight per MPa decrease in Ψ (Williams et al., 2010). These findings are similar to the 50% average decrease in berry weight per MPa decrease in Ψ found by a recent meta-analysis (Mirás-Avalos and Intrigliolo, 2017). However, Mirás-Avalos and Intrigliolo (2017) found that this relationship was variety dependent. When taken together studies suggest that, on average, a moderate decrease in Ψ of 0.2 MPa results in a 10% yield penalty. The severity, duration, and timing of the water deficit greatly impact the berry’s response in terms of size and metabolic adjustment. For example, there is evidence that the reduction of berry size and yield is greater with pre-veraison water deficits (Hardie and Considine, 1976; Matthews and Anderson, 1989).
Water deficit and berry metabolism
Water deficit greatly affects berry metabolism. The recent meta-analysis of Mirás-Avalos and Intrigliolo (2017) indicates that sugars and organic acids negatively and positively correlate, respectively, with Ψ. However, inconsistent results have been reported among studies. We closely analysed 18 studies performed on four major red grape varieties (Cabernet Sauvignon, Merlot, Syrah, and Tempranillo). In these studies, water potentials for well-irrigated (i.e. control) and deficit-irrigated vines were compared and water deficit levels corresponded to either moderate (−0.9<Ψ stem<−1.1 MPa) or severe water deficit (−1.1<Ψ stem<−1.4 MPa) (Fig. 6; Supplementary Table S1 at JXB online). Moderate deficit significantly affected sugar concentration at harvest in only 7 out of 19 experiments (seasons were independently considered within each study). In all those cases, the deficit increased the concentration (5% on average). Similar results were obtained with severe deficit (7 out of 18 studies indicated a significant increase by 7.8% in sugar concentration under deficit). Similarly, few experiments identified an effect of water deficit on titratable acidity (5 out of 15 for moderate deficit and 6 out of 15 for severe deficit). In all cases but one, titratable acidity was reduced (approx. −12% for both moderate and severe deficit). This analysis indicates that varieties respond differently to water deficit (for instance, Merlot was more sensitive than Cabernet Sauvignon; Supplementary Table S1) and that seasonal conditions affect the responses as suggested by Herrera et al. (2017).
Fig. 6.
Meta-analysis of 18 published studies investigating berry (and sometimes wine) composition changes under water deficit. Experiments were divided into those applying either a moderate (pink bars) or a severe (red bars) water deficit; the corresponding Ψ stem ranges (values represent mean Ψ stem across the entire study period) are noted in the figure. The proportion of study years demonstrating statistically significate effects are noted above each bar in parentheses. The bars represent the average percentage change (for only those studies showing statistically significant changes) of the compound relative to non-stressed controls. Each season within a study was treated separately and all references and detailed data can be found in Supplementary Table S1.
Like the osmotic adjustment observed in leaves under drought (discussed above), berries also accumulate osmolytes in response to water deficit. Nitrogen metabolism seems to play a central role in this process in which water deficit increases the concentration of the amino acids proline, leucine, isoleucine, and valine in parallel with the up-regulation of genes related to their biosynthesis (Deluc et al., 2009; Savoi et al., 2017; Canoura et al., 2018). The accumulation of these osmolytes in response to water deficit can vary between varieties (Deluc et al., 2009; Bouzas-Cid et al., 2018). The role of amino acids in adjusting berry osmotic potential under drought has to be integrated with our current knowledge of the predominant molecules driving berry osmotic potential during ripening: malate, tartrate, glucose, fructose, and potassium before veraison, and mostly glucose and fructose after veraison (Wada et al., 2008).
Flavonoids
Several specialized (also known as secondary) berry metabolites strongly respond to abiotic stressors such as water deficit. Among these metabolites, flavonoids and volatile organic compounds (discussed below) are the most important since they largely contribute to the grape and wine flavor and quality.
Water deficit results in red wines with a higher concentration of anthocyanins, more intense pigmentation, and color shifts towards bluer hues (e.g. Herrera et al., 2015; Cáceres-Mella et al., 2018) due to increases in the anthocyanin concentration in grapes and wines (e.g. Matthews and Anderson, 1988; Chapman et al., 2004; Castellarin et al., 2007a, b; Deluc et al., 2009; Koundouras et al., 2009; Intrigliolo et al., 2012; Savoi et al., 2017). This trend was consistent among the grape varieties in our meta-analysis (Fig. 6; Supplementary Table S1). However, there are examples of studies that have shown no significant changes in anthocyanin levels under water deficit, so this response, although common, is not universal (Bonada et al., 2015; Herrera et al., 2017; Brillante et al., 2018). From the numerous studies on the subject, it emerges that seasonal weather conditions, grapevine variety, and the magnitude and timing of the water deficit dictate the precise effects on berry and wine anthocyanins (Koundouras et al., 2009; Bucchetti et al., 2011; Shellie and Bowen, 2014; Pinasseau et al., 2017; Buesa et al., 2019). For instance, water deficit had a greater impact on anthocyanin accumulation in Syrah than in Cabernet Sauvignon berries and induced the production of acylated anthocyanins in Cabernet Sauvignon while reducing the same compounds in Syrah (Hochberg et al., 2015a). Consistent with the observed changes in metabolism, several genes of the phenylpropanoid and flavonid pathway are up-regulated under water deficit (Castellarin et al., 2007a, b; Deluc et al., 2009; Martínez-Lüscher et al., 2014; Savoi et al., 2017). Some of these genes control the branching of the pathway. The increased expression of 3′,5′-flavonoid hydroxylases and methyltransferases under water deficit resulted in a relative increase in the concentration of tri-substituted and methoxylated anthocyanins, respectively (Castellarin et al., 2007a, b; Martínez-Lüscher et al., 2014; Savoi et al., 2017). Higher levels of methylation increase the stability of anthocyanin (Sadilova et al., 2006), and higher levels of hydroxylation shift the pigment hue to more purple-blue (Tanaka and Brugliera, 2013).
The impact of drought on berry flavan-3-ols and proanthocyanidins (also known as tannins) still remains unclear as contrasting results have been reported among studies (Castellarin et al., 2007a; Deluc et al., 2009; Ollé et al., 2011; Hochberg et al., 2015a; Casassa et al., 2015; Savoi et al., 2017). Pre- and post-veraison application of water deficit increased proanthocyanindin levels in Syrah and Cabernet Sauvignon berries, but only transiently, and at harvest no differences were observed (Castellarin et al., 2007a; Ollé et al., 2011). Other studies in Cabernet Sauvignon showed that water deficit increased proanthocyanidin content corresponding to the up-regulation of the flavan-3-ol- and proanthocyanindin-related genes VviLAR2 and VviMYBPA1 (Casassa et al., 2015; Cáceres-Mella et al., 2017). A recent study that compared the berry phenolic profile of 279 Vitis vinifera varieties to water deficit indicated that berry polyphenol response is not consistent among varieties, with different molecular families affected either positively or negatively between the different varieties (Pinasseau et al., 2017). However, a general increase in the mean degree of polymerization and percentage tri-hydroxylation of proanthocyanindins was observed, and similar effects on the degree of polymerization were observed in other studies (Ollé et al., 2011; Cáceres-Mella et al., 2017; García-Esparza et al., 2018). In contrast, Yu et al. (2016) showed that in Merlot berries there was no effect of water deficit on the skin proanthocyanidin concentration and degree of polymerization.
Flavonols are another major class of flavonoids that accumulate in grapes. Most studies have shown no effect of water deficit on flavonol concentration (Castellarin et al., 2007a; Martínez-Lüscher et al., 2014; Yu et al., 2016; Savoi et al., 2017); nevertheless, several studies have shown transient increases in the expression of the flavonol synthase (VviFLS) genes under water deficit (Ojeda et al., 2002; Castellarin et al., 2007b; Savoi et al., 2016), and increases in flavonol concentration during berry ripening have also been reported (Ojeda et al., 2002; Deluc et al., 2009; Savoi et al., 2016). In addition to having direct effects on berry metabolism, water deficit reduces canopy growth, potentially increasing the exposure of clusters to sunlight (Castellarin et al., 2007b). Flavonol biosynthesis is particularly responsive to light and UV exposure (reviewed in Teixeira et al., 2013), so inconsistencies among studies might be related to differences in the impact of the water deficit on canopy structure. Moreover, Deluc et al. (2009) suggested that the increase in flavonol production observed in white varieties (e.g. Chardonnay) exposed to water stress might be an alternative strategy for photo-protection as white berries lack anthocyanins.
Inconsistencies among studies have also been found when analysing the impact of water deficit on stilbene accumulation, indicating a potential variety-specific response. Deluc et al. (2011) showed that water deficit increased the accumulation of trans-piceid, a glucosylated form of resveratrol, in Cabernet Sauvignon but not Chardonnay. Other studies have also reported variety-specific effects of water deficit on increases in stilbene concentration (Versari et al., 2001; Vezzulli et al., 2007). However, decreases in stilbene biosynthesis under water deficit have also been demonstrated, perhaps resulting from the competition for precursors between the stilbenoid and flavonoid pathway (Hochberg et al., 2015a; Savoi et al., 2017).
Volatile organic compounds
Wine aroma is affected by the vine water status (Chapman et al., 2004; Bonada et al., 2015; Herrera et al., 2015; Picard et al., 2017), but despite the relevance of the topic for commercial production, the impact of water deficit on the accumulation of wine aroma precursors has rarely been investigated. In red grapes, water deficit results in wines with a higher concentration of volatiles (Talaverano et al., 2017) and distinctive organoleptic features such as a higher level of red/black berry, jam/cooked berry, dried fruit, and raison aromas (Chapman et al., 2004; Bonada et al., 2015; Herrera et al., 2015). Riesling wines produced under a moderate water deficit had more pronounced citrus, apple, and pear aromas (Marciniak et al., 2013).
Terpenoids are a major class of secondary metabolites that include several key aromatics for grapes and wines, such as monoterpenes, sesquiterpenes, and C13-norisoprenoids, which contribute to floral and fruity aromas. For example, monoterpenes are responsible for the typical aromas of Muscat, Riesling, Viognier, and Gewürztraminer (Robinson et al., 2014; Siebert et al., 2018). Water deficit increased the concentration of terpene alcohols in both white and red grapes (Song et al., 2012; Savoi et al., 2016), partially through the up-regulation of key methylerythritol phosphate pathway (MEP) genes including terpene synthases (Deluc et al., 2009; Savoi et al., 2016). Interestingly, many of these responsive genes were enriched for drought-related regulatory elements in their promoter regions (Savoi et al., 2016). However, the terpene response to water deficit might not be common for all varieties as recent studies also report decreases in terpene concentration under water deficit (Brillante et al., 2018; Bouzas-Cid et al., 2018). Carotenoids and their volatile C13-norisoprenoid degradation products were also increased by water deficit (Bindon et al., 2007; Song et al., 2012). The increase of C13-norisoprenoids may be due to a larger production of the carotenoid precursors (Bindon et al., 2007) and/or a larger breakdown of carotenoids, as the gene encoding a key norisoprenoid synthesis enzyme, cleavage dioxygenase, was up-regulated by water deficit (Savoi et al., 2016).
Methoxy-pyrazines are compounds that impart a herbaceous or ‘green’ aroma to grapes and wines. Research has found that water deficit can reduce the levels of 3-isobutyl-2-methoxy-pyrazine in Syrah wine (Brillante et al., 2018). Despite the lack of information on whether and how water deficit affects methoxy-pyrazine production and/or degradation, we hypothesize that water deficit indirectly reduces methoxy-pyrazine by limiting canopy growth, increasing cluster exposure, and thus increasing light intensity and temperature (Ryona et al., 2008; Šuklje et al., 2012; Mosetti et al., 2016; Gregan and Jordan, 2016). Water deficit also decreases other compounds imparting herbaceous aromas, such as the C6 compounds hexanal, trans-2-hexenal, and 1-hexanol (Song et al., 2012; García-Esparza et al., 2018). However, an increase of such compounds under water deficit or specific regulated deficit irrigation strategies was also shown (Talaverano et al., 2018; Vilanova et al., 2019). Other grape volatile aldehydes and alcohols that impart both green and fruity aromas, such as 2-heptenal, 1-octen-3-ol, 1-octen-3-one, 2-octenal, nonenal, and nonanol, were induced under water deficit (Savoi et al., 2016, 2017).
Interaction with other factors: environment and phenology
Disagreement among studies indicates that varieties often display different berry metabolic responses to the same abiotic stresses (Deluc et al., 2009, 2011; Hochberg et al., 2015a; Savoi et al., 2017). These inconsistencies could be due to multiple factors. Studies have shown that the timing of water deficit differentially impacts grape and wine quality (Matthews and Anderson, 1988; Castellarin et al., 2007a). Early water deficits prior to veraison have a larger effect on berry size and thus a stronger potential for increasing the concentration of the phenolic compounds (Matthews and Anderson, 1988; Wong et al., 2016). Interestingly, studies also indicate that water deficit continues to effect the biosynthesis of anthocyanins even after the deficit was relieved (Castellarin et al., 2007a), suggesting that the signaling pathways and hormones involved in the berry response remain activated beyond the duration of the stress.
Variety (Deluc et al., 2009; Hochberg et al., 2015a), rootstock (Berdeja et al., 2015), soil structure (Tramontini et al., 2014), and other environmental variables such as light and temperature (Herrera et al., 2017) also play important roles in modulating the composition and quality of grapes grown under water deficit (Zarrouk et al., 2016). The complex interaction between factors is well illustrated by the example of grape berry phenolics. Water deficit enhances berry color, but high berry temperature induces the degradation of anthocyanins, limiting and sometimes even reversing the positive effects of water deficit (Bonada et al., 2015; Zarrouk et al., 2016; Cooley et al., 2017; Herrera et al., 2017). These interactions extend to wine sensory traits, including modulating tannin structure as well as floral and fruity aromas (Bonada et al., 2015).
In order to improve our knowledge of berry metabolic response to water deficit, and the underlying mechanisms in relation to variety and environment, controlled environmental studies should be performed in which varieties are compared in identical conditions with appropriate experimental designs. Field studies, particularly in commercial vineyards, provide a wealth of knowledge, but within this setting, it is difficult to compare multiple genotypes, or characterize the interaction of irrigation regimes with other environmental variables.
Water deficit signaling in berries
Research suggests that both ABA-dependent and ABA-independent mechanisms are involved in the regulatory network controlling changes in berry metabolism under water deficit. ABA-dependent pathways are evident considering the role of ABA as a cornerstone in controlling the onset of ripening in non-climacteric fruits such as grape (Wheeler et al., 2009; Lacampagne et al., 2010; Giribaldi et al., 2010; Gambetta et al., 2010) and its role in regulating phenolic biosynthesis even under non-stressed conditions (e.g. Gagné et al., 2011; Villalobos-González et al., 2016). In berries, water deficit increases the expression of ABA biosynthetic genes (Deluc et al., 2009; Savoi et al., 2017) and ABA concentration (Deluc et al., 2009; Niculcea et al., 2014). The promoters of many water deficit-induced genes are enriched for ABA-related cis-regulatory elements (Savoi et al., 2016) including those for biosynthesis of aroma compounds such as terpenes (discussed above). In addition to ABA-dependent pathways, studies also suggest that ABA-independent pathways act in response to water deficit in the grape berry. Notably, many components of the ethylene signaling pathway are strongly up-regulated by a water deficit during ripening (Savoi et al., 2017), suggesting ethylene may play an important role in both climacteric and non-climacteric fruit ripening (Sun et al., 2010; Chen et al., 2018). It remains to be determined how these molecular signals, which could be endogenous (arising through de novo synthesis in berries) or exogenous (arriving from the parent plant), are integrated and how the hydraulic connection of the berry with the parent plant affects this integration.
Integrating berries with the vine: relationships between source–sink and water
In the previous sections we have discussed the effects of water deficit on grape berry growth and metabolism, which in turn should impact the source–sink relations of the plant. The relationships between source–sink and plant water use are poorly studied, but it has been suggested that fruit load can impact gas exchange of the parent plant (Körner, 2015), specifically that decreasing fruit load (i.e. increasing source to sink ratio) would result in a decrease in gs and assimilation. Under water deficit, reductions in growth are greater than reductions in assimilation, which leads to the accumulation of sugars in leaves (Muller et al., 2011). This increased sugar accumulation in leaves could ultimately down-regulate gs and leaf hydraulic conductance (Kelly et al., 2013, 2017). This hypothesis is supported from many studies that found that fruit removal led to lower gas exchange in plum (Gucci et al., 1991), olive (Bustan et al., 2016), avocado (Silber et al., 2013), and coffee (Franck et al., 2006). In many other species, sink limitation through girdling restricted gas exchange, providing additional support for the hypothesis. In grapevines, Williams et al. (2000) showed that girdling reduced gs from 500 to 300 mmol m−2 s−1 and increased Ψ leaf by 0.3 MPa. Similarly, cluster thinning decreased assimilation (Iacono et al., 1995), and in the opposite sense, decreasing source to sink ratio by defoliating two-thirds of the leaves increased stomatal conductance by 20% (Hunter and Visser, 1989). Other studies in grape have found no effect of fruit load on vine gas exchange. Fruit removal at different stages of development in plants growing in lysimeters did not affect whole plant transpiration (Y. Netzer et al., personal communication). Additionally, several studies that compared gas exchange before and after harvest did not find major differences in gs (e.g. Munitz et al., 2017). This limited number of studies highlight our inability to resolve berry effects on grapevine water demand and the need for more studies that combine carbon allocation with hydraulics.
Conclusions
Our knowledge on grapevine drought stress physiology has increased tremendously in recent years. However, we still lack a mechanistic understanding of many drought responses, which prevents accurate modeling under future climatic scenarios. The remaining questions are numerous. For example, what are the genetic determinants of key drought tolerance traits such as Emax, gs regulation, Ψ TLP acclimation, rooting behavior, and the plasticity of xylem structure that directly impact productivity and vulnerability? How plastic are these traits within a single genotype? To what extent are key drought tolerance traits interconnected genetically and to what extent can they be disentangled for breeding purposes? Can scion–rootstock interactions be better exploited to modulate drought tolerance? Future research needs to aim to answer these questions and should consider interactions with other stressors (e.g. heatwaves, radiation, biotic stressors) that are likely to co-occur in the field.
Climate change is threatening our eco- and agrosystems and we still lack the knowledge to accurately predict how crops will respond to these new challenges. For viticulture, the threat of climate change produces anxiety for individual growers and winemakers, and regional and national governments, all of which rely on this economically and culturally important crop. A clear understanding of grapevine responses to water deficit is critical in addressing these concerns, especially in increasing the efficiency and resiliency of viticultural practices and guiding the development of drought-tolerant varieties and rootstocks.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Data and references of the 18 published studies used in a meta-analysis investigating berry and wine composition changes under water deficit.
Acknowedgements
The authors would like to thank Markus Keller for providing the grape berry photographs shown in Fig. 5. This work was supported by the EU, ERA-NET, ARIMNET2 project, Opportunities for an Environmental-friendly Viticulture (EnViRoS), the Natural Science and Engineering Research Council of Canada (RGPIN-2015-04760), and the Canada Research Chair (950-230913).
References
- Alsina MM, De Herralde F, Aranda X, Savé R, Biel C. 2007. Water relations and vulnerability to embolism in eight grapevine cultivars. Vitis 46, 1–6. [Google Scholar]
- Alsina MM, Smart DR, Bauerle T, de Herralde F, Biel C, Stockert C, Negron C, Save R. 2011. Seasonal changes of whole root system conductance by a drought-tolerant grape root system. Journal of Experimental Botany 62, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alston JM, Sambucci O. 2019. Grapes in the world economy. In: Cantu D, Walker MA, eds. The grape genome. Cham: Springer, 1–24. [Google Scholar]
- Ayuda M-I, Esteban E, Martín-Retortillo M, Pinilla V. 2020. The blue water footprint of the Spanish wine industry: 1930–2015. Working paper no. 248. New York: American Association of Wine Economists. [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L. 2012. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecology Letters 15, 393–405. [DOI] [PubMed] [Google Scholar]
- Bartlett MK, Zhang Y, Kreidler N, Sun S, Ardy R, Cao K, Sack L. 2014. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecology Letters 17, 1580–1590. [DOI] [PubMed] [Google Scholar]
- Beis A, Patakas A. 2012. Relative contribution of photoprotection and anti-oxidative mechanisms to differential drought adaptation ability in grapevines. Environmental and Experimental Botany 78, 173–183. [Google Scholar]
- Berdeja M, Nicolas P, Kappel C, Dai ZW, Hilbert G, Peccoux A, Lafontaine M, Ollat N, Gomès E, Delrot S. 2015. Water limitation and rootstock genotype interact to alter grape berry metabolism through transcriptome reprogramming. Horticulture Research 2, 15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindon KA, Dry PR, Loveys BR. 2007. Influence of plant water status on the production of C13-norisoprenoid precursors in Vitis vinifera L. Cv. Cabernet Sauvignon grape berries. Journal of Agricultural and Food Chemistry 55, 4493–4500. [DOI] [PubMed] [Google Scholar]
- Blanke MM, Leyhe A. 1987. Stomatal activity of the grape berry cv. Riesling, Müller-Thurgau and Ehrenfelser. Journal of Plant Physiology 127, 451–460. [Google Scholar]
- Bonada M, Jeffery DW, Petrie PR, Moran MA, Sadras VO. 2015. Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines. Australian Journal of Grape and Wine Research 21, 240–253. [Google Scholar]
- Bondada BR, Matthews MA, Shackel KA. 2005. Functional xylem in the post-veraison grape berry. Journal of Experimental Botany 56, 2949–2957. [DOI] [PubMed] [Google Scholar]
- Boneh U, Biton I, Zheng C, Schwartz A, Ben-Ari G. 2012. Characterization of potential ABA receptors in Vitis vinifera. Plant Cell Reports 31, 311–321. [DOI] [PubMed] [Google Scholar]
- Bota J, Tomás M, Flexas J, Medrano H, Escalona JM. 2016. Differences among grapevine cultivars in their stomatal behavior and water use efficiency under progressive water stress. Agricultural Water Management 164, 91–99. [Google Scholar]
- Bouzas-Cid Y, Trigo-Córdoba E, Falqué E, Orriols I, Mirás-Avalos JM. 2018. Influence of supplementary irrigation on the amino acid and volatile composition of Godello wines from the Ribeiro Designation of Origin. Food Research International 111, 715–723. [DOI] [PubMed] [Google Scholar]
- Brillante L, Martínez-Lüscher J, Kurtural SK. 2018. Applied water and mechanical canopy management affect berry and wine phenolic and aroma composition of grapevine (Vitis vinifera L., cv. Syrah) in Central California. Scientia Horticulturae 227, 261–271. [Google Scholar]
- Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV. 2003. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant, Cell & Environment 26, 443–450. [Google Scholar]
- Brodribb TJ, Sussmilch F, McAdam SAM. 2020. From reproduction to production, stomata are the master regulators. The Plant Journal 101, 756–767. [DOI] [PubMed] [Google Scholar]
- Bucchetti B, Matthews MA, Falginella L, Peterlunger E, Castellarin SD. 2011. Effect of water deficit on Merlot grape tannins and anthocyanins across four seasons. Scientia Horticulturae 128, 297–305. [Google Scholar]
- Buckley TN. 2019. How do stomata respond to water status? New Phytologist 224, 21–36. [DOI] [PubMed] [Google Scholar]
- Buesa I, Caccavello G, Basile B, Merli MC, Poni S, Chirivella C, Intrigliolo DS. 2019. Delaying berry ripening of Bobal and Tempranillo grapevines by late leaf removal in a semi-arid and temperate-warm climate under different water regimes. Australian Journal of Grape and Wine Research 25, 70–82. [Google Scholar]
- Bustan A, Dag A, Yermiyahu U, Erel R, Presnov E, Agam N, Kool D, Iwema J, Zipori I, Ben-Gal A. 2016. Fruit load governs transpiration of olive trees. Tree Physiology 36, 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttrose MS. 1974. Fruitfulness in grapevines: effects of water stress. Vitis 12, 299–305. [Google Scholar]
- Cáceres-Mella A, Ribalta-Pizarro C, Villalobos-González L, Cuneo IF, Pastenes C. 2018. Controlled water deficit modifies the phenolic composition and sensory properties in Cabernet Sauvignon wines. Scientia Horticulturae 237, 105–111. [Google Scholar]
- Cáceres-Mella A, Talaverano MI, Villalobos-González L, Ribalta-Pizarro C, Pastenes C. 2017. Controlled water deficit during ripening affects proanthocyanidin synthesis, concentration and composition in Cabernet Sauvignon grape skins. Plant Physiology and Biochemistry 117, 34–41. [DOI] [PubMed] [Google Scholar]
- Canoura C, Kelly MT, Ojeda H. 2018. Effect of irrigation and timing and type of nitrogen application on the biochemical composition of Vitis vinifera L. cv. Chardonnay and Syrah grapeberries. Food Chemistry 241, 171–181. [DOI] [PubMed] [Google Scholar]
- Casassa LF, Keller M, Harbertson JF. 2015. Regulated deficit irrigation alters anthocyanins, tannins and sensory properties of cabernet sauvignon grapes and wines. Molecules 20, 7820–7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarin SD, Gambetta GA, Wada H, Krasnow MN, Cramer GR, Peterlunger E, Shackel KA, Matthews MA. 2016. Characterization of major ripening events during softening in grape: turgor, sugar accumulation, abscisic acid metabolism, colour development, and their relationship with growth. Journal of Experimental Botany 67, 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarin SD, Matthews MA, Di Gaspero G, Gambetta GA. 2007a Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227, 101–112. [DOI] [PubMed] [Google Scholar]
- Castellarin SD, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, Di Gaspero G. 2007b Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant, Cell & Environment 30, 1381–1399. [DOI] [PubMed] [Google Scholar]
- Chapman DM, Matthews MA, Guinard JX. 2004. Sensory attributes of Cabernet Sauvignon wines made from vines with different crop yields. American Journal of Enology and Viticulture 55, 325–334. [Google Scholar]
- Charrier G, Delzon S, Domec JC, et al. 2018. Drought will not leave your glass empty: Low risk of hydraulic failure revealed by long-term drought observations in world’s top wine regions. Science Advances 4, eaao6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier G, Torres-Ruiz JM, Badel E, et al. 2016. Evidence for hydraulic vulnerability segmentation and lack of xylem refilling under tension. Plant Physiology 172, 1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelet DS, Rost TL, Shackel KA, Matthews MA. 2008. The peripheral xylem of grapevine (Vitis vinifera). 1. Structural integrity in post-veraison berries. Journal of Experimental Botany 59, 1987–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Zarrouk O, Francisco R, Costa JM, Santos T, Regalado AP, Rodrigues ML, Lopes CM. 2010. Grapevine under deficit irrigation: hints from physiological and molecular data. Annals of Botany 105, 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Grimplet J, David K, et al. 2018. Ethylene receptors and related proteins in climacteric and non-climacteric fruits. Plant Science 276, 63–72. [DOI] [PubMed] [Google Scholar]
- Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE. 2018. Triggers of tree mortality under drought. Nature 558, 531–539. [DOI] [PubMed] [Google Scholar]
- Choat B, Drayton WM, Brodersen C, Matthews MA, Shackel KA, Wada H, McElrone AJ. 2010. Measurement of vulnerability to water stress-induced cavitation in grapevine: a comparison of four techniques applied to a long-vesseled species. Plant, Cell & Environment 33, 1502–1512. [DOI] [PubMed] [Google Scholar]
- Choat B, Gambetta GA, Shackel KA, Matthews MA. 2009. Vascular function in grape berries across development and its relevance to apparent hydraulic isolation. Plant Physiology 151, 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley NM, Clingeleffer PR, Walker RR. 2017. Effect of water deficits and season on berry development and composition of Cabernet Sauvignon (Vitis vinifera L.) grown in a hot climate. Australian Journal of Grape and Wine Research 23, 260–272. [Google Scholar]
- Costa JM, Vaz M, Escalona J, Egipto R, Lopes C, Medrano H, Chaves MM. 2016. Modern viticulture in southern Europe: vulnerabilities and strategies for adaptation to water scarcity. Agricultural Water Management 164, 5–18. [Google Scholar]
- Coupel-Ledru A, Lebon É, Christophe A, Doligez A, Cabrera-Bosquet L, Péchier P, Hamard P, This P, Simonneau T. 2014. Genetic variation in a grapevine progeny (Vitis vinifera L. cvs Grenache×Syrah) reveals inconsistencies between maintenance of daytime leaf water potential and response of transpiration rate under drought. Journal of Experimental Botany 65, 6205–6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupel-Ledru A, Tyerman SD, Masclef D, Lebon E, Christophe A, Edwards EJ, Simonneau T. 2017. Abscisic acid down-regulates hydraulic conductance of grapevine leaves in isohydric genotypes only. Plant Physiology 175, 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho MH. 2008. Drought stress and reactive oxygen species. Plant Signaling & Behavior 3, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Santo S, Palliotti A, Zenoni S, et al. 2016. Distinct transcriptome responses to water limitation in isohydric and anisohydric grapevine cultivars. BMC genomics 17, 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin F. 1898. Observations on stomata. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 190, 531–621. [Google Scholar]
- Dayer S, Herrera JC, Dai Z, Burlett R, Lamarque LJ, Delzon S, Bortolami G, Cochard H, Gambetta GA. 2020. The sequence and thresholds of leaf hydraulic traits underlying grapevine varietal differences in drought tolerance. Journal of Experimental Botany 71, 4333–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer S, Prieto JA, Galat E, Perez Peña J. 2013. Carbohydrate reserve status of Malbec grapevines after several years of regulated deficit irrigation and crop load regulation. Australian Journal of Grape and Wine Research 19, 422–430. [Google Scholar]
- Dayer S, Reingwirtz I, McElrone AJ, Gambetta GA. 2019. Response and recovery of grapevine to water deficit: from genes to physiology. In: Cantu D, Walker MA, eds. The grape genome. Cham: Springer, 223–245. [Google Scholar]
- Degu A, Hochberg U, Wong DCJ, Alberti G, Lazarovitch N, Peterlunger E, Castellarin SD, Herrera JC, Fait A. 2019. Swift metabolite changes and leaf shedding are milestones in the acclimation process of grapevine under prolonged water stress. BMC Plant Biology 19, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc LG, Decendit A, Papastamoulis Y, Mérillon JM, Cushman JC, Cramer GR. 2011. Water deficit increases stilbene metabolism in Cabernet Sauvignon berries. Journal of Agricultural and Food Chemistry 59, 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc LG, Quilici DR, Decendit A, Grimplet J, Wheatley MD, Schlauch KA, Mérillon JM, Cushman JC, Cramer GR. 2009. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 10, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos N, Tindjau R, Wong DCJ, Matzat T, Haslam T, Song C, Gambetta GA, Kunst L, Castellarin SD. 2020. Drought stress modulates cuticular wax composition of the grape berry. Journal of Experimental Botany 71, 3126–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupis G, Bosabalidis AM, Patakas A. 2016. Comparative effects of water deficit and enhanced UV-B radiation on photosynthetic capacity and leaf anatomy traits of two grapevine (Vitis vinifera L.) cultivars. Theoretical and Experimental Plant Physiology 28, 131–141. [Google Scholar]
- Downton WJS, Millhouse J. 1983. Turgor maintenance during salt stress prevents loss of variable fluorescence in grapevine leaves. Plant Science Letters 31, 1–7. [Google Scholar]
- During H. 1984. Evidence for osmotic adjustment to drought in grapevines. Vitis 23, 1–10. [Google Scholar]
- Escalona JM, Pou A, Tortosa I, Hernández-Montes E, Tomás M, Martorell S, Bota J, Medrano H. 2016. Using whole-plant chambers to estimate carbon and water fluxes in field-grown grapevines. Theoretical and Experimental Plant Physiology 28, 241–254. [Google Scholar]
- FAO-OIV 2016. FAO-OIV Focus 2016. Table and dried grapes. Rome: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Flexas J, Bota J, Cifre J, et al. 2004. Understanding down-regulation of photosynthesis under water stress: future prospects and searching for physiological tools for irrigation management. Annals of Applied Biology 144, 273–283. [Google Scholar]
- Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. 2002. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology 29, 461–471. [DOI] [PubMed] [Google Scholar]
- Flexas J, Escalona JM, Medrano H. 1998. Down-regulation of photosynthesis by drought under field conditions in grapevine leaves. Functional Plant Biology 25, 893–900. [Google Scholar]
- Flexas J, Galmés J, Gallé A, Gulías J, Pou A, Ribas-Carbo M, Tomàs M, Medrano H. 2010. Improving water use efficiency in grapevines: Potential physiological targets for biotechnological improvement. Australian Journal of Grape and Wine Research 16, 106–121. [Google Scholar]
- Flexas J, Medrano H. 2002. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Annals of Botany 89, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck N, Vaast P, Génard M, Dauzat J. 2006. Soluble sugars mediate sink feedback down-regulation of leaf photosynthesis in field-grown Coffea arabica. Tree Physiology 26, 517–525. [DOI] [PubMed] [Google Scholar]
- Gagné S, Cluzet S, Mérillon J-M, Gény L. 2011. ABA initiates anthocyanin production in grape cell cultures. Journal of Plant Growth Regulation 30, 1–10. [Google Scholar]
- Gambetta GA. 2016. Water stress and grape physiology in the context of global climate change. Journal of Wine Economics 11, 168–180. [Google Scholar]
- Gambetta GA, Knipfer T, Fricke W, McElrone AJ. 2017. Aquaporins and root water uptake. In: Chaumont F, Tyerman SD, eds. Plant aquaporins. Cham: Springer, 133–153. [Google Scholar]
- Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD. 2010. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 232, 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Esparza MJ, Abrisqueta I, Escriche I, Intrigliolo DS, Álvarez I, Lizama V. 2018. Volatile compounds and phenolic composition of skins and seeds of ‘Cabernet Sauvignon’ grapes under different deficit irrigation regimes. Vitis 57, 83–91. [Google Scholar]
- Geilfus CM. 2017. The pH of the apoplast: dynamic factor with functional impact under stress. Molecular Plant 10, 1371–1386. [DOI] [PubMed] [Google Scholar]
- Giribaldi M, Gény L, Delrot S, Schubert A. 2010. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. Journal of Experimental Botany 61, 2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowdy M, Destrac-Irvine A, Haines M, Gambetta G, Pieri P, Marguerit E, van Leeuwen C. 2018. Varietal responses to soil water deficit: first results from a common-garden vineyard near Bordeaux France. E3S Web of Conferences 50, 01043. [Google Scholar]
- Greenspan MD, Shackel KA, Matthews MA. 1994. Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant, Cell & Environment 17, 811–820. [Google Scholar]
- Greer DH, Rogiers SY. 2009. Water flux of Vitis vinifera L. cv. Shiraz bunches throughout development and in relation to late-season weight loss. American Journal of Enology and Viticulture 60, 155–163. [Google Scholar]
- Gregan SM, Jordan B. 2016. Methoxypyrazine accumulation and O-methyltransferase gene expression in sauvignon blanc grapes: the role of leaf removal, light exposure, and berry development. Journal of Agricultural and Food Chemistry 64, 2200–2208. [DOI] [PubMed] [Google Scholar]
- Grimes DW, Williams LE. 1990. Irrigation effects on plant water relations and productivity of Thompson Seedless grapevines. Crop Science 30, 255–260. [Google Scholar]
- Guan XQ, Zhao SJ, Li DQ, Shu HR. 2004. Photoprotective function of photorespiration in several grapevine cultivars under drought stress. Photosynthetica 42, 31–36. [Google Scholar]
- Gucci R, Xiloyannis C, Flore JA. 1991. Gas exchange parameters, water relations and carbohydrate partitioning in leaves of field-grown Prunus domestica following fruit removal. Physiologia Plantarum 83, 497–505. [Google Scholar]
- Hannah L, Roehrdanz PR, Ikegami M, Shepard AV, Shaw MR, Tabor G, Zhi L, Marquet PA, Hijmans RJ. 2013. Climate change, wine, and conservation. Proceedings of the National Academy of Sciences, USA 110, 6907–6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie WJ, Considine JA. 1976. Response of grapes to water-deficit stress in particular stages of development. American Journal of Enology and Viticulture 27, 55–61. [Google Scholar]
- Herrera JC, Bucchetti B, Sabbatini P, Comuzzo P, Zulini L, Vecchione A, Peterlunger E, Castellarin SD. 2015. Effect of water deficit and severe shoot trimming on the composition of Vitis vinifera L. Merlot grapes and wines. Australian Journal of Grape and Wine Research 21, 254–265. [Google Scholar]
- Herrera JC, Hochberg U, Degu A, Sabbatini P, Lazarovitch N, Castellarin SD, Fait A, Alberti G, Peterlunger E. 2017. Grape metabolic response to postveraison water deficit is affected by interseason weather variability. Journal of Agricultural and Food Chemistry 65, 5868–5878. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Albuquerque C, Rachmilevitch S, Cochard H, David-Schwartz R, Brodersen CR, McElrone A, Windt CW. 2016a Grapevine petioles are more sensitive to drought induced embolism than stems: evidence from in vivo MRI and microcomputed tomography observations of hydraulic vulnerability segmentation. Plant, Cell & Environment 39, 1886–1894. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Bonel AG, David-Schwartz R, Degu A, Fait A, Cochard H, Peterlunger E, Herrera JC. 2017a Grapevine acclimation to water deficit: the adjustment of stomatal and hydraulic conductance differs from petiole embolism vulnerability. Planta 245, 1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg U, Degu A, Cramer GR, Rachmilevitch S, Fait A. 2015a Cultivar specific metabolic changes in grapevines berry skins in relation to deficit irrigation and hydraulic behavior. Plant Physiology and Biochemistry 88, 42–52. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Degu A, Fait A, Rachmilevitch S. 2013a Near isohydric grapevine cultivar displays higher photosynthetic efficiency and photorespiration rates under drought stress as compared with near anisohydric grapevine cultivar. Physiologia Plantarum 147, 443–452. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Degu A, Gendler T, Fait A, Rachmilevitch S. 2015b The variability in the xylem architecture of grapevine petiole and its contribution to hydraulic differences. Functional Plant Biology 42, 357. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Degu A, Toubiana D, Gendler T, Nikoloski Z, Rachmilevitch S, Fait A. 2013b Metabolite profiling and network analysis reveal coordinated changes in grapevine water stress response. BMC Plant Biology 13, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg U, Herrera JC, Cochard H, Badel E. 2016b Short-time xylem relaxation results in reliable quantification of embolism in grapevine petioles and sheds new light on their hydraulic strategy. Tree Physiology 36, 748–755. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Herrera JC, Degu A, Castellarin SD, Peterlunger E, Alberti G, Lazarovitch N. 2017b Evaporative demand determines the relative transpirational sensitivity of deficit-irrigated grapevines. Irrigation Science 35, 1–9. [Google Scholar]
- Hochberg U, Rockwell FE, Holbrook NM, Cochard H. 2018. Iso/anisohydry: a plant-environment interaction rather than a simple hydraulic trait. Trends in Plant Science 23, 112–120. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Windt CW, Ponomarenko A, Zhang YJ, Gersony J, Rockwell FE, Holbrook NM. 2017c Stomatal closure, basal leaf embolism, and shedding protect the hydraulic integrity of grape stems. Plant Physiology 174, 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook NM, Zwieniecki MA. 2005. Vascular transport in plants. Burlington, San Diego, London: Elsevier Academic Press. [Google Scholar]
- Hsiao TC, Acevedo E, Fereres E, Henderson DW. 1976. Water stress, growth, and osmotic adjustment. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 273, 479–500. [Google Scholar]
- Hunter JJ, Visser JH. 1989. The effect of partial defoliation, leaf position and developmental stage of the vine on the photosynthetic activity of Vitis vinifera L. cv Cabernet Sauvignon. South African Journal of Enology & Viticulture 10. [Google Scholar]
- Iacono F, Bertamini M, Scienza A, Coombe BG. 1995. Differential effects of canopy manipulation and shading of Vitis vinifera L. cv. Cabernet Sauvignon. Leaf gas exchange, photosynthetic electron transport rate and sugar accumulation in berries. Vitis 34, 201–206. [Google Scholar]
- Intergovernmental Panel on Climate Change 2014. Climate change 2014 – impacts, adaptation and vulnerability: regional aspects. New York: Cambridge University Press. [Google Scholar]
- Intrigliolo DS, Pérez D, Risco D, Yeves A, Castel JR. 2012. Yield components and grape composition responses to seasonal water deficits in Tempranillo grapevines. Irrigation Science 30, 339–349. [Google Scholar]
- Johnson DM, Wortemann R, McCulloh KA, Jordan-Meille L, Ward E, Warren JM, Palmroth S, Domec JC. 2016. A test of the hydraulic vulnerability segmentation hypothesis in angiosperm and conifer tree species. Tree Physiology 36, 983–993. [DOI] [PubMed] [Google Scholar]
- Keller M, Smith JP, Bondada BR. 2006. Ripening grape berries remain hydraulically connected to the shoot. Journal of Experimental Botany 57, 2577–2587. [DOI] [PubMed] [Google Scholar]
- Keller M, Zhang Y, Shrestha PM, Biondi M, Bondada BR. 2015. Sugar demand of ripening grape berries leads to recycling of surplus phloem water via the xylem. Plant, Cell & Environment 38, 1048–1059. [DOI] [PubMed] [Google Scholar]
- Kelly G, Moshelion M, David-Schwartz R, Halperin O, Wallach R, Attia Z, Belausov E, Granot D. 2013. Hexokinase mediates stomatal closure. The Plant Journal 75, 977–988. [DOI] [PubMed] [Google Scholar]
- Kelly G, Sade N, Doron-Faigenboim A, et al. 2017. Sugar and hexokinase suppress expression of PIP aquaporins and reduce leaf hydraulics that preserves leaf water potential. The Plant Journal 91, 325–339. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Eustis A, Brodersen C, Walker AM, McElrone AJ. 2015a Grapevine species from varied native habitats exhibit differences in embolism formation/repair associated with leaf gas exchange and root pressure. Plant, Cell & Environment 38, 1503–1513. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fei J, Gambetta GA, McElrone AJ, Shackel KA, Matthews MA. 2015b Water transport properties of the grape pedicel during fruit development: insights into xylem anatomy and function using microtomography. Plant Physiology 168, 1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. 2015. Paradigm shift in plant growth control. Current Opinion in Plant Biology 25, 107–114. [DOI] [PubMed] [Google Scholar]
- Koundouras S, Hatzidimitriou E, Karamolegkou M, Dimopoulou E, Kallithraka S, Tsialtas JT, Zioziou E, Nikolaou N, Kotseridis Y. 2009. Irrigation and rootstock effects on the phenolic concentration and aroma potential of Vitis vinifera L. cv. cabernet sauvignon grapes. Journal of Agricultural and Food Chemistry 57, 7805–7813. [DOI] [PubMed] [Google Scholar]
- Lacampagne S, Gagné S, Gény L. 2010. Involvement of abscisic acid in controlling the proanthocyanidin biosynthesis pathway in grape skin: new elements regarding the regulation of tannin composition and leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) activities and expression. Journal of Plant Growth Regulation 29, 81–90. [Google Scholar]
- Lavoie-Lamoureux A, Sacco D, Risse PA, Lovisolo C. 2017. Factors influencing stomatal conductance in response to water availability in grapevine: a meta-analysis. Physiologia Plantarum 159, 468–482. [DOI] [PubMed] [Google Scholar]
- Lebon E, Dumas V, Pieri P, Schultz HR. 2003. Modelling the seasonal dynamics of the soil water balance of vineyards. Functional Plant Biology 30, 699. [DOI] [PubMed] [Google Scholar]
- Levin AD, Williams LE, Matthews MA. 2019. A continuum of stomatal responses to water deficits among 17 wine grape cultivars (Vitis vinifera). Functional Plant Biology 47, 11–25. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li X, Chen G, Li M, Liu M, Liu D. 2015. Epidermal micromorphology and mesophyll structure of Populus euphratica heteromorphic leaves at different development stages. PLoS ONE 10, e0137701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovisolo C, Lavoie-Lamoureux A, Tramontini S, Ferrandino A. 2016. Grapevine adaptations to water stress: new perspectives about soil/plant interactions. Theoretical and Experimental Plant Physiology 28, 53–66. [Google Scholar]
- Lovisolo C, Perrone I, Carra A, Ferrandino A, Flexas J, Medrano H, Schubert A. 2010. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update. Functional Plant Biology 37, 98–116. [Google Scholar]
- Lovisolo C, Perrone I, Hartung W, Schubert A. 2008. An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytologist 180, 642–651. [DOI] [PubMed] [Google Scholar]
- Marciniak M, Reynolds AG, Brown R. 2013. Influence of water status on sensory profiles of Ontario Riesling wines. Food Research International 54, 881–891. [Google Scholar]
- Maréchaux I, Bartlett MK, Iribar A, Sack L, Chave J. 2017. Stronger seasonal adjustment in leaf turgor loss point in lianas than trees in an Amazonian forest. Biology letters 13, 20160819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Lüscher J, Sánchez-Díaz M, Delrot S, Aguirreolea J, Pascual I, Gomès E. 2014. Ultraviolet-B radiation and water deficit interact to alter flavonol and anthocyanin profiles in grapevine berries through transcriptomic regulation. Plant & Cell Physiology 55, 1925–1936. [DOI] [PubMed] [Google Scholar]
- Martin-StPaul N, Delzon S, Cochard H. 2017. Plant resistance to drought depends on timely stomatal closure. Ecology Letters 20, 1437–1447. [DOI] [PubMed] [Google Scholar]
- Martorell S, Medrano H, Tomàs M, Escalona JM, Flexas J, Diaz-Espejo A. 2015. Plasticity of vulnerability to leaf hydraulic dysfunction during acclimation to drought in grapevines: an osmotic-mediated process. Physiologia Plantarum 153, 381–391. [DOI] [PubMed] [Google Scholar]
- Matthews MA, Anderson MM. 1988. Fruit ripening in Vitis vinifera L.: responses to seasonal water deficits. American Journal of Enology and Viticulture 39, 313–320. [Google Scholar]
- Matthews MA, Anderson MM. 1989. Reproductive development in grape (Vitis vinifera L.): responses to seasonal water deficits. American Journal of Enology and Viticulture 40, 52–60. [Google Scholar]
- Maurel C, Prado K. 2017. Aquaporins and leaf water relations. In: Chaumont F, Tyerman SD, eds. Plant aquaporins. Cham: Springer, 155–165. [Google Scholar]
- McAdam SAM, Brodribb TJ. 2015. The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiology 167, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ. 2016. Linking turgor with ABA biosynthesis: implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiology 171, 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ. 2018. Mesophyll cells are the main site of abscisic acid biosynthesis in water-stressed leaves. Plant Physiology 177, 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist 178, 719–739. [DOI] [PubMed] [Google Scholar]
- Medrano H, Escalona JM, Cifre J, Bota J, Flexas J. 2003. A ten year study on the physiology of two Spanish grapevine cultivars under field conditions: effects of water availability from leaf photosynthesis to grape yield and quality. Functional Plant Biology 30, 607–619. [DOI] [PubMed] [Google Scholar]
- Medrano H, Tortosa I, Montes E, Pou A, Balda P, Bota J, Escalona JM. 2018. Genetic improvement of grapevine (Vitis vinifera L.) water use efficiency. In: Tejero IFC, Zuazo VHD eds. Water scarcity and sustainable agriculture in semiarid environment: tools, strategies, and challenges for woody crops. London, San Diego, Cambridge, Kidlington: Academic Press, 377–401. [Google Scholar]
- Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR. 2009. Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Functional Ecology 23, 922–930. [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. 2010. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment 33, 453–467. [DOI] [PubMed] [Google Scholar]
- Mirás-Avalos JM, Intrigliolo DS. 2017. Grape composition under abiotic constrains: water stress and salinity. Frontiers in Plant Science 8, 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosetti D, Herrera JC, Sabbatini P, Green A, Alberti G, Peterlunger E, Lisjak K, Castellarin SD. 2016. Impact of leaf removal after berry set on fruit composition and bunch rot in ‘Sauvignon blanc’. Vitis 55, 57–64. [Google Scholar]
- Muller B, Pantin F, Génard M, Turc O, Freixes S, Piques M, Gibon Y. 2011. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. Journal of Experimental Botany 62, 1715–1729. [DOI] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI. 2015. Mechanisms of abscisic acid-mediated control of stomatal aperture. Current Opinion in Plant Biology 28, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munitz S, Netzer Y, Schwartz A. 2017. Sustained and regulated deficit irrigation of field-grown Merlot grapevines. Australian Journal of Grape and Wine Research 23, 87–94. [Google Scholar]
- Munitz S, Netzer Y, Shtein I, Schwartz A. 2018. Water availability dynamics have long-term effects on mature stem structure in Vitis vinifera. American Journal of Botany 105, 1443–1452. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yamaguchi-Shinozaki K. 2013. ABA signaling in stress-response and seed development. Plant Cell Reports 32, 959–970. [DOI] [PubMed] [Google Scholar]
- Nardini A, Savi T, Trifilò P, Lo Gullo MA. 2017. Drought stress and the recovery from xylem embolism in woody plants. Progress in Botany 79, 197–231. [Google Scholar]
- Niculcea M, López J, Sánchez-Díaz M, Carmen Antolín M. 2014. Involvement of berry hormonal content in the response to pre- and post-veraison water deficit in different grapevine (Vitis vinifera L.) cultivars. Australian Journal of Grape and Wine Research 20, 281–291. [Google Scholar]
- Ojeda H, Andary C, Kraeva E, Carbonneau A, Deloire A. 2002. Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv. Shiraz. American Journal of Enology and Viticulture 53, 261–267. [Google Scholar]
- Ollé D, Guiraud JL, Souquet JM, Terrier N, Ageorges A, Cheynier V, Verries C. 2011. Effect of pre- and post-veraison water deficit on proanthocyanidin and anthocyanin accumulation during Shiraz berry development. Australian Journal of Grape and Wine Research 17, 90–100. [Google Scholar]
- Ouyang W, Struik PC, Yin X, Yang J. 2017. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. Journal of Experimental Botany 68, 5191–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B. 2013. The dual effect of abscisic acid on stomata. New Phytologist 197, 65–72. [DOI] [PubMed] [Google Scholar]
- Patakas A, Nikolaou N, Zioziou E, Radoglou K, Noitsakis B. 2002. The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Science 163, 361–367. [Google Scholar]
- Patakas A, Noitsakis B. 1999. Osmotic adjustment and partitioning of turgor responses to drought in grapevines leaves. American Journal of Enology and Viticulture 50, 76–80. [Google Scholar]
- Peccoux A, Loveys B, Zhu J, Gambetta GA, Delrot S, Vivin P, Schultz HR, Ollat N, Dai Z. 2018. Dissecting the rootstock control of scion transpiration using model-assisted analyses in grapevine. Tree Physiology 38, 1026–1040. [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sancho-Knapik D, Martín P, Saz MÁ, Gea-Izquierdo G, Cañellas I, Gil-Pelegrín E. 2015. Evidence of vulnerability segmentation in a deciduous Mediterranean oak (Quercus subpyrenaica E. H. del Villar). Trees 29, 1917–1927. [Google Scholar]
- Penman HL. 1948. Natural evaporation from open water, hare soil and grass. Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences 193, 120–145. [DOI] [PubMed] [Google Scholar]
- Petruzzellis F, Savi T, Bacaro G, Nardini A. 2019. A simplified framework for fast and reliable measurement of leaf turgor loss point. Plant Physiology and Biochemistry 139, 395–399. [DOI] [PubMed] [Google Scholar]
- Picard M, van Leeuwen C, Guyon F, Gaillard L, de Revel G, Marchand S. 2017. Vine water deficit impacts aging bouquet in fine red Bordeaux wine. Frontiers in Chemistry 5, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinasseau L, Vallverdú-Queralt A, Verbaere A, et al. 2017. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Frontiers in Plant Science 8, 1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivovaroff AL, Sack L, Santiago LS. 2014. Coordination of stem and leaf hydraulic conductance in southern California shrubs: a test of the hydraulic segmentation hypothesis. New Phytologist 203, 842–850. [DOI] [PubMed] [Google Scholar]
- Pou A, Flexas J, Alsina Mdel M, et al. 2008. Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). Physiologia Plantarum 134, 313–323. [DOI] [PubMed] [Google Scholar]
- Robinson AL, Boss PK, Solomon PS, Trengove RD, Heymann H, Ebeler SE. 2014. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. American Journal of Enology and Viticulture 65, 1–24. [Google Scholar]
- Rodrigues M, Chaves M, Wendler R, David M, Quick W, Leegood R, Stitt M, Pereira J. 1993. Osmotic adjustment in water stressed grapevine leaves in relation to carbon assimilation. Functional Plant Biology 20, 309. [Google Scholar]
- Rogiers SY, Greer DH, Hatfield JM, Hutton RJ, Clarke SJ, Hutchinson PA, Somers A. 2012. Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiology 32, 249–261. [DOI] [PubMed] [Google Scholar]
- Rogiers SY, Hatfield JM, Jaudzems VG, White RG, Keller M. 2004. Grape berry cv. Shiraz epicuticular wax and transpiration during ripening and preharvest weight loss. American Journal of Enology and Viticulture 55, 121–127. [Google Scholar]
- Ryona I, Pan BS, Intrigliolo DS, Lakso AN, Sacks GL. 2008. Effects of cluster light exposure on 3-isobutyl-2-methoxypyrazine accumulation and degradation patterns in red wine grapes (Vitis vinifera L. cv. Cabernet Franc). Journal of Agricultural and Food Chemistry 56, 10838–10846. [DOI] [PubMed] [Google Scholar]
- Sadilova E, Stintzing FC, Carle R. 2006. Thermal degradation of acylated and nonacylated anthocyanins. Journal of Food Science 71, C504–C512. [Google Scholar]
- Savi T, Petruzzellis F, Martellos S, Stenni B, Dal Borgo A, Zini L, Lisjak K, Nardini A. 2018. Vineyard water relations in a karstic area: deep roots and irrigation management. Agriculture, Ecosystems & Environment 263, 53–59. [Google Scholar]
- Savoi S, Wong DC, Arapitsas P, Miculan M, Bucchetti B, Peterlunger E, Fait A, Mattivi F, Castellarin SD. 2016. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biology 16, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoi S, Wong DCJ, Degu A, Herrera JC, Bucchetti B, Peterlunger E, Fait A, Mattivi F, Castellarin SD. 2017. Multi-omics and integrated network analyses reveal new insights into the systems relationships between metabolites, structural genes, and transcriptional regulators in developing grape berries (Vitis vinifera L.) exposed to water deficit. Frontiers in Plant Science 8, 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HR. 2003. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant, Cell & Environment 26, 1393–1406. [Google Scholar]
- Schultz HR, Matthews MA. 1993. Growth, osmotic adjustment, and cell-wall mechanics of expanding grape leaves during water deficits. Crop Science 33, 287. [Google Scholar]
- Scoffoni C, Rawls M, McKown A, Cochard H, Sack L. 2011. Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiology 156, 832–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatil-Cohen A, Attia Z, Moshelion M. 2011. Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: a target of xylem-borne ABA? The Plant Journal 67, 72–80. [DOI] [PubMed] [Google Scholar]
- Shelden MC, Vandeleur R, Kaiser BN, Tyerman SD. 2017. A comparison of petiole hydraulics and aquaporin expression in an anisohydric and isohydric cultivar of grapevine in response to water-stress induced cavitation. Frontiers in Plant Science 8, 1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellie KC, Bowen P. 2014. Isohydrodynamic behavior in deficit-irrigated Cabernet Sauvignon and Malbec and its relationship between yield and berry composition. Irrigation Science 32, 87–97. [Google Scholar]
- Siebert TE, Barker A, Pearson W, Barter SR, de Barros Lopes MA, Darriet P, Herderich MJ, Francis IL. 2018. Volatile compounds related to ‘stone fruit’ aroma attributes in Viognier and Chardonnay Wines. Journal of Agricultural and Food Chemistry 66, 2838–2850. [DOI] [PubMed] [Google Scholar]
- Silber A, Israeli Y, Levi M, et al. 2013. The roles of fruit sink in the regulation of gas exchange and water uptake: a case study for avocado. Agricultural Water Management 116, 21–28. [Google Scholar]
- Soar CJ, Spei J, Maffei SM, Penrose AB, McCarthy MG, Loveys BR. 2006. Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Australian Journal of Grape and Wine Research 12, 2–12. [Google Scholar]
- Sofo A, Manfreda S, Fiorentino M, Dichio B, Xiloyannis C. 2008. The olive tree: a paradigm for drought tolerance in Mediterranean climates. Hydrolology & Earth System Sciences 12, 293–301. [Google Scholar]
- Song J, Shellie KC, Wang H, Qian MC. 2012. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). Food Chemistry 134, 841–850. [DOI] [PubMed] [Google Scholar]
- Šuklje K, Lisjak K, Česnik HB, Janeš L, Du Toit W, Coetzee Z, Vanzo A, Deloire A. 2012. Classification of grape berries according to diameter and total soluble solids to study the effect of light and temperature on methoxypyrazine, glutathione, and hydroxycinnamate evolution during ripening of Sauvignon blanc (Vitis vinifera L.). Journal of Agricultural and Food Chemistry 60, 9454–9461. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang M, Ren J, Qi J, Zhang G, Leng P. 2010. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biology 10, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaverano I, Ubeda C, Cáceres-Mella A, Valdés ME, Pastenes C, Peña-Neira Á. 2018. Water stress and ripeness effects on the volatile composition of Cabernet Sauvignon wines. Journal of the Science of Food and Agriculture 98, 1140–1152. [DOI] [PubMed] [Google Scholar]
- Talaverano I, Valdés E, Moreno D, Gamero E, Mancha L, Vilanova M. 2017. The combined effect of water status and crop level on Tempranillo wine volatiles. Journal of the Science of Food and Agriculture 97, 1533–1542. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Brugliera F. 2013. Flower colour and cytochromes P450. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368, 20120432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F, Simonneau T. 1998. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49, 419–432. [Google Scholar]
- Teixeira A, Eiras-Dias J, Castellarin SD, Gerós H. 2013. Berry phenolics of grapevine under challenging environments. International Journal of Molecular Sciences 14, 18711–18739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas TR, Matthews MA, Shackel KA. 2006. Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant, Cell & Environment 29, 993–1001. [DOI] [PubMed] [Google Scholar]
- Tilbrook J, Tyerman SD. 2009. Hydraulic connection of grape berries to the vine: varietal differences in water conductance into and out of berries, and potential for backflow. Functional Plant Biology 36, 541. [DOI] [PubMed] [Google Scholar]
- Tomás M, Medrano H, Brugnoli E, Escalona JM, Martorell S, Pou A, Ribas-Carbó M, Flexas J. 2014. Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency. Australian Journal of Grape and Wine Research 20, 272–280. [Google Scholar]
- Tombesi S, Frioni T, Poni S, Palliotti A. 2018. Effect of water stress “memory” on plant behavior during subsequent drought stress. Environmental and Experimental Botany 150, 106–114. [Google Scholar]
- Tombesi S, Nardini A, Farinelli D, Palliotti A. 2014. Relationships between stomatal behavior, xylem vulnerability to cavitation and leaf water relations in two cultivars of Vitis vinifera. Physiologia Plantarum 152, 453–464. [DOI] [PubMed] [Google Scholar]
- Tombesi S, Nardini A, Frioni T, Soccolini M, Zadra C, Farinelli D, Poni S, Palliotti A. 2015. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Scientific Reports 5, 12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontini S, Döring J, Vitali M, Ferrandino A, Stoll M, Lovisolo C. 2014. Soil water-holding capacity mediates hydraulic and hormonal signals of near-isohydric and near-anisohydric Vitis cultivars in potted grapevines. Functional Plant Biology 41, 1119–1128. [DOI] [PubMed] [Google Scholar]
- Trifiló P, Raimondo F, Savi T, Lo Gullo MA, Nardini A. 2016. The contribution of vascular and extra-vascular water pathways to drought-induced decline of leaf hydraulic conductance. Journal of Experimental Botany 67, 5029–5039. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Tyree MT. 2000. Plant hydraulic conductance measured by the high pressure flow meter in crop plants. Journal of Experimental Botany 51, 823–828. [PubMed] [Google Scholar]
- Tyerman SD, Tilbrook J, Pardo C, Kotula L, Sullivan W, Steudle E. 2008. Direct measurement of hydraulic properties in developing berries of Vitis vinifera L. cv Shiraz and Chardonnay. Australian Journal of Grape and Wine Research 10, 170–181. [Google Scholar]
- Tyree MT, Ewers FW. 1991. The hydraulic architecture of trees and other woody plants. New Phytologist 119, 345–360. [Google Scholar]
- Tyree MT, Sperry JS. 1989. Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Plant Molecular Biology 40, 19–36. [Google Scholar]
- Tyree MT, Zimmermann MH. 2002. Xylem structure and the ascent of sap. Berlin, Heidelberg: Springer-Verlag. [Google Scholar]
- van Leeuwen C, Pieri P, Gowdy M, Ollat N, Roby J-P. 2019. Reduced density is an environmental friendly and cost effective solution to increase resilience to drought in vineyards in a context of climate change. OENO One 53, 129–146. [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD. 2009. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant physiology 149, 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versari A, Parpinello GP, Tornielli GB, Ferrarini R, Giulivo C. 2001. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. Journal of Agricultural and Food Chemistry 49, 5531–5536. [DOI] [PubMed] [Google Scholar]
- Vezzulli S, Civardi S, Ferrari F, Bavaresco L. 2007. Methyl jasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. American Journal of Enology and Viticulture 54, 63–66. [Google Scholar]
- Vilanova M, Rodríguez-Nogales JM, Vila-Crespo J, Yuste J. 2019. Influence of water regime on yield components, must composition and wine volatile compounds of Vitis vinifera cv. Verdejo. Australian Journal of Grape and Wine Research 25, 83–91. [Google Scholar]
- Villalobos-González L, Muñoz-Araya M, Franck N, Pastenes C. 2019. Controversies in midday water potential regulation and stomatal behavior might result from the environment, genotype, and/or rootstock: evidence from Carménère and Syrah grapevine varieties. Frontiers in Plant Science 10, 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos-González L, Peña-Neira A, Ibáñez F, Pastenes C. 2016. Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: gene expression and metabolite content. Plant Physiology and Biochemistry 105, 213–223. [DOI] [PubMed] [Google Scholar]
- Vitali M, Cochard H, Gambino G, Ponomarenko A, Perrone I, Lovisolo C. 2016. VvPIP2;4N aquaporin involvement in controlling leaf hydraulic capacitance and resistance in grapevine. Physiologia Plantarum 158, 284–296. [DOI] [PubMed] [Google Scholar]
- Wada H, Matthews MA, Shackel KA. 2009. Seasonal pattern of apoplastic solute accumulation and loss of cell turgor during ripening of Vitis vinifera fruit under field conditions. Journal of Experimental Botany 60, 1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Shackel KA, Matthews MA. 2008. Fruit ripening in Vitis vinifera: apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta 227, 1351–1361. [DOI] [PubMed] [Google Scholar]
- Warren CR, Aranda I, Cano FJ. 2012. Metabolomics demonstrates divergent responses of two Eucalyptus species to water stress. Metabolomics 8, 186–200. [Google Scholar]
- Wheeler JK, Sperry JS, Hacke UG, Hoang N. 2005. Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport. Plant, Cell & Environment 28, 800–812. [Google Scholar]
- Wheeler S, Loveys B, Ford C, Davies C. 2009. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Australian Journal of Grape and Wine Research 15, 195–204. [Google Scholar]
- Williams LE, Ayars JE. 2005. Grapevine water use and the crop coefficient are linear functions of the shaded area measured beneath the canopy. Agricultural and Forest Meteorology 132, 201–211. [Google Scholar]
- Williams LE, Grimes DW, Phene CJ. 2010. The effects of applied water at various fractions of measured evapotranspiration on reproductive growth and water productivity of Thompson Seedless grapevines. Irrigation Science 28, 233–243. [Google Scholar]
- Williams LE, Matthews MA. 1990. Grapevine. In: Stewart BA, Nielsen DR, eds. Irrigation of agricultural crops. Madison: ASA-CSSA-SSSA, 1019–1055. [Google Scholar]
- Williams LE, Retzlaff WA, Yang W, Biscay PJ, Ebisuda N. 2000. Effect of girdling on leaf gas exchange, water status, and non-structural carbohydrates of field-grown Vitis vinifera L. (cv. Flame Seedless). American Journal of Enology and Viticulture 51, 49–54. [Google Scholar]
- Wong DC, Lopez Gutierrez R, Dimopoulos N, Gambetta GA, Castellarin SD. 2016. Combined physiological, transcriptome, and cis-regulatory element analyses indicate that key aspects of ripening, metabolism, and transcriptional program in grapes (Vitis vinifera L.) are differentially modulated accordingly to fruit size. BMC Genomics 17, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DCJ, Zhang L, Merlin I, Castellarin SD, Gambetta GA. 2018. Structure and transcriptional regulation of the major intrinsic protein gene family in grapevine. BMC Genomics 19, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Cook MG, Yacco RS, Watrelot AA, Gambetta G, Kennedy JA, Kurtural SK. 2016. Effects of leaf removal and applied water on flavonoid accumulation in grapevine (Vitis vinifera L. cv. Merlot) berry in a hot climate. Journal of Agricultural and Food Chemistry 64, 8118–8127. [DOI] [PubMed] [Google Scholar]
- Zarrouk O, Costa JM, Francisco R, Lopes C, Chaves MM. 2016. Drought and water management in Mediterranean vineyards. In: Gerós H, Chaves MM, Gil HM, Delrot S, eds. Grapevine in a changing environment: a molecular and ecophysiological perspective. Chichester: John Wiley & Sons, 38–67. [Google Scholar]
- Zhang Y, Keller M. 2015. Grape berry transpiration is determined by vapor pressure deficit, cuticular conductance, and berry size. American Journal of Enology and Viticulture 66, 454–462. [Google Scholar]
- Zhang Y, Keller M. 2017. Discharge of surplus phloem water may be required for normal grape ripening. Journal of Experimental Botany 68, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Dai Z, Vivin P, Gambetta GA, Henke M, Peccoux A, Ollat N, Delrot S. 2018. A 3-D functional-structural grapevine model that couples the dynamics of water transport with leaf gas exchange. Annals of Botany 121, 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Génard M, Poni S, Gambetta GA, Vivin P, Vercambre G, Trought MCT, Ollat N, Delrot S, Dai Z. 2019. Modelling grape growth in relation to whole-plant carbon and water fluxes. Journal of Experimental Botany 70, 2505–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Huang C, Su Y, Sato M. 2014. 3D ground penetrating radar to detect tree roots and estimate root biomass in the field. Remote Sensing 6, 5754–5773. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.