Abstract
Objective:
In adult female rodents, ovarian estradiol (E2) regulates body weight, adiposity, energy balance, physical activity, glucose-insulin homeodynamics, and lipid metabolism, while protecting against diet-induced obesity. The same E2 actions are presumed to occur in primates, but confirmatory studies have been lacking.
Methods:
We investigated the consequences of ovariectomy (OVX) and E2 replacement in female marmoset monkeys on major metabolic and morphometric endpoints. Sexual behavior and uterine diameters were assessed as positive controls for E2 treatment efficacy. Metabolic parameters were measured 1 mo prior to OVX, and 3 and 6 mo thereafter. During OVX, animals received empty or E2-containing silastic s.c. implants. To test the interaction between E2 and diet, both treatment groups were assigned to either a higher fat diet (HFD) or a low-fat diet (LFD).
Results:
As anticipated, OVX animals exhibited diminished frequency (p=0.04) of sexually receptive behavior and increased rejection behavior (p=0.04) towards their male partners compared to E2-treated OVX females. OVX also decreased (p=0.01) uterine diameter. There was no treatment effects on total caloric intake. There were no significant effects of OVX, E2 treatment, or diet on body weight, body composition, energy expenditure, physical activity, fasting glucose, or glucose tolerance. Regardless of E2 treatment, serum triglycerides were higher (p=0.05) in HFD than LFD females. Postmortem qPCR analysis of hypothalamic tissues revealed higher mRNA expression (p<0.001) for PGR in E2-treated monkeys versus OVX controls, but no differences between groups in other selected metabolic genes. There was a decrease in mRNA expression of PGC1a (PPARGC1A), HTR1A and HTR5A in HFD compared to LFD females.
Conclusions:
Our findings, overall, document a greatly diminished role for ovarian E2 in the metabolic physiology of a female primate, and encourage consideration that primates, including humans, evolved metabolic control systems regulated by extra-ovarian E2 or are generally less subject to E2 regulation.
Introduction
In many rodent biomedical research models, ovarian estradiol (E2) regulates body weight and composition, energy balance, and insulin-glucose homeodynamics. E2 regulated control mechanisms are particularly pronounced in rodents, since ovariectomy (OVX)-mediated E2 depletion is reliably accompanied by increased body weight and visceral adiposity, reduced physical activity and energy expenditure (1), as well as diminished glucose tolerance and insulin sensitivity. All these effects are prevented or reversed by physiological E2 replacement (2,3). Furthermore, while intact female mice are resistant to high-fat diet-induced obesity and its associated sequelae, in contrast to male mice, OVX-mediated E2 depletion abolishes this protection (4,5). Virtually all of these E2 actions are mediated by estrogen receptor alpha (ERα; ESR1), as mice bearing null mutations of the ESR1 gene exhibit body weight, adiposity and energy metabolism phenotypes that largely mimic those observed in long-term OVX mice (6–8).
Substantial progress has been made towards clarifying molecular and cellular mechanisms engaging ovarian E2 regulation of energy homeostasis in rodents, particularly those actions that are transduced by hypothalamic neurons expressing ERα (9) (10). Female-specific stimulatory effects of E2 on energy expenditure, for example, have been shown to be transduced in ERα expressing neurons of the ventromedial nucleus (VMN) of the hypothalamus (11) by non-classical ERα signaling (10) coupled to activation of PI3-kinase (12). E2 has also been shown to regulate gene expression associated with regulation of food intake and energy expenditure in the hypothalamus, largely through ERα activation (9) (13). It has yet to be substantiated, however, whether the same mechanisms obtain more broadly among non-rodent biomedical research models, and in humans. In addition to the role of ovarian E2, high fat diets in female rodents, as well as the marmoset monkey, have had effects on neuromodulators of satiety. In female rodents, pharmacological activation of 5HT1A and 5HT2c receptors induce hyperphagia, notably however, only the 5HT2c receptor is consistently accompanied with an obese phenotype (Lam et al 2010). In the female marmoset monkey, an identical diet to the higher fat diet used in this study, mRNA of serotonin production related genes are attenuated in the presence of E2 (Bethea et al 2015). The hypothalamic mRNA expression of serotonin receptors, particularly 1A and 2C response to either ovariectomy or a higher fat diet in female primates, including women, has not yet been identified prior to this study.
Metabolic functions of ovarian E2 in women, in contrast to rodents, have been difficult to define, partly due to logistical and ethical constraints in designing definitive experiments with rigorous control. Numerous studies have attempted to dissociate the effects of normal aging versus declining E2 levels on adiposity, energy balance and cardiometabolic health in menopausal women (14) (15) (16). In general, these studies support the idea that menopause per se is associated with increasing abdominal obesity, and that visceral fat accumulation may, in part, be secondary to an acceleration of aging-related decline in fat oxidation and metabolic energy expenditure (17) (18) (19). While these changes parallel those observed in OVX rodents (20), a causal relationship between declining ovarian E2 in menopause and altered body composition and energy balance has been difficult to confirm. Most randomized controlled studies have demonstrated that both oral and transdermal E2 therapy in postmenopausal women is associated with a reduction in central adiposity and increased lean body mass (21) (22), as well as reduced insulin resistance and fasting glucose, new-onset diabetes, blood lipids, blood pressure, and adhesion molecules and procoagulant factors (23). Of the few studies of energy expenditure after menopausal hormone replacement therapy (HRT) comprising a variety of estrogenic formulations, some demonstrate increases in fat oxidation and energy expenditure (24) (25), while others reveal acute decreases in fat mass, lipid oxidation and energy expenditure (26). There are similarly conflicting data on the effects of HRT on insulin sensitivity, with some suggesting beneficial effects (23) while others find no consistent improvement (27) (28) (29). Differences in ages of the subjects, as well as in amount, composition, timing, duration and route of administration of HRT, and difficulties in controlling for diet, life-style and environmental factors, likely contribute to inconsistencies among clinical studies.
Female nonhuman primates possess reproductive and metabolic control systems that most closely parallel those in women, including ~28-day ovulatory cycles that feature E2 surges during the peri-ovulatory phase followed by a definitive luteal phase. Nonhuman primates have therefore served as important biomedical research models with which to study the actions of E2 on sexual behavior and the control of ovarian cyclicity. Comprehensive studies of the roles played by ovarian E2 on energy balance and body composition in nonhuman primates, by contrast, are scarce. OVX-mediated E2 depletion has small effects on female body weight, with no change in body mass index (BMI) in female rhesus macaques (30), and no effect on female body weight in cynomolgus macaques (31) (32). While a putative selective estrogen receptor modifier (SERM) promotes weight loss in OVX rhesus monkeys (33), E2 replacement therapy has no effect on body weight in OVX cynomolgus macaques (32) (34). The effects of OVX and E2 replacement on energy expenditure, physical activity, body composition, glucoregulation and lipid metabolism have yet to be comprehensively assessed in any female nonhuman primate to determine if the metabolic actions of ovarian E2 in adult female primates resemble those identified in rodents.
To investigate the metabolic functions of ovarian E2 in a female nonhuman primate, we selected the common marmoset monkey (Callithrix jacchus), a higher primate modestly susceptible to diet-induced obesity (35,36). We explored whether OVX-mediated E2 depletion imparts a greater susceptibility to diet-induced obesity as observed in female mice (4,5). Apart from practical reasons to perform this study in marmosets (i.e., ease of handling, compressed life cycle), we chose to study this New World (NW) monkey so as to permit analysis of female energy expenditure and physical activity levels in male-female pairs that maintain a wide range of species-specific social and sexual interactions. Marmosets also exhibit predictable sexual behavior responses to OVX and E2 treatments that provided valid quantitation in the present studies confirming efficacy of E2 regimens in eliciting anticipated biological responses. There is one notable difference, however, between circulating E2 levels in NW compared to Old World (OW) female primates, including women. In pre- and post-OVX female NW monkeys such as female marmosets, circulating levels of E2 are substantially higher compared to E2 levels in female OW monkeys (37). The elevated circulating levels of E2 in NW monkeys may reflect compensatory responses to comprehensive target organ resistance to steroid hormone action (38) (39).
We report here that ovarian E2 sustains female marmoset sexual behavior that is otherwise diminished at 6 mo following OVX, as anticipated from previous studies (40). Neither OVX alone nor E2 replacement, however, produce significant changes in any of the metabolic and morphometric parameters quantified in the same females, regardless of diet, suggesting major differences in metabolic regulation by ovarian E2 in at least one primate species versus its rodent counterparts.
Methods
Animals
Sixteen adult female common marmosets (2–6 years of age) from the Wisconsin National Primate Research Center colony were evenly randomized based on age, body weight and fasting triglyceride values into 4 groups (Supplementary Table 1): E2 replaced + low fat control diet, LFD, (E2+LFD), E2 replaced + higher fat diet, HFD, (E2+HFD), E2 depleted + LFD (OVX+LFD), E2 depleted +HFD (OVX+HFD). Following onset of experiment, one female was excluded from the study (from E2+LFD group) due to diet noncompliance. Animals were maintained in these groups for 6 months after which they went to necropsy to permit tissue collection for gene expression analysis.
All animals lived with a cagemate in 0.60m x 0.91m x 1.83m enclosures and were maintained with 12-hour (h) lighting, ambient temperature of ~27°C and humidity of ~50%. This study was reviewed and approved by the Graduate School Animal Care and Use Committee of the University of Wisconsin, Madison and was performed consistent with the USDA Animal Welfare Act and regulations and the Guide for the Care and Use of Laboratory Animals. The animal care and use program at the University of Wisconsin maintains a Public Health Services Assurance, and is fully accredited by AAALAC.
Prior to study onset, animals were fed Mazuri Callitrichid High Fiber Diet #5MI6 (Purina Mills International, St. Louis, MO) comprising approximately 53% carbohydrate, 20% protein, 6% fat and 10% fiber by weight, with a metabolizable energy of 3.3 kcal/g (approximately 61%, 23% and 16% kcal from carbohydrate, protein and fat, respectively). All animals were then switched to either a low-fat diet (LFD) or higher fat diet (HFD) 1 month prior to baseline assessments and ovariectomy to allow for dietary acclimation. Both the LFD and HFD were semi-purified customized diets designed by Teklad Custom Research Diets (Madison, WI), and primarily comprised lactalbumin, dextrin, sucrose, soybean oil and cellulose with additional vitamins and minerals, as described in (41). The HFD was supplemented with anhydrous milk fat and additional sucrose, at the expense of a lower dextrin content, to increase the sucrose and fat content of the HFD by 2-fold and 2.4-fold, respectively, relative to the LFD. The LFD (TD.110278) comprised 64.3% carbohydrate, 14% protein, 5.6% fat and 5.0% fiber by weight, with a caloric density of 3.6 kcal/g (70.7%, 15.4% and 13.9% kcal from carbohydrate, protein and fat, respectively). The HFD (TD.110277) consisted of 56.5% carbohydrate, 15.4% protein, 12.7% fat and 5.0% fiber by weight, with a caloric density of 4.0 kcal/g (56.2%, 15.3% and 28.4% kcal from carbohydrate, protein and fat, respectively). Animals were separated from their cagemates for ~1 hour in the morning and ~1 hour in the afternoon for feeding to allow for accurate assessment of individual animals’ food intake. All food (base diet plus enrichment) eaten was quantified daily throughout the study.
Ovariectomy and estrogen replacement
Following baseline assessments, bilateral OVX was performed in all females to provide gonadal hormone deficiency. Cloprostenol (Estrumate®, 0.75–1.50 μg intramuscular injection for two successive days approximately 11–60 days after ovulation), an analog of prostaglandin-F2-alpha, was administered prior to OVX to facilitate scheduling of OVX during the follicular phase. At the time of OVX, either empty or E2-filled (to maintain consistent peri-ovulatory E2 levels, E2 replaced) silastic capsules were implanted subcutaneously. Silastic capsules were removed and replaced at 3 months post-OVX to maintain consistent E2 levels. At this time, empty capsules in the OVX groups were replaced as well to maintain consistent conditions among the groups.
As an indicator of functional E2 depletion, uterine dimensions were obtained monthly by transabdominal ultrasonography. Using the scanner’s calibrated, digitized calipers, uterine trans-fundus length (transverse uterine diameter) and dorso-ventral uterine diameter were measured from transverse views, and fundus-cervix length was measured from sagittal views.
Hormone Assay
For steroid hormone analyses, plasma samples underwent extraction and subsequent analysis on a QTRAP 5500 quadruple linear ion trap mass spectrometer (AB Sciex) equipped with an atmospheric pressure chemical ionization source (LC-MS/MS) (37). The system included two Shimadzu LC20ADXR pumps and a Shimadzu SIL20ACXR autosampler. A sample of 30 μl was injected onto a Phenomenex Kinetex 2.6u C18 100A, 100 × 2.1 mm column (Phenomenex) for separation using a mobile phase: water with 1% formic acid (Solution A) and acetonitrile with 1% formic acid (Solution B), at a flow rate of 200 μl/min. After 3 min, Solution B was increased over the course of 0.1 min to 3% and this was maintained for 3 min, followed by another 0.1 min step-up to 50% Solution B that was maintained for 2.9 min. Subsequent 0.1 min step-ups raised Solution B to 67% for 15 min and then 100% for 10 min. The system was finally returned to initial conditions of 3% Solution B over 0.1 min for the remaining 9.9 min of each sample run. Mass spectrometer results were generated in positive-ion mode with the following optimized voltages: corona discharge current, 3 V; entrance potential, 10 V. The source temperature was 500°C. The gas settings were as follows: curtain gas, 30 psi; nebulizing gas, 20 psi; collisionally activated dissociation gas, medium. Quantitative results were recorded as multiple reaction monitoring (MRM) area counts after determination for the response factor for each compound and internal standard. Each steroid had a MRM used for quantitation and 1 or 2 additional MRMs as qualifiers. The lower limits of quantitation (LLOQ) was 2.7 pg/mL for E2. Linearity was r > 0.9990 and the curve fit was linear with 1/x weighting. None of the compounds of interest were detected in blank or double blank samples. Inter-assay coefficient of variation was determined by a pool of marmoset serum. Intra- and Inter- assay CoVs, respectively, were 4% and 9%.
Behavioral Observations
Following treatment onset, pairs were acclimated to the testing cages, as previously described (42). Behavior testing took place five months post treatment onset. There were two 15-minute tests per week for two weeks. Well-established (>6 months) male-female pairmates were used. Pairs were deprived of visual and olfactory contact with one another for 90 minutes immediately before behavioral observations were taken. Following the 90-minute separation period, males were placed in a holding box for 5 minutes before being allowed into the main testing cage with the female. Observations were recorded digitally using JWatcher and via manual scoring of behaviors for 15 minutes. Each test was recorded and inter- and intra-observer reliability was 80% or greater.
Body composition
Animals were weighed weekly. Body dimensions were assessed monthly in awake, manually restrained animals. Measurements included abdomen, chest, arm and leg circumference (by tape measure), and crown-rump length (by osteometric board). Body mass index (BMI, body weight [kg]/height[m2]) was calculated from crown rump length. At baseline, 3 and 6 months post-OVX, total body composition was further assessed by dual-energy x-ray absorptiometry (DXA, iDXA, GE/Lunar Corp., Madison, WI) on sedated animals.
Locomotor activity
Monthly a small accelerometer (Actiwatch Mini, CamNtech Ltd., Cambridge UK) was added to each animal’s standard collar. Activity and intensity of movement were recorded over an ~ two-week period after which the accelerometers were removed. The accelerometer sampled activity counts every 30 seconds and these data were averaged for every hour, day (during lights on), night (during lights off), morning (0600–1200 h), afternoon (1200–1800 h), and 24h.
Energy expenditure by D2180 (doubly labeled water [DLW])
Total energy expenditure (TEE) was determined at baseline and 3 and 6 months post-OVX by the doubly labeled water technique (D218O). On day 1 of this procedure, immediately following a pre-dose urine collection, sterile deuterium oxide and oxygen-18 labeled water (D2180) mixed with normal saline was administered via intraperitoneal injection. The respective non-radioactive isotope doses were ~0.16 and 0.24 g/kg body weight. On days 2 through 4, additional urine samples were collected. For urine collection, each marmoset was manually captured and released into a small urine-collection chamber (15 × 20 × 15 cm), constructed of stainless steel mesh and Plexiglass, which was positioned inside the animal’s home cage. The marmoset remained in the chamber until a sample was produced (~10 minutes). Urine was stored at −20ºC in cryogenically stable tubes until analysis by isotope ratio mass spectrometry as previously described (43) and CO2 production was calculated according to the equation of (44). Data is represented as TEE/free fat mass (FFM) for each animal at each time point recorded.
Glucoregulation
Glucoregulation was assessed at baseline and 3 and 6 months post-OVX in overnight fasted, awake animals by oral glucose tolerance test. Following a baseline blood sample, animals were given an oral dose (5 ml/kg) of 40% sucrose. Blood samples were then collected at 15, 30, 60 and 120 minutes following sucrose administration and assessed for glucose. Glucose was measured by glucometer (Accu-Check Aviva, Roche Diagnostics, Indianapolis, IN). The trapezoidal rule was used to calculate area under the curve (AUC).
Lipid Metabolism Measure
A standard lipid panel (UW-Meriter hospital clinics, Madison, WI) was used to measure circulating lipids (cholesterol, triglycerides, HDL and LDL) in fasted serum samples collected at baseline during oral glucose tolerance testing.
Gene Expression Analysis
Medial basal hypothalamus tissues were dissected and frozen at necropsy. Total RNA was isolated using the AllPrep DNA/RNA/miRNA Universal kit (Qiagen) and cDNA synthesized using the Multiscribe High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Real-time quantitative PCR was performed on a StepOnePlus instrument (Applied Biosystems) using Power SYBR Green PCR Master Mix (Applied Biosystems). Gene expression changes are normalized to TBP as a reference gene and expressed relative to the E2+LFD group. Primer sequences were designed using NCBI Primer-Blast (45) and are listed in Supplemental Table 2.
Statistical Analysis
Data collected was analyzed utilizing SPSS software. Repeated measures ANOVA were used to analyze data collection that repeated throughout the duration of the study. Behavioral observations and gene expression data were analyzed with a two-way ANOVA test. Statistical significance was determined at p<0.05.
Results
Reproductive Physiology
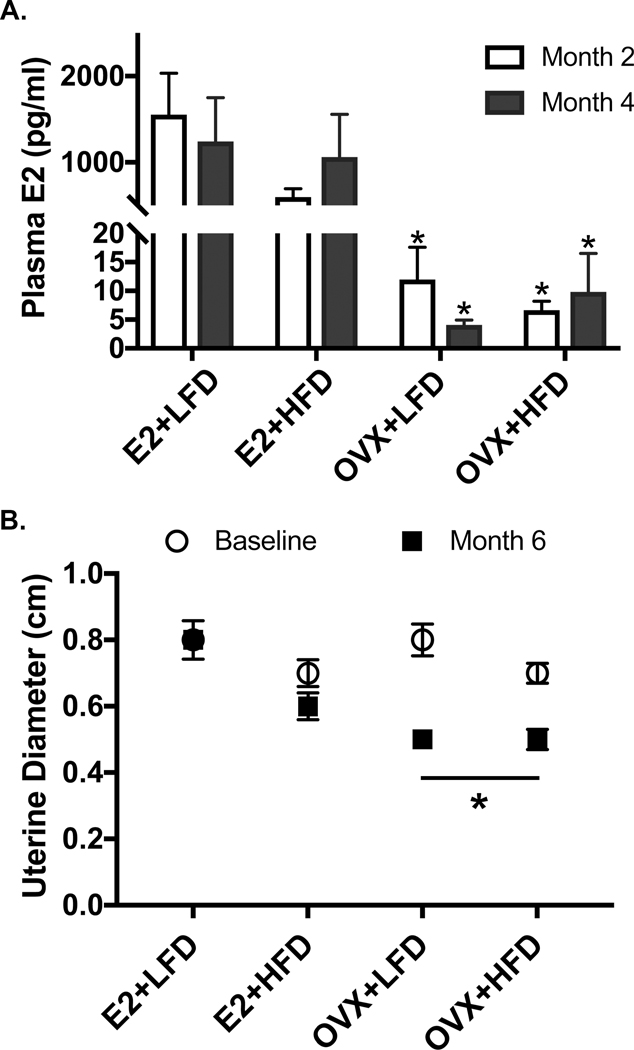
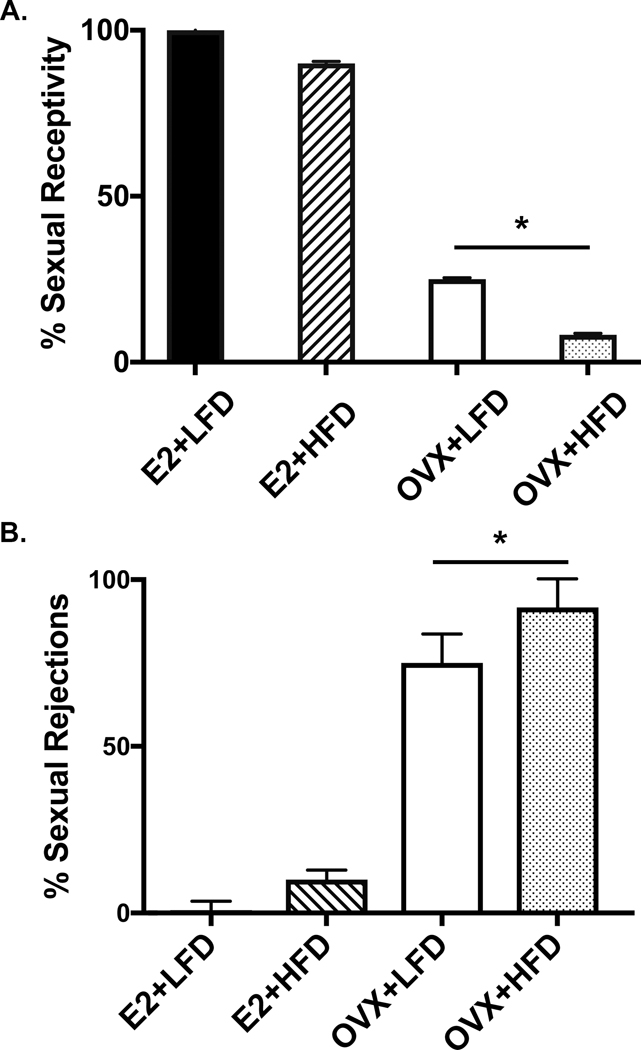
OVX effectively diminished E2 levels, regardless of diet condition. Females that received E2 replacement sustained peri-ovulatory phase levels of E2 over the course of the study, as shown in Figure 1A (p=0.003). The achieved level of circulating E2 in the E2 replaced females maintained pre-OVX uterine diameter, a key indicator of effective ovarian E2 replacement, whereas OVX resulted in a diminished uterine diameter over the 6-month experiment (p=0.01, Figure 1B). Additionally, E2 replacement physiologically supported the expression of female sexual behaviors in E2 replaced females. Figure 2 shows that by 5 mo post OVX, depletion of E2 leads to an expected decrease (~50%) in sexually receptive behavior (A; p=0.04) and a complementary increase in species-characteristic sexual rejection behavior towards the male partner’s mounting behavior (B; p=0.04).
Figure 1A-B:
(A) Estradiol, E2, was diminished in both LFD and HFD OVX groups. Peri-ovulatory levels of E2 (1.0 ± 0.3 ng/ml at 2 months; 1.1±0.3 ng/ml at 4 months) were obtained with subcutaneous capsules throughout the study. (B) After 6 months of treatment onset, uterine diameter was diminished in OVX females (p<0.01). E2 replacement maintained pre-OVX uterine diameter.
Figure 2A-B:
(A) After 5 months of treatment onset, OVX, regardless of diet condition, induced typically diminished expression of sexually receptive behaviors (p=0.04) compared to E2 treated females. (B) OVX females, complimentary to the receptivity decline, exhibited an increase in rejection behavior towards their male partners (p=0.04).
Metabolic Physiology
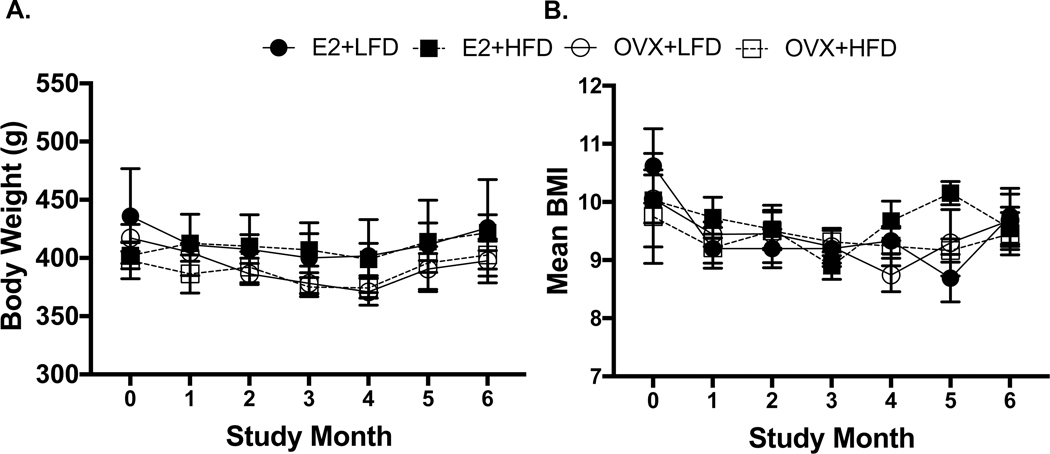
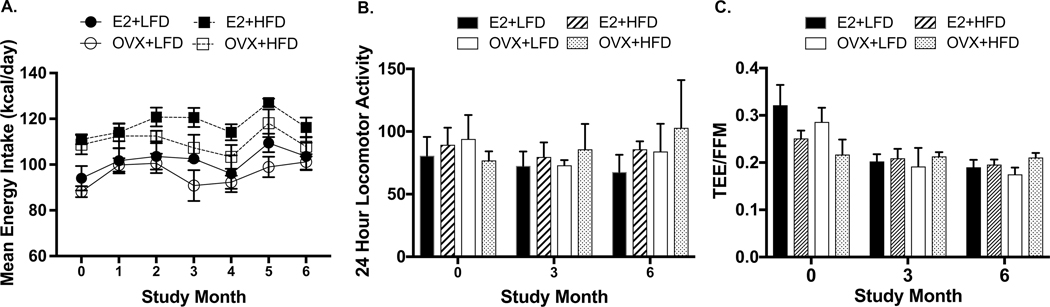
Contrary to reproductive responses to OVX, there were minimal effects of both E2 depletion and a higher fat diet (HFD) on metabolic function in female marmosets over 6 months. Similar body weights (Figure 3A; p=0.97) and BMI (Figure 3B; p=0.58) were observed across all treatment groups throughout the study. Total body and regional fat mass, measured by DXA were also similar among all females over the 6 months (Supplemental Table 3, total body fat, p=0.82; chest, p=0.69; abdomen, p=0.87; legs, p=0.68). Additionally, measures of energy intake and expenditure were similar across all female groups (Figure 4 A-C). These measures included caloric intake (A, p=0.95), locomotor activity (B, p=0.81) and doubly-labeled water estimates of energy expenditure (C, TEE/FFM, p=0.88). Fasted serum lipids, including cholesterol measures (cholesterol: p=0.16; HDL: p=0.66; LDL: p=0.71; Chol:HDL Ratio, p=0.76) (Table 1) were similarly comparable across all female groups. In contrast to all other metabolic measures, circulating triglyceride levels did produce an interaction between time and diet (p=0.04). As the study progressed, females fed the HFD exhibited elevated triglyceride levels compared with females receiving the LFD. Fasting blood glucose levels and AUC glucose from GTTs were not different between the treatment groups and did not change (fasting glucose, p=0.054; AUC glucose, p=0.91) throughout the study (Table 1).
Figure 3A-B:
Across all study groups, body weights and BMI measures remained similar throughout the study duration (A: p=0.97; B: p=0.58, respectively).
Figure 4A-C:
Energy intake, assessed by food intake (A, p=0.95), and energy expenditure, estimated through activity collars (B, p=0.81) and by doubly labeled water expressed as total energy expenditure/free fat mass (TEE/FFM)(C, p=0.88), did not differ between female groups.
Table 1:
Fasted measurements of serum cholesterol (total, LDL and HDL), and triglycerides were taken across the 6-month study. The only effect observed occurred at Month 3: higher triglycerides in HFD females (p=0.05), independent of E2 treatment. This effect was no longer significant at 6 months; however, there was a trend towards HFD elevation of triglycerides. There were no cholesterol differences between treatment groups. There were also no changes in fasted blood glucose and glucoregulatory function between groups throughout the study. All groups showed similar responses in glucose tolerance test (p=0.91)
| Cholesterol (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | Cholesterol:HDL | Triglycerides (mg/dl) | Glucose (mg/dl) | Glucose AUC (mg/dl) | |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| E2+LFD | 138±1 | 55±5 | 59±6 | 2.6±0.3 | 121±20 | 92±9 | 20983±4969 |
| E2+HFD | 157±12 | 67±14 | 62±5 | 2.5±0.3 | 139±13 | 86±8 | 17227±1112 |
| OVX+LFD | 170±15 | 64±11 | 74±6 | 2.8±0.3 | 161±22 | 77±6 | 20820±3081 |
| OVX+HFD | 142±14 | 59±6 | 55±14 | 2.4±0.3 | 137±31 | 94±13 | 20155±2566 |
| Month 3 | |||||||
| E2+LFD | 114±2 | 45±2 | 51±2 | 2.5±0.1 | 90±6 | 101±17 | 23404±8695 |
| E2+HFD | 166±6 | 72±12 | 69±7 | 2.5±−0.3 | 126±2 | 83±5 | 20001±3384 |
| OVX+LFD | 132±15 | 50±6 | 52±10 | 2.7±0.2 | 146±16 | 79±6 | 21383±2048 |
| OVX+HFD | 145±18 | 74±17 | 40±4 | 2.1±0.2 | 157±21 | 111±21 | 21248±1872 |
| Month 6 | |||||||
| E2+LFD | 155±14 | 65±8 | 70±4 | 2.4±0.1 | 99±15 | 112±6 | 25931±8856 |
| E2+HFD | 170±11 | 74±6 | 71±10 | 2.3±0.1 | 123±9 | 97±8 | 21053±2981 |
| OVX+LFD | 154±16 | 66±7 | 66±8 | 2.4±0.2 | 111±22 | 88±6 | 23012±2746 |
| OVX+HFD | 179±22 | 82±15 | 57±19 | 2.3±0.1 | 200±58 | 118±15 | 23307±3060 |
Hypothalamic Gene Expression
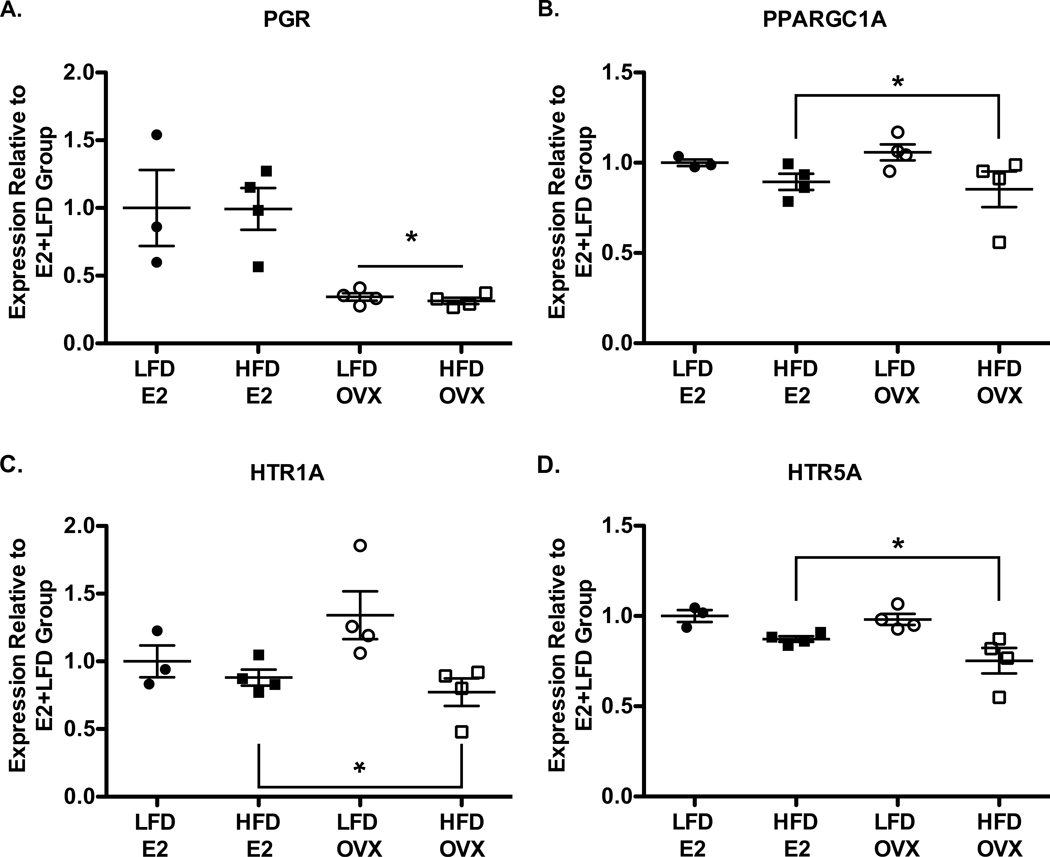
Gene expression analysis, via qPCR, of the medial-basal hypothalamus showed that E2 depletion, regardless of diet, resulted in a decrease in the expression of PGR, which encodes progesterone receptor (Figure 5A, p=0.01). Other selected, E2-regulated, neuroendocrine and behaviorally-related genes did not exhibit any E2 dependent changes in hypothalamic mRNA expression (Supplemental Table 4). In response to HFD, there was a decrease in mRNA expression for PPARGC1A, which encodes PGC1α (Figure 5B, p=0.03), and the serotonin receptors HTR1A (Figure 5C, p=0.01) and HTR5A (Figure 5D, p=0.01). Other metabolically-related genes, however, exhibited no expression differences between treatment groups (Supplemental Table 4).
Figure 5 A-D:
qPCR-determined mRNA expression of progesterone receptor (PGR) (A) was diminished in OVX compared to E2 replaced females, regardless of diet condition (p<0.01). There were, however, decreases in PGC1α(PPARGC1A) (B, p=0.03), HTR1A (C, p=0.03) and HTR5A (D, p=0.01) mRNA expression in response to HFD despite an absence of obvious changes in body weight and body composition. The decreased expression of 5HTR1A, 5HTR5A, and PPARGC1A are independent of any detectable effect of ovariectomy.
Discussion
In women, the natural decline in ovarian E2 and fertility during the menopausal transition is associated with detrimental metabolic changes, including increased body weight, central adiposity and impaired glucoregulation (14). In developing countries, obesity and diabetes are prevalent among women (15) due to many factors, including increasingly sedentary lifestyles and diet changes involving higher fat and sugar content. When metabolic detriments overlap with menopause, they place women at high risk for type 2 diabetes mellitus, T2DM, compared with healthy, pre-menopausal women (46). There is, however, a lack of evidence identifying ovarian E2 as a causal agent of metabolic dysregulation in postmenopausal women, and whether changes in physical activity, metabolic rate and food intake occur because of a decline in E2 signaling.
In the present study, we used a nonhuman primate model, the female marmoset monkey, to understand the effects of both OVX-mediated E2 depletion and a diet higher in fat and moderately high in sugar on various aspects of metabolic function. Marmosets demonstrate modest susceptibility to diet-induced obesity from either a high-sugar or high-fat diet (35,36). Ovary intact female mice are protected against diet-induced obesity and metabolic disturbances relative to OVX mice (4,5). We therefore hypothesized that in OVX female marmosets, E2 depletion will permit the HFD, which has increased sucrose and fat content relative to the LFD, to promote increased body weight and adiposity, together with decreased glucose tolerance. The results, however, do not implicate ovarian E2 as a major contributor to female primate metabolic health. We found a lack of change in energy balance in female marmosets due to either OVX and/or HFD. There were no differences in feeding behavior, or in locomotor activity and energy expenditure, in response to either OVX or diet. Unsurprisingly, given the lack of change in energy balance, these female marmosets did not exhibit increases in body weight, BMI or subsequent changes in adiposity and glucose metabolism, regardless of E2 or diet condition. These results do not implicate ovarian E2 as a major contributor to female primate metabolic health.
In female rodents (47), OVX can transiently increase food intake and decrease metabolic energy expenditure and locomotor activity. In rhesus macaques one study has documented a 3% increase in body weight at 6 wk post-OVX and effect that was exacerbated by a typical high fat diet containing approximately 35% of calories from fat (30). No changes in body weight were reported in cynomolgus macaques maintained on a normal fat diet (~11% calories from fat) after long-term OVX (31) (32). However, the effects of a high fat diet in the cynomolgus has not been studied. While neural mechanisms governing food intake are not well characterized in female nonhuman primates, in rodents, changes in hypothalamic mRNA expression of orexigenic AgRP/NPY and anorexigenic POMC/CART neuropeptides are associated with changes in feeding behavior and energy expenditure, particularly regarding E2 changes across the estrous cycle (48). In the present female marmoset study, however, changes in hypothalamic mRNA expression of these selected genes were absent (Supplementary Table 4), thus providing evidence that in a female nonhuman primate model, ovarian E2 does not alter mRNA expression of known neuropeptides involved in regulating calorie consumption and energy expenditure. Interestingly, in monkeys receiving HFD, and independent of E2 treatment, mRNA expression of PPARGC1A was downregulated. PPARGC1A encodes PGC1α, a co-activator primarily implicated in regulating thermogenesis and mitochondrial biogenesis in hepatocytes, adipocytes and skeletal muscle tissue (49). Prior to this study, the regulation of hypothalamic PPARGC1A mRNA expression has only been examined in mice in response to ‘Western style diets’ and obesity. In male, but not female mice, hypothalamic PGC1α has been shown to be a regulator of hypothalamic ERα and its mRNA expression is diminished in response to a ‘Western diet’ (50). In contrast, in the present nonhuman primate study, HFD induced a decrease in hypothalamic PPARGC1A expression in female marmosets, independent of E2 condition, and without accompanying decrease in the expression of ESR1, which encodes ERα (Supplemental Table 4). Together, these findings suggest that, unlike female rodents, E2 does not protect female nonhuman primates from altered lipid metabolism due to HFD. The absence of a subsequent decrease in ESR1 expression in female marmosets in response to diet induced downregulation of PPARGC1A expression further points to an ovarian E2 independent mechanism of metabolic regulation in this study.
Interestingly, HFD decreased hypothalamic gene expression for serotonin receptors 1A and 5A (HTR1A, HTR5A). Pharmacological blockade of HTR1A in female rodents has been implicated in increasing food consumption (51). In the present study, however, there were no changes in food consumption observed in monkeys fed HFD, despite the decrease in 5HT1A receptor expression. Notably, there was no difference in hypothalamic mRNA expression of 5HT2c receptor despite a dramatic increase in food intake behavior observed in both the genetic deletion of this receptor as well as pharmacological agonist treated female mice (Lam et al 2010). HTR5A gene expression, nevertheless, was not previously studied in animal models with regard to feeding behavior and appetite, however in this study is downregulated by the higher fat diet. The diet-related decrease in gene expression for HTR1A and HTR5A in this study may indicate an underlying dysregulation of satiety possibly in response to altered metabolic function in the brain, even in the absence of obvious food intake changes and obesity. In the marmoset monkey, serotonin production has been shown to be diminished in the dorsal ralphe nucleus in response to HFD (41). In the hypothalamus, given the data presented here, it appears that serotonin action may also be diminished via decreased receptor expression, independent of ovarian E2. HTR5A has been found to decrease in the rat dentate gyrus in response to diet induced obesity, but the hypothalamic expression of this receptor has not been examined. It is also worthwhile to note that the limitations of our experimental food intake measures may have confounded our ability to accurately measure feeding regulation. We measured food intake only during 1 hour feeding periods, thus it is possible that food intake would be better represented through free feeding measurements. Together with our earlier report (41) this study is the first to identify diet induced changes of PPARGC1a, as well as serotonergic production and action, in a female primate model. These findings suggest that in female marmoset monkeys, HFD induces neural molecular changes in the hypothalamus and dorsal raphe (41) prior to any increase in body and fat mass.
Despite a lack of E2 specific regulation of body weight, body composition and glucose and energy homeostasis, E2 replacement in this study maintained other female-typical biological functions in female marmosets, including receptive sexual behavior (40). In the present study, the pairmates used were well-established pairs that lived together continuously prior to testing and throughout the testing periods. This paradigm is very applicable to relationship paradigms in humans. E2 replacement, in the present study, was also shown to induce an increase in hypothalamic mRNA expression of PGR, which encodes the progesterone receptor. This has not been examined previously in marmoset monkeys, but is another well-established function of E2 in both rodents (53) and other nonhuman primates (54). Additionally, E2 replacement was able to maintain the diameter of the uterus found in ovary intact female marmosets, in contrast to the diminished diameter found in OVX females. Taken together, these results provide evidence of sufficient replacement of the ovarian contribution to circulating E2 levels in our female marmoset subjects.
It is worthwhile to note that NW primates, such as marmoset monkeys, have species-specific differences in steroid hormone metabolism compared with OW primates, including humans. There are naturally occurring states of hypoestrogenism, such as anovulatory, reproductively subordinate states (42) (55) in female marmoset monkeys. This is one possible explanation for the lack of metabolic response to OVX observed in this study. Subordinate females with very low E2 do not exhibit changes in body weight (42) or even bone density (Colman, personal communication). Reproductive subordination in the female marmoset is a naturally-occurring phenomenon that suggests an evolutionary advantage of this species to maintaining adult female body weight and bone density in a low estrogenic state. This is markedly different from other mammalian species, including other primates, in which detriments to bone metabolism and possibly energy homeostasis occurs in the presence of low or insufficient circulating levels of E2, normally contributed by the ovaries (56). Thus suggesting NW primates or at least the marmoset compensatory increase in bioavailability of estradiol to cope with the natural occurrence of hypoestrogenism observed in subordinate females. There is also the possibility that female marmoset monkeys have a heightened sensitivity to E2. In the OVX state, female marmosets exhibit low, but detectable E2 levels (37), and thus it is possible that the female marmoset has evolved to regulate normal metabolic function with less circulating E2.
Another possible explanation for the lack of metabolic response to OVX is that, in primates, including women, it is possible that extra-ovarian E2 produced in tissues such as adipose (57) and the hypothalamus (58), may be sufficient to regulate metabolic function. It has been shown that extra-ovarian estradiol can exert negative feedback effects in the marmoset monkey (37) which suggests that extra-ovarian estradiol in the female marmosets in this study may also contribute to maintaining body weight and adiposity. This effect may not be limited to the marmoset monkey. In rodent models, the obesity associated with ablation of ERα (10) (11) appears to exceed that induced by OVX alone (1) (59). This may suggest that even in the rodent, extra-ovarian E2 may protect against obesity. Nevertheless, it is clear from this study is that in female marmoset monkeys, ovarian E2 is not necessary to maintain homeostatic regulation of metabolism even when fed a HFD.
While this study suggests that female marmoset monkeys are resistant to metabolic changes due to HFD, and also to OVX, it is not without its limitations. The HFD and LFD diet compositions used are both relatively low in fat compared to Western-style diets. Taken together with the moderate (~10%) calorie increase of the HFD, such dietary intervention may have been insufficient to induce changes in body composition and adiposity. It is possible that a longer duration of time on HFD, or an increased fat content of the HFD, would elicit increases in body weight and adiposity, and subsequently induce altered glucoregulation in these female nonhuman primates. The marmoset LFD and HFD diets used in this study are both semi-purified diets, as opposed to standard commercial marmoset diets that are often hybrid diets comprising a mix of natural and purified ingredients. Although the marmosets in this study readily consumed both the LFD and HFD diets, it is possible that the dietary composition of the semi-purified diets may need to be optimized to improve palatability and to promote an increased caloric intake sufficient for positive energy balance. It has been previously shown that feeding marmosets a high-glucose diet (Mazuri 5MI5) elicited earlier and more sustained increases in both fat mass and hyperglycemia relative to feeding a high-fat diet (36). Marmosets may therefore prefer diets high in simple sugars (especially sucrose), as opposed to increased fat content (60). Our findings, nevertheless, demonstrate an absence of ovarian E2 influence on homeostatic regulation of female metabolism in a nonhuman primate.
Supplementary Material
Supplemental Table 1: Females in all groups were of similar ages and body weights prior to study onset.
Supplemental Table 3: DXA scans were utilized to determine body composition. There were no differences in adiposity between female groups.
Supplemental Table 2: Primers used for qPCR gene expression analysis.
Supplemental Table 4: qPCR-determined mRNA expression of selected metabolic, reproductive, and behaviorally related genes are shown here. Significant effects are shown in the text and included here. mRNA of additional selected genes were not difference among groups.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22(3):583–93. [DOI] [PubMed] [Google Scholar]
- 2.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–17. [DOI] [PubMed] [Google Scholar]
- 3.Yonezawa R, Wada T, Matsumoto N, Morita M, Sawakawa K, Ishii Y, et al. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. Am J Physiol Endocrinol Metab. 2012;303(4):E445–56. [DOI] [PubMed] [Google Scholar]
- 4.Hong J, Stubbins RE, Smith RR, Harvey AE, Núñez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 2009;17;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludgero-Correia A Jr, Aguila MB, Mandarim-de-Lacerda CA, Faria TS. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition. 2012;28(3):316–23. [DOI] [PubMed] [Google Scholar]
- 6.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49(3):588–97. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144(1):230–9. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, et al. Genetic rescue of nonclassical ERalpha signaling normalizes energy balance in obese Eralpha-null mutant mice. J Clin Invest. 2011;121(2):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104(7):2501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito K, He Y, Yang Y, Zhu L, Wang C, Xu P, et al. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Sci Rep. 2016;6:23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Mol Endocrinol. 2006;20(9):2080–92. [DOI] [PubMed] [Google Scholar]
- 14.Clegg D, Hevener AL, Moreau KL, Morselli E, Criollo A, Van Pelt RE, et al. Sex Hormones and Cardiometabolic Health: Role of Estrogen and Estrogen Receptors. Endocrinology. 2017;158(5):1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Safi ZA, Polotsky AJ. Obesity and menopause. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):548–53. [DOI] [PubMed] [Google Scholar]
- 16.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, et al. Understanding weight gain at menopause. Climacteric. 2012;15(5):419–29. [DOI] [PubMed] [Google Scholar]
- 17.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55(5):950–4. [DOI] [PubMed] [Google Scholar]
- 18.Genazzani AR, Gambacciani M. Effect of climacteric transition and hormone replacement therapy on body weight and body fat distribution. Gynecol Endocrinol. 2006;22(3):145–50. [DOI] [PubMed] [Google Scholar]
- 19.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegorzewska IN, Walters K, Weiser MJ, Cruthirds DF, Ewell E, Larco DO, et al. Postovariectomy weight gain in female rats is reversed by estrogen receptor alpha agonist, propylpyrazoletriol. Am J Obstet Gynecol. 2008;199(1):67 e1–5. [DOI] [PubMed] [Google Scholar]
- 21.Gambacciani M, Ciaponi M, Cappagli B, Genazzani AR. Effects of low-dose continuous combined conjugated estrogens and medroxyprogesterone acetate on menopausal symptoms, body weight, bone density, and metabolism in postmenopausal women. Am J Obstet Gynecol. 2001;185(5):1180–5. [DOI] [PubMed] [Google Scholar]
- 22.Lahmann PH, Lissner L, Gullberg B, Berglund G. Sociodemographic factors associated with long-term weight gain, current body fatness and central adiposity in Swedish women. Int J Obes Relat Metab Disord. 2000;24(6):685–94. [DOI] [PubMed] [Google Scholar]
- 23.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–54. [DOI] [PubMed] [Google Scholar]
- 24.Chmouliovsky L, Habicht F, James RW, Lehmann T, Campana A, Golay A. Beneficial effect of hormone replacement therapy on weight loss in obese menopausal women. Maturitas. 1999;32(3):147–53. [DOI] [PubMed] [Google Scholar]
- 25.dos Reis CM, de Melo NR, Meirelles ES, Vezozzo DP, Halpern A. Body composition, visceral fat distribution and fat oxidation in postmenopausal women using oral or transdermal oestrogen. Maturitas. 2003;46(1):59–68. [DOI] [PubMed] [Google Scholar]
- 26.O’Sullivan AJ, Hoffman DM, Ho KK. Estrogen, lipid oxidation, and body fat. N Engl J Med. 1995;333(10):669–70. [DOI] [PubMed] [Google Scholar]
- 27.Duncan AC, Lyall H, Roberts RN, Petrie JR, Perera MJ, Monaghan S, et al. The effect of estradiol and a combined estradiol/progestagen preparation on insulin sensitivity in healthy postmenopausal women. J Clin Endocrinol Metab. 1999;84(7):2402–7. [DOI] [PubMed] [Google Scholar]
- 28.Mattiasson I, Rendell M, Tornquist C, Jeppsson S, Hulthen UL. Effects of estrogen replacement therapy on abdominal fat compartments as related to glucose and lipid metabolism in early postmenopausal women. Horm Metab Res. 2002;34(10):583–8. [DOI] [PubMed] [Google Scholar]
- 29.Sites CK, L’Hommedieu GD, Toth MJ, Brochu M, Cooper BC, Fairhurst PA. The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(5):2701–7. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan EL, Daniels AJ, Koegler FH, Cameron JL. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes Res. 2005;13(12):2072–80. [DOI] [PubMed] [Google Scholar]
- 31.Sandoval-Guzman T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16(2):146–53. [DOI] [PubMed] [Google Scholar]
- 32.Wagner JD, Clarkson TB, St Clair RW, Schwenke DC, Shively CA, Adams MR. Estrogen and progesterone replacement therapy reduces low density lipoprotein accumulation in the coronary arteries of surgically postmenopausal cynomolgus monkeys. J Clin Invest. 1991;88(6):1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan EL, Shearin J, Koegler FH, Cameron JL. Selective estrogen receptor modulator promotes weight loss in ovariectomized female rhesus monkeys (Macaca mulatta) by decreasing food intake and increasing activity. Am J Physiol Endocrinol Metab. 2012;302(7):E759–67. [DOI] [PubMed] [Google Scholar]
- 34.Cefalu WT, Wagner JD, Bell-Farrow AD, Wang ZQ, Adams MR, Toffolo G, et al. The effects of hormonal replacement therapy on insulin sensitivity in surgically postmenopausal cynomolgus monkeys (Macaca fascicularis). Am J Obstet Gynecol. 1994;171(2):440–5. [DOI] [PubMed] [Google Scholar]
- 35.Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus). Obesity (Silver Spring). 2009;17(8):1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wachtman LM, Kramer JA, Miller AD, Hachey AM, Curran EH, Mansfield KG. Differential contribution of dietary fat and monosaccharide to metabolic syndrome in the common marmoset (Callithrix jacchus). Obesity (Silver Spring). 2011;19(6):1145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraynak M, Flowers MT, Shapiro RA, Kapoor A, Levine JE, Abbott DH. Extraovarian gonadotropin negative feedback revealed by aromatase inhibition in female marmoset monkeys. Am J Physiol Endocrinol Metab. 2017;313(5):E507–E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gacad MA, Chen H, Arbelle JE, LeBon T, Adams JS. Functional characterization and purification of an intracellular vitamin D-binding protein in vitamin D-resistant new world primate cells. Amino acid sequence homology with proteins in the hsp-70 family. J Biol Chem. 1997;272(13):8433–40. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Hewison M, Hu B, Sharma M, Sun Z, Adams JS. An Hsp27-related, dominant-negative-acting intracellular estradiol-binding protein. J Biol Chem. 2004;279(29):29944–51. [DOI] [PubMed] [Google Scholar]
- 40.Kendrick KM, Dixson AF. Effects of oestradiol 17B, progesterone and testosterone upon proceptivity and receptivity in ovariectomized common marmosets (Callithrix jacchus). Physiol Behav. 1985;34(1):123–8. [DOI] [PubMed] [Google Scholar]
- 41.Bethea CL, Reddy AP, Flowers M, Shapiro RA, Colman RJ, Abbott DH, et al. High fat diet decreases beneficial effects of estrogen on serotonin-related gene expression in marmosets. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbott DH, Saltzman W, Schultz-Darken NJ, Tannenbaum PL. Adaptations to subordinate status in female marmoset monkeys. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(3):261–74. [DOI] [PubMed] [Google Scholar]
- 43.Chery I, Zahariev A, Simon C, Blanc S. Analytical aspects of measuring (2)H/(1)H and (18)O/(16)O ratios in urine from doubly labelled water studies by high-temperature conversion elemental analyser-isotope-ratio mass spectrometry. Rapid Commun Mass Spectrom. 2015;29(7):562–72. [DOI] [PubMed] [Google Scholar]
- 44.Schoeller DA, Leitch CA, Brown C. Doubly labeled water method: in vivo oxygen and hydrogen isotope fractionation. Am J Physiol. 1986;251(6 Pt 2):R1137–43. [DOI] [PubMed] [Google Scholar]
- 45.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brand JS, van der Schouw YT, Onland-Moret NC, Sharp SJ, Ong KK, Khaw KT, et al. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care. 2013;36(4):1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol. 2010;166(3):520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci U S A. 2009;106(37):15932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30(4):145–51. [DOI] [PubMed] [Google Scholar]
- 50.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, et al. Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Rep. 2014;9(2):633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 2009;587(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shabbir F, Patel A, Mattison C, Bose S, Krishnamohan R, Sweeney E, et al. Effect of diet on serotonergic neurotransmission in depression. Neurochem Int. 2013;62(3):324–9. [DOI] [PubMed] [Google Scholar]
- 53.Sa SI, Fonseca BM. Dynamics of progesterone and estrogen receptor alpha in the ventromedial hypothalamus. J Endocrinol. 2017;233(2):197–207. [DOI] [PubMed] [Google Scholar]
- 54.Urbanski HF, Mueller K, Bethea CL. Effect of an obesogenic diet on circadian activity and serum hormones in old monkeys. Endocr Connect. 2017;6(6):380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbott DH, Hodges JK, George LM. Social status controls LH secretion and ovulation in female marmoset monkeys (Callithrix jacchus). J Endocrinol. 1988;117(3):329–39. [DOI] [PubMed] [Google Scholar]
- 56.Masarachia PJ, Pennypacker BL, Pickarski M, Scott KR, Wesolowski GA, Smith SY, et al. Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res. 2012;27(3):509–23. [DOI] [PubMed] [Google Scholar]
- 57.DiSilvestro D, Petrosino J, Aldoori A, Melgar-Bermudez E, Wells A, Ziouzenkova O. Enzymatic intracrine regulation of white adipose tissue. Horm Mol Biol Clin Investig. 2014;19(1):39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol. 2012;33(4):364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blaustein JD, Gentry RT, Roy EJ, Wade GN. Effects of ovariectomy and estradiol on body weight and food intake in gold thioglucose-treated mice. Physiol Behav. 1976;17(6):1027–30. [DOI] [PubMed] [Google Scholar]
- 60.Tardif SD, Richter CB. Competition for a desired food in family groups of the common marmoset (Callithrix jacchus) and the cotton-top tamarin (Saguinus oedipus). Lab Anim Sci. 1981;31(1):52–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Females in all groups were of similar ages and body weights prior to study onset.
Supplemental Table 3: DXA scans were utilized to determine body composition. There were no differences in adiposity between female groups.
Supplemental Table 2: Primers used for qPCR gene expression analysis.
Supplemental Table 4: qPCR-determined mRNA expression of selected metabolic, reproductive, and behaviorally related genes are shown here. Significant effects are shown in the text and included here. mRNA of additional selected genes were not difference among groups.