Abstract
Adipose-derived stem/stromal cells (ASCs) have been previously used for bone repair. However, significant cell heterogeneity exists within the ASC population, which has the potential to result in unreliable bone tissue formation and/or low efficacy. Although the use of cell sorting to lower cell heterogeneity is one method to improve bone formation, this is a technically sophisticated and costly process. In this study, we tried to find a simpler and more deployable solution—blocking antiosteogenic molecule Dickkopf-1 (DKK1) to improve osteogenic differentiation. Human adipose-derived stem cells were derived from = 5 samples of human lipoaspirate. In vitro, anti-DKK1 treatment, but not anti-sclerostin (SOST), promoted ASC osteogenic differentiation, assessed by alizarin red staining and real-time polymerase chain reaction (qPCR). Increased canonical Wnt signaling was confirmed after anti-DKK1 treatment. Expression levels of DKK1 peaked during early osteogenic differentiation (day 3). Concordantly, anti-DKK1 supplemented early (day 3 or before), but not later (day 7) during osteogenic differentiation positively regulated osteoblast formation. Finally, anti-DKK1 led to increased transcript abundance of the Wnt inhibitor SOST, potentially representing a compensatory cellular mechanism. In sum, DKK1 represents a targetable “molecular brake” on the osteogenic differentiation of human ASC. Moreover, release of this brake by neutralizing anti-DKK1 antibody treatment at least partially rescues the poor bone-forming efficacy of ASC.
Keywords: perivascular stem cell, adipose-derived stem cells, Dickkopf-1, sclerostin
Introduction
Adipose-derived stem cells (ASCs) have been used for two decades to induce bone repair in both research and clinical settings [1,2]. ASCs can accelerate tissue regeneration through multiple mechanisms, including direct tissue formation, stimulation of progenitor cell proliferation and differentiation, modulation of the immune system, and induction of vascularization (see Zuk [3] for a review).
Despite many communications demonstrating the ability of ASCs to contribute to bone healing [4–6], low bone-forming efficacy and batch-to-batch variability have been identified [5,7]. One potential limiting factor is cellular heterogeneity, which is present within ASC preparations [7,8]. Indeed, nonprogenitor cells and endothelial cells have been observed to decrease the osteogenic efficacy of ASCs in some contexts [9–11]. Recent studies have concluded that without additional biological supplements, ASCs have limited potential for use in bone tissue engineering [12,13].
Several methods have been utilized to purify mesenchymal stem cell fractions within adipose tissue [7,14–18]. One method that our group has used is to fluorescence activated cell sorting (FACS) purify stromal vascular fraction (SVF) based on positive selection of the perivascular antigens CD34 or CD146 [7,15]. These cells, termed human perivascular stem cells (PSCs), have higher innate bone-forming potential compared to ASCs [7,19]. Other antigens used to purify human ASCs have been examined, for example, use of CD90, CD105, or CD73 [14,16–18,20].
However, the use of cell purification methods for tissue engineering is a relatively complex and costly procedure. A much simpler solution would be to target those signaling pathways differentially expressed between unpurified stromal cell population and purified progenitor cell fractions, and leverage this information for improved cell-mediated bone repair using ASCs.
Extracellular Wnt antagonists regulate bone formation either by directly binding to Wnt ligands, such as secreted frizzled-related proteins, or by competing with Wnt ligands for binding to co-receptors lipoprotein-related protein 5 and 6 (LRP5 and LRP6) expressed on the cell surface, such as sclerostin (SOST) and Dickkopf-1 (DKK1–1) [21]. Genetic deletion of sclerostin results in increased bone formation in both mice and humans [22,23]. Antibodies that neutralize sclerostin have previously been shown to have the potential to promote bone formation [24,25].
DKK1 is a well-known secreted protein that acts as antagonist of Wnt signaling [26]. Its impact on bone physiology was first sparked by the identification of loss of function mutations in LRP5, responsible for the disease osteoporosis pseudo glioma [27]. Knockout of DKK1 results in a dose-dependent increase of bone mass in mice [28–30]. Many preclinical studies have shown that DKK1 neutralizing antibodies (anti-DKK1) stimulate bone formation at both cortical and trabecular sites, effectively combating ovariectomy-induced bone loss in mice and increasing bone mineral density in nonhuman primates [31–33].
The importance of DKK1 in fracture repair has also been established [33,34]. Previously, it had been reported that long bone injury induces canonical Wnt signaling activation among osteoprogenitor cell populations [35]. Conversely, failed fracture healing (such as in human nonunion fractures) shows elevated levels of DKK1 among stromal cells of the fracture site [36]. In mouse studies, adenoviral delivered DKK1 has been observed to result in impaired fracture healing [35], associated with accumulation of undifferentiated stromal cells [37]. Recently, systemic anti-DKK1 treatment has improved fracture healing in two independent mouse long bone fracture models [34,38]. Despite this accumulating translational evidence, the use of anti-DKK1 treatment in the context of cell-mediated bone repair is an entirely novel avenue of investigation.
Previously, we observed that DKK1 was highly overexpressed in ASCs compared to purified PSCs. To test the potential use of DKK1 neutralization to augment unpurified adipose-derived stromal cell (termed ASC)-mediated bone repair, anti-DKK1 neutralizing antibody was applied to ASCs in vitro. In this study, we confirmed that DKK1 expression is enriched among human ASCs during early osteogenic differentiation. The effect of anti-DKK1 on human adipose-derived MSC biology was assessed. We identified an overall pro-osteogenic effect of anti-DKK1 in human ASCs, defined the intersample variability of responsiveness to DKK1 neutralization, and deciphered potential mechanisms of this variation.
Materials and Methods
Isolation of human ASCs from human adipose tissue
Under IRB approval with a waiver of informed consent, human lipoaspirate was acquired from five healthy adult donors. Patient demographics can be found in Supplementary Table S1. Before processing, fat tissue had been stored at 4°C for <48 h. According to previously published methods [7], ASCs were obtained by collagenase digestion.
Lipoaspirate was rinsed with equal volume of phosphate-buffered saline (PBS). The rinsed lipoaspirate was digested with 1 mg/mL type II collagenase in Dulbecco's modified Eagle's medium (DMEM) containing 3.5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) at 37°C for 70 min under agitation. Adipose cells were separated and eliminated by centrifugation. Cell particles are resuspended and incubated in red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM ethylenediaminetetraacetic acid) at room temperature for 10 min. After centrifugation, the cells were resuspended in PBS and sifted at 40 μm.
Cells were cultured at 37°C in a humidified atmosphere, which contained 95% air and 5% CO2. Standard growth medium consisted of DMEM (Gibco, Grand Island, NY), 15% fetal bovine serum (FBS; Gibco), 1% penicillin/streptomycin (Gibco), and 2 mg/mL human basic fibroblast growth factor (R&D System, Minneapolis, MN).
For PSC FACS, uncultured ASCs were further processed using a mixture of the following directly conjugated antibodies: anti-CD34-R-phycoerythrin (1:50; BD Pharmingen, San Diego, CA), anti-CD45-allophycocy-anin-cyanin 7 (1:100; BD Pharmingen), anti-CD146-fluorescein isothiocyanate (1:100; Bio-Rad, Hercules, CA), and anti-CD31-allophycocyanin-cyanin 7 (1:100; Bi-Rad). See Supplementary Table S2 for antibody information. All incubations were performed at 4°C for 20 min. In this manner, a combined population of microvessel pericytes (CD146+CD34−CD45−CD31−) and adventitial cells (CD34+CD146−CD45−CD31−) were isolated to constitute the PSC population.
Proliferation assay
After 48–96 h, cell proliferation was measured with the CellTiter96® Aqueous One Solution Cell Proliferation Assay kit (MTS, G358A; Promega, Madison, WI), in which 2,000 cells were cultured in 96-well dishes. Briefly, 200 μL of MTS solution was added into each well. After incubation for 1 h at 37°C, the absorbance was measured at 490 nm with an Epoch microspectrophotometer (Bio-Tek, Winooski, VT). N = 3 wells were used in each group, and all studies were performed in biologic triplicate.
RNA isolation and real-time polymerase chain reaction
To analyze gene expression, TRIzol (Life Technology, Waltham, MA) was adopted for total RNA isolation. According to the manufacturer's instructions, RNA was reverse transcribed into cDNA through iScript cDNA Synthesis Kit (Bio-Rad). Real-time polymerase chain reaction (qPCR) was performed with SYBR Green PCR Master Mix (Life Technology). Primer information is presented in Supplementary Table S3. N = 3 wells were used in each group, and all studies were performed in biologic triplicate.
Osteogenic differentiation
ASCs or PSCs were cultured in osteogenic differentiation medium that was composed of DMEM, 10% FBS, 1% penicillin/streptomycin with 100 nM dexamethasone, 10 mM β glycerophosphate, and 50 μM ascorbic acid (Sigma-Aldrich). Anti-DKK1, anti-SOST (Bio-Rad), and/or recombinant DKK1 (R&D Systems) were added to osteogenic differentiation medium at defined concentrations. IgG was used as an isotype control. See Supplementary Table S2 for antibody information. Medium was changed every 3 days. To detect mineralization, cultures were stained with alizarin red S (Sigma-Aldrich) up to 14 days of differentiation. 0.1 N sodium hydroxide was used to dissolve the calcium precipitate and quantified by absorbance at 548 nm.
Western blot analysis
Proteins were separated by SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and were transferred onto a nitrocellulose membrane. Blots were probed with primary antibodies to β-catenin (1:1,000; Cell Signaling Technology, Danvers, MA) and glyceraldehyde 3-phophate dehydrogenase (1:2,000; Cell Signaling Technology) overnight at 4°C. Afterward, these blots were incubated with goat anti-rabbit secondary antibodies that were conjugated with horseradish peroxidase and visualized by the ChemiDoc Touch Imaging System (Bio-Rad).
Statistical analysis
Results are expressed as the mean ± standard deviation. Statistical analysis was performed with a Student's t-test for a two-sample comparison, an analysis of one-way ANOVA with Dunnett's multiple comparisons test, or two-way ANOVA with Sidak's multiple comparisons test (GraphPad Software 6.0). *P < 0.05, **P < 0.01, and ***P < 0.001 were considered significant.
Results
Effect of anti-DKK1 on osteogenic differentiation of human ASCs
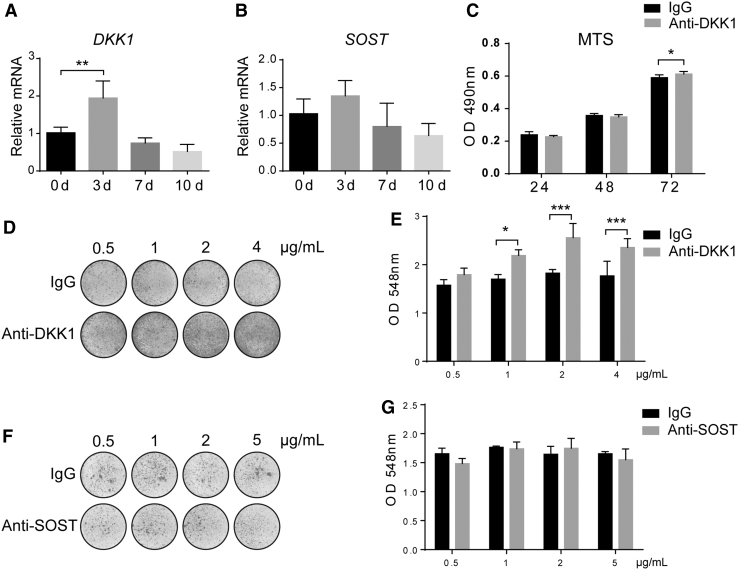
Gene expression of the Wnt signaling antagonists DKK1 and SOST was first assessed across time during the osteogenic differentiation of human ASCs by qPCR (Fig. 1A, B). Results showed that the levels of DKK1 transcripts peaked early in osteogenic differentiation (90% increase on day 3, **P < 0.01) and returned to slightly below baseline on days 7 and 10 of differentiation (50% reduction from baseline expression on day 10). The levels of SOST transcripts followed a similar trend with a slight increase on day 3 of osteogenic differentiation (31% increase, which did not reach statistical significance), followed by a reduction by 10 days (38% reduction from baseline on day 10).
FIG. 1.
Anti-DKK1, but not anti-SOST, enhances the osteogenic differentiation of human ASCs. (A, B) Gene expression of DKK1 and SOST across time during the osteogenic differentiation of human ASC (0–10 days of differentiation). Each group of different time points was compared with that of 0 days. (C) Effects of anti-DKK1 on ASC proliferation, assessed by MTS assay at 24–72 h (1 μg/mL). (D, E) Effects of anti-DKK1 on ASC osteogenic differentiation, assessed by alizarin red staining and quantification after 7 days of differentiation (0.5–4 μg/mL). Whole well images shown. (F, G) Effects of anti-SOST on ASC osteogenic differentiation, assessed by alizarin red staining and quantification after 7 days of differentiation (0.5–5 μg/mL). All experiments performed with an appropriate isotype IgG control. Experiments performed in at least experimental and biological triplicate. Error bars represent one standard deviation. *P < 0.05; **P < 0.01; ***P < 0.001. ASC, adipose-derived stem/stromal cell; DKK1, Dickkopf 1; SOST, sclerostin.
The cellular effects of neutralizing antibodies to either DKK1 or SOST were next assessed. In each case, control cells were treated with an IgG isotype control at the same concentration. When neutralizing anti-DKK1 antibody was used to treat ASCs during culture, a slightly higher proliferative rate was observed, assessed by MTS assays (2% increase at 72 h, Fig. 1C). In addition, anti-DKK1 treatment promoted osteogenic differentiation dose dependently, starting from the 1 μg/mL (Fig. 1D, E). This was detected by alizarin red staining (Fig. 1D) for bone nodule deposition and quantification (Fig. 1E, 13%–40% increase from 0.5 to 4 μg/mL). In contrast, across a wide range of concentrations, anti-SOST did not increase ASC osteogenic differentiation at any dosage (Fig. 1F, G).
Previously, we observed that DKK1 was less expressed in purified PSCs in comparison to ASCs. To know whether PSCs have different response to anti-DKK1 compared to ASCs, we performed FACS to further process SVF using the detection of two perivascular antigens (CD34 and CD146) to obtain PSCs as previously described (Supplementary Fig. S1A–D) [7]. Next, we detected the effect of anti-DKK1 in PSC osteogenic differentiation. In contrast to ASCs, anti-DKK1 did not induce a significant increase in PSC osteogenic differentiation (Supplementary Fig. S1E, F).
Anti-DKK1 induces changes in osteogenic and Wnt signaling-related genes in human ASCs
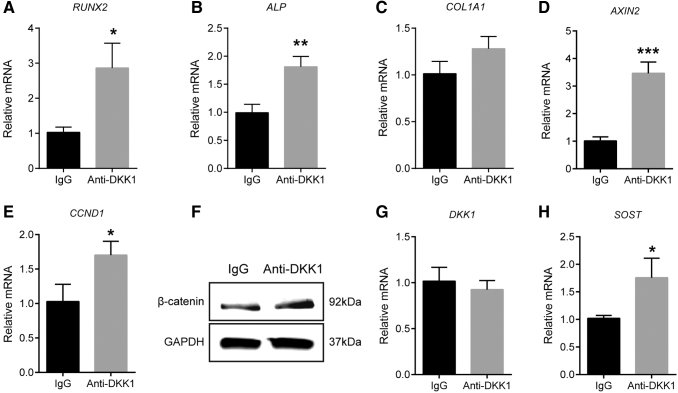
Overall, we observed an increase in ASC osteogenesis with anti-DKK1, but not anti-SOST treatment. To confirm and expand on this observation, we next assessed changes in osteogenic gene expression by qPCR with anti-DKK1 or isotype control treatment (Fig. 2A–C). Results showed a significant increase in osteogenic gene expression among anti-DKK1-treated samples in comparison to IgG isotype control. This included increased expression of the master osteogenic transcription factor RUNX2 (runt-related transcription factor 2, 1.8-fold increase, Fig. 2A) and the enzyme ALP (alkaline phosphatase, 82% increase, Fig. 2B). A trend toward an increase in the bone matrix encoding gene COL1A1 (collagen type I alpha 1) was also observed (26% increase, Fig. 2C).
FIG. 2.
Anti-DKK1 induces changes in osteogenic and Wnt signaling-related genes in human ASCs. (A–C) Gene expression during osteogenic differentiation with anti-DKK1 treatment for 3 days, including (A) RUNX2, (B) ALP, and (C) COL1A1. (D, E) Wnt signaling gene expression with anti-DKK1 (1 μg/mL) treatment for 3 days, including (D) AXIN2 and (E) CCND1. (F) Total β-catenin expression with anti-DKK1 treatment for 1 day by Western blot. (G, H) Gene expression of Wnt signaling antagonists with anti-DKK1 (1 μg/mL) treatment for 3 days, including (G) DKK1 and (H) SOST. All experiments performed with an appropriate isotype IgG control. Experiments performed in at least experimental and biological triplicate. Error bars represent one standard deviation. *P < 0.05; **P < 0.01; ***P < 0.001. ALP, alkaline phosphatase; AXIN2, axis inhibition protein 2; CCND1, cyclin D1; COL1A1, collagen type I alpha 1; RUNX2, runt-related transcription factor 2.
Next, we assessed gene expression of markers indicative of overall canonical Wnt signaling activity, including AXIN2 (axis inhibition protein 2) and CCND1 (cyclin D1) (Fig. 2D, E). Confirming bioactivity of anti-DKK1, both gene transcripts were more highly expressed among anti-DKK1-treated cells in comparison to isotype control (2.4-fold and 65% increase in AXIN2 and CCND1 transcripts, respectively). Furthermore, Western blot for total β-catenin expression confirmed activation of canonical Wnt signaling pathway after anti-DKK1 treatment (Fig. 2F).
Neutralization of a Wnt antagonist such as DKK1 may lead to compensatory changes, such as increased transcription of the same or other Wnt antagonists. Such compensatory changes have been reported with anti-DKK1 treatment in rodent models [33]. To investigate, we examined gene expression of both DKK1 and SOST after anti-DKK1 treatment. Results indicated that anti-DKK1 induced no change in DKK1 expression (Fig. 2G). In contrast, anti-DKK1 led to a significant increase in SOST transcript abundance (Fig. 2H, 72% increase in SOST transcripts).
Timing of DKK1 neutralization on ASC osteogenesis
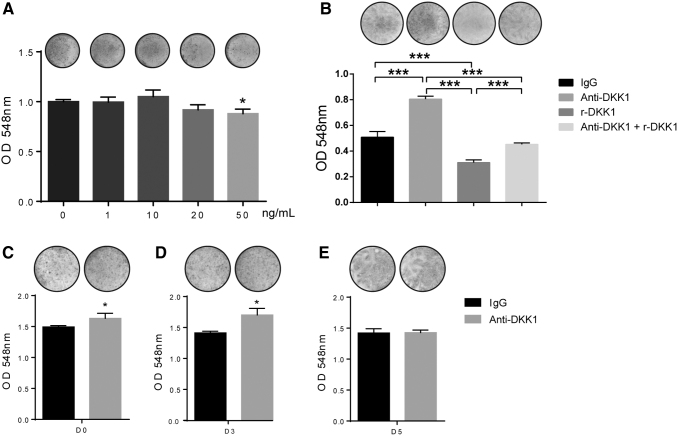
To confirm the anti-DKK1 neutralizing function further, we employed recombinant DKK1 to impair osteogenic differentiation in ASCs. Results demonstrated that DKK1 treatment decreased mineralization at a concentration of 50 ng/mL, but not at lower concentrations (Fig. 3A). Next, anti-DKK1 was supplemented with DKK1 during osteogenic differentiation conditions. In this study, anti-DKK1 rescued the impairment of osteogenesis induced by recombinant DKK1 (Fig. 3B).
FIG. 3.
Effects of DKK1 and timing of anti-DKK1 neutralization on ASC osteogenesis. (A) Alizarin red staining (above) and quantification (below) on day 7 of osteogenic differentiation with recombinant DKK1 (1–50 ng/mL). Whole well images shown. (B) Alizarin red staining (above) and quantification (below) on day 7 of osteogenic differentiation with DKK1 (50 ng/mL) with or without anti-DKK1 (2 μg/mL). (C–E) Effects of anti-DKK1 treatment beginning at different time points of osteogenic differentiation. (C) Anti-DKK1 treatment (1 μg/mL) initiated on day 0 of osteogenic differentiation, with alizarin red staining (above) and quantification (below) performed on day 7 of differentiation. (D) Anti-DKK1 treatment (1 μg/mL) initiated on day 3, with alizarin red staining (above) and quantification (below) performed on day 7 of differentiation. (E) Anti-DKK1 treatment initiated on day 5 (1 μg/mL), with alizarin red staining (above) and quantification (below) on day 7 of differentiation. All experiments performed with an appropriate isotype IgG control. Experiments performed in at least experimental and biological triplicate. Error bars represent one standard deviation. *P < 0.05; ***P < 0.001.
After baseline effects of anti-DKK1 treatment were determined, the effects of timing of DKK1 neutralization on ASC osteogenesis were assessed (Fig. 3C–E). Anti-DKK1 supplemented at early time points of differentiation (treatment beginning at either days 0 or 3) led to an increase in ASC osteogenic differentiation (Fig. 3C, D). This was quantified to determine a 9% and 20% increase in mineralization if anti-DKK1 treatment was initiated on days 0 and 3, respectively. In contrast, initiating anti-DKK1 treatment at the later time point of day 5 of differentiation had no significant effect on mineralization (Fig. 3E). This observation was concordant with the peak of expression levels of DKK1 during early osteogenic differentiation (see again Fig. 1A).
The expressions of basal DKK1 and SOST correlate with anti-DKK1-induced osteogenic differentiation in human ASCs
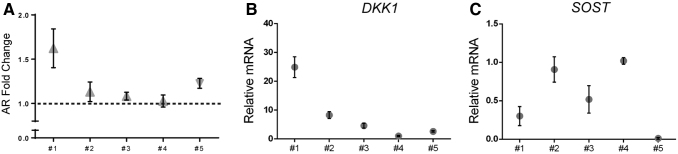
We observed an elevation of SOST expression after anti-DKK1 treatment, a phenomenon that potentially represented a compensatory cellular mechanism as has been demonstrated in other contexts [25,28]. Therefore, we examined the correlation of basal DKK1 and SOST expression with responsiveness to anti-DKK1 in terms of osteogenic differentiation within five distinct human ASC preparations (Fig. 4A–C).
FIG. 4.
Anti-DKK1-induced osteogenic differentiation in human ASCs is related to both basal DKK1 and SOST expression. (A) Fold change in alizarin red staining among anti-DKK1 versus IgG (1 μg/mL) within five distinct human ASC preparations. See Supplementary Table S1 for a description of patient demographics. (B) Relative baseline DKK1 expression among each ASC preparation, as determined by qPCR. (C) Relative baseline SOST expression among each ASC preparation, as determined by qPCR. Each data point represents a mean value, while error bars represent one standard deviation. qPCR, real-time polymerase chain reaction.
Some variability in responsiveness to anti-DKK1 was observed. Specifically, osteogenic differentiation was most prominently induced in patient samples #1 and 5, while it was less robust in patient samples #2–4 (Fig. 4A). Interestingly, high responsiveness of patient sample #1 correlated with high basal expression of DKK1 transcripts, as assessed by qPCR (Fig. 4B). Conversely, high SOST transcripts were observed in patient samples #2–4 (Fig. 4C), which showed comparatively lower responsiveness to anti-DKK1 treatment. Thus, high basal DKK1 and low basal SOST expression correlate with a pro-osteogenic effect of anti-DKK1 in human ASCs.
Discussion
In this study, anti-DKK1 treatment promoted ASC osteogenic differentiation. As expression levels of DKK1 peaked during early osteogenic differentiation, anti-DKK1 supplemented early, but not late, during osteogenic differentiation positively regulated osteoblast formation. Furthermore, the capacity of anti-DKK1 to enhance osteogenic differentiation varied between cell batches. Finally, anti-DKK1 led to increased transcript abundance of the Wnt inhibitor SOST, potentially representing a compensatory cellular mechanism.
ASCs that reside within vessel walls can accelerate bone regeneration [3–6]. ASCs have been used in both research and clinical settings [1,2]. Unfortunately, significant cell heterogeneity exists within the ASC population, including high numbers of nonstem cells, nonviable cells, and endothelial cells that may inhibit osteogenesis [9,11], which may result in low bone-forming efficacy and batch-to-batch variability.
Preclinical studies have shown that DKK1 neutralizing antibodies can stimulate bone formation [31–33]. Moreover, failed fracture healing (such as in human nonunion fractures) shows elevated levels of DKK1 among stromal cells of the fracture [36]. Also DKK1 was upregulated transiently in the early stages of adipogenesis, and knockdown of DKK1 can inhibit adipogenic differentiation of ASCs [39]. Thus, DKK1 enrichment among ASCs could be a plausible molecular mechanism for its poor bone forming efficacy for several reasons. However, heterogeneity in ASC varies from batch to batch, which may contribute to variable response to anti-DKK1.
Besides mesenchymal stem cell populations, various types of cell types express DKK1 and potentially respond to DKK1 neutralization [40]. Endothelial cells, in which the majority of DKK1 was expressed other than smooth muscle cells and macrophages, are commonly found in the aortic plaques [41]. DKK1 promotes human umbilical vein endothelial cell (HUVEC) apoptosis through activating JNK signaling and inhibiting canonical Wnt signaling, and then activating the IRE1α and eif2α/CHOP pathways [41]. In aortic endothelial cells, DKK1 can upregulate ALP enzyme activity and enhances mineralization by Smad activation [42]. Therefore, it is rational to speculate that the contamination of endothelial cells in the ASCs may have an adverse effect on osteogenesis induced by anti-DKK1.
Apart from endothelial cells, platelets have been found to be a major source of DKK1 in the circulatory system [43]. Platelet-derived DKK1 has been reported to be involved in the inflammation responding to tissue damage [43–45]. Neutralization of DKK1 attenuates neutrophil influx into the lungs during acute pulmonary inflammation [44]. DKK1 induces pathological type 2 (Th2) cell-mediated immune response through the MAPK and mTOR signaling pathway. Moreover pharmacological inhibition of DKK1 impaired Th2 cell cytokine production and leukocyte infiltration in house dust mite-induced asthma or Leishmania major infection models [45].
Moreover, DKK1 expression is elevated in serum, synovial tissue, and cartilage of arthritis models. DKK1 neutralization decreased osteoclast formation in the inflamed joint, but induces osteophyte formation [46,47]. Therefore, in an inflammatory environment, anti-DKK1 has potential to inhibit inflammation as well as to promote osteogenesis.
DKK1 and SOST are both Wnt inhibitors by inhibiting LRP5/6. The anabolic effects of bone were well established by blocking DKK1 or SOST [25,28,48,49]. However in some circumstances, DKK1, is inefficacious for building bone. For example, blocking DKK1cannot improve bone in adult estrogen-deficient rats [33]. Moreover, blocking either DKK1 or SOST could increase the other one expression and combined blocking of DKK1and SOST produced a synergistic effect on bone gain [25,49]. An engineered bispecific heterodimeric antibody (Hetero-DS), by which both SOST and DKK1 were targeted, has been established. Hetero-DS shows greater activity for bone repair comparing with either anti-DKK1 or anti-SOST alone, and it can robustly increase Wnt signaling target gene and markers of osteogenesis [49].
However, our results show that the dynamic regulation of SOST during osteogenic differentiation in ASCs is not high. Indeed, SOST is primarily considered to be an osteocyte-derived protein, rather than an MSC-derived protein [50,51]. This cell-specific expression, and/or lack of dynamic upregulation during ASC osteogenesis, may represent possible reasons for the nonresponsiveness of ASC to anti-SOST treatment. In addition, our results also show a potential compensatory mechanism between DKK1 and SOST, yet the molecular mechanism in ASCs is still not clear. However, one major determinant in anti-DKK1 efficacy in our study was the level of SOST transcripts in each cell preparation.
There are some limitations to this study. First, all human samples were only from female patients. Evidence suggests that ASC osteogenic differentiation potential differs on the basis of gender. Specifically, male-derived ASCs isolated from superficial adipose layer have greater capability of osteogenic differentiation than female-derived ASCs [52]. The mechanisms regulating ASC osteogenesis may differ to some degree between male and female donors.
Second, all donors had a narrow age range (42–57 years old). Age has clear effects on ASC osteogenic differentiation potential. For example, one research group described a distinct relationship between donors' age, ranging from 20 to 58 years old, and osteogenic potential of ASCs. Older donors, whose ages range from 40 to 49, exhibited a decrease in matrix calcification compared to younger donors. However, donors over 50 years of age showed an increase of matrix calcification potential compared to others [53]. This finding suggests likewise that age of donor may result in different underlying regulatory mechanisms in terms of ASC osteogenic capability.
In conclusion, DKK1 represents a targetable “molecular brake” on the osteogenic differentiation of ASCs, and that “release” of this brake by use of neutralizing anti-DKK1 antibody treatment will “rescue” the poor bone-forming efficacy of ASCs.
In future studies, the combination of anti-DKK1 antibody with ASCs may be applied in vivo for bone regeneration. In future studies, it will be important to determine the most appropriate mode of delivery for anti-DKK1 treatment. Both local anti-DKK1 implantation or systemic anti-DKK1 treatment are potential modalities for DKK1 neutralization. In addition, anti-DKK1 treatment in combination with cell therapies may be particularly valuable in certain other clinical contexts, for example, in patients with senile osteoporosis, or even in patients with multiple myeloma, in which levels of DKK1 are known to be increased [54].
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health, Department of Defense, or U.S. Army. We thank the JHU microscopy core facility, JHMI deep sequencing and microarray core facility, and Hao Zhang within the JHU Bloomberg Flow Cytometry and Immunology Core.
Author Disclosure Statement
A.W.J. is a paid consultant for Novadip. This arrangement has been reviewed and approved by the JHU in accordance with its conflict of interests polices. A.W.J. receives funding for unrelated research from MTF Biologics and Novadip. B.P. is the inventor of perivascular stem cell-related patents held by the UC Regents. All other authors declare no conflicts of interest.
Funding Information
A.W.J. was supported by the NIH/NIAMS (R01 AR070773 and K08 AR068316), NIH/NIDCR (R21 DE027922), Department of Defense (W81XWH-18-1-0121, W81XWH-18-1-0336, and W81XWH-18-10613), American Cancer Society (Research Scholar Grant, RSG-18-027-01-CSM), the Maryland Stem Cell Research Foundation, and MTF Biologics. In addition, MTF Biologics donated reagents for the study.
Supplementary Material
References
- 1. Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, Hedrick MH, Berthold L and Howaldt HP (2004). Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 32:370–373 [DOI] [PubMed] [Google Scholar]
- 2. Cowan CM, Shi Y-Y, Aalami OO, Chou Y-F, Mari C, Thomas R, Quarto N, Contag CH, Wu B and Longaker MT (2004). Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22:560–567 [DOI] [PubMed] [Google Scholar]
- 3. Zuk P. (2013). Adipose-derived stem cells in tissue regeneration: a review. ISRN Stem Cells 12:822 [Google Scholar]
- 4. Grottkau BE and Lin Y (2013). Osteogenesis of adipose-derived stem cells. Bone Res 1:133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Müller AM, Mehrkens A, Schäfer DJ, Jaquiery C, Güven S, Lehmicke M, Martinetti R, Farhadi I, Jakob M, Scherberich A and Martin I (2010). Toward an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cells Mater 19:127–135 [DOI] [PubMed] [Google Scholar]
- 6. Cheung WK, Working DM, Galuppo LD and Leach JK (2010). Osteogenic comparison of expanded and uncultured adipose stromal cells. Cytotherapy 12:554–562 [DOI] [PubMed] [Google Scholar]
- 7. James AW, Zara JN, Corselli M, Askarinam A, Zhou AM, Hourfar A, Nguyen A, Megerdichian S, Asatrian G, et al. (2012). An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med 1:673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, MRitt JPF and van Milligen FJ (2006). Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8:166–177 [DOI] [PubMed] [Google Scholar]
- 9. Meury T, Verrier S and Alini M (2006). Human endothelial cells inhibit BMSC differentiation into mature osteoblasts in vitro by interfering with osterix expression. J Cell Biochem 98:992–1006 [DOI] [PubMed] [Google Scholar]
- 10. Clarkin CE, Garonna E, Pitsillides and AA Wheeler-Jones CPD (2008). Heterotypic contact reveals a COX-2-mediated suppression of osteoblast differentiation by endothelial cells: a negative modulatory role for prostanoids in VEGF-mediated cell: cell communication? Exp Cell Res 314:3152–3161 [DOI] [PubMed] [Google Scholar]
- 11. Rajashekhar G, Traktuev DO, Roell WC, Johnstone BH, Merfeld-Clauss S, Van Natta B, Rosen ED, March KL, and Clauss M (2008). IFATS collection: adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells 26:2674–2681 [DOI] [PubMed] [Google Scholar]
- 12. Xu J, Wang Y, Hsu CY, Gao Y, Meyers CA, Chang L, Zhang L, Broderick K, Ding C, et al. (2019). Human perivascular stem cell-derived extracellular vesicles mediate bone repair. Elife 8:e48191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levi B, Nelson ER, Li S, James AW, Hyun JS, Montoro DT, Lee M, Glotzbach JP, Commons GW and Longaker MT (2011). Dura mater stimulates human adipose-derived stromal cells to undergo bone formation in mouse calvarial defects. Stem Cells 29:1241–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwalie PC, Dong H, Zachara M, Russeil J, Alpern D, Akchiche N, Caprara C, Sun W, Schlaudraff KU, et al. (2018). A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559:103–108 [DOI] [PubMed] [Google Scholar]
- 15. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313 [DOI] [PubMed] [Google Scholar]
- 16. Ishimura D, Yamamoto N, Tajima K, Ohno A, Yamamoto Y, Washimi O and Yamada H (2008). Differentiation of adipose-derived stromal vascular fraction culture cells into chondrocytes using the method of cell sorting with a mesenchymal stem cell marker. Tohoku J Exp Med 216:149–156 [DOI] [PubMed] [Google Scholar]
- 17. Chung MT, Liu C, Hyun JS, Lo DD, Montoro DT, Hasegawa M, Li S, Sorkin M, Rennert R, et al. (2013). CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 19:989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davies OG, Cooper PR, Shelton RM, Smith AJ and Scheven BA (2015). Isolation of adipose and bone marrow mesenchymal stem cells using CD29 and CD90 modifies their capacity for osteogenic and adipogenic differentiation. J Tissue Eng 6:2041731415592356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, et al. (2012). Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med 1: 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Q, Qi LJ, Guo ZK, Li H, Zuo HB and Li NN (2013). CD73+ adipose-derived mesenchymal stem cells possess higher potential to differentiate into cardiomyocytes in vitro. J Mol Histol 44:411–422 [DOI] [PubMed] [Google Scholar]
- 21. Baron R and Kneissel M (2013). WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192 [DOI] [PubMed] [Google Scholar]
- 22. Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, et al. (2002). Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, et al. (2008). Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869 [DOI] [PubMed] [Google Scholar]
- 24. Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, et al. (2010). Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25:948–959 [DOI] [PubMed] [Google Scholar]
- 25. Witcher PC, Miner SE, Horan DJ, Bullock WA, Lim KE, Kang KS, Adaniya AL, Ross RD, Loots GG and Robling AG (2018). Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight 3:e98673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cruciat CM and Niehrs C (2013). Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb Perspect Biol 5:a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, et al. (2001). LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523 [DOI] [PubMed] [Google Scholar]
- 28. Morvan F, Boulukos K, Clément-Lacroix P, Roman SR, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, . (2006). Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934–945 [DOI] [PubMed] [Google Scholar]
- 29. Morse A, Cheng TL, Schindeler A, McDonald MM, Mohanty ST, Kneissel M, Kramer I and Little DG (2018). Dkk1 KO mice treated with sclerostin antibody have additional increases in bone volume. Calcif Tissue Int 103:298–310 [DOI] [PubMed] [Google Scholar]
- 30. McDonald MM, Morse A, Schindeler A, Mikulec K, Peacock L, Cheng T, Bobyn J, Lee L, Baldock PA, et al. (2018). Homozygous Dkk1 knockout mice exhibit high bone mass phenotype due to increased bone formation. Calcif Tissue Int 102:105–116 [DOI] [PubMed] [Google Scholar]
- 31. Glantschnig H, Scott K, Hampton R, Wei N, McCracken P, Nantermet P, Zhao JZ, Vitelli S, Huang L, et al. (2011). A rate-limiting role for Dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J Pharmacol Exp Ther 338:568–578 [DOI] [PubMed] [Google Scholar]
- 32. Glantschnig H, Hampton RA, Lu P, Zhao JZ, Vitelli S, Huang L, Haytko P, Cusick T, Ireland C, et al. (2010). Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem 285:40135–40147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li X, Grisanti M, Fan W, Asuncion FJ, Tan H-L, Dwyer D, Han C-Y, Yu L, Lee J, et al. (2011). Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J Bone Miner Res 26:2610–2621 [DOI] [PubMed] [Google Scholar]
- 34. Jin H, Wang B, Li J, Xie W, Mao Q, Li S, Dong F, Sun Y, Ke HZ, et al. (2015). Anti-DKK1 antibody promotes bone fracture healing through activation of β-catenin signaling. Bone 71:63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JB, Leucht P, Lam K, Luppen C, Ten Berge D, Nusse R and Helms JA (2007). Bone regeneration is regulated by Wnt signaling. J Bone Miner Res 22:1913–1923 [DOI] [PubMed] [Google Scholar]
- 36. Bajada S, Marshall MJ, Wright KT, Richardson JB and Johnson WEB (2009). Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone 45:726–735 [DOI] [PubMed] [Google Scholar]
- 37. Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R and Alman BA (2007). Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med 4:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Komatsu DE, Mary MN, Schroeder RJ, Robling AG, Turner CH and Warden SJ (2010). Modulation of Wnt signaling influences fracture repair. J Orthop Res 28:928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park JR, Jung JW, Lee YS and Kang KS (2008). The roles of Wnt antagonists Dkk1 and sFRP4 during adipogenesis of human adipose tissue-derived mesenchymal stem cells. Cell Prolif 41:859–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chae WJ and Bothwell ALM (2019). Dickkopf1:an immunomodulatory ligand and Wnt antagonist in pathological inflammation. Differentiation 108:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di M, Wang L, Li M, Zhang Y, Liu X, Zeng R, Wang H, Chen Y, Chen W, Zhang Y and Zhang M (2017). Dickkopf1 destabilizes atherosclerotic plaques and promotes plaque formation by inducing apoptosis of endothelial cells through activation of ER stress. Cell Death Dis 8:e2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng SL, Shao JS, Behrmann A, Krchma K and Towler DA (2013). Dkk1 and Msx2-Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol 33:1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, et al. (2009). Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol 9:1228–1234 [DOI] [PubMed] [Google Scholar]
- 44. Guo Y, Mishra A, Howland E, Zhao C, Shukla D, Weng T and Liu L (2015). Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood 126:2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chae WJ, Ehrlich AK, Chan PY, Teixeira AM, Henegariu O, Hao L, Shin JH, Park JH, Tang WH, et al. (2016). The Wnt antagonist Dickkopf-promotes pathological type 2 cell-mediated inflammation. Immunity 44:246–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, et al. (2007). Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13:156–163 [DOI] [PubMed] [Google Scholar]
- 47. Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ and Shaughnessy JD (2009). The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 113:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kamiya N. (2012). The role of BMPs in bone anabolism and their potential targets SOST and DKK1. Curr Mol Pharmacol 5:153–163 [DOI] [PubMed] [Google Scholar]
- 49. Florio M, Gunasekaran K, Stolina M, Li X, Liu L, Tipton B, Salimi-Moosavi H, Asuncion FJ, Li C, et al. (2016). A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun 7:11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Bezooijen RL, Roelen BAJ, Visser A, Van Der Wee-Pals L, De Wilt E, Karperien M, Hamersma H, Papapoulos SE, Ten Dijke and C P Löwik WGM (2004). Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weivoda MM, Youssef SJ and Oursler MJ (2017). Sclerostin expression and functions beyond the osteocyte. Bone 96:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aksu AE, Rubin JP, Dudas JR and Marra KG (2008). Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg 60:306–322 [DOI] [PubMed] [Google Scholar]
- 53. Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P and Zuk P (2009). The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med 3:290–301 [DOI] [PubMed] [Google Scholar]
- 54. Béréziat V, Mazurier C, Auclair M, Ferrand N, Jolly S, Marie T, Kobari L, Toillon I, Delhommeau F, et al. (2019). Systemic dysfunction of osteoblast differentiation in adipose-derived stem cells from patients with multiple myeloma. Cells 8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.