Abstract
Graves’ orbitopathy (GO) is characterised in early stages by orbital fibroblast inflammation, which can be aggravated by oxidative stress and often leads to fibrosis. Protein tyrosine protein 1B (PTP1B) is a regulator of inflammation and a therapeutic target in diabetes. We investigated the role of PTP1B in the GO mechanism using orbital fibroblasts from GO and healthy non-GO subjects. After 24 hours of transfection with PTPN1 siRNA, the fibroblasts were exposed to interleukin (IL)-1β, cigarette smoke extract (CSE), H2O2, and transforming growth factor (TGF)-β stimulations. Inflammatory cytokines and fibrosis-related proteins were analysed using western blotting and/or enzyme-linked immunosorbent assay (ELISA). Reactive oxygen species (ROS) release was detected using an oxidant-sensitive fluorescent probe. IL-1β, tumor necrosis factor (TNF)-α, bovine thyroid stimulating hormone (bTSH), high-affinity human stimulatory monoclonal antibody of TSH receptor (M22), and insulin-like growth factor-1 (IGF-1) significantly increased PTP1B protein production in GO and non-GO fibroblasts. PTPN1 silencing significantly blocked IL-1β-induced inflammatory cytokine production, CSE- and H2O2-induced ROS synthesis, and TGF-β-induced expression of collagen Iα, α-smooth muscle actin (SMA), and fibronectin in GO fibroblasts. Silencing PTPN1 also decreased phosphorylation levels of Akt, p38, and c-Jun N-terminal kinase (JNK) and endoplasmic reticulum (ER)-stress response proteins in GO cells. PTP1B may be a potential therapeutic target of anti-inflammatory, anti-oxidant and anti-fibrotic treatment of GO.
Introduction
Graves’ orbitopathy (GO) is an orbital involvement of autoimmune thyroid disease; it occurs in approximately 50% of Grave’s disease and 2% of chronic thyroiditis [1, 2]. Clinical manifestations include lid swelling, lid retraction, proptosis, and ocular movement limitations; 3–5% of patients have a severe form, including pain, inflammation, and compressive optic neuropathy [1–3]. Orbital fibroblasts dysregulated by autoantibodies to thyrotropin receptor and IGF-1 receptor (IGF-1R) play a key role in the pathogenesis of GO. Furthermore, orbital fibroblasts differentiate into adipocytes, accumulating fatty tissue and producing hyaluronan, which leads to the typical findings of GO [3, 4]. Mononuclear cells, such as macrophages, T cells, and B cells, infiltrating the orbital tissue orchestrate the pathogenic inflammatory processes; moreover, ROS have been found to aggravate GO [5]. As inflammation in the orbital tissue is the primary aetiology of GO, glucocorticoids have been used as first-line treatment; however, their systemic complications have prompted researchers to explore alternative treatment options.
Protein tyrosine phosphatase 1B (PTP1B), encoded by the PTPN1 gene, is a ubiquitously expressed non-receptor protein tyrosine phosphatase enzyme that dephosphorylates tyrosine-phosphorylated proteins. It is expressed in multiple tissues, including the skeletal muscle, liver, adipose tissue, and brain [6]. PTP1B is a negative regulator of the leptin and insulin signalling pathways. It has been investigated as a therapeutic agent in diabetes and obesity following observations that mice with whole-body deletion of PTP1B exhibited increased insulin sensitivity and reduced weight gain when reared on a high-fat diet [7, 8]. PTP1B is also known to be involved in immune cell signalling [9, 10] by regulating cytokines via dephosphorylation of janus kinase (JAK)2, signal transducer and activator of transcription (STAT)5, and tyrosine kinase (TYK)2 [11, 12]. However, depending on the cell or tissue type, PTP1B displays different specific roles; for example, PTP1B knockout (KO) induced no change in inflammation in neuronal cells but resulted in upregulation of inflammation in B cells [13].
As the role of PTP1B in GO has not yet been studied, we characterised the effect of PTP1B in Graves’ orbital fibroblasts in response to proinflammatory and oxidative stress challenges, which simulate the main pathogenesis of GO.
Materials and methods
Reagents and chemicals
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin, and gentamycin were purchased from Hyclone Laboratories, Inc. (Logan, UT, USA). Recombinant human IL-1β, TNF-α and IGF-1 were purchased from R&D Systems (Minneapolis, UT, USA). M22 was purchased from RSR Ltd. (Llanishen, Cardiff, UK) and bTSH was obtained from Genzyme Corporation (Cambridge, MA, USA). TGF-β and antibodies for PTP1B, phosphorylated (p)-Akt, total (t)-Akt, p-extracellular signal-related kinase (ERK), t-ERK, p-p38, t-p38, p-JNK, t-JNK, cyclooxygenase (Cox)-2, intercellular adhesion molecule (ICAM)-1, p-nuclear factor (NF)-κB, t-NF-κB, binding immunoglobulin protein (BiP), p-eukaryotic translation initiation factor (eIF)2α, t-eIF2α, p-inositol-requiring enzyme (IRE)1α, t-IRE1α, p-protein kinase R-like endoplasmic reticulum kinase (PERK), t-PERK, activating transcription factor (ATF)4, ATF6α, and C/EBP-homologous protein (CHOP) were obtained from Cell Signaling Technology (Beverly, MA, USA), the antibody for fibronectin was obtained from BD Bioscience (Franklin Lake, NJ, USA), and the α-SMA antibody was obtained from Sigma-Aldrich (Saint Louis, MO, USA). collagen Iα, and anti–β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Tissue and cell preparation
Orbital adipose tissues were harvested from surgical by-products during orbital decompression surgery in 4 patients with GO (3 males, 1 female; age 37–55 years). At the time of the surgery, patients were in a stable euthyroid state and their clinical activity scores (CAS) were 3 or less. Normal control adipose tissues were obtained from 3 patients with no history of GO (1 male, 2 females; age 36–52 years) during lid blepharoplasty (Table 1). All patients provided written informed consent. This study was approved by the institutional review board of Severance Hospital, Yonsei University College of Medicine (Seoul, Korea) and followed the tenets of the Declaration of Helsinki.
Table 1. Clinical information of patient samples used in this in vitro study.
| Age (years) | Sex | CAS | Smoker | Duration of GO (years) | Proptosis R/L (mm) | Surgery performed |
|---|---|---|---|---|---|---|
| GO patients | ||||||
| 37 | F | 3/7 | N | 2.0 | 21/21 | Decompression |
| 42 | M | 1/7 | Y | 2.1 | 21/21 | Decompression |
| 52 | M | 0/7 | N | 4.2 | 21/20 | Decompression |
| 55 | M | 1/7 | N | 3.0 | 23/23 | Decompression |
| Non-GO control subjects | ||||||
| 52 | F | n/a | N | n/a | n/a | Lower lid blepharoplasty |
| 36 | M | n/a | N | n/a | n/a | Upper lid blepharoplasty |
| 51 | F | n/a | N | n/a | n/a | Upper lid blepharoplasty |
Abbreviations: GO, Graves’ orbitopathy; CAS, clinical activity scores; Y, yes; N, No; n/a; not applicable; F, female; M, male; R, right eye; L, left eye.
Orbital fibroblasts were cultured according to the method used in our previous study [14]. Briefly, the tissue was minced and placed in DMEM:F12 (1:1) containing 20% FBS, penicillin (100 U/mL), and gentamicin (20 μg/mL). Following fibroblast growth, the cells were passaged serially treating them with trypsin/ethylenediaminetetraacetic acid (EDTA). Strains were stored in liquid nitrogen. Only strains between the 3rd and 6th passages were used for experiments.
Silencing of PTP1B
Small interfering RNA (siRNA) designed to silence the PTP1B gene (PTPN1) and negative control #1 siRNA were purchased from Ambion/Applied Biosystems (s11507; Ambion, Austin, Texas, USA). Transfection was performed with Lipofectamine RNAiMAX (Invitrogen) according to the instructions of the supplier. Cells (1 × 105 cells/well) were supplemented with 10% FBS and antibiotics and maintained for a 24-hour period.
Western blot
Orbital fibroblasts treated with each reagent were washed with phosphate-buffered saline (PBS) and lysed in cell lysis buffer on ice in accordance with the methods described in our previous study [14]. Cell lysates were centrifuged to produce homogenous cell fractions, which were then boiled in buffer. Proteins were resolved by 10% SDS-PAGE, transferred to polyvinylidene fluoride membranes (Immobilon; Millipore Corp., Billerica, MA, USA), and treated with primary antibodies overnight in Tris-buffered saline and Tween 20 (TBST). Immunoreactive bands were detected with horseradish peroxidase and its secondary antibody. The bound peroxidase was visualised by chemiluminescence (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA) and exposure to X-ray film (Amersham Pharmacia Biotech, Inc.). Protein was quantified via densitometry and normalised to the level of β-actin in each sample.
Enzyme-linked immunosorbent assay
To quantify the levels of IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1), supernatants collected from cell culture were evaluated using an ELISA kit (R&D system, Minneapolis, UT, USA) according to the manufacturer’s protocol. Absorbance of the samples was measured at 450 nm using microplate reader (Molecular Devices, Sunnyvale, CA, USA) and the percentage of binding was calculated.
Real-time PCR
RNA was extracted from orbital tissues, which were homogenized with a tissue homogenizer (Precellys® 24; Bertin Instruments, Montigny-le-Bretonneux, France) using a Precellys lysing kit (Bertin Instruments) with TriZol (Invitrogen, Carlsbad, CA, USA) and cDNA was synthesized. PCR was performed using TaqMan universal PCR master mix in an ABI 7300 real-time PCR thermocycler (Applied Biosystems, Carlsbad, CA, USA). Primers for Toll-like receptor (TLR) 2, 4, and 9 were as follows: TLR2; 5’-GAC TTC TCC CAT TTC CGT CT-3’ (forward), 5’-CAG GTA GGT CTT GGT GTT CA-3’ (reverse), TLR4; 5’-GAC CTT TCC AGC AAC AAG ATT C-3’ (forward), 5’-GAG AGA TTG AGT AGG GGC ATT T-3’ (reverse), TLR9; 5’-GGC TGG TAT AAA AAT CTT ACT TCC TC-3’ (forward), 5’-CAC ACT CGA GGT CCC TTC C-3’ (reverse); these primers were designed using Applied Biosystems software and GAPDH were No. Hs99999905_m1. The results were expressed using the 2−ΔΔCt method, as relative exponential changes of threshold cycle (Ct) relative to the control group. The amplified band was quantified through densitometry and normalized against GAPDH. RT-PCR was performed in duplicate in three cells from different individuals.
Intracellular ROS measurement
CSE and H2O2 were used to induce ROS production as previously reported [15]. CSE was prepared from two commercially available cigarettes containing 8.0 mg tar and 0.7 mg nicotine (Marlboro 20 class A; Philip Morris Korea, Inc., Seoul, Korea) as described previously [15]. Here, 2% CSE and 200 μM H2O2 were used to generate oxidative stress in cells without reducing cell viability. Cells (1 × 105 cells/well) were exposed to oxidative stressors, washed with PBS, and incubated with the oxidant-sensitive fluorescent probe 5-(and 6)-carboxy-2’,7’-dichloro-dihydrofluorescein diacetate (H2DCFDA; Invitrogen, Eugene, OR, USA) for 30 minutes. The cells were then trypsinised, washed, and resuspended in PBS. To measure fluorescence intensity, a flow cytometer (ELITE flow cytometer; Coulter Cytometry, Inc., Hialeah, FL, USA) was used, and fluorescent photos were taken using an IX71-F22PH inverted fluorescence microscope (Olympus Corp., Tokyo, Japan).
Cell viability and apoptosis assays
Cell viability after PTPN1 blockade was evaluated with a 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. Orbital fibroblasts from GO and non-GO patients with sicontrol or siPTPN1 transfection were seeded in 24-well culture plates (1 × 105 cells/well) and were treated with IL-1β (10 ng/mL) for 24 and 48 hours. After treatment, cells were washed and incubated with 5 mg/mL MTT solution for 3 hours at 37 °C and then stabilized in ice-cold isopropanol. Microplate reader (EL 340 Bio Kinetics Reader; Bio-Tek Instruments, Winooski, VT, USA) was used to measure the absorbance of the dye at 490 nm.
An annexing V-FITC kit (R&D systems, Minneapolis, MN, USA) was used to assess apoptotic cells with PTPN1 blockade. Cells were washed with PBS and incubated with annexin V labeled with FITC and propidium iodide (PI) for 15 minutes at room temperature. Apoptotic cells were analyzed using a fluorescence-activated cell sorter (FACS, Becton Dickinson-FACScalibur, Cockeysville, MD, USA). In total, 10,000 cells were excited at 488 nm and emission was measured at 515–545 nm and 600 nm to assess FITC and PI, respectively.
Statistical analysis
IBM SPSS Statistics for Windows v 20.0 (IBM Corp., Armonk, NY) was used for statistical analyses. All experiments were performed in duplicate on three samples from different individuals, and the results are expressed as the mean values ± standard deviation. The Mann–Whitney U-test and Kruskal–Wallis test were used for nonparametric data, and the Kolmogorov–Smirnov test was used for data that was not normally distributed. A p-value less than 0.05 was considered statistically significant.
Results
TSH and IGF-1 increased PTP1B production in orbital fibroblasts
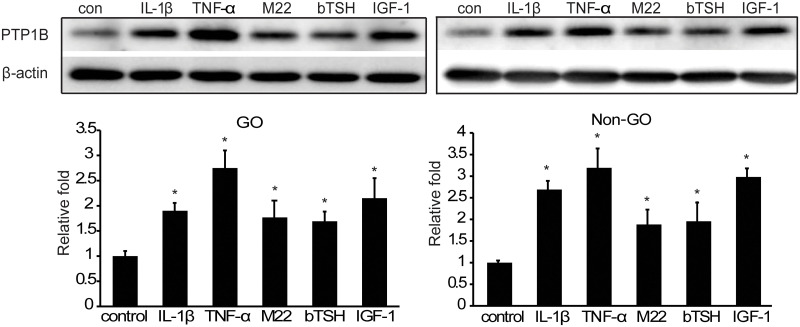
Fibroblasts were stimulated with IL-1β (10 ng/mL) or TNF-α (10 ng/mL) for 48 hours to evaluate PTP1B expression changes in response to inflammatory stimulation in GO and non-GO cells, as previously reported [14, 16, 17]. We also treated cultures with IGF-1 (100 ng/mL), M22 (10 ng/mL), or bTSH (2.5 mU/mL). PTP1B protein production was increased significantly by all these stimulants in both GO and non-GO cells (p<0.05) (Fig 1).
Fig 1. PTP1B protein expression stimulated by proinflammatory cytokines, TSH Receptor (TSHR), and IGF-1R ligands.
Orbital fibroblasts cultured from GO and non-GO tissues were treated with IL-1β (10 ng/mL), TNF-α (10 ng/mL), M22 (10 ng/mL), bTSH (2.5 mU/mL), or IGF-1 (100 ng/mL) for 48 hours. Cell lysates were subjected to western blot. IL-1β, TNF-α, M22, bTSH, and IGF-1 significantly increased the expression of PTP1B protein in both GO and non-GO fibroblasts. Experiments were performed in three GO and non-GO cells from different individuals. Data in the columns indicate the mean density ratio ± SD by densitometry normalised to the level of β-actin in the same sample. Representative gel images are shown. Differences between treated and untreated cells are indicated (*p<0.05). See S3 Fig for uncropped blots.
PTP1B inhibition reduces IL-1β-induced inflammation in GO orbital fibroblasts
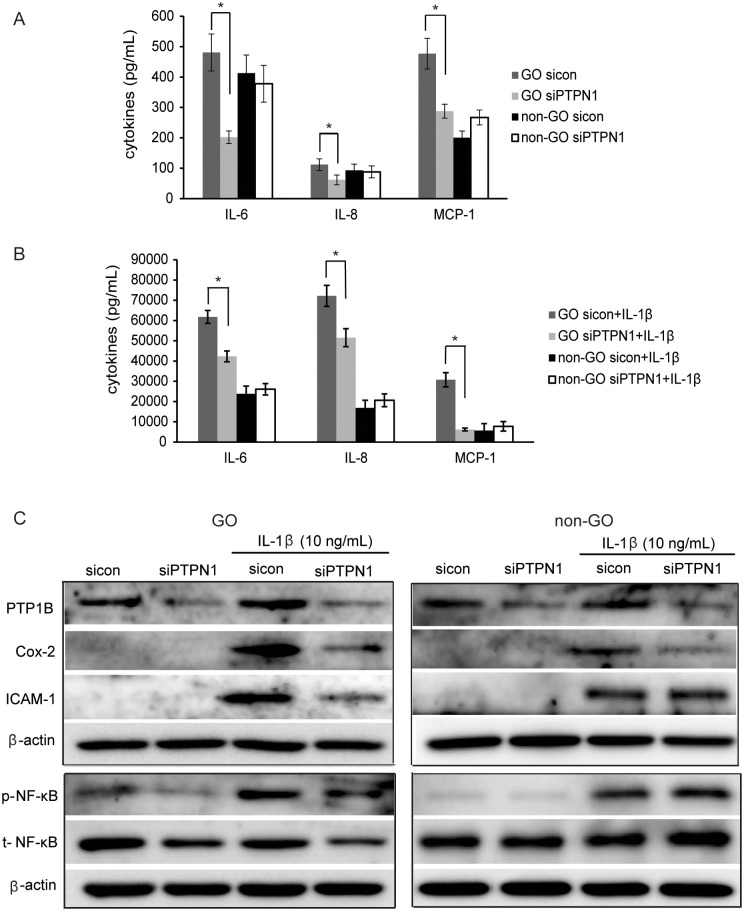
To clarify the role of PTP1B in inflammatory stimulation, PTPN1 was silenced via transfection of PTPN1-targeting siRNA (siPTPN1); silencing efficiency of siPTPN1 compared with sicontrol (sicon in figures) was verified using western blot. We then treated PTPN1-silenced GO and non-GO cells with IL-1β (10 ng/mL) for 48 hours and measured the production of pro-inflammatory cytokines, IL-6, IL-8 and MCP-1 using ELISA. GO cells secreted more proteins of IL-6, IL-8 and MCP-1 than non-GO cells under basal and IL-1β stimulated condition. Down-regulation of PTP1B by siPTPN1 inhibited the secretion of IL-6, IL-8, and MCP-1 in GO cells with or without IL-1β (*p<0.05), but not in non-GO cells (Fig 2A and 2B). In western blot analyses, IL-1β-induced Cox-2 and ICAM-1 protein production was reduced in siPTPN1-treated GO cells and only Cox-2 expression was attenuated in siPTPN1-treated non-GO cells (Fig 2C). Stimulation of fibroblasts with IL-1β (10 ng/mL) for 1 hour increased the p-NF-κB production; however, this upregulation was suppressed by PTPN1 silencing in GO cells.
Fig 2. Effect of PTP1B suppression on proinflammatory cytokine production.
After transfection of GO and non-GO fibroblasts with siPTPN1 for 24 hours, cells were treated with IL-1β (10 ng/mL) for 1 hour or 48 hours for the evaluation of NF-κB or inflammatory cytokine production, respectively. Experiments were performed in three GO and non-GO cells from different individuals. In sicontrol cells and siPTPN1 cells, IL-6, IL-8, and MCP-1 protein release was analysed under basal condition (A) and after 48 hours of IL-1β (10 ng/mL) stimulation (B) using ELISA (*p<0.05, sicontrol versus siPTPN1 cells). (C) Protein expression levels of ICAM-1, Cox-2, and p-NF-κB in sicontrol and siPTPN1 cells with or without IL-1β (10 ng/mL) stimulation were evaluated using western blot analysis. Representative bands are shown. See S3 Fig for uncropped blots.
TLRs participate in autoimmune diseases [18] and TLR-related signaling activates NF-κB to secrete inflammatory cytokines [19]. After transfection of GO and non-GO fibroblasts with siPTPN1 for 24 hours, TLR2, 4, and 9 mRNA expression levels were identified through RT-PCR analysis. TLR2, 4, and 9 were suppressed through PTPN1 inhibition in GO fibroblasts. In non-GO fibroblasts, TLR4 and 9 were suppressed through PTPN1 inhibition (*p<0.05) (S1 Fig).
PTP1B inhibition reduces ROS generation upon CSE and H2O2 stimulation
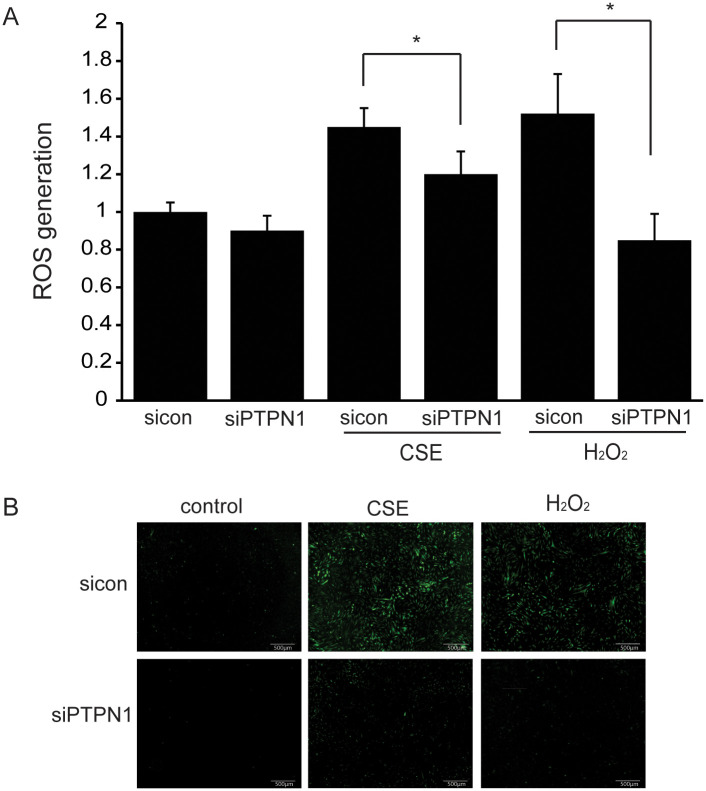
GO orbital fibroblasts were treated with 2% CSE or 200 μM H2O2 for 30 minutes. In PTPN1-silenced GO cells, CSE- or H2O2-induced intracellular ROS generation was reduced significantly as detected by H2DCFDA (*p<0.05) (Fig 3A and 3B).
Fig 3. Effect of PTP1B suppression on CSE- or H2O2-induced intracellular ROS generation.
GO cells were transfected with sicontrol or siPTPN1 for 24 hours. Cells were pretreated with H2DCFDA for 30 minutes in all GO fibroblast groups. Then, cells were exposed to 2% CSE or 200 μM H2O2 for 30 minutes. Experiments were performed using three cells from different individual samples. (A) Intracellular ROS generation was quantified by flow-cytometry with H2DCFDA. The bar graphs show mean ratios ± SD relative to the sicontrol without CSE or H2O2 treatment. (*p<0.05, sicontrol versus siPTPN1) (B) ROS production was observed using an inverted fluorescence microscope at 40x magnifications.
PTP1B suppression results in an anti-fibrotic effect
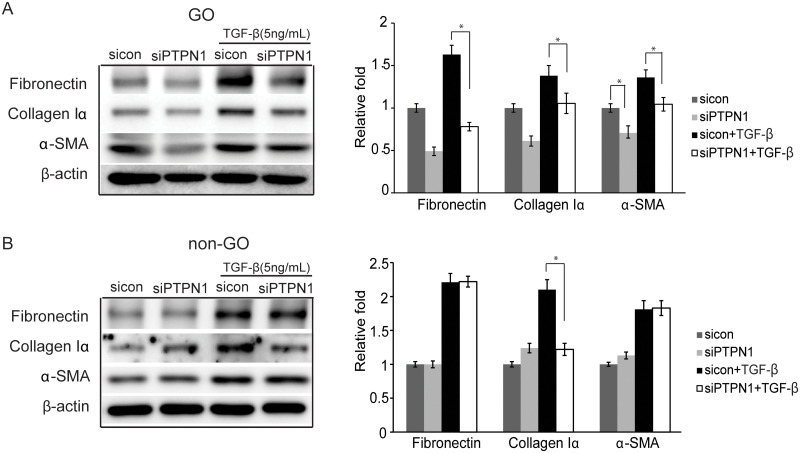
We also investigated whether PTP1B suppression has an effect on the expression of fibrosis-related proteins. Fibronectin, collagen Iα, and α-SMA protein expression was evaluated after 24 hours of TGF-β (5 ng/mL) stimulation in sicontrol cells and siPTPN1 cells. Enhanced expression of fibronectin, collagen Iα, and α-SMA following TGF-β treatment was significantly inhibited in the siPTPN1-transfected GO cells (*p<0.05) (Fig 4A). In non-GO cells, only collagen Iα was inhibited after PTPN1 blocking (*p<0.05) (Fig 4B).
Fig 4. Effect of PTP1B suppression on the fibrosis of orbital fibroblasts.
Orbital fibroblasts from GO and non-GO patients transfected with sicontrol or siPTPN1 for 24 hours were exposed to TGF-β (5 ng/mL) for 24 hours. Cell lysates of GO fibroblasts (A) and non-GO fibroblasts (B) were subjected to western blot analysis of fibronectin, collagen Iα, and α-SMA protein expression. Experiments were performed in three GO cell strains and non-GO cell strains taken from different individuals. Data in the columns indicate the mean density ratio ± SD by densitometry normalised to the level of β-actin in the same sample. Representative gel images are shown. Differences between sicontrol and siPTPN1 are indicated (*p<0.05). See S3 Fig for uncropped blots.
Role of PTP1B suppression in ER stress
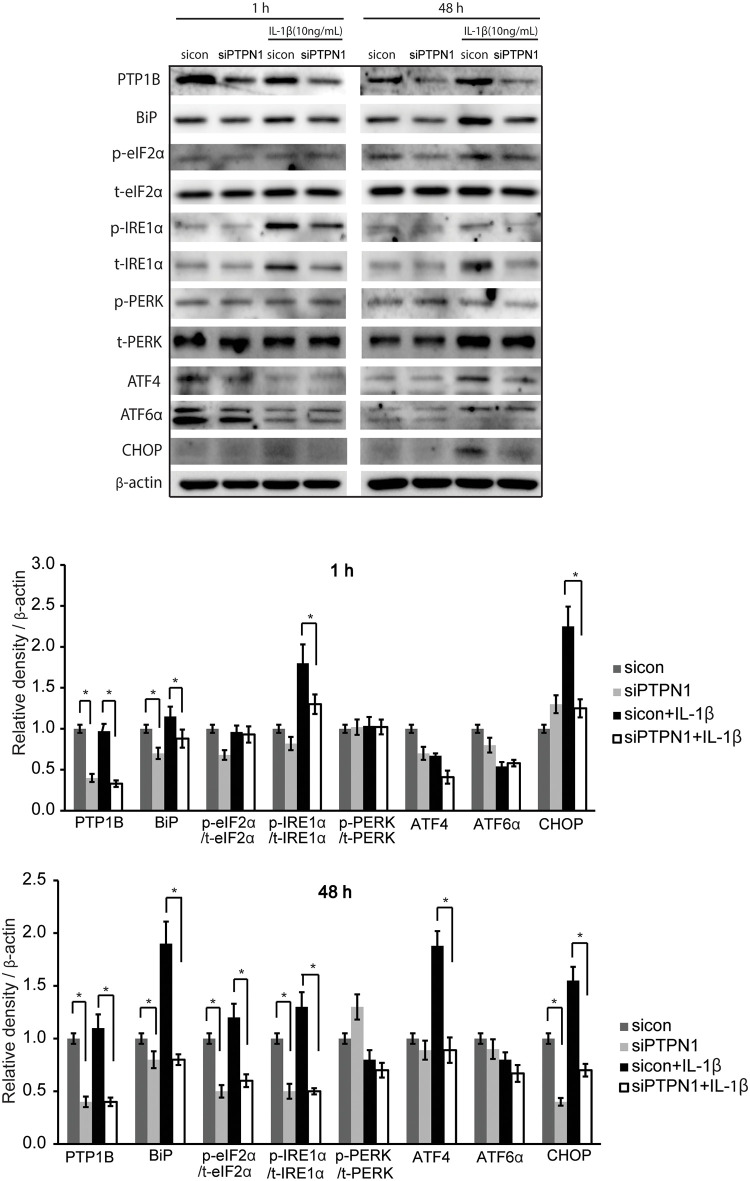
PTP1B is a negative regulator of insulin signalling and is localised on the ER membrane. ER stress was induced in GO cells by treatment with IL-1β (10 ng/mL) for 1 hour and 48 hours. Silencing of PTPN1 with siRNA significantly suppressed the protein expression of BiP, p-IRE1α, and CHOP induced by 1 hour IL-1β treatment as well as the protein expression of BiP, p-eIF2α, p-IRE1α, ATF4, and CHOP induced by 48 hours of IL-1β treatment in GO cells (*p<0.05). The expression levels of PERK and ATF6α were not changed by silencing of PTPN1 (Fig 5).
Fig 5. Role of PTP1B suppression on ER stress.
GO fibroblasts were transfected with sicontrol or siPTPN1 for 24 hours and exposed to IL-1β (10 ng/mL) for 1 hour or 48 hours. The GO fibroblasts were lysed and proteins related ER stress such as BiP, CHOP, ATF4, ATF6α, phosphorylated and total eIF2α, IRE1α, and PERK were analysed by western blot. Experiments were performed in three GO cells from different individuals. (A) Representative gel images are shown. (B,C) Data in the columns represent the mean density ratio ± SD by densitometry normalised to the level of β-actin in the same sample for 1 hour and 48 hours, respectively. Differences between sicontrol and siPTPN1 are indicated (*p<0.05). See S3 Fig for uncropped blots.
We investigated the effect of PTPN1 silencing on cell viability and apoptosis whether the suppression of ER stress protein by PTP1B suppression was associated with apoptosis [20, 21]. Upon IL-1β stimulation for 48 hours, GO fibroblasts displayed increased proliferative activity (*p<0.05). Treatment with IL-1β for 24 hours and 48 hours in both sicontrol and siPTPN1-transfected cells did not change cell viability in both GO and non-GO fibroblasts (S2A Fig). The sicontrol and siPTPN1-transfected cells were subjected to an Annexin V apoptosis assay after incubation for 24 and 48 hours. PTPN1 inhibition did not significantly increase apoptosis or necrosis in GO and non-GO fibroblasts (S2B Fig).
Effect of PTP1B suppression on signal pathway molecules
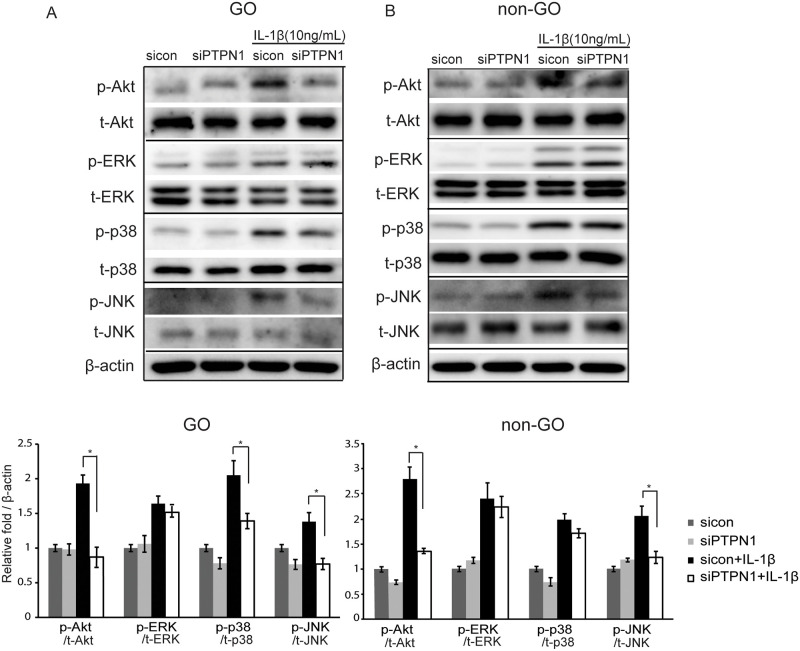
The phosphoinosidie 3-kinase (PI3K)-Akt pathway has been reported to be involved in GO [22, 23] and mitogen-activated protein kinase (MAPK) is well-known for its involvement in inflammatory diseases [24, 25]. After treatment of sicontrol fibroblasts with IL-1β (10 ng/mL) for 1 hour, Akt, ERK, p38, and JNK phosphorylation increased significantly in both GO and non-GO cells. However, when PTP1B was suppressed by siPTPN1 transfection, Akt, p38, and JNK phosphorylation was significantly reduced in GO cells (*p<0.05) (Fig 6A); Akt and JNK phosphorylation was inhibited in non-GO cells (*p<0.05) (Fig 6B).
Fig 6. Effect of PTP1B suppression on signalling pathways in orbital fibroblasts.
GO and non-GO cells were transfected with sicontrol or siPTPN1 for 24 hours and then exposed to IL-1β (10 ng/mL) for 1 hour. Phosphorylated and total Akt, ERK, p38, and JNK were determined by western blotting in GO fibroblasts (A) and non-GO fibroblasts (B). Experiments were performed in three GO and non-GO cells from different individuals. Representative bands are shown. Data in the columns indicate the mean density ratio ± SD by densitometry normalised to the level of β-actin in the same sample. Differences between sicontrol and siPTPN1 are indicated (*p<0.05). See S3 Fig for uncropped blots.
Discussion
In this study, we investigated the role of PTP1B in the pathogenesis of GO and evaluated whether suppression of PTP1B could reverse inflammation, oxidative stress, and fibrosis in GO cells. PTPN1 silencing significantly inhibited proinflammatory cytokine production, ROS generation induced by H2O2 or CSE, and the expression of proteins related to TGF-β-induced fibrosis in GO cells. Furthermore, PTPN1 silencing resulted in the amelioration of ER stress and suppression of the phosphorylation of Akt, p38, and JNK signalling pathway molecules without affecting cell viability. Protein tyrosine phosphorylation is regulated through coordination between protein tyrosine kinases and phosphatases; disturbance of the balance between the two enzymes results in aberrant tyrosine phosphorylation, which is implicated in several human diseases, including cancer, diabetes, and inflammation [26]. This study is, to our knowledge, the first to find that PTP1B suppression has a protective effect in vitro against GO pathologic mechanisms.
Recently, bidirectional crosstalk between TSHR and IGF-1R was demonstrated in GO fibroblasts. Here, we evaluated whether PTP1B was stimulated by M22, bTSH, IGF-1, IL-1β, or TNF-α in GO fibroblasts. There have been no previous studies linking TSHR and PTP1B signalling. In this study, we demonstrated that the treatment of orbital fibroblasts with TSHR or IGFR ligands upregulated the expression of PTP1B in both GO and non-GO cultures, although the mechanism is still unclear. IGF-1 activity is reported to be negatively controlled by the action of PTP1B [27] and inhibiting PTP1B is considered to have neuroprotective effects in diabetic retinopathy by modulating IGF-1 activity [28]. Proinflammatory cytokines have previously been shown to increase PTP1B levels, similar to our findings in orbital fibroblasts. TNF-α treatment increased PTP1B levels by 2- to 5-fold in adipocytes and hepatocytes; moreover, administration of TNF-α to mice resulted in elevated PTP1B mRNA levels in the adipose tissue, skeletal muscle, hypothalamic arcuate nucleus, and liver [29].
Orbital inflammation progresses by the infiltration of mononuclear cells, such as B-lymphocytes, macrophages, and especially T-lymphocytes, into the orbital tissue in GO [30, 31]. Furthermore, as previously reported [32], GO fibroblast was more inflammatory by nature and were sensitive to stimulation. Herein, compared to non-GO cells, GO fibroblasts displayed increased secretion of pro-inflammatory cytokines upon IL-1β stimulation and even in naïve conditions. PTPN1 silencing in GO cells reduced proinflammatory proteins, including IL-6, IL-8, MCP-1, Cox-2, ICAM1, and p-NF-κB. However, in non-GO fibroblasts, proinflammatory proteins except for Cox-2 were not significantly curtailed upon PTP1B suppression. PTP1B seems to play a specific role in GO cells, which serves as a positive regulator of inflammation.
There are still controversies regarding the role of PTP1B in the inflammatory mechanism. It has been shown that PTP1B plays a key role in converting dendritic cells to an active and mature state and activating T cells by displaying podosomes [33]. In PTP1B-deficient mice with induced colitis, inflammation was suppressed by the production of myeloid-derived suppressor cells, which have characteristic T cell-suppressant functions [34]. PTP1B blockage in microglial cells inhibited the production of lipopolysaccharide-induced proinflammatory molecules, such as IL-1β, TNF-α, Cox-2, and iNOS [35], and protected microglial cells against hypothalamic inflammation by activating the JAK2-STAT3 signalling pathway [36]. In contrast, there are several opposing reports insisting that PTP1B is a negative regulator of inflammation. PTP1B knockout in the spleen leukocytes of mice resulted in increased chemotaxis and transendothelial migration and exacerbated allergic inflammation [37]. Acute pancreatitis was enhanced in PTP1B knockout mice via increased production of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α [38]. PTP1B is ubiquitously expressed and performs different functions in various tissues. Therefore, modulation of PTP1B has different results depending on the cell type in vitro as well as the specific tissue in vivo [6, 11].
PTP1B has been reported to promote fibrosis in many other cell types. Genetic deletion of PTP1B and administration of PTP1B inhibitors reduced cardiac fibrosis in chronic heart failure mice [39]. In the endothelial cell-specific PTP1B KO mouse, cardiac fibrosis was reduced via elevation in caveolin-1, which inhibited ROS generation and suppressed TGF-β signalling [40]. In addition, PTP1B plays a regulatory role in liver fibrosis. PTP1B blocking using siRNA inhibited the proliferation and activation of hepatic stellate cells induced by TGF-β1 [41]. PTP1B-depleted hepatocytes showed resistance to TGF-β through the activation of NADPH oxidase 1 [42]; moreover, hepatic fibrosis in response to cholestatic liver injury was ameliorated by the same mechanism [43]. In our study on GO orbital fibroblasts, PTPN1 silencing down-regulated all fibrosis-related proteins induced by TGF-β, which was not evident in non-GO fibroblasts.
Systemic hyperthyroidism and T cell infiltration into orbital tissue leads to ROS production, and oxidative stress from cigarette smoking can aggravate GO severity by increasing T cell proliferation, adipogenesis, and glycosaminoglycan production in orbital fibroblasts [44–46]. Our study shows that PTPN1 silencing ameliorated ROS generation in both CSE- and H2O2-stimulated cells. Regarding oxidative stress, PTP1B deficiency in skeletal muscle cells resulted in the reduction of ceramide and ROS levels, whereas overexpression of PTP1B showed the opposite effect [47]. PTP1B deletion reduced mitochondrial dysfunction by increasing the expression of SIRT1 and reducing the phosphorylation of p65. PTP1B inhibition in mouse hepatocytes ameliorated acetaminophen-induced cell death and reduced GSH depletion, ROS generation, and activation of JNK and p38. PTP1B inhibition also enhanced the expression of the Nrf2-target gene heme oxygenase-1 (HO-1), suggesting that PTP1B plays a key regulatory role in the antioxidant system [48].
The changes in the phosphorylation level of multiple transcription factors following PTP1B inhibition in GO fibroblasts indicate that a more complex network of signalling pathways may exist. Kumar et al. discovered that an autoantibody to TSHR stimulated the PI3K/Akt pathway downstream and induced adipogenesis in orbital preadipocytes in GO [22]. Herein, PTP1B inhibition suppressed IL-1β-induced Akt, JNK phosphorylation in GO and non-GO fibroblasts. However, p38 MAPK phosphorylation was reduced by PTP1B inhibition only in GO fibroblasts. The p38 and JNK pathways, along with the MAPK pathway, are well-known for mediating the transcription and translation of inflammatory cytokines; moreover, they have been considered as targets of anti-inflammatory therapy [24, 25]. In several other disease-specific cell models, JNK and p38 phosphorylation was ameliorated by PTP1B inhibition [47, 48].
The downregulation of inflammation and its associated transcription factors with siPTPN1 may be related to a blocked ER stress response. It has been well-documented that the ER stress response, also known as the unfolded protein response (UPR), activates inflammation by inducing the NF-κB and JNK pathways [49, 50]. The upregulation of UPR molecules in autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel syndrome, has shown this relationship [50, 51]. PTP1B is located in the cytoplasmic region of the ER and plays an active role in the UPR. Gu et al. reported that PTP1B KO embryonic fibroblasts showed reduced IRE1 signalling, specific XBP-1 splicing, and JNK pathway activation; moreover, they were resistant to ER stress-induced apoptosis [52]. Similarly, liver-specific PTP1B deletion impaired the UPR by suppressing the phosphorylation of UPR molecules such as PERK, eIF2α, p38, JNK, and CHOP under a high fat-induced ER stress environment [53, 54]. Additionally, mouse myoblasts transfected with PTP1B siRNA exhibited attenuated phosphorylation of eIF2α and JNK1/2 [55]. Our results demonstrated that PTPN1 silencing blocks part of the UPR cascade by reducing the expression of proteins such as BiP, eIF2α, IRE1α, and CHOP. However, PTPN1 silencing did not affect apoptosis and cell viability.
To our knowledge, this study demonstrates for the first time that PTP1B mediates inflammatory reactions in GO fibroblasts. We found that PTPN1 silencing attenuated inflammation, fibrosis, and ROS generation, which could possibly be associated with the suppression of the ER stress response pathway and Akt, JNK, and p38 phosphorylation in GO cells. Inhibiting PTP1B might be a novel therapeutic option for the treatment of GO related to inflammatory and oxidative stressors. In addition, the inhibitory effect of PTP1B modulation on TGF-β induced fibrosis could also contribute to controlling fibrotic events in GO. Further studies are necessary to prove the in vivo effect of PTP1B inhibition in GO in clinically applications.
Supporting information
GO and non-GO fibroblasts were transfected with sicontrol or siPTPN1 for 24hours. TLR2, 4, and 9 expression levels were quantified with RT-PCR. Data in the columns indicate relative TLR mRNA fold of siPTPN1 cells normalised to sicontrol of each cell type (*p<0.05, sicontrol versus siPTPN1). Experiments were performed in three GO and non-GO fibroblasts from different individuals.
(TIF)
Orbital fibroblasts from GO and non-GO patients were transfected with sicontrol and siPTPN1 for 24 hours. (A) After the transfection, cells were cultured in different conditions (with or without IL-1β) for 24 hours and 48 hours. Then, the viability of the cells was assessed with MTT assay. Difference between sicontrol and sicontrol with IL-1β stimulation in GO was indicated (*p<0.05). (B) The transfected cells were subjected to Annexin V apoptosis assay after incubating for 24 hours and 48 hours. Assays were performed using three GO and non-GO cells from different individuals.
(TIF)
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT (NRF-2016M3A9E9941746). JSY received the grant (www.nrf.re.kr). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Garrity JA, Bahn RS. Pathogenesis of graves ophthalmopathy: implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006;142(1):147–153. 10.1016/j.ajo.2006.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiromatsu Y, Eguchi H, Tani J, Kasaoka M, Teshima Y. Graves’ ophthalmopathy: epidemiology and natural history. Intern Med. 2014;53(5):353–360. 10.2169/internalmedicine.53.1518 [DOI] [PubMed] [Google Scholar]
- 3.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726–738. 10.1056/NEJMra0905750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Coenen MJ, Scherer PE, Bahn RS. Evidence for Enhanced Adipogenesis in the Orbits of Patients with Graves’ Ophthalmopathy. J Clin Endocrinol Metab. 2004;89(2):930–935. 10.1210/jc.2003-031427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burch HB, Lahiri S, Bahn RS, Barnes S. Superoxide radical production stimulates retroocular fibroblast proliferation in graves’ ophthalmopathy. Exp Eye Res. 1997;65(2):311–316. 10.1006/exer.1997.0353 [DOI] [PubMed] [Google Scholar]
- 6.Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam Horm. 2013;91:405–424. 10.1016/B978-0-12-407766-9.00017-1 [DOI] [PubMed] [Google Scholar]
- 7.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–1548. 10.1126/science.283.5407.1544 [DOI] [PubMed] [Google Scholar]
- 8.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20(15):5479–5489. 10.1128/mcb.20.15.5479-5489.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adachi M, Sekiya M, Arimura Y, Takekawa M, Itoh F, Hinoda Y, et al. Protein-tyrosine phosphatase expression in pre-B cell NALM-6. Cancer Res. 1992;52(3):737–740. [PubMed] [Google Scholar]
- 10.Yi T, Cleveland JL, Ihle JN. Identification of novel protein tyrosine phosphatases of hematopoietic cells by polymerase chain reaction amplification. Blood. 1991;78(9):2222–2228. [PubMed] [Google Scholar]
- 11.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276(51):47771–47774. 10.1074/jbc.C100583200 [DOI] [PubMed] [Google Scholar]
- 12.Aoki N, Matsuda T. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J Biol Chem. 2000;275(50):39718–39726. 10.1074/jbc.M005615200 [DOI] [PubMed] [Google Scholar]
- 13.Samantha LS, Martina P, Cristina MG, Mirela D. Protein Tyrosine Phosphatase 1B (PTP1B in the immune system. Inflammation & Cell Signaling. 2015;2(2):e965. [Google Scholar]
- 14.Yoon JS, Lee HJ, Choi SH, Chang EJ, Lee SY, Lee EJ. Quercetin inhibits IL-1beta-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves’ orbitopathy. PLoS One. 2011;6(10):e26261 10.1371/journal.pone.0026261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon JS, Lee JH, Chae MK, Lee SY, Lee EJ. Cigarette smoke extract-induced adipogenesis in graves’ orbital fibroblasts is inhibited by quercetin via reduction in oxidative stress. J Endocrinol. 2013;216(2):145–156. 10.1530/JOE-12-0257 [DOI] [PubMed] [Google Scholar]
- 16.Chen B, Tsui S, Smith TJ. IL-1β Induces IL-6 Expression in Human Orbital Fibroblasts: Identification of an Anatomic-Site Specific Phenotypic Attribute Relevant to Thyroid-Associated Ophthalmopathy. J Immunol. 2005;175(2):1310–1319. 10.4049/jimmunol.175.2.1310 [DOI] [PubMed] [Google Scholar]
- 17.Ludgate M, Baker G. Unlocking the immunological machanisms of orbital inflammation in thyroid eye disease. Clin Exp Immunol. 2002;127(2):193–198. 10.1046/j.1365-2249.2002.01792.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drexler SK, Foxwell BM. The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 2010;42(4):506–518. 10.1016/j.biocel.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 19.Lacheta D, Miskiewicz P, Gluszko A, Nowicka G, Struga M, Kantor I, et al. Immunological aspects of graves’ ophthalmopathy. Biomed Res Int. 2019;5:1–12. 10.1155/2019/7453260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. 10.1038/nrd2755 [DOI] [PubMed] [Google Scholar]
- 21.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. 10.1038/sj.cdd.4401373 [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Nadeem S, Stan MN, Coenen M, Bahn RS. A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves’ ophthalmopathy. J Mol Endocrinol. 2011;46(3):155–163. 10.1530/JME-11-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko J, Kim JY, Lee EJ, Yoon JS. Inhibitory Effect of Idelalisib, a Selective Phosphatidylinositol 3-Kinase delta Inhibitor, on Adipogenesis in an In Vitro Model of Graves’ Orbitopathy. Invest Ophthalmol Vis Sci. 2018;59(11):4477–4485. 10.1167/iovs.18-24509 [DOI] [PubMed] [Google Scholar]
- 24.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. 10.1126/science.1072682 [DOI] [PubMed] [Google Scholar]
- 25.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754(1–2):253–262. 10.1016/j.bbapap.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 26.Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 2001;13(2):182–195. 10.1016/s0955-0674(00)00196-4 [DOI] [PubMed] [Google Scholar]
- 27.Buckley DA, Cheng A, Kiely PA, Tremblay ML, O’Connor R. Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-1-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol. 2002;22(7):1998–2010. 10.1128/mcb.22.7.1998-2010.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arroba AI, Valverde ÁM. Inhibition of Protein Tyrosine Phosphatase 1B Improves IGF-I Receptor Signaling and Protects Against Inflammation-Induced Gliosis in the Retina. Invest Ophthalmol Vis Sci. 2015;56(13):8031–8044. 10.1167/iovs.15-17234 [DOI] [PubMed] [Google Scholar]
- 29.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283(21):14230–14241. 10.1074/jbc.M800061200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Bahn RS. Relative overexpression of macrophage-derived cytokines in orbital adipose tissue from patients with graves’ ophthalmopathy. J Clin Endocrinol Metab. 2003;88(9):4246–4250. 10.1210/jc.2003-030380 [DOI] [PubMed] [Google Scholar]
- 31.Smith TJ. Novel aspects of orbital fibroblast pathology. J Endocrinol Invest. 2004;27(3):246–253. 10.1007/BF03345273 [DOI] [PubMed] [Google Scholar]
- 32.Novaes P, Diniz Grisolia AB, Smith TJ. Update on thyroid-associated ophthalmopathy with a special emphasis on the ocular surface. Clin Diabetes Endocrinol. 2016;2:19 10.1186/s40842-016-0037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin-Granados C, Prescott AR, Le Sommer S, Klaska IP, Yu T, Muckersie E, et al. A key role for PTP1B in dendritic cell maturation, migration, and T cell activation. J Mol Cell Biol. 2015;7(6):517–528. 10.1093/jmcb/mjv032 [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Wang B, Zhang W, Wei Y, Bian Z, Zhang CY, et al. Protein Tyrosine Phosphatase 1B Deficiency Ameliorates Murine Experimental Colitis via the Expansion of Myeloid-Derived Suppressor Cells. PLoS one. 2013;8(8):e70828 10.1371/journal.pone.0070828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song GJ, Jung M, Kim JH, Park H, Rahman MH, Zhang S, et al. A novel role for protein tyrosine phosphatase 1B as a positive regulator of neuroinflammation. J Neuroinflammation. 2016;13(1):86 10.1186/s12974-016-0545-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsunekawa T, Banno R, Mizoguchi A, Sugiyama M, Tominaga T, Onoue T, et al. Deficiency of PTP1B Attenuates Hypothalamic Inflammation via Activation of the JAK2-STAT3 Pathway in Microglia. EBioMedicine. 2017;16:172–183. 10.1016/j.ebiom.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berdnikovs S, Pavlov VI, Abdala-Valencia H, McCary CA, Klumpp DJ, Tremblay ML, et al. PTP1B deficiency exacerbates inflammation and accelerates leukocyte trafficking in vivo. J Immunol. 2012;188(2):874–884. 10.4049/jimmunol.1004108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bettaieb A, Koike S, Chahed S, Bachaalany S, Griffey S, Sastre J, et al. Pancreatic Protein Tyrosine Phosphatase 1B Deficiency Exacerbates Acute Pancreatitis in Mice. Am J Pathol. 2016;186(8):2043–2054. 10.1016/j.ajpath.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez E, Vercauteren M, Kurtz B, Ouvrard-Pascaud A, Mulder P, Henry JP, et al. Reduction of heart failure by pharmacological inhibition or gene deletion of protein tyrosine phosphatase 1B. J Mol Cell Cardiol. 2012;52(6):1257–1264. 10.1016/j.yjmcc.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 40.Gogiraju R, Schroeter MR, Bochenek ML, Hubert A, Munzel T, Hasenfuss G, et al. Endothelial deletion of protein tyrosine phosphatase-1B protects against pressure overload-induced heart failure in mice. Cardiovasc Res. 2016;111(3):204–216. 10.1093/cvr/cvw101 [DOI] [PubMed] [Google Scholar]
- 41.Chen PJ, Cai SP, Yang Y, Li WX, Huang C, Meng XM, et al. PTP1B confers liver fibrosis by regulating the activation of hepatic stellate cells. Toxicol Appl Pharmacol. 2016;292:8–18. 10.1016/j.taap.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 42.Ortiz C, Caja L, Bertran E, Gonzalez-Rodriguez A, Valverde AM, Fabregat I, et al. Protein-tyrosine phosphatase 1B(PTP1B) deficiency confers resistance to transforming growth factor-b(TGF-b)-induced suppressor effects in hepatocytes. J Biol Chem. 2012;287(19):15263–15274. 10.1074/jbc.M111.303958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Ruiz I, Blanes Ruiz N, Rada P, Pardo V, Ruiz L, Blas-Garcia A, et al. Protein tyrosine phosphatase 1b deficiency protects against hepatic fibrosis by modulating nadph oxidases. Redox boil. 2019;26:101263 10.1016/j.redox.2019.101263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venditti P, Di Meo S. Thyroid hormone-induced oxidative strees. Cell Mol Life Sci. 2006;63(4):414–434. 10.1007/s00018-005-5457-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song Y, Driessens N, Costa M, De Deken X, Detours V, Corvilian B, et al. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab. 2007;92(10):3764–3773. 10.1210/jc.2007-0660 [DOI] [PubMed] [Google Scholar]
- 46.Marcocci C, Leo M, Altea MA. Oxidative stress in graves’ disease. Eur Thyroid J. 2012;1(2):80–87. 10.1159/000337976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taheripak G, Bakhtiyari S, Rajabibazi M, Pasalar P, Meshkani R. Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells. Free Radic Biol Med. 2013;65:1435–1446. 10.1016/j.freeradbiomed.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 48.Mobasher MA, Gonzalez-Rodriguez A, Santamaria B, Ramos S, Martin MA, Goya L, et al. Protein tyrosine phosphatase 1B modulates GSK3beta/Nrf2 and IGFIR signaling pathways in acetaminophen-induced hepatotoxicity. Cell Death Dis. 2013;4:e626 10.1038/cddis.2013.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. 10.1038/nature07203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16(8):469–484. 10.1038/nri.2016.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q, Korner H, Wu H, Wei W. Endoplasmic reticulum stress in autoimmune diseases. Immunobiology. 2019;28:151881 10.1016/j.imbio.2019.11.016 [DOI] [PubMed] [Google Scholar]
- 52.Gu F, Nguyen DT, Stuible M, Dube N, Tremblay ML, Chevet E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem. 2004;279(48):49689–49693. 10.1074/jbc.C400261200 [DOI] [PubMed] [Google Scholar]
- 53.Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, et al. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58(3):590–599. 10.2337/db08-0913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agouni A, Mody N, Owen C, Czopek A, Zimmer D, Bentires-Alj M, et al. Liver-specific deletion of protein tyrosine phosphatase (PTP) 1B improves obesity- and pharmacologically induced endoplasmic reticulum stress. Biochem J. 2011;438(2):369–378. 10.1042/BJ20110373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panzhinskiy E, Hua Y, Culver B, Ren J, Nair S. Endoplasmic reticulum stress upregulates protein tyrosine phosphatase 1B and impairs glucose uptake in cultured myotubes. Diabetologia. 2013;56(3):598–607. 10.1007/s00125-012-2782-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GO and non-GO fibroblasts were transfected with sicontrol or siPTPN1 for 24hours. TLR2, 4, and 9 expression levels were quantified with RT-PCR. Data in the columns indicate relative TLR mRNA fold of siPTPN1 cells normalised to sicontrol of each cell type (*p<0.05, sicontrol versus siPTPN1). Experiments were performed in three GO and non-GO fibroblasts from different individuals.
(TIF)
Orbital fibroblasts from GO and non-GO patients were transfected with sicontrol and siPTPN1 for 24 hours. (A) After the transfection, cells were cultured in different conditions (with or without IL-1β) for 24 hours and 48 hours. Then, the viability of the cells was assessed with MTT assay. Difference between sicontrol and sicontrol with IL-1β stimulation in GO was indicated (*p<0.05). (B) The transfected cells were subjected to Annexin V apoptosis assay after incubating for 24 hours and 48 hours. Assays were performed using three GO and non-GO cells from different individuals.
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.