Abstract
Increases in global temperature are already having a significant impact on our climate. The hydrofluorocarbon (HFC) propellants used today in pressurized metered-dose inhalers (pMDIs) have global warming potential (GWP) many times that of carbon dioxide. Their use, together with all other emissive uses of HFCs, is being phased down under the Montreal protocol. This has prompted calls to switch patients to dry powder inhalers (DPIs). This paper presents a new analysis of the top 15 respiratory drug markets by drug class. It shows that a switch to DPIs would be economically feasible for most countries and most drugs. However, a wholesale switch of reliever medications, notably short-acting β-agonists, would lead to significant increases in the cost of these life-saving medications. Reviewing the evidence, whilst most patients are capable of using DPIs, the very young, very old and those undergoing an acute exacerbation still require a pMDI. Thus, there is a clinical and economic need to have both pMDIs and DPIs available. At the same time, it is projected that the reduction in non-medical uses of propellants is likely to give rise to a 5-fold increase in their cost for pMDI uses and is likely to hit the Western world in 2025. This may lead to a price increase in reliever medication that will make it unaffordable for the poorer communities in some markets. At the same time, opportunities to save money by developing new formulations using propellants with lower GWP, such as HFC 152a or HFO 1234ze(E), are described. Two companies have made this commitment, but neither currently have a strong presence in reliever medication. For them, or other companies, now is the time to act; 2025 is not far away in terms of product development timescales and the climate cannot wait.
Keywords: climate change, global warming, F-gases, propellants, respiratory drug market, inhalers
Introduction
Since their introduction in 1956, the pressurized metered-dose inhaler (pMDI) has been the dominant method of treating respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). According to the IQVIA database (Durham, NC), in the 12 months to June 2019, there were over 480M packs prescribed, equating to 2400 doses taken every second somewhere in the world ! When originally developed, they utilized chlorofluorocarbon (CFC) propellants. However, due to their effect of depleting stratospheric ozone, the industry invested hundreds of millions of dollars in replacing pMDI products with alternative, non-ozone-depleting hydrofluorocarbon (HFC) propellants, as well as developing novel delivery systems such as dry powder inhalers (DPIs) and soft-mist inhalers (SMIs).1 Nonetheless, the pMDI still accounts for two-thirds of all doses prescribed.
However, the industry now faces a similar challenge because of concerns over global warming.2 The Intergovernmental Panel on Climate Change (IPCC), the United Nations body that assesses the science related to climate change, has attributed much of the rise in global temperatures to anthropogenic sources of the emission of “greenhouse gases”, which include the HFC propellants used in pressurized metered-dose inhalers (pMDIs). These gases can survive for long periods in the upper atmosphere allowing solar radiation to penetrate to the earth’s surface, but trapping reflected emissions and re-radiating the energy back towards the earth.3
Climate change carries with it many risks, including catastrophic weather events, famine, adverse health outcomes and displacement of whole communities.4 As a consequence, it is increasingly at the forefront of public and political discussions, so whether it is from customers, regulators or environmental pressure groups, the industry once again faces the question of how best to respond. The choices are to do nothing and allow usage to dwindle over time, undergo a reformulation exercise once again, or to move the market into dosage forms with lower global warming potential (GWP). Each has potential consequences. pMDIs are inherently cheaper to produce than DPIs today, so payors may not be able to afford to switch patients into DPIs. However, the cost of propellants will rise as other non-medical uses of HFC decline, and so they will become less profitable. Nonetheless, not all patients are necessarily suited to using a DPI, so the need for pMDIs is likely to remain. If so, there remain technical questions over how they might be reformulated. This paper considers each of these factors in proposing a call for action to the industry.
Regulatory Environment
Through the highly successful operation of the Montreal Protocol, the emissive use of CFCs has ceased for most uses, including in pMDIs. This has not only had positive impacts on stratospheric ozone levels; the effect of switching to HFCs with lower GWP than CFCs (Table 1) has reduced the emission of greenhouse gases by the equivalent of 11 GTonnes CO2 per year, an amount similar to the annual emission of carbon dioxide produced by burning fossil fuels to generate heat and electricity at the start of this decade. This remains the single biggest initiative to reduce greenhouse gas emissions.
Table 1.
Properties of Some Fluorinated Aerosol Propellants
| Propellant | Formula | Ozone Depleting Potential | Global Warming Potential (CO2 = 1) | B. Pt oC | Density g/mL | Viscosity cP | Dipole Moment Debye | Solubility of Water in Propellant ppm |
|---|---|---|---|---|---|---|---|---|
| CFC 11 | CFCl3 | 1 | 4,660 | 23.7 | 1.49 | 0.43 | 0.45 | 100 |
| CFC 12 | CF2Cl2 | 1 | 10,800 | −29.8 | 1.33 | 0.26 | 0.51 | 90 |
| HFC 134a | CF3-CFH2 | 0 | 1,300 | −26.2 | 1.23 | 0.21 | 2.06 | 2,200 |
| HFC 227a | CF3-CFH-CF3 | 0 | 3,350 | −16.5 | 1.41 | 0.26 | 1.46 | 610 |
| HFC 152a | CF2H-CH3 | 0 | 138 | −24.7 | 0.91 | 0.24 | 2.26 | 2,200 |
| HFO1234ze(E) | CF3CH=CHF | 0 | <1 | −18.9 | 1.29 | 0.20 | 1.44 | 225 |
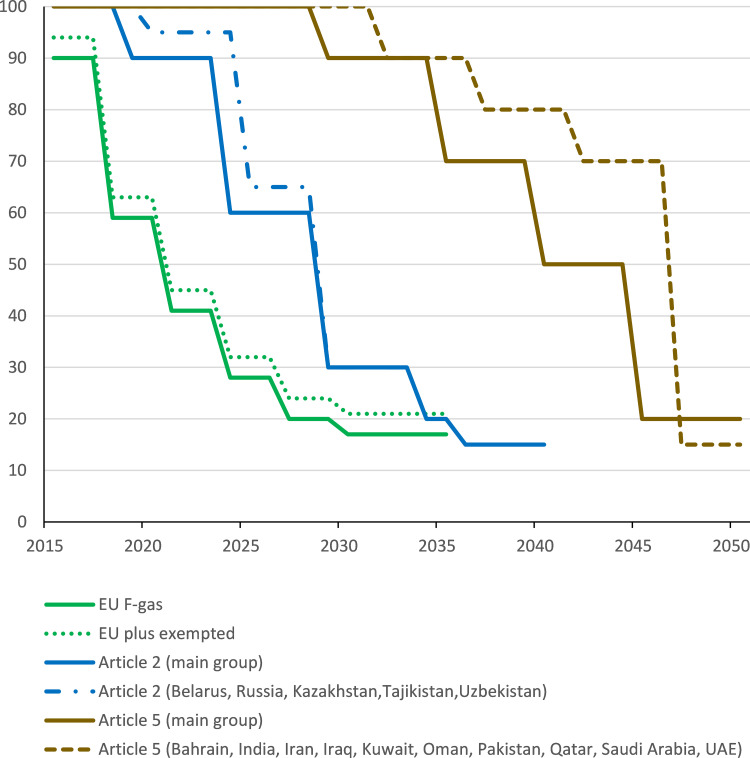
It has been estimated that in 2014, HFC emissions from MDIs represented only 0.03% of annual global greenhouse gas emissions.5 Nonetheless, its uses are regulated as part of a basket of fluorinated gases (so-called “F-gases”) which also include perfluorocarbons, sulphur hexafluoride and nitrogen trifluoride. The European Union had already begun to regulate their use as early as 2006, with members implementing a 10% reduction from 2015 and at least a 40% reduction from 2018 (see Figure 1).6 However, certain uses were exempted from this phase down, including HFC propellants used in pMDIs. By 2015, over 90% of the global F-gas emissions were attributed to HFCs, so given its success in transition from CFCs to HFCs, an amendment was passed to the Montreal Protocol in Kigali in 2016 to manage the phase-down of HFCs, which has so far been ratified by 65 countries.7 The Kigali Amendment entered into force on 1 January 2019, and, and commits each party to phasing down the total emissions of F-Gases over the next 30 years, individual country targets varying across the globe (Figure 1). Under Kigali, the remainder of the Western World (Article 2 countries) have reduced emission targets that began in 2019; the targets for Article 5 countries begin in 2029.8
Figure 1.
Phase down of HFC use under the EU F-gas regulations6 and the Kigali amendment to the Montreal Protocol8.
By 2015 over 90% of F-gas contributions to greenhouse emissions were HFCs. However, the majority were used in refrigeration or air conditioning applications, aerosol usage accounting for less than 10%. Thus, the emissive use of pMDIs contributed only 2.3% of the F-gas contribution to greenhouse emissions.2 Nevertheless, demand for pMDIs and other aerosols will grow with increasing population and disease prevalence. This has been modelled under a business-as-usual scenario by the UN Technical and Economic Assessment Panel.9 The panel project that contribution to the greenhouse gas emissions from the Western World could grow to about 15% of the total HFCs by 2035 as other uses are eliminated.2 With a delayed phase-down, the corresponding proportion for emerging economies, plus countries such as India and China would still be only around 3% at this time. Whilst there is obvious uncertainty in projecting out so far into the future, use of HFCs in pMDIs is unlikely to be greatly affected by the timetable for the overall phase-down of HFCs under the Kigali Amendment. Thus, the pharmaceutical industry would appear to have sufficient time to observe the situation and decide upon a course of action.
Environmental Pressures
Few can be unaware of the position that environmental pressure groups take on this issue. In response, national governments are introducing targets that may be more restrictive or faster than the regulations dictate. For example, the UK Government’s Environmental Audit Committee has set the UK National Health Service (NHS) the challenge of reducing the GWP impact from inhaler use by 50% before 2028.10 However, such views may not address the needs of individual patients.11 The United Kingdom has a high proportion of inhaled medicines as pMDIs, which contribute an estimated 3.9% of the carbon footprint of the National Health Service. In turn, the UK National Institute for Health and Care Excellence (NICE) published a Patient Decision Aid on asthma inhalers in 2019 that emphasizes carbon footprint as a criterion in the choice of inhaler, thereby favoring a switch to DPIs or re-usable SMIs.12 However, there are many other ways in which the environmental impact of inhaler use can be reduced, including improved disease control and increased recycling.13
Furthermore, the pharmaceutical industry and their suppliers are increasingly conscious of their corporate social responsibility and are committed to reducing carbon emissions across their operations. GlaxoSmithKline (GSK) have calculated that 28% of their entire carbon footprint derives from patient use of their inhalers.14 GSK also reported that the lifecycle carbon footprint of their DPIs (Diskus® and Ellipta®) is 24 times lower than pMDIs, whilst an analysis of a Chiesi pMDI and equivalent DPI suggested only a 12-fold difference.15 It should be noted that some companies will gain commercial advantage from a switch to DPIs, which may lead to a commercial motivation behind some publications.16 Furthermore, carbon footprint is only one aspect of environmental impact; it has been estimated that DPIs may have greater impact than pMDIs on human toxicity, marine eutrophication and fossil fuel depletion.17 In response, two companies (AstraZeneca and Chiesi Farmaceutici) have announced commitments to launch next generation pMDIs containing low-GWP propellants by 2025.18,19 These developments may cause some surprise, given that, as discussed above, pMDIs only account for 0.03% of greenhouse gas emissions. However, it is interesting to note that both Starbucks and Disney have banned the use of plastic drinking straws, even although reportedly these only contribute a similarly small fraction of the total plastic in the environment.20 Given the Montreal Protocol was signed in 1987, but the first non-CFC pMDI was not launched until 1995, these targets may be ambitious, but it is very encouraging to see such initiatives. Every journey begins with a single step.
Price Pressures
Given that new low-GWP products are still some years away, it is important to consider what else might be done, and whether a more immediate switch to lower-GWP DPIs is economically feasible. This could make the pMDI market less attractive in the future, reducing the likelihood that producers would invest in a new propellant. There has been a recent analysis of the cost to the National Health Service in England of a wholesale switch from using pMDIs (currently 85% of the doses in the whole of the UK from pMDIs and DPIs combined) into DPIs.21 This study concluded that there would be a £ 127M rise in prescribing costs per annum, representing around an additional 10% cost of respiratory therapy, if DPIs were prescribed in the same proportion of brands per drug class as in 2017. There was also an analysis performed suggesting there could actually be a cost saving if all patients were switched to the cheapest available DPI in that class of molecules. However, in cost-constrained times, it would be expected that doctors are already prescribing the lowest cost inhaler suitable for that patient, so this seems a highly unlikely scenario. Not every class of molecule resulted in a cost increase, and it was noted that these data did not translate to other healthcare systems across the globe.
To investigate the global economic impact of switching from pMDIs to DPIs, an analysis of prescribing data sourced from the IQVIA database was performed, using the moving annual average for the 12 months up to, and including, the end of June 2019. The analysis was performed for the Top 15 global markets by value, which together account for 90% of the global spend on respiratory drugs for asthma and COPD, looking at the following categories of drug:
• Short-acting β-agonist (SABA)
• Short-acting muscarinic antagonist (SAMA)
• Short-acting combination SABA/SAMA
• Inhaled corticosteroid (ICS)
• Long-acting β-agonist (LABA)
• Long-acting muscarinic antagonist (LAMA)
• Long-acting combination of LABA/ICS
• Long-acting combination of LABA/LAMA
• Triple combination of ICS/LABA/LAMA (triple)
• Cromones
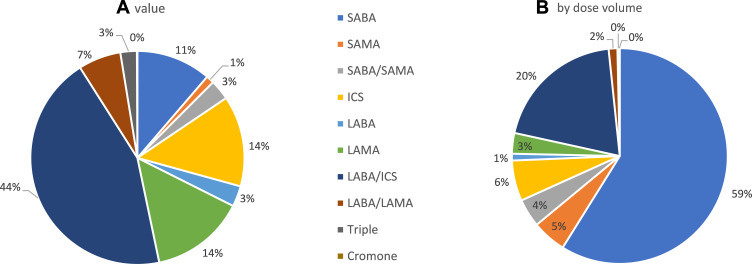
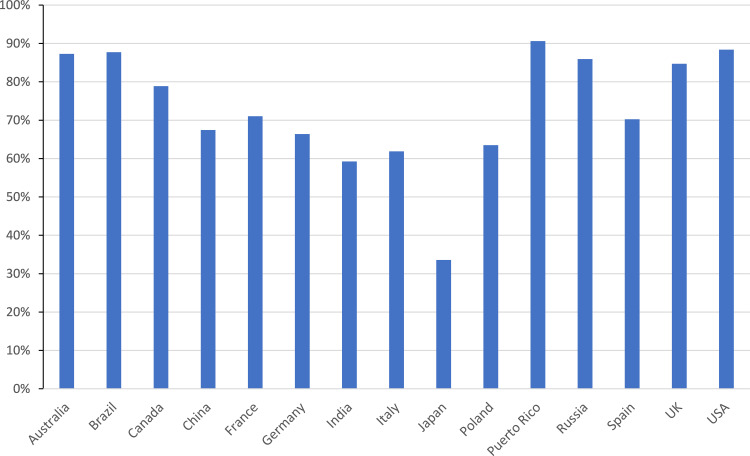
The overall value that each of these classes represent is shown in Figure 2A from which it may be seen that LABA/ICS combinations dominate the global market followed by LAMA and ICS mono-products. In contrast, when one considers the breakdown by number of doses prescribed in Figure 2B, SABAs dominate accounting for over half of inhaled medication globally. The data for cromones were excluded from subsequent analysis, as they represent only 0.1% of the value of the respiratory market and 0.2% by volume, with over 90% of the usage being in Germany. Furthermore, as may be seen from Figure 3, in all of the markets analyzed, cromones are only available in pMDI format, so a switch is not possible.
Figure 2.
Breakdown of drug class usage in the top 15 markets (A) by value and (B) by dose volume.
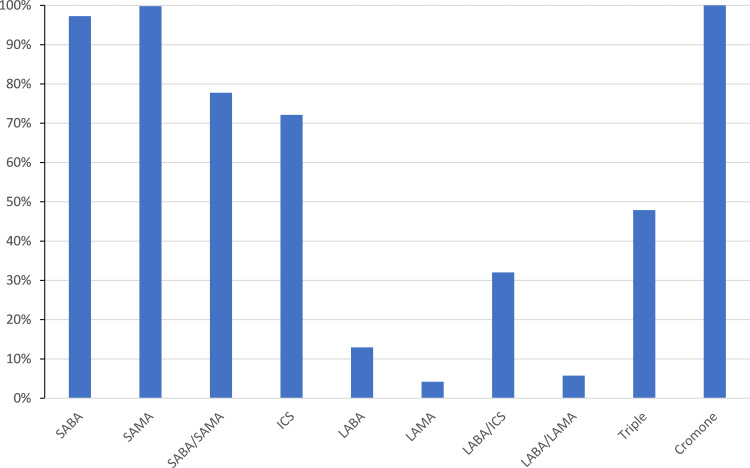
Figure 3.
The proportion of inhaler doses delivered by pMDI.
For each drug class and country, the total value of the doses prescribed was divided by the total number of doses (pack size multiplied by unit sales) to obtain the average sales price (ASP). When soft mist inhalers (SMIs) were available, these were combined with DPIs to get a mean ASP for the entire non-pMDI inhaler market segment. Also, for pMDIs, the total number of doses for each class of medication was divided by 2, because these are almost all two puffs per dose. However, this assumption was not applied to relievers (SABA, SAMA and SABA/SAMA), as the instructions are usually take as needed, and so one puff per dose was assumed. It was then possible to calculate the net change in prescribing cost if all doses taken from a pMDI in that drug class were replaced by the average cost of a DPI dose. This was then expressed as a ratio of the shift in prescribing cost for that entire class of medication, including any nebulized drug products, in order to see the local country impact of a shift in practice for a particular drug class. These data are presented in Table 2, together with the impact on the total respiratory drug costs for that market. In order to highlight key findings, those cases where the cost for a drug class increases by more than 10%, the text is marked in red, whilst if the drug costs drop by more than 10%, it is highlighted in blue. Where there are not equivalent products in both pMDI and DPI format, this is also indicated. In a fifth of instances, there are no pMDIs to replace, mostly LABAs, LAMAs and their combination (Figure 3), whilst in a similar proportion, there are not available DPIs as an alternative (mostly relievers). The total value of each market is also listed.
Table 2.
The % Change in Prescription Costs to Switch a Drug Class from pMDI to All DPI at the Average Sales Price per Dose of the DPI
| USA | Germany | UK | Japan | China | France | Canada | Spain | Italy | Brazil | India | Australia | Poland | Russia | Puerto Rico | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SABA | 114% | 147% | 290% | 132% | 213% | 171% | 566% | 304% | 277% | X | 102% | 170% | 290% | X | 105% |
| SAMA | X | X | X | X | X | X | X | X | N/A | X | 101% | X | X | X | X |
| SABA/SAMA | o | 205% | N/A | N/A | X | 596% | o | N/A | X | X | 121% | N/A | X | X | o |
| ICS | 93% | 81% | 121% | 90% | 100% | 101% | 60% | 99% | 107% | 104% | 101% | 64% | 80% | 100% | 100% |
| LABA | o | 92% | 92% | o | o | 100% | o | 107% | 99% | o | 102% | o | 95% | 95% | o |
| LAMA | o | o | o | 100% | o | o | o | o | o | o | 148% | o | o | o | o |
| LABA/ICS | 111% | 91% | 85% | 102% | 100% | 97% | 88% | 92% | 100% | 100% | 102% | 89% | 93% | 99% | 117% |
| LABA/LAMA | 105% | o | o | o | o | o | o | o | o | o | 147% | o | o | o | 104% |
| Triple | 100% | 155% | 148% | o | N/A | 142% | o | 182% | 161% | N/A | 152% | o | X | N/A | o |
| TOTAL | 106% | 102% | 107% | 101% | 113% | 107% | 110% | 104% | 106% | 101% | 108% | 103% | 96% | 99% | 107% |
| Total excl. relievers | 105% | 97% | 95% | 100% | 100% | 99% | 85% | 97% | 106% | 101% | 106% | 92% | 91% | 99% | 107% |
| Market size $M | $24,774 | $1394 | $1,293 | $1,280 | $1,220 | $894 | $846 | $751 | $685 | $335 | $315 | $314 | $271 | $219 | $201 |
Notes: Drug class abbreviations (SABA, SAMA etc) as defined in the text: Red text indicates a greater than 10% cost increase, blue text a greater than 10% cost reduction: Text in italics denotes the overall size of the respiratory drug market in that country.
Abbreviations: X, no equivalent DPI; o, no MDI to replace; N/A, no drugs in class in inhaler on market.
It can be readily seen from Table 2 that in every market, the drug costs would rise if SABA pMDIs were replaced with DPIs, except in Brazil, where there is no equivalent DPI registered. In only 2 of the remaining markets would the cost rise by less than 10%. Indeed, in Canada the salbutamol DPI is 6 times more expensive per dose than the pMDI. For the remaining medications used as reliever therapy, there are either no equivalent DPIs, or in Germany, France and India where an alternative to a SABA/SAMA pMDI exists, the cost of a switch would also be significantly higher. Any alternative to replace this usage in an economic fashion needs to address the extremely low price of this drug in pMDI products (ASP of $0.01 per dose in the UK). The triple combinations exhibit a similar pattern, but at the moment only represent 0.3% of the total number of doses taken, so the impact today is minimal. However, this is a new class of drugs entering the market, and so will have increasing impact on budgetary considerations over the next few years, given that they already account for 2.5% of total spend (Figure 2).
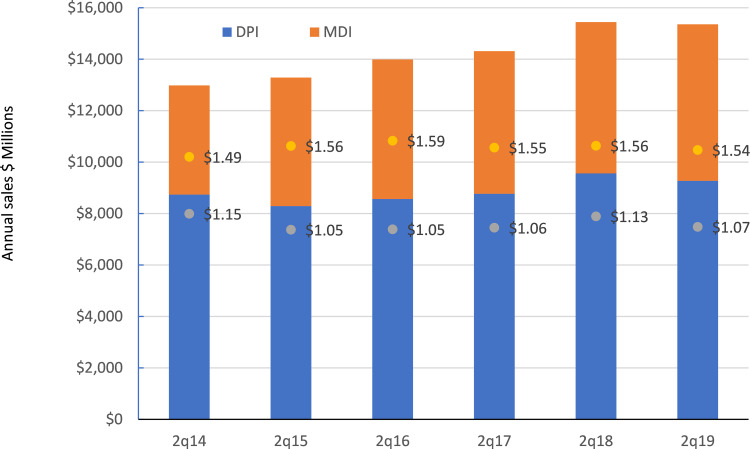
In contrast, for the LABA/ICS combination which is the highest value class of drugs and the second highest in terms of number of doses, costs would only rise significantly in the USA and Puerto Rico. In 8 of the 15 markets costs would decrease, by more than 10% for the UK, Canada and Australia. This is probably due to the recent introduction of many new combinations from both innovator and branded generic companies.22 As predicted, the market for this class of drug has become more competitive with the total value declining over the last year (Figure 4). Of interest, the gap between the ASP for the pMDI and DPI has narrowed over the last 4 years, which if this trend continues, will result in less of a saving in a switch from pMDI to DPI in the future.
Figure 4.
The evolution of the LABA/ICS market over the last 6 years. (Data labels indicate the Average Sales Price, ASP).
For the remaining drug classes, the situation is fairly neutral with little overall change in costs for a particular drug class. There are a few instances where costs will rise significantly on switching, such as ICS in the UK or LABA/LAMA combinations in India. Equally there are a few where the costs would drop significantly, such as ICS in Australia, Canada, Germany and Poland. Of more significance is the change to the overall prescribing costs. This will depend on the individual mix of drug classes in each country and what proportion of those are in pMDI format. The overall breakdown of pMDI use by country is shown in Figure 5. Solely in China would the overall prescription costs rise by more than 10%, although only in Poland and Russia would a DPI only treatment regime be cheaper. However, as noted above, much of the cost driver is from pMDIs used to deliver reliever medication. Therefore, Table 2 also includes an analysis of how costs would change if only the long-acting prophylactic therapies were switched to DPI. No country would see a rise in cost for these drug classes of more than 7%, whilst 8 of the 15 would see a drop in overall cost. Therefore, there are no financial barriers to switching patients into DPI therapies for long-acting drugs.
Figure 5.
Proportion of inhaler doses delivered by pMDI in top 15 markets.
There are limitations to this approach. Firstly, the database has some issues when it comes to data collection, particularly in countries where there can be limited prescribing information available electronically. Furthermore, it does not capture devices supplied under tender, a common practice in emerging economies, especially for salbutamol. However, whilst the global numbers may underestimate the total number of pMDIs sold by over 25%, the cost analysis remains robust for most of the top 15 markets studied. The greatest uncertainty is likely to be for the Chinese and Indian markets, where accurate records are harder to establish. Also, China is unique in preferring nebulized therapy for ICS to DPI or pMDI; if all pMDI use were to be switched to nebulized drugs rather than DPIs, then the cost for that class would rise by 21% and the total cost of respiratory products would rise by 24% rather than 13% discussed above. The assumption that all preventer pMDIs are two puffs per dose, whereas reliever medication can be taken as one puff per dose may not hold in all markets, thereby influencing the calculation of ASP per dose. Lastly, these data project what might happen if every patient and every dose was switched, and so represents an extreme and currently unachievable situation. Nonetheless, it is clear that cost should not be a barrier to reducing carbon footprint for most classes of medication, but that there remains an issue with affordability when it comes to reliever medications, which unfortunately represent the bulk of HFC emissive use.
The Role of pMDIs in Clinical Practice
Each type of inhaler requires instruction in its use. Interventions to monitor and improve technique are varied, and have mixed success.23 Indeed, device switching has been shown to lead to poorer outcomes, leading to a recommendation that any device switch should be agreed with the patient.24,25 It has been concluded that in the light of currently available evidence, it would seem reasonable to restrict regular (preventer) inhaled medication to a single type of device (pMDIs or DPIs) whenever possible.26 Nearly all patients will have at least some familiarity with pMDIs from use with reliever medication, suggesting this might be the preferred option. For example, despite the high DPI usage, a recent study reported that Japanese patients may still prefer a pMDI to a DPI.27 Furthermore, there are clearly local factors which can influence prescriber choice, given that the proportion of inhaler doses (excluding nebulized) given by pMDI ranges from 34% in Japan to 88% in the US (Figure 5). Differing health policies, costs, health insurance issues, commercial aspects and prescribers’ and patients’ preferences can cause significant variation in prescribing practice, even within the European Union.28 Thus, to simply switch from pMDI to DPI will require a major change in prescribing practice, and will involve a significant investment in patient education.
For an effective switch, not only must an inhaler be affordable, it must be appropriate to the needs of the patient for whom it is prescribed. The DPI is prescribed globally for only 3% of the doses of SABAs, which are the mainstay of reliever medication today (Figure 5). This is large because the pMDI delivered dose is independent of inspiratory effort at a time when patients are struggling to breathe. Recently, the Global Initiative for Asthma have updated their guidelines to recommend either low dose inhaled corticosteroid (ICS) alongside a SABA, or as needed low-dose combinations of ICS with formoterol.29 Nonetheless, during an exacerbation, GINA guidelines still recommend the use of repeat doses of SABA, usually administered via a pMDI with spacer. It is not possible to determine how much SABA use is first-line therapy and how much is used in an acute situation. Nonetheless, given that the ASP for ~60 Bn doses of salbutamol dispensed annually across the globe is around $ 0.06 per dose, and that for LABA/ICS is at least $ 1.07 per dose (Figure 4), the cost to the payors of such a switch would run into tens of billions of dollars, at least doubling the total costs for respiratory drugs. Furthermore, the global initiative for chronic Obstructive Lung Disease (GOLD) guidelines for COPD regard stand-alone bronchodilators as central to symptom management and whilst long-acting bronchodilators are recommended for maintenance treatment, SABAs, with or without SAMAs are recommended to treat an acute exacerbation.30
Arguments about the best inhaler for patients continue with studies advocating patients can use pMDIs effectively and others advocating that patients are able to use DPIs more effectively.31 The most appropriate inhaler should be identified for each patient to ensure optimal care. It is clear that the most common errors relate to breathing pattern rather than manipulation of the device, and such errors increase with age.32 For pMDIs the patient needs to coordinate the actuation of the spray with inhalation, for SMIs the relatively long duration of spray requires a slow inhalation, whilst for DPIs, an optimal dose of drug to the lungs requires a deep and forceful inhalation. There are groups of patients who struggle to generate sufficient inspiratory flows to get adequate delivery from a DPI. One review of the literature found values ranging between 19% and 78% of COPD patients unable to generate 60 LPM peak inspiratory flow (PIF).33 Indeed, it has been found that COPD patients unable to generate such a level of PIF when discharged from hospital following a severe exacerbation, were more likely than those with better lung capacity to readmit.34 In addition to the impact of disease, it is known that aging impairs the ability to inhale quickly; for example, increasing age and increasing disease severity in COPD independently reduce PIF through different DPIs.35 Similarly, DPIs do not have market authorisations for use in children under 4, due to their inability to generate sufficient inspiratory flow. Thus, there are groups of patients amongst the very young, very old and very ill, for whom DPIs are contra-indicated.
Should there be a loss of control on switching, this has both environmental and economic consequences. It has been suggested that the carbon footprint from excess healthcare use involving a hospitalization could equate to 6 months’ use of a pMDI.18 It has also been estimated that for every 20% of the patient population experiencing treatment failure there would be an additional 4100 and 5223 exacerbations of COPD and asthma, respectively, per million population with associated hospitalization rates of 287 and 141 for COPD and asthma, respectively.36 So, combining these estimates, with 5.4M asthmatics and 1.2M COPD patients in the UK, a 20% failure rate would equate to 1700 exacerbations or the equivalent carbon footprint of 10,000 pMDIs.37 Given there were 53M pMDIs prescribed in 2019, the impact is negligible. From an economic standpoint, it has been estimated in the US that an asthma exacerbation costs $ 3000, and a COPD hospitalization, $9900.38,39 Applying the UK hospitalization rate estimates to the 24.7M asthmatics and 12.8M COPD patients in the US, would give rise to additional costs of $ 39M, compared to respiratory drug costs of $ 24Bn; again, an insignificant impact.40 Of course, this does not take account of social costs (days off work, school, etc.), which for asthma as a disease can amount to 3.5% of the medical costs.41 Nonetheless, based on these rough estimations and despite the individual human suffering, it would not appear that loss of control would be of major concern from an economic or environmental perspective.
Technical Solutions for pMDIs with Low GWP
On the basis that pMDIs are required both from a clinical and an economic perspective, at least for reliever medication, there is a need to find a low-GWP replacement. Historically, to find replacements for CFCs, two consortia were formed from a number of pharmaceutical companies to generate a package of safety data on pharmaceutical grade propellants to meet regulatory requirements (IPACT I and IPACT II).42 HFC 134a was approved for use in pMDIs by the EU in 1994 and HFC 227ea in 1995, but it took a further decade to complete the phase out of CFCs in that region. At present, there is no equivalent initiative and no industry-wide approach to this problem. Whilst hydrocarbons and dimethyl ether are used in consumer and topical aerosols, both are very flammable, have potential cardiac side-effects and some have taste issues. There are also developments in valve technology to create a metering valve capable of using compressed gases such as CO2.43 Non-metered variants of this technology have recently reached the market.44 Not-in-kind alternatives, such as SMIs also address the issue of effort-independence. Several companies are pursuing this approach, including Boehringer Ingelheim, Merxin, Pharmaero and Well-Bridge. However, given their mechanical complexity, and the need to maintain a microbial-free environment, these would appear unlikely to meet the challenge of an affordable reliever medication.
This leaves only two potential alternate propellants for pMDIs with significantly lower GWP, HFO 1234ze(E) and HFC 152a (see Table 1). The former is the more attractive from an environmental perspective, as it has the lowest GWP. HFO 1234ze(E) also appears closer to the HFCs 134a and 227ea in key physical properties (density, vapor pressure, moisture solubility, dipole moment). That may make the path to development faster if the materials and processes developed for HFC can be transferred directly across. However, as new propellants, it is extremely likely the Regulators will expect long-term human safety data to be collected before granting market authorization. This will undoubtedly be critical path activity. Given that neither is widely available in pharmaceutical-grade material, nor yet has a comprehensive inhalation safety data package, may make the projected launch date of 2025 a stretch target.
At present, HFO 1234ze(E) uses appear to be more focused on refrigeration and on novelty aerosols, such as party streamers. Although there are patent applications relating to its use as a medical propellant, there have been few publications on pMDI applications other than one or two academic research papers. There have also been some concerns expressed over its safety profile for inhaler use.45 However, it is known to be in active development, with product launches potentially possible in a similar timeframe to 152a below.
On the other hand, HFC 152a is well publicized to be in active development for applications in pMDIs.46 HFC 152a is used as a precursor in the chemical synthesis of polymers, and so is comparable in price to HFC 134a. Data on prototype formulations have shown good pharmaceutical performance despite a significant density difference between the propellant and most commonly prescribed respiratory drugs.47 Nonetheless, there may still be significant valve development required. Product chemical stability and compatibility with existing pMDI components also appear promising. Koura (formerly Mexichem Fluor) announced that the FDA has cleared the company’s IND for Zephex HFA 152a MDI propellant, and the company plans to complete clinical trials of the propellant for safety and tolerability by the end of March 2020.48 Nonetheless, 152a has been associated with deaths of abuse caused by deliberate inhalation of this gas from consumer aerosols, but with the smaller fill weights in pMDIs, greater chemical purity and availability only by prescription, it remains to be seen whether this remains a risk from a medical product.49
HFC 152a was also being considered alongside HFCs 134a and 227ea at the time of the CFC-transition but was not adopted at the time, possibly because the propellant is flammable, with a lower explosive limit (LEL) of 3.9% by volume in air at room temperature (isobutane is 1.8%). Unlike some ethanolic pMDI formulations, it does not cause flame extension from a standard flame test. Nonetheless, safe manufacturing processes for HFC 152a will still need to be developed. Given that many millions of aerosol cans are safely filled with isobutane, the technology exists to overcome this problem. Normally, a paste is filled into the can, a valve crimped on and then the cans pass out to an isolated explosion shed where an automated, highly ventilated gassing facility adds the liquefied propellant. Cold fill is not recommended for flammable aerosols, so to replicate this in pMDIs, ethanol could be used. However, not all drugs are compatible with ethanol due to formulation instability.50 Furthermore, it is also flammable with an LEL of 3%, so is likely to raise additional safety concerns if used with HFC 152a. To address the risks, batch sizes could be kept small, or mixing also moved to the explosion shed. As an alternative, one potential solution is to develop filling methods that involve dry powder dosing into the can; a particularly attractive approach is to use tablets which disperse into stable suspensions when the propellant is added.51 Apart from the obvious reduction in risk, this has the advantages that there are no issues of drug loss during filling, nor formulation instability due to the presence of ethanol, and it is readily scalable from pilot- to full-scale production.
Potential Commercial Drivers for Change
The pharmaceutical industry has spent hundreds of millions of dollars in developing new formulations, device hardware, manufacturing processes and clinical data to support the phase-out of CFCs. In order to see a return on this investment, there is inevitable reluctance to repeat this process afresh. Furthermore, if the market is going to switch into DPIs, there may not be an opportunity to recoup further investment in a new pMDI entering a declining market. Apart from the environmental pressure groups and government mandated targets to reduce carbon footprints, some pharmaceutical companies who only have a DPI offering for a particular drug class will try to influence the market of the importance of GWP in device selection.
Nonetheless, there are other economic factors which will influence the market dynamics. Despite the regulatory environment, the biggest single factor which drove the timing of transition away from CFCs was the increasing price of propellant, and associated CFC components. As it became more expensive to manufacture CFC pMDIs than HFC, industry swapped and so the CFC pMDIs disappeared from the supply chain. It is highly likely that this will also drive the timing of a move to low-GWP pMDIs. HFC 134a propellant accounts for around 30% of the $ 0.80 it costs to manufacture a pMDI.52 With fewer non-medical uses for HFC 227ea, the cost is 3–4 times that of HFC 134a, a gap that is currently widening as these industrial uses are replaced by propellants with lower GWP. Since pharmaceutical grade propellant is typically 2–4 Euro/kg more expensive than industrial, there may already be a strong commercial driver to seek an alternative to HFC 227ea.
There was also a warning of things to come. In the EU, the price of industrial grade HFC 134a rose rapidly towards the end of 2018 in anticipation of the 40% reduction in quotas (see Figure 1), peaking at a 5-fold increase compared to 2015 levels. This trend was largely reversed in 2019 due to imports of propellant from outside of the EU, which were allowed due to uncertainty whether the imports qualified for exemptions. It is anticipated this may be resolved within the EU, causing prices to rise once more. Under the Kigali Amendment, the 40% reduction is repeated across the Western World (Article 2 Parties) in 2025 (Figure 1). There are no exemptions under this protocol, and so it is highly likely that these 5-fold price increases will hold. Furthermore, as the industrial uses for these HFCs are switched to low-GWP alternatives, production will be switched from continuous to batch campaigns. As a consequence, there will be additional costs of underused assets, with increasing maintenance bills, and lower purchasing power on feedstocks like hydrogen fluoride, which all have to be amortized across the reducing tonnes made. Even if there are no other price rises, but propellant costs rise 6-fold in 2025, the cost to manufacture an HFC 134a pMDI would rise almost 3-fold to $2.88, whilst that for a 227ea pMDI would rise almost 4-fold to $5.40.53 Thus, 2025 would appear to be a tipping point for pMDI cost of goods in the Western World.
For long-acting drugs, there may be sufficient profit margin in the product price for a company to absorb much of the rise in cost of goods. Nonetheless, the potential magnitude of the saving by switching to a low-GWP makes re-investment an attractive proposition. Given Chiesi’s current growth in annual sales and a quoted €350M investment, it can be calculated that even if they do not gain market share, they will see payback through propellant cost savings within 4 years.19 However, for reliever medication, where the ASP today is $0.06 per dose, the profit margin will be more than completely eroded. Most likely, the cost of drugs will rise in line with the estimated cost of switching to DPIs (Table 2). Alternatively, suppliers in Asia and the Far East, where propellant price increases under Kigali start much later, will need to gain Market Authorizations for their products in the Western World. Of course, this simply delays the problem, as ultimately propellant prices will rise in these countries as well. Today, salbutamol dominates the market, with over 90% of all SABA doses. It is supplied by GlaxoSmithKline and Teva (together providing over 4/5 in the Western top 15 markets), who together with Mylan and Novartis account for over 90% of salbutamol pMDIs. It would be an act of corporate social responsibility and sound commercial sense for at least one of these major companies to ensure a continuing supply of reliever inhalers with lower GWP and similar price to that of today. Capturing a much larger proportion of the reliever medication market based on an ability to keep prices low represents a tremendous advantage. Furthermore, because the volume of SABAs to be manufactured is at least 5 times that of any other drug class, economies of scale will transfer into lower cost of goods of any other pMDI product produced by that company.
Conclusions
The emissive use of HFCs as propellants in pMDIs is regulated under the Kigali Amendment to the Montreal protocol. It is only one of many uses of F-gases impacted by the phase-down, and as such would not appear to be greatly impacted by the phase-down targets, at least for the next 15 years. However, pressures to reduce carbon footprint, together with the highly probable increase in cost of medical grade propellant in the Western world may force a re-evaluation of treatment regimes. For any change to occur, it must be both clinically and economically feasible. Many patients can use DPIs successfully, and for most classes of drugs, there will be little change in prescription costs. However, not all patients can use DPIs, notably the very young, very old and those undergoing an acute exacerbation. Reliever medication presents a particular challenge, as DPIs are not recommended, and there is every possibility that prices will have to rise by 2025. In many markets, this may make this life-saving medicine unaffordable for the poorer communities.
Thus, there is a clear need for alternatives to the current reliever pMDIs that are both affordable and with a lower GWP. The most promising option in the near-term is to reformulate salbutamol with either HFC 152a or HFO 1234ze(E). Not only will this ensure continuity of affordable care, there are sound commercial reasons for a company to make this investment. Two companies have made this commitment, but neither currently have a strong presence in reliever medication. For them, or other companies, now is the time to act; 2025 is not far away in terms of product development timescales and the climate cannot wait.
Acknowledgments
I am very grateful to Tim Noakes, of Koura, whose counsel proved invaluable in the discussions of propellant pricing, and also to Pierre Carlotti, Claire Jahan and their colleagues at Aptar, for assistance in analyzing the IQVIA database. Lastly, I would like to acknowledge the hard work and dedication of current and past colleagues on the Medical and Technical Options Committee of the UN Environmental Programme.
Disclosure
The author consults with a variety of clients, some of whom may be commercially impacted by regulations impacting the use of propellants in MDIs. He also has minority ownership of shares in pharmaceutical companies who develop, manufacture and sell respiratory products that are impacted by such regulations. He received no financial support in relation to the writing of this article.
References
- 1.Stein SW, Thiel CG. The history of therapeutic aerosols; a chronological review. J Aerosol Med Pulmon Drug Deliv. 2017;30:20–41. doi: 10.1089/jamp.2016.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritchard JN. The environmental impact of propellants – what now? In: Drug Delivery to the Lungs. 10-13 Bristol, UK: The Aerosol Society; 2019. [Google Scholar]
- 3.Intergovernmental Panel on Climate Change Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, Qin D, Plattner G-K, et al., Editors. Cambridge, United Kingdom: Cambridge University Press; 2013. 1535 pp [Google Scholar]
- 4.World Meteorological Organization. WMO Statement on the state of the global climate in 2018. Available from: https://library.wmo.int/doc_num.php?explnum_id=5789. Accessed July 17, 2020.
- 5.United National Environment Programme. Assessment report of the technology and economic assessment panel; 2014. Available from: https://ozone.unep.org/sites/default/files/2019-05/TEAP_Assessment_report_2014.pdf. Accessed July 17, 2020.
- 6.European Commission. Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006 Text with EEA Relevance. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0842&qid=1594982827385&from=EN. Accessed July 17, 2020.
- 7.European Environment Agency. Emissions and supply of fluorinated greenhouse gases in Europe. Available from: https://www.eea.europa.eu/data-and-maps/indicators/emissions-and-consumption-of-fluorinated-2/assessment-2. Accessed July 17, 2020.
- 8.United Nations Environment Program. The Kigali Amendment to the Montreal Protocol: HFC Phase-Down. OzonAction Fact Sheet OZFS/16/11_1. Paris, France: United Nations Environmental Program; 2016. [Google Scholar]
- 9.United Nations Technical and Economic Assessment Panel. Decision XXVII/4 task force update report; further information on alternatives to ozone depleting substances Volume I. Nairobi, Kenya; United Nations Environment Programme; 2016. Available from: https://ozone.unep.org/sites/default/files/2019-05/TEAP_TFXXVII-4_Report_September2016.pdf. Accessed July 17, 2020. [Google Scholar]
- 10.Sustainable development Unit, National Health Service, United Kingdom. Available from: https://www.sduhealth.org.uk/nhs%20long%20term%20plan/carbon-reduction/anaesthetics-and-inhalers.aspx. AccessedJuly 17, 2020.
- 11.Usmani OS, Scullion J, Keeley D. Our planet or our patients-is the sky the limit for inhaler choice? Lancet Respir Med. 2019;7:11–13. doi: 10.1016/S2213-2600(18)30497-1 [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Clinical and Healthcare Excellence. Patient decision aid; inhalers for Asthma; 2019. Available from: https://www.nice.org.uk/guidance/ng80/resources/inhalers-for-asthma-patient-decision-aid-pdf-6727144573. Accessed July 17, 2020.
- 13.Keeley D, Scullion JE, Usmani O. Minimising the environmental impact of inhaled therapies: problems with policy on low carbon inhalers. Eur Respir J. 2020;55:2001122. doi: 10.1183/13993003.00048-2020 [DOI] [PubMed] [Google Scholar]
- 14.GlaxoSmithKline. Understanding our 2017 value chain carbon footprint. Available from: https://www.gsk.com/en-gb/responsibility/environment/carbon. Accessed July 17, 2020.
- 15.Panigone S, Sandri F, Ferri R, et al. Environmental impact of inhalers for respiratory diseases: decreasing the carbon footprint while preserving patient-tailored treatment. BMJ Open Resp Res. 2020;7:e000571. doi: 10.1136/bmjresp-2020-000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partridge MR. Green respiratory healthcare: need for proportionality. Thorax. 2020;75:369. doi: 10.1136/thoraxjnl-2019-214315 [DOI] [PubMed] [Google Scholar]
- 17.Jeswani HK, Azapagic A. Life cycle environmental impacts of inhalers. J Cleaner Prod. 2019;237:117733. doi: 10.1016/j.jclepro.2019.117733 [DOI] [Google Scholar]
- 18.AstraZeneca. Investing in a sustainable future for patients with respiratory disease. Available from: https://www.astrazeneca.com/media-centre/articles/2020/investing-in-a-sustainable-future-for-patients-with-respiratory-disease.html. Accessed July 17, 2020.
- 19.Farmaceutici C. Chiesi outlines €350 million investment and announces first carbon minimal pressurised metered dose inhaler (pMDI) for Asthma and COPD 04/ 12/2019. Available from: https://www.chiesi.com/en/chiesi-outlines-350-million-investment-and-announces-first-carbon-minimal-pressurised-metered-dose-inhaler-pmdi-for-asthma-and-copd/. Accessed July 17, 2020.
- 20.Viswanathan R. Why Starbucks, Disney, and the EU are all shunning plastic straws. Available from: https://www.vox.com/2018/6/25/17488336/plastic-straw-ban-ocean-pollution. Accessed July 17, 2020.
- 21.Wilkinson AJK, Braggins R, Steinbach I, et al. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open. 2019;9:e028763. doi: 10.1136/bmjopen-2018-028763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritchard JN. What are meaningful device improvements for patients, providers and payers In: Dalby RN, Byron PR, Peart J, et al. editors. Respiratory Drug Delivery. River Grove, IL: Davis Healthcare International;2014;1: 217–228. [Google Scholar]
- 23.Normansell R, Kew KM, Mathioudakis AG. Interventions to improve inhaler technique for people with asthma. Cochrane Database Systematic Rev. 2017;3 Art. No.: CD012286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas M, Price D, Chrystyn H, et al. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009;9:1–10. doi: 10.1186/1471-2466-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjermer L. The importance of continuity in inhaler device choice for asthma and chronic obstructive pulmonary disease. Respiration. 2014;88:346–352. doi: 10.1159/000363771 [DOI] [PubMed] [Google Scholar]
- 26.Levy M, Dekhuijzen PNR, Barnes PJ, et al. Inhaler technique: facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). Primary Care Respir Med. 2016;26:16017. doi: 10.1038/npjpcrm.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muraki M, Gose K, Hanada S, et al. Which inhaled corticosteroid and long-acting β-agonist combination is better in patients with moderate-to-severe asthma, a dry powder inhaler or a pressurized metered-dose inhaler? Drug Deliv. 2017;24:1395–1400. doi: 10.1080/10717544.2017.1378937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir Med. 2011;105:1099–1103. doi: 10.1016/j.rmed.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 29.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2020 report. Available from: https://ginasthma.org/gina-reports/. Accessed July 17, 2020.
- 30.Global initiative for chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2020 Report. Available from: https://goldcopd.org/gold-reports/. Accessed July 17, 2020..
- 31.Bonini M, Usmani O. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1–9. [Google Scholar]
- 32.Liang C-Y, Chen Y-J, Sheu S-M, et al. Misuse of inhalers among COPD patients in a community hospital in Taiwan. Int J COPD. 2018;13:1309–1316. doi: 10.2147/COPD.S158864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thoracic Soc. 2017;14:1103–1107. doi: 10.1513/AnnalsATS.201702-156PS [DOI] [PubMed] [Google Scholar]
- 34.Loh CH, Peters SP, Lovings TM, et al. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14:1305–1311. doi: 10.1513/AnnalsATS.201611-903OC [DOI] [PubMed] [Google Scholar]
- 35.Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; Time for re-evaluation? Age Ageing. 2007;36:213–218. doi: 10.1093/ageing/afl174 [DOI] [PubMed] [Google Scholar]
- 36.Jenkins D, Johal J, Mahon J. P228 Analysis of the potential clinical impact of an environmentally driven transition from pressurised metered dose inhalers (pMDIs) to dry powder inhalers (DPIs). Thorax. 2019;74:A213-A214. [Google Scholar]
- 37.British Lung Foundation. Available from: https://statistics.blf.org.uk/copd. Accessed July 17, 2020.
- 38.Sullivan PW, Ghushchyan VH, Campbell JD, et al. Measuring the cost of poor asthma control and exacerbations. J Asthma. 2017;54:24–31. doi: 10.1080/02770903.2016.1194430 [DOI] [PubMed] [Google Scholar]
- 39.Ur Rehman A, Hassali MAA, Muhammad SA, et al. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: results from a systematic review of the literature. Expert Rev Pharmacoecon Outcomes Res. 2019. doi: 10.1080/14737167.2020.1678385 [DOI] [PubMed] [Google Scholar]
- 40.Center for Disease Control National Center for Health Statistics. Fast stats. Available from: https://www.cdc.gov/nchs/fastats. Accessed July17, 2020.
- 41.Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008–2013. Ann Amer Thoracic Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC [DOI] [PubMed] [Google Scholar]
- 42.Solvay Special Chemicals. HFA propellants for medical use. Available from: https://www.solvay.us/en/binaries/35442-237443.pdf. Accessed July 17, 2020.
- 43.Friel M, inventor; Aer Beatha Ltd assignee: canister and valve. United States Patent Application US20190315560A1. 2019. October 17.
- 44.Goldberg T. Strength to strength. World Aerosols. 2019;32–33. [Google Scholar]
- 45.Lindley AA, Noakes TJ. Consideration of hydrofluoroolefins (HFOs) as potential candidate medical propellants. Mexichem Fluor, 2010. Available from: https://www.zephex.co.uk/wp-content/uploads/2020/01/HFOs-as-candidate-medical-propellants.pdf. Accessed July 17, 2020 [Google Scholar]
- 46.Corr S, Noakes T. Pressurized metered dose inhaler propellants: going forward In: Dalby RN, Byron PR, Peart J, et al. editors. Respiratory Drug Delivery. River Grove, IL: DHI Publishing;2017;2: 255–258. [Google Scholar]
- 47.Corr S, Noakes TJ, inventors; Mexichem Amanco Holding SA de CV assignee. Compositions comprising salbutamol sulphate. United States Patent US9114164B2. 2015. August 25
- 48.Koura. ‘Green’ medical propellant receives FDA approval to proceed to clinical trials. Available from: https://www.prnewswire.com/news-releases/green-medical-propellant-receives-fda-approval-to-proceed-to-clinical-trials-300998598.html. Accessed July 17, 2020.
- 49.Vance C, Swalwell C, McIntyre IM. Deaths involving 1,1-difluoroethane at the San Diego County medical examiner’s office. J Anal Toxicol. 2012;36:626–633. doi: 10.1093/jat/bks074 [DOI] [PubMed] [Google Scholar]
- 50.Toon RC, Preedy EC, Prokopovich P. Formulating drugs for inhalers and stability issues. Eurasian Chem Tech J. 2012;14:271–286. doi: 10.18321/ectj124 [DOI] [Google Scholar]
- 51.Taylor G, Warren S, Tran C inventors; Cardiff Scintigraphics Ltd assignee. Pressurized metered dose inhalers and method of manufacture. United States Patent 9981092B2. 2018. May 29.
- 52.Howlett D. Balancing the needs of patients, regulators, and industry to satisfy the global demand for inhaled products In: Dalby RN, Peart J, Young PM, et al. editors. Respiratory Drug Delivery Asia 2018. River Grove: IL; DHI Publishing; 2018:119–128. [Google Scholar]
- 53.Pritchard JN. F-gas regulations and beyond: clinical and economic factors that will drive propellant transition In: Dalby RN, Byron PR, Peart J, et al. editors. Respiratory Drug Delivery . River Grove, IL: DHI Publishing;2020;1: 183–193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Meteorological Organization. WMO Statement on the state of the global climate in 2018. Available from: https://library.wmo.int/doc_num.php?explnum_id=5789. Accessed July 17, 2020.
- United National Environment Programme. Assessment report of the technology and economic assessment panel; 2014. Available from: https://ozone.unep.org/sites/default/files/2019-05/TEAP_Assessment_report_2014.pdf. Accessed July 17, 2020.
- European Commission. Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006 Text with EEA Relevance. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0842&qid=1594982827385&from=EN. Accessed July 17, 2020.
- European Environment Agency. Emissions and supply of fluorinated greenhouse gases in Europe. Available from: https://www.eea.europa.eu/data-and-maps/indicators/emissions-and-consumption-of-fluorinated-2/assessment-2. Accessed July 17, 2020.
- Sustainable development Unit, National Health Service, United Kingdom. Available from: https://www.sduhealth.org.uk/nhs%20long%20term%20plan/carbon-reduction/anaesthetics-and-inhalers.aspx. AccessedJuly 17, 2020.
- National Institute for Clinical and Healthcare Excellence. Patient decision aid; inhalers for Asthma; 2019. Available from: https://www.nice.org.uk/guidance/ng80/resources/inhalers-for-asthma-patient-decision-aid-pdf-6727144573. Accessed July 17, 2020.
- GlaxoSmithKline. Understanding our 2017 value chain carbon footprint. Available from: https://www.gsk.com/en-gb/responsibility/environment/carbon. Accessed July 17, 2020.
- AstraZeneca. Investing in a sustainable future for patients with respiratory disease. Available from: https://www.astrazeneca.com/media-centre/articles/2020/investing-in-a-sustainable-future-for-patients-with-respiratory-disease.html. Accessed July 17, 2020.
- Farmaceutici C. Chiesi outlines €350 million investment and announces first carbon minimal pressurised metered dose inhaler (pMDI) for Asthma and COPD 04/ 12/2019. Available from: https://www.chiesi.com/en/chiesi-outlines-350-million-investment-and-announces-first-carbon-minimal-pressurised-metered-dose-inhaler-pmdi-for-asthma-and-copd/. Accessed July 17, 2020.
- Viswanathan R. Why Starbucks, Disney, and the EU are all shunning plastic straws. Available from: https://www.vox.com/2018/6/25/17488336/plastic-straw-ban-ocean-pollution. Accessed July 17, 2020.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2020 report. Available from: https://ginasthma.org/gina-reports/. Accessed July 17, 2020.
- Global initiative for chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2020 Report. Available from: https://goldcopd.org/gold-reports/. Accessed July 17, 2020..
- British Lung Foundation. Available from: https://statistics.blf.org.uk/copd. Accessed July 17, 2020.
- Center for Disease Control National Center for Health Statistics. Fast stats. Available from: https://www.cdc.gov/nchs/fastats. Accessed July17, 2020.
- Solvay Special Chemicals. HFA propellants for medical use. Available from: https://www.solvay.us/en/binaries/35442-237443.pdf. Accessed July 17, 2020.
- Koura. ‘Green’ medical propellant receives FDA approval to proceed to clinical trials. Available from: https://www.prnewswire.com/news-releases/green-medical-propellant-receives-fda-approval-to-proceed-to-clinical-trials-300998598.html. Accessed July 17, 2020.