Abstract
A 75-year-old man presented to a French hospital with a 4-day fever after returning from a coronavirus disease-19 (COVID-19) cluster region. A reverse-transcription polymerase chain reaction test was positive for severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) using a nasopharyngeal swab sample. After he returned home and a telephone follow-up, he was found deceased 9 days after first showing symptoms. Whole-body, non-enhanced, post-mortem computed tomography (PMCT) and a forensic autopsy were performed approximately 48 h after death, with sanitary precautions. The PMCT showed bilateral and diffuse crazy-paving lung opacities, with bilateral pleural effusions. Post-mortem virology studies detected the presence of SARS-CoV-2 (B.1 lineage) in the nasopharynx, plasma, lung biopsies, pleural effusion and faeces confirming the persistence of viral ribonucleic acid 48 h after death. Microscopic examination showed that severe lung damage was responsible for his death. The main abnormality was diffuse alveolar damage, associated with different stages of inflammation and fibrosis. This case is one of the first to describe complete post-mortem data for a COVID-19 death and highlights the ability of PMCT to detect severe involvement of the lungs before autopsy in an apparently natural death. The present pathology results are concordant with previously reported findings and reinforce the disease pathogenesis hypothesis of combined viral replication with an inappropriate immune response.
Keywords: COVID-19, SARS-CoV-2 coronavirus, Post-mortem, Autopsy, Pathology, Post-mortem computed tomography
Introduction
Most patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) experience a mild form of the illness, but 5% experience a severe form resulting in 1.4% mortality [1].
The pathophysiological mechanisms of these severe infections are poorly understood [2]. SARS-CoV-2 infection has been reported to induce both direct organ damage [3, 4] and an inappropriate immune response that results in viral sepsis [5]. Infectious SARS-CoV-2 virus particles have been isolated from respiratory samples [6], but to date, there are few post-mortem reports about the possibility of broad dissemination of viral particles and possible impact on different organs [7, 8]. Such findings are essential for a better understanding of the disease and to provide new perspectives for future treatment (e.g. anti-viral and immunomodulator) trials. Here we report the full autopsy results for a 75-year-old man deceased from coronavirus disease-19 (COVID-19).
Clinical report
A 75-year-old man was admitted to a COVID-19 testing unit in a French regional hospital early in the pandemic. He presented for virus screening after experiencing 4 days of fever after returning from a disease cluster area. A family member was also hospitalized for SARS-CoV-2 infection. He had no relevant medical history, and his only medication was a laxative.
Upon arrival, blood pressure, heart rate and cardio-pulmonary auscultation were normal. The patient did not complain of dyspnoea, and oximetry with room air was 95%. He presented with hyperthermia (38 °C), moderate asthenia, diarrhoea and drowsiness. His body-mass index was normal (25 kg/m2). SARS-CoV-2 screening was performed by nasopharyngeal swab and COVID-19 was confirmed 24 h later by real-time reverse-transcription polymerase-chain reaction (RT-PCR). The threshold for hospitalization was not met, so the patient returned home with clinical monitoring instructions and follow-up by telephone. The blood sample taken 3 days before death showed lymphopenia (770/mm3) and an increase in C-reactive protein (163 mg/L). Polymorphonuclear neutrophils were slightly increased (7.38 109/L), and electrolytes were normal.
The telephone follow-up reported symptom stability and no fever on the seventh day following the first symptoms. On the ninth day after illness onset, the patient was found deceased in his bed at home by a close relative. A forensic investigation was required by a French prosecutor.
The present report was written in accordance with the Helsinki Declaration on Medical Research.
Post-mortem investigations
The first investigation was whole-body, non-enhanced, post-mortem computed tomography (PMCT) imaging approximately 24 h after death, with specific disinfection of the CT scanner. PMCT was performed using an Aquilion Prime scanner (Canon Medical Systems Corporation, Otawara, Tochigi, Japan), with soft tissue, bone and pulmonary parenchyma reconstructions. Scan parameters were 135 k, modulated mAs and a 1-mm slice thickness. A radiologist trained for pulmonary diseases and a forensic radiologist examined the images using a Carestream PACS Station (Carestream Health, Rochester, NY, USA) with multiplanar reconstructions.
After the PMCT, a complete forensic autopsy was performed by a forensic pathologist and a forensic assistant, in a negative room pressure. Access to the autopsy room was strongly limited during the examination according to published recommendations [9]. Personal protective equipment included a filtering facepiece type 2 (FFP2) medical mask, a scrub hat, two pairs of surgical gloves separated by a pair of cut-resistant gloves and a Tyvek® DuPont coverall covered by a surgical gown, protective eyewear and rubber boots. Normal autopsy technique was used, except that the trachea was clamped as a precaution before extracting the heart and lungs as a block. Organs were studied both in situ and individually on a dissection table.
The following samples were taken. (1) For virology purposes: a nasopharyngeal swab, percutaneous lumbar puncture sample, transthoracic blood puncture sample, rectal swab, pleural effusion sample, lung biopsy sample. (2) For bacteriology purposes: blood samples for culture and a lumbar puncture sample. (3) For histology: samples of the liver, kidneys, small intestine, colon, brain, heart and lungs were fixed in formalin.
Specialized disinfection of the autopsy room and the material it contained was performed using a disinfecting detergent (Anios Oxy’Floor, Anios Laboratories, Lille-Hellemmes, France) on the floors, the ceiling and surfaces. Every piece of equipment, used or unused, was discarded after the autopsy. The samples were conditioned in triple packaging, which was disinfected before being taken out of the autopsy room.
Pathological examination was performed on each organ. Microscopic examinations of the lungs were performed on the central and peripheral areas of each lobe, using sections stained with haematoxylin and eosin and a CD3 (T cell marker) immunohistochemical staining.
Real-time RT-PCR was performed using the nasopharyngeal swab, plasma, pleural effusion, lung biopsy, cerebrospinal fluid and faecal swab samples. Nasopharyngeal swab, pleural effusion and plasma sample were also sent to the National Reference Center for Respiratory Viruses (Lyon, France) for sequencing. An amplicon-based approach developed by the ARTIC network (https://artic.network/ncov-2019) and combined with Oxford Nanopore Technologies sequencing (Oxford, UK) was used.
Results
PMCT findings
Brain, cervical and abdominal areas showed no abnormalities other than normal post-mortem changes. No signs of putrefaction or traumatic injuries to bone or soft tissues were observed.
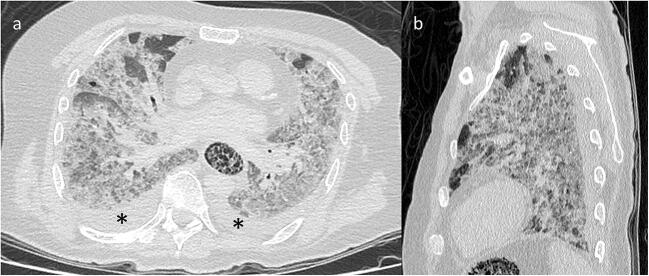
Examination of the thorax revealed bilateral pleural effusions but no pericardial effusion. A bilateral, multilobar crazy-paving pattern was observed in the lungs, defined by ground-glass opacity and intralobular and perilobular reticulations (Fig. 1). Approximately 85% of lung parenchyma was affected. The subpleural parenchyma was slightly consolidated, but the anterior portion of each lung field was spared. The trachea and bronchi were filled with liquid. The oesophagus presented with global and marked distension with undetermined spongiform contents. A few centimetre-sized nodes were present in the mediastinum, notably in the pre-tracheal area.
Fig. 1.
Post-mortem computed tomography and pulmonary parenchyma reconstruction. Axial (a) and sagittal (b) views showing diffuse, bilateral and panlobar ground-glass opacities associated with interlobular and intralobular septal thickening, subpleural consolidations and bilateral pleural effusions (asterisk). The anterior portions of both lungs were more likely to be spared
Autopsy findings
The post-mortem autopsy delay was estimated to be 48 h. External examination of the body showed no traumatic injuries to the skin. Internal exploration of the chest confirmed the bilateral pleural effusions totaling 200 mL of serohaematic fluid. The heart weighted 470 g and showed a moderate dilatation of the right ventricle, without increase of the myocardial wall thickness. Gross examination showed non-obstructive atherosclerotic plaques in the coronary arteries and the aortic bifurcation. Both lungs were slightly dense, but the liver, spleen, intestines, kidneys, pancreas and brain were macroscopically normal.
Microbiological analysis
SARS-CoV-2 was detected in all the samples except in cerebrospinal fluid. To quantify SARS-CoV-2 viral load, we used an external standard curve that revealed a higher viral load in respiratory samples (7.05 log10 copies/106 cells in nasopharyngeal swab, 5.52 log10 copies/106 cells in lung biopsy and 5.15 log10 copies/106 cells in pleural effusion sample) than in plasma (4.5 log10 copies/106 cells). The percentage of SARS-CoV-2 genome covered was 99.6%, 98.9% and 92.2% for the nasopharyngeal swab, pleural effusion and plasma sample, respectively. Multiple sequence alignment revealed that consensus sequences generated from the three samples were identical. Using the pangolin web application (https://pangolin.cog-uk.io), the sequences were assigned to the B.1 lineage currently circulating in Europe.
Bacteriological blood analysis identified gram-positive cocci (Staphylococcus schelfeiferi and S. epidermidis) considered to be consequent to post-mortem proliferation.
Pathological findings
The right and left lungs weighed 1044 g and 834 g, respectively. The main lung injury was bilateral diffuse alveolar damage. It appeared heterogeneous with identifiable stages: an acute stage (Fig. 2) defined by scattered or diffuse hyaline membranes, associated in some areas with alveolar oedema, an alveolar eosinophil exudate and a few vacuolated macrophages; and a more organized stage (Fig. 3) defined by parenchymal collapse and enlargement of alveolar septa, filled with incorporated alveolar fibrin deposits, relatively pronounced hyperplasia of type-2 pneumocytes, very sparse multinucleated giant cells and minor fibroblast proliferation. Squamous cell metaplasia was not observed. Immunohistology identified slight-to-moderate interstitial or perivascular infiltration by inflammatory TCD3+ lymphocytes. Alveolar infiltration by neutrophils was not observed and both capillaries and arterioles were thrombosis-free.
Fig. 2.
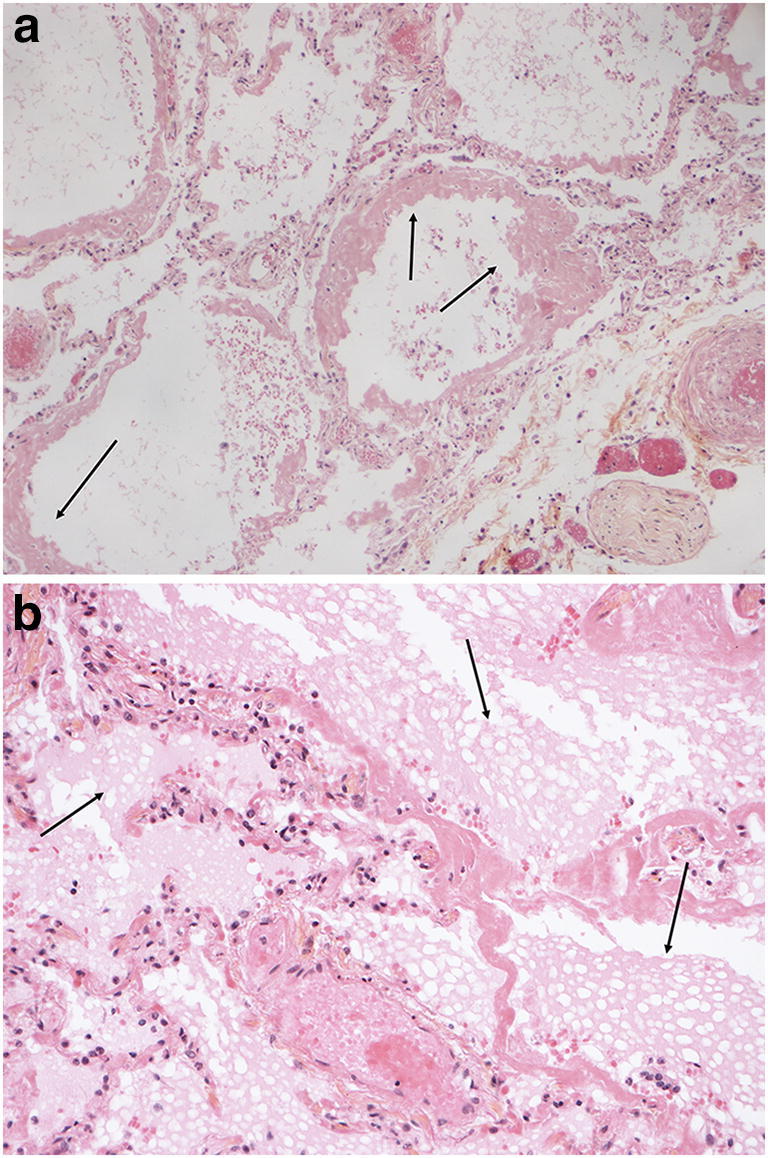
Microscopic findings in the lungs. (HES, magnification × 20). Diffuse alveolar damage in the acute (exudative) stage. a Hyaline membranes (arrow). b Hyaline membranes and alveolar vacuolated exudates (arrow)
Fig. 3.
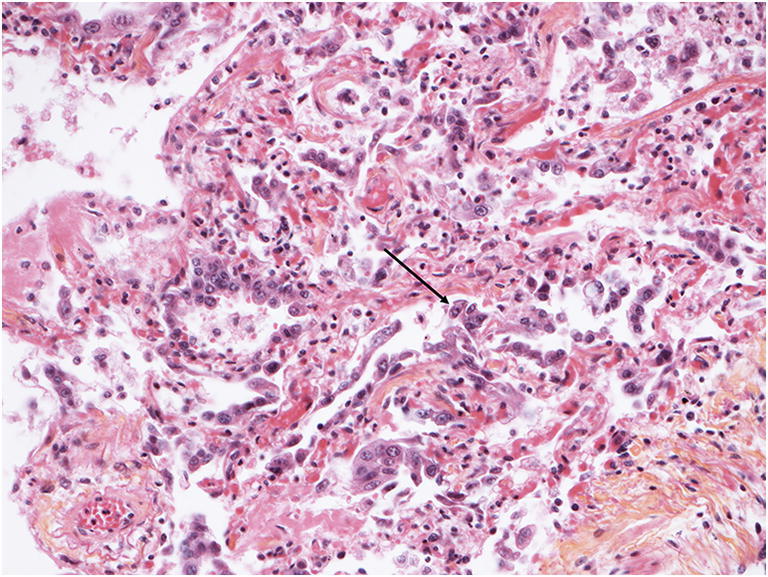
Microscopic findings in the lungs. (HES, magnification × 20). Diffuse alveolar damage in a more organized stage. Note the enlargement of the alveolar septa, fibrin deposition and incorporation, intraalveolar exudates and a type-2 pneumocyte hyperplasia (arrow) representing patchy, interstitial chronic inflammation
Other pathological findings included mild hepatic steatosis and confirmed a mild coronary artery atherosclerosis. Heart sections showed no evidence of myocarditis but displayed some scattered wavy fibres. No other microscopic anomalies were found in other organs, especially brain and kidney vasculitis or disseminated intravascular coagulation.
Discussion
Currently, post-mortem COVID-19 pathology and virology findings are rare. Clinical and forensic COVID-19 autopsies are usually not required for such deaths, especially when such diagnoses are made before death and given the considerable logistical constraints deployed to limit the risk of virus transmission. Here we report complete post-mortem data following the natural evolution of untreated COVID-19.
Considering the subject’s clinical history and absence of other acute or chronic diseases detected by histological examination, the main hypothesis was that death resulted from the consequences of SARS-CoV-2 infection. Diffuse alveolar damage led to hypoxemia, resulting in polypnea. Cardiac arrest may have occurred when compensatory mechanisms were exhausted. Cardiac abnormalities, asymptomatic during the individual’s lifetime, may also have been responsible for a poorer hemodynamic response to hypoxia. Electrolyte disturbances may have been associated. Pulmonary embolism, frequently observed during the evolution of the disease [10], was excluded by both gross and microscopic examinations.
The histological findings were consistent with those of previous studies [7, 8, 11], and very similar to those viral pneumonia findings associated with Middle East respiratory syndrome and SARS-CoV-1 [12]. In addition to diffuse alveolar damage, different microscopic patterning was observed that may illustrate the progression and pathogenesis of the disease. In some areas, an acute exudate pattern (fibrin deposition and/or alveolar exudates) appeared to be the consequence of early-stage damage to the capillary endothelium and alveolar epithelium. In other areas, there was a more organized pattern, with enlargement of alveolar septa and pronounced desquamated type-2 pneumocyte hyperplasia, fibrin deposition/incorporation into alveolar septa and some interstitial fibrosis with minimal fibroblast proliferation. These changes suggest the beginning of a healing process 1 week after disease progression. Moreover, the presence of a T lymphocyte infiltrate may reflect an early immune response that increases extracellular matrix remodelling and contributed to the fibrosis. These results reinforce the hypothesis of direct pathogenesis due to viral replication combined with an excessive immune response [13, 14]. The autopsy allowed an analysis of almost all the organs, but there was no evidence for multi-organ damage.
Concerning the post-mortem virology data, this case demonstrated that RNA from SARS-CoV-2 was still detectable in blood, faeces, the lungs and the upper airways more than 48 h after death. Given the current status of viral persistence on inanimate surfaces [15], this finding should be interpreted cautiously: viral presence is not the same as viral infectivity. Only viral cultures from post-mortem samples would reliably demonstrate viral survival in the deceased. No support was found for neurological involvement during the infection [16, 17] as virus was not detected in cerebrospinal fluid. Sequencing brought no arguments for a variation of the viral sequence between airway and plasma. The European lineage was the one expected in this part of the world [18].
In addition, we have demonstrated the first post-mortem imaging of a COVID-19 deceased person. Normally, there are many post-mortem changes, and some can mimic pulmonary diseases observed in the living [19]. The most frequent post-mortem changes in the lungs are diffuse ground-glass opacities sometimes associated with posterior consolidation. These features are very close to those described in COVID-19 chest CT scans and could imitate or mask them [20]. For this reason, PMCT would probably be inefficient for detecting moderate or asymptomatic COVID-19 in a deceased person. In the present case, the predominant pattern described as crazy paving was indicative of COVID-19, especially as there was no other pre-existing pulmonary pathology [21–23]. The very extensive distribution in both lungs was reminiscent of previously described white lungs in severe cases [24]. Pleural effusion is an uncommon finding in moderate COVID-19 but is associated with severe and refractory COVID-19 pneumonia [25]. In contrast, tracheal and bronchial findings should not be used for diagnosis as they are subject to many post-mortem changes due to passive mobilization of gastric fluid [26]. The present data highlight the potential for PMCT to orient a pathologist investigating an apparently natural death, measures for preventing contamination of personnel and facilities and guidance for forensic pathologists to harvest appropriate virology and histology samples.
Conclusion
Collecting post-mortem information adds to the knowledge base for this new virus. This report provides additional descriptions of microscopic findings in COVID-19 and illustrates different stages of disease progression. It also highlights the possible role of PMCT for detecting severe and potentially fatal COVID-19, provided that images are read by a trained forensic radiologist. It also confirms virology data on post-mortem viral persistence in the respiratory tract and supports recommendations for infection prevention when performing an autopsy. Further cases are needed for a better understanding of, treatment of, and hopefully prevention of COVID-19.
Acknowledgements
The authors gratefully acknowledge Jean-Louis VAN GRIMBERGHE and Dr. David LEBOSSE for their support.
Abbreviations
- COVID-19
Coronavirus disease-19
- HES
Haematoxylin and eosin stain
- MERS
Middle East respiratory syndrome
- PMCT
Post-mortem computed tomography
- RNA
Ribonucleic acid
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SARS-CoV 2
Severe acute respiratory syndrome–associated coronavirus 2
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guan W, Ni Z, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamming I, Timens W, Bulthuis M, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD, Thackray LB, Young MD, Mason RJ, Ambrosino DM, Wentworth DE, DeMartini JC, Holmes KV. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin G-L, McGinley JP, Drysdale SB, Pollard AJ (2018) Epidemiology and immune pathogenesis of viral sepsis. Front Immunol 9. 10.3389/fimmu.2018.02147 [DOI] [PMC free article] [PubMed]
- 6.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao S-Y (2020) Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed]
- 9.Hanley B, Lucas SB, Youd E, Swift B, Osborn M (2020) Autopsy in suspected COVID-19 cases. J Clin Pathol jclinpath: 2020–206522. 10.1136/jclinpath-2020-206522 [DOI] [PubMed]
- 10.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E (2020) Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology 201544. 10.1148/radiol.2020201544 [DOI] [PMC free article] [PubMed]
- 11.Carsana L, Sonzogni A, Nasr A, et al (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis S1473309920304345. 10.1016/S1473-3099(20)30434-5 [DOI] [PMC free article] [PubMed]
- 12.Franks TJ, Chong PY, Chui P, Galvin JR, Lourens RM, Reid AH, Selbs E, Mcevoy CPL, Hayden CDL, Fukuoka J, Taubenberger JK, Travis WD. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo AW, Tang NL, Toh K-F. How the SARS coronavirus causes disease: host or organism? J Pathol. 2006;208:142–151. doi: 10.1002/path.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Liu L, Zhang D, et al (2020) SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet S014067362030920X. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed]
- 15.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun S0889159120303573. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed]
- 17.Li Y, Bai W, Hashikawa T (2020) The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J Med Virol jmv.25728. 10.1002/jmv.25728
- 18.van Dorp L, Acman M, Richard D, Shaw LP, Ford CE, Ormond L, Owen CJ, Pang J, Tan CCS, Boshier FAT, Ortiz AT, Balloux F. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol. 2020;83:104351. doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filograna L, Thali MJ. Post-mortem CT imaging of the lungs: pathological versus non-pathological findings. Radiol Med (Torino) 2017;122:902–908. doi: 10.1007/s11547-017-0802-2. [DOI] [PubMed] [Google Scholar]
- 20.Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao W, Zhong Z, Xie X, Yu Q, Liu J (2020) Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am J Roentgenol 1–6. 10.2214/AJR.20.22976 [DOI] [PubMed]
- 22.Xu Y-H, Dong J-H, An W-M, Lv X-Y, Yin X-P, Zhang J-Z, Dong L, Ma X, Zhang H-J, Gao B-L. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Inf Secur. 2020;80:394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo P, Xing Y, Xiao Y et al (2020) Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis:ciaa270. 10.1093/cid/ciaa270
- 26.Knight BH. The significance of the postmortem discovery of gastric contents in the air passages. Forensic Sci. 1975;6:229–234. doi: 10.1016/0300-9432(75)90014-x. [DOI] [PubMed] [Google Scholar]