Abstract.
The efficacy of commonly used antibiotics for treating severe cholera has been compromised over time because of the reduced antibiotic susceptibility. This study aimed to describe the rate of detection of Vibrio cholerae O1 from fecal samples and antimicrobial susceptibility profiles of V. cholerae O1 serotypes to commonly used antibiotics. During January 2000–December 2018, V. cholerae O1 was detected in fecal samples of 7,472 patients. Vibrio cholerae O1 Inaba serotype was predominant, ranging from 60% to 86% during the period 2000–2006 except for 2003 and 2005 when the Ogawa serotype was predominant. Later on, the Ogawa serotype became predominant from 2007 to 2015, fluctuating between 52% and 100%. However, in 2016 and 2017, isolation rates declined to 2% and 1%, respectively, but surged again to 75% in 2018. Nearly 100% of V. cholerae O1 strains were sensitive to tetracycline during 2000–2004. Thereafter, a declining trend of sensitivity was observed to be continued and dropped down to < 6% during 2012–2017 and again increased to 76% in 2018. Susceptibility to azithromycin and ciprofloxacin was nearly 100%, and susceptibility to cotrimoxazole and furazolidone was 01% throughout the study period. We also found the emergence of resistance to erythromycin in 2005 and sensitivity to cotrimoxazole in 2018. Thus, the rapid decline of the sensitivity of V. cholerae O1 to tetracycline and a reversed peak after 6 years need continued monitoring and reporting.
INTRODUCTION
Cholera is known to be caused by the toxigenic strains of Vibrio cholerae, which belongs to O1 or O139 serogroups. Vibrio cholerae O1 is also classified into two biotypes, Classical and El Tor, and two surviving serotypes, Inaba and Ogawa (another unstable serotype is Hikojima).1 The disease is caused by ingestion of V. cholerae, commonly present in contaminated water and food.2 Globally, epidemics and endemics of cholera have been reported in 47 countries.3 Bangladesh is well known for endemicity of cholera that breaks out in epidemic proportion almost every year. Two peaks are observed each year in Dhaka, one in the hot summer and another during the fall.4 Cholera is characterized by the passage of voluminous watery stools and most often accompanied by profuse vomiting; thus, the patient becomes dehydrated, may even develop hypovolemic shock, and, if not properly treated, death may occur within few hours of onset.5 The most important part of the treatment of cholera is rehydration therapy and replacement of comparable amount of water and salts lost in stool. The rehydration and replacement is performed using intravenous (I.V) fluid or oral rehydration salt solution as recommended by the WHO.6 Antibiotic treatment is recommended to reduce the total stool volume and limit the duration of fecal excretion of V. cholerae.1,2
Tetracycline is a broad-spectrum antibiotic compound that has a common basic structure and is either isolated directly from several species of Streptomyces bacteria or produced semi-synthetically from those isolated compounds. Doxycycline is one of the semi-synthetic, second-generation, long-acting tetracyclines. Erythromycin and azithromycin are macrolides (bacteriostatic, inhibit bacterial protein synthesis, a class of natural product, and consist of a large macrocyclic ring). Tetracycline, doxycycline, and erythromycin were the antibiotics of choice for treating severe cholera in Bangladesh before late 2004, except young children and pregnant women.1,2,4,7 However, the effectiveness of these commonly used antimicrobials in treating cholera suddenly lost credibility because of development of resistance. Later on, ciprofloxacin became the drug of choice in treating cases of cholera caused by V. cholerae O1 or O139 serotypes because of its significant susceptibility. Whatsoever, soon the clinical outcome turned out to be poor because of the observed substantially decreased susceptibility and a higher increase in minimum inhibitory concentration (MIC) of the drug.7 Currently, single oral dose of azithromycin is the antimicrobial of choice for managing cholera in children and adults in Bangladesh.8,9 Recent published literature exhibits worldwide public health threats and concerns for the consequences of antimicrobial resistance. Such expanding resistant infections are overburdening the economy of individuals and health systems persistently. Individuals with resistant infections are more prone to hospital visits, longer hospital stays, deferred recovery, excess medical cost, and higher case fatality.10 Increasing antimicrobial resistance in V. cholerae infections has become an emerging public health concern in cholera-endemic settings of low- and middle-income countries. Changes in susceptibility of various commonly used antimicrobials in cholera have been reported over time in the recent past. This warrants the necessity of regular monitoring and reporting of the current sensitivity status of various antibiotics so that appropriate drug therapy can be prescribed in treating cases of cholera following a more realistic approach. Thus, such efforts would help clinicians to detect the antimicrobial resistance pattern of cholera. Simultaneously, this would help the policy makers and public health experts to revise the treatment guideline of cholera and keep them updated about the current strains. Ultimately, this will help clinicians in treating cholera cases with appropriate antibiotics.
This study was undertaken to describe the antimicrobial susceptibility patterns of V. cholerae O1 serotypes Inaba and Ogawa against commonly used antibiotics (tetracycline, ciprofloxacin, azithromycin, cotrimoxazole, furazolidone, and erythromycin) for the treatment of cholera in patients who sought clinical care from the Dhaka Hospital of International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), over the last 19 years.
METHODS
Study site.
The Dhaka Hospital of the icddr,b, established in 1962, is a specialized research and training health facility which primarily provides cost-free care to all patients, mostly from poor socioeconomic strata, presenting with diarrheal illnesses. For the last 10 years, patient attendance ranged between 140,000 and 170,000 each year. The catchment area consisted of the Dhaka metropolitan city and its outer edge, although some patients seek care from rural areas, too. Since 1979, the icddr,b has been running a hospital-based diarrheal disease surveillance system (DDSS) that systematically samples patients to collect information using a structured questionnaire. Relevant information includes socioeconomic and demographic characteristics, housing and environment situation, feeding behaviors of infants and young children, and use of drugs and oral rehydration therapy at home before seeking care from the facility. Information on presenting features, nutritional measurements, and treatments received during hospital stay and their outcome are also recorded on prescribed forms.
Extensive microbiological assessments of fecal samples (culture and ELISA) are performed to identify diarrheal pathogens. The diarrheal disease surveillance system also monitors antimicrobial susceptibility of common bacterial pathogens, including V. cholerae. The activity provides valuable information to hospital clinicians in their clinical decision-making processes and enables the icddr,b to detect the emergence of new pathogens as well as early identification of outbreaks and their locations, thereby alerting the host government to take adequate and appropriate preventive and control measures. The system also monitors changes in patients’ characteristics and antimicrobial susceptibility of bacterial pathogens. These population-based surveillance data constitute an important database for conducting epidemiological studies, validating the result of clinical studies, developing new research ideas and study designs, improving patient treatment strategies, and introducing preventive programs. All these activities have been approved by the Research Review Committee and Ethical Review Committee, collectively known as the Institutional Review Board of the icddr,b.11
Study period.
Relevant data collected from all patients enrolled in the DDSS of the Dhaka Hospital of the icddr,b between January 2000 and December 2018 were used to form the database for this analysis.
Study population.
The study population consisted of patients of all ages admitted with diarrheal disease (three or more loose stools per 24 hours) and enrolled in the DDSS of the Dhaka Hospital of the icddr,b during the study period.
Laboratory methods.
A single, fresh, stool specimen was collected from all enrolled patients and submitted immediately to the clinical microbiology laboratory in Dhaka. All stool samples were routinely screened for common enteric pathogens, including Enterotoxigenic Escherichia coli,12 V. cholerae,13 Shigella spp.,13 and rotavirus14 following standard laboratory procedures. Isolation, identification, serogrouping, and biotyping of V. cholerae were performed using standard laboratory procedures.15,16 Susceptibility to antimicrobials was determined by the standard disc diffusion method on Muller–Hinton agar with commercial discs (BD, Becton, Dickinson, and Company, Franklin Lakes, NJ), and the results were reported as S, I, R (sensitive, intermediate, and resistant) by a method based on the cutoff of the zone size for different antibiotics according to the latest available Clinical and Laboratory Standards Institute guidelines for V. cholerae.13,17
Data analysis.
Data analysis was performed using the Statistical Package for the Social Sciences, version 20.0 Windows (SPSS, Chicago, IL). Data were summarized. Statistical analyses included descriptive methods, including percentages of detection, serotype distribution, and susceptibility of tetracycline, azithromycin, ciprofloxacin, cotrimoxazole, furazolidone, and erythromycin.
RESULTS
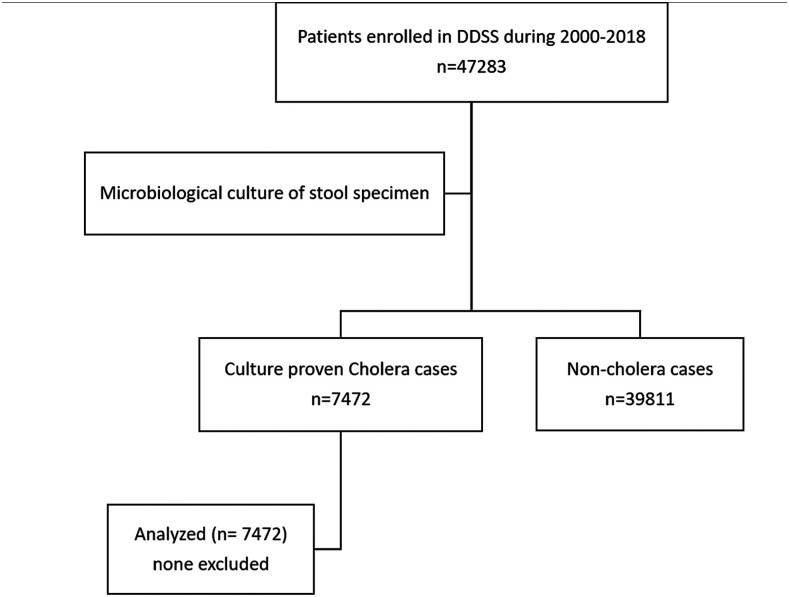
A total of 47,283 diarrheal patients (irrespective of age and gender) were enrolled in the DDSS during the study period; among them, 7,472 (16%) had microbiologically proven cholera (Figure 1). Of the 7,472 patients, 4,391 (59%) were adults and 3,081 (41%) were children (< 18 years). Among the children, 1,553 (21%) were younger than 5 years. The male-to-female ratio in both adults and under-five children was 1.3:1.
Figure 1.
Study flowchart summarizing the selection and inclusion processes of the study.
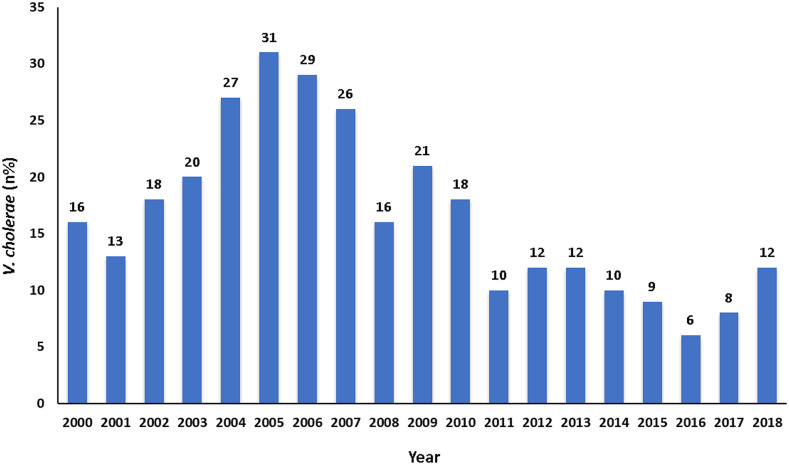
Figure 2 shows the overall patterns of the isolation rate of V. cholerae O1. The rate of isolation ranged between 13% and 31% during 2000–2005, and it was 31% in 2005 (which was higher during the study period). In the successive years, the isolation rate of V. cholerae O1 gradually decreased, and it was only 6% in 2016. Thereafter, an increasing trend was observed, and it was 12% in 2018.
Figure 2.
Rate of isolation of Vibrio cholerae O1 by standard laboratory methods at Dhaka Hospital from 2000 to 2018. This figure appears in color at www.ajtmh.org.
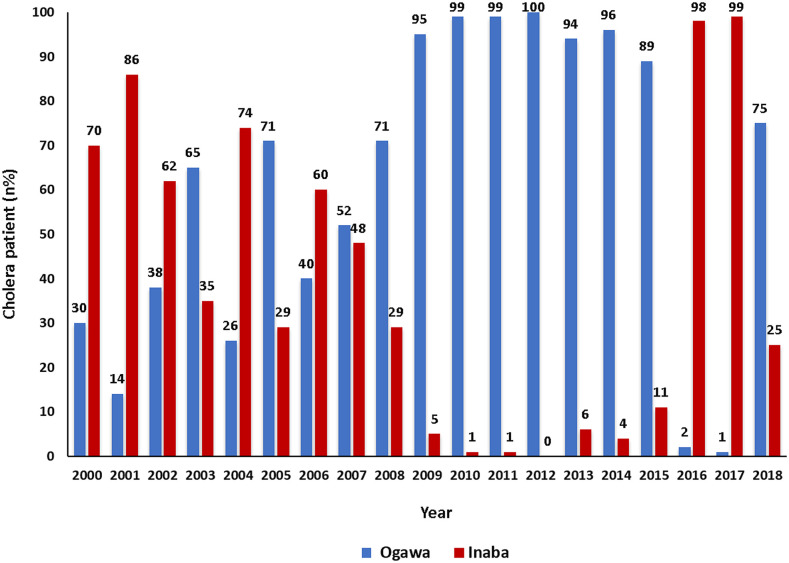
Figure 3 shows the pattern of detection rates of V. cholerae O1 in Dhaka Hospital. Throughout January 2000 and December 2006, V. cholerae O1 Inaba was the predominant serotype; however, in 2003 and 2005, the prime serotype was V. cholerae O1 Ogawa. During January 2007–December 2015, Ogawa was the predominant serotype (ranged from 52% to 100%). However, in 2016 and 2017, a sharp decline in the isolation rate of V. cholerae O1 Ogawa (2% in 2016 and 1% in 2017) was observed, followed by a sharp increase in the detection of Ogawa serotype in 2018, and it was as high as 75%. A reversal in the detection of Inaba strains was noted, and the rates were 98% and 99% in 2016 and 2017, respectively. However, the detection rate declined to 25% in 2018.
Figure 3.
Rate of isolation of Vibrio cholerae O1 (Ogawa and Inaba) by standard laboratory methods at Dhaka Hospital from 2000 to 2018. This figure appears in color at www.ajtmh.org.
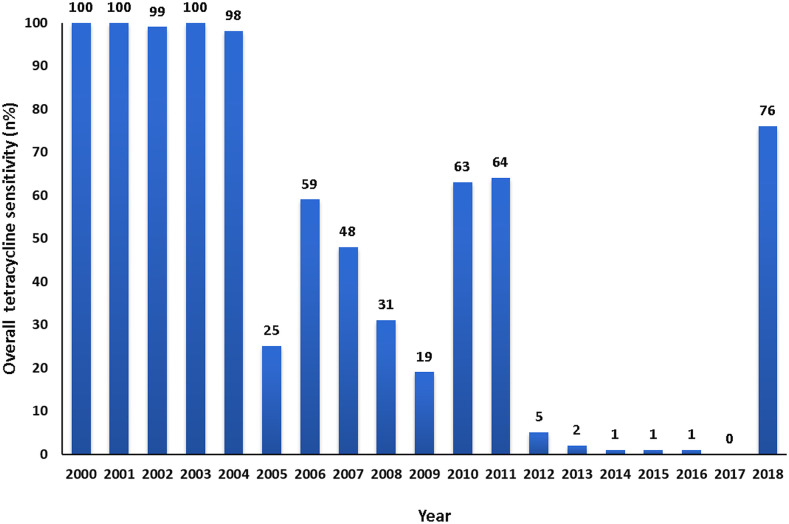
The isolated V. cholerae O1 strains were examined for susceptibility to tetracycline. It was nearly 100% throughout 2000 and 2004. Afterward, a declining trend was observed. In 2009, only 19% of the isolates of V. cholerae O1 were sensitive to tetracycline, but during 2010 and 2011, susceptibility to tetracycline increased to 63% and 64%, respectively. During the next 6 years, there was a decline in tetracycline susceptibility, and it decreased to as low as 0.4% in 2017. However, a dramatic change in susceptibility patterns was observed throughout the year 2018, and sensitivity to tetracycline reached as high as 76% in 2018 (Figure 4).
Figure 4.
Sensitivity pattern of Vibrio cholerae O1 to tetracycline by standard disc diffusion methods on Muller–Hinton agar with commercial discs from 2000 to 2018. This figure appears in color at www.ajtmh.org.
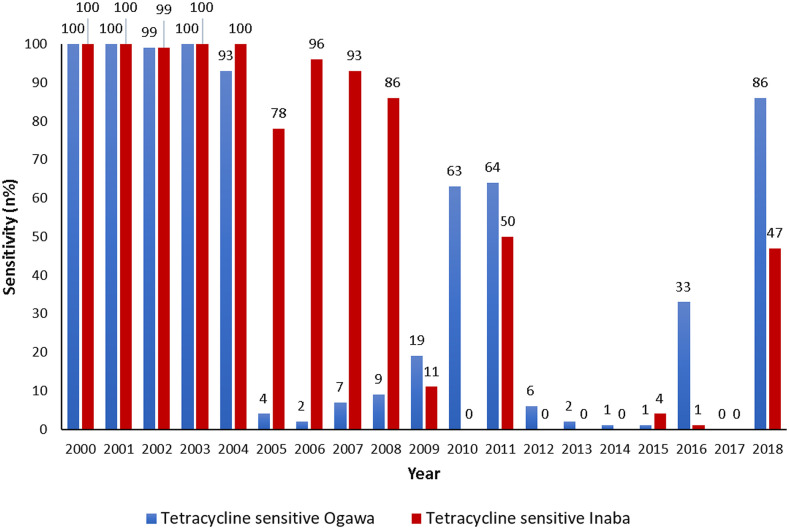
The susceptibility pattern of V. cholerae O1 Ogawa and Inaba to tetracycline was similar during the period January 2000 through December 2004, and it was nearly 100%. Thereafter, a sharp decline in the sensitivity pattern of V. cholerae O1 Ogawa was observed, which continued for the next few years. Figure 5 shows the reversal of the susceptibility pattern of V. cholerae O1 Ogawa serotype (86%) to tetracycline in Dhaka in the year 2018. Such reversal of the susceptibility pattern of serotype Inaba to tetracycline was also found to be as high as 47% in 2018.
Figure 5.
Sensitivity pattern of Vibrio cholerae O1 (Ogawa and Inaba) to tetracycline by standard disc diffusion methods on Muller–Hinton agar with commercial discs from 2000 to 2018. This figure appears in color at www.ajtmh.org.
Vibrio cholerae O1 isolates were also examined for susceptibility to azithromycin, ciprofloxacin, cotrimoxazole, furazolidone, and erythromycin. The susceptibility pattern was uniform for azithromycin and ciprofloxacin throughout the study period, which was nearly 100%, except for V. cholerae O1 Ogawa in 2010; during that period, the susceptibility to azithromycin was 69% (Table 1). A reverse scenario was observed for cotrimoxazole and furazolidone. The susceptibility was 01% for both the antibiotics, although a rising susceptibility pattern was observed for V. cholerae O1 Inaba to cotrimoxazole, which was nearly 76% in 2018. We stopped consistently testing for sensitivity to furazolidone after 2008 because of its high resistance levels. Erythromycin showed a higher rate of susceptibility up to 2005 and then a sharp decline to nearly 0% in the rest of the study period (Table 1).
Table 1.
Sensitivity pattern of Vibrio cholerae O1 (Inaba and Ogawa) to azithromycin, ciprofloxacin, cotrimoxazole, furazolidone, and erythromycin during 2000–2018
| Azithromycin | Ciprofloxacin | Cotrimoxazole | Furazolidone | Erythromycin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inaba | Ogawa | Inaba | Ogawa | Inaba | Ogawa | Inaba | Ogawa | Inaba | Ogawa | |
| 2000 | Not done | Not done | 99.6% (236/237) | 100% (104/104) | 0.0% (0/237) | 1.0% (1/104) | 0.0% (0/237) | 1.0% (1/104) | 99.6% (236/237) | 99% (103/104) |
| 2001 | 100% (200/200) | 100% (34/34) | 0.5% (1/200) | 0.0% (0/34) | 0.0% (0/200) | 0.0% (0/34) | 100% (200/200) | 100% (34/34) | ||
| 2002 | 100% (226/226) | 100 (136/136) | 0.9% (2/226) | 0.7% (1/136) | 0.4% (1/226) | 0.0% (0/136) | 99.6% (225/226) | 99.3% (135/136) | ||
| 2003 | 100% (122/122) | 100% (232/232) | 0.8% (1/122) | 0.4% (1/232) | 0.0% (0/122) | 0.0% (0/232) | 100% (122/122) | 99.6% (231/232) | ||
| 2004 | 100% (447/447) | 100% (160/160) | 0.2% (1/447) | 1.2% (2/160) | 0.0% (0/447) | 0.0% (0/160) | 99.8% (446/447) | 92.5% (148/160) | ||
| 2005 | 100% (196/196) | 100% (488/488) | 2.0% (4/196) | 0.4% (2/488) | 0.5% (1/196) | 0.4% (2/488) | 79.1% (155/196) | 24.6% (120/488) | ||
| 2006 | 100% (372/372) | 100% (245/245) | 0.8% (3/372) | 0.4% (1/245) | 0.0% (0/372) | 0.0% (0/245) | 0.0% (0/372) | 1.6% (4/245) | ||
| 2007 | 100% (301/301) | 99.7% (330/331) | 0.0% (0/301) | 0.0% (0/331) | 0.0% (0/301) | 0.0% (0/331) | 0.0% (0/301) | 0.0% (0/331) | ||
| 2008 | 100% (113/113) | 100% (276/276) | 0.0% (0/113) | 2.2% (6/276) | 0.0% (0/113) | 0.0% (0/276) | 0.0% (0/113) | 0.0% (0/276) | ||
| 2009 | 100% (27/27) | 99.7% (571/573) | 0.0% (0/27) | 0.7% (4/573) | Not done | Not done | 0.0% (0/27) | 0.0% (0/573) | ||
| 2010 | 100% (3/3) | 68.6% (262/382) | 100% (3/3) | 99.6% (509/511) | 0.0% (0/3) | 1.6% (8/511) | 0.0% (0/3) | 0.4% (2/511) | ||
| 2011 | 100% (2/2) | 100% (249/249) | 100% (2/2) | 99.6% (248/249) | 0.0% (0/2) | 3.6% (9/249) | 0.0% (0/2) | 0.4% (1/249) | ||
| 2012 | 100% (1/1) | 98.5% (325/330) | 100% (1/1) | 99.1% (327/330) | 0.0% (0/1) | 1.2% (4/330) | 0.0% (0/1) | 0.9% (3/330) | ||
| 2013 | 100% (15/15) | 99.2% (254/256) | 100% (15/15) | 99.2% (254/256) | 0.0% (0/15) | 1.2% (3/256) | 0.0% (0/15) | 0.8% (2/256) | ||
| 2014 | 100% (12/12) | 98.9% (263/266) | 100% (12/12) | 98.9% (263/266) | 0.0% (0/12) | 0.4% (1/266) | 0.0% (0/12) | 0.4% (1/266) | ||
| 2015 | 100% (27/27) | 99.1% (210/212) | 100% (27/27) | 99.5% (211/212) | 3.7% (1/27) | 0.0% (0/212) | 0.0% (0/27) | 0.0% (0/212) | ||
| 2016 | 98.7% (148/150) | 100% (3/3) | 98.7% (148/150) | 100% (3/3) | 1.3% (2/150) | 0.0% (0/3) | 0.7% (1/150) | 0.0% (0/3) | ||
| 2017 | 99.6% (251/252) | 100% (2/2) | 100% (252/252) | 100% (2/2) | 0.8% (2/252) | 0.0% (0/2) | 0.0% (0/252) | 0.0% (0/2) | ||
| 2018 | 98.9% (90/91) | 98.5% (266/270) | 96.7% (88/91) | 98.5% (266/270) | 75.8% (69/91) | 2.6% (7/270) | 1.9% (1/54) | 0.0% (0/226) | ||
DISCUSSION
Various studies have reported changing drug susceptibility patterns of bacterial pathogens which are increasingly becoming more resistant to commonly used antimicrobial agents.18,19 Such increasing antimicrobial resistance poses a detrimental effect on global health and the country’s development in general. These undesirable effects are taking place regardless of age, gender, and geographic location.20,21 The emergence and spread of resistance are more extensive where antibiotics are readily available for human and animal use without any prescription.22,23 Moreover, in countries lacking standard antibiotic treatment guidelines, antibiotics are overused by the general population because of their ready availability from drug stores without any prescription and frequent over-prescribing by health workers and veterinarians.24 Contaminated soil and water of surroundings can cause the spread of drug-resistant bacteria present in animal feces.23 Treatment for a wide range of infectious diseases has become less effective in many parts of the world because of rapidly increasing antimicrobial resistance against conventional antibiotics.25 Development of antibiotic resistance occurs either spontaneously via random mutation of genes as a natural selection process or by transfer of the genetic information from bacteria by plasmid exchanges following a horizontal manner (individual to individual). It has been reported that the absence of antibiotic pressure causes loss of a resistance allele that leads to reversal of antibiotic resistance.10,21 Studies have indicated that V. cholerae can acquire resistance genes from resistant bacteria present in the environment either by close contact or from commensals or other bacterial pathogens present in the human gut. Thus, antimicrobial resistance can render the treatment of cases of cholera difficult.10,26
A study conducted in the Alborz Province of Iran during the cholera outbreak of 2011 reported that among the rectal swab specimens of diarrheal patients examined (n = 9,844), 244 (2.5%) reported growth of V. cholerae O1. All cases belonged to serotype Ogawa.27 The antibiotic susceptibility to tetracycline in that study was 81.2%, which was similar to the findings of our study in 2018. A comparable result was found in Mozambique during cholera outbreaks that occurred from 2012 to 2015. It was revealed that in 2012, 25% of the V. cholerae O1 El Tor Ogawa isolates were resistant to tetracycline, which increased to 100% in the case of doxycycline in 2015.28 Another study conducted in a rural area of Mozambique during 2002–2004 showed that 97.3% of V. cholerae O1 Ogawa isolates were resistant to tetracycline. None of the isolates was resistant to ciprofloxacin.29 On the other hand, in our study, the sensitivity pattern of V. cholerae O1 Ogawa to tetracycline reversed. In Kelantan, Malaysia, during 1992 and 1994, all the V. cholerae isolates tested (n = 37) for drug susceptibility were sensitive to tetracycline. During the 1998 outbreak, the treating physicians observed that the duration of illness was prolonged in some of the cases, which prompted antibiotic sensitivity testing and revealed that only 12.5% (3/24) of isolates of V. cholerae were sensitive to tetracycline.30
In December 1979, V. cholerae O1 resistant to tetracycline, ampicillin, kanamycin, streptomycin, and cotrimoxazole (trimethoprim–sulfamethoxazole) was observed in cholera patients seeking care from the Matlab Hospital of the icddr,b, located in rural Bangladesh.31 In November 2004, once more multidrug-resistant strains of V. cholerae (strains resistant to furazolidone, cotrimoxazole [trimethoprim– sulphamethoxazole], tetracycline, and erythromycin) were isolated in Dhaka Hospital among both Ogawa (13%) and Inaba (5%) serotypes. By February 2005, all the Ogawa isolates became multidrug resistant. For the first time, a unique multidrug resistance due to resistance to erythromycin among V. cholerae O1 in Bangladesh was encountered.7 A study noted a consistent increase in the median MIC (ciprofloxacin) of V. cholerae O1 strains detected at the Dhaka Hospital of the icddr,b over the years: 0.003 µg/mL in 1994, 0.023 µg/mL in 2001, and 0.38–0.5 µg/mL in 2005. These observations were alarming because any further increase in the MIC may cause ciprofloxacin to be more ineffective in the clinical response of cholera patients infected with multidrug-resistant strains of V. cholerae O1.7,32
Periodic interconversion (genetic reversion) of V. cholerae strains have been reported between Inaba and Ogawa serotypes. Such conversion of Ogawa to Inaba is common; however, conversion from Inaba to Ogawa is less frequent. During two consecutive cholera seasons (1989–90) in Calcutta, India, serotype Inaba dominated in 1989 and Ogawa in 1990. The present study observed that V. cholerae Inaba serotype suddenly disappeared, whereas Ogawa strains have emerged along with widespread tetracycline susceptibility patterns that were similar to that of Inaba observed in the recently prevailing V. cholerae O1 Inaba El Tor biotype. Such genetic conversion has been reported in other cholera-endemic countries of the developing world. In addition, the absence of drug pressure due to conventional antibiotics no longer being used in the management of cholera cases might have contributed to the reversal of the antibiotic resistance patterns. In August 2006, a re-emergence of the Inaba serotype and a sharp reduction in the detection of the Ogawa serotype were observed in the Dhaka Hospital of urban Bangladesh.7,33,34
According to researchers of different countries of the world, an appropriate antibiotic for cholera reduces the total diarrheal stool volume, I.V fluid requirement, duration of diarrhea, and excretion of V. cholerae in the stool.35–37 Moreover, as an adjunct to rehydration therapy, the effective role of tetracycline in different doses in Bangladeshi cholera patients was also observed. Researchers have indicated that although multiple doses of tetracycline are the best preferred, single dose remains a reasonable alternative in the clinical management of cases of cholera.38 Single-dose therapy is reported to be less expensive and has better patient compliance and simplicity in dispensing, particularly during the epidemic situation with an escalating patient load.38
A single 300-mg dose of doxycycline, a second-generation, long-acting tetracyclines, has also been found to be as effective as multiple doses of tetracycline in the treatment of cholera.39 A clinical trial in Bangladesh reported a higher rate of clinical (cessation of watery stool within 48 hours of the initiation of the study drug without recurrence in the subsequent 72 hours) and bacteriological cure (inability to isolate V. cholerae from stool or rectal swab samples after study day 2) with single-dose (1 g) ciprofloxacin than with single-dose doxycycline (300 mg) among patients infected with V. cholerae. According to a clinical trial in Bangladesh, single-dose azithromycin (1 g) was effective in the treatment of severe cholera in adults. Patients who received azithromycin had a shorter duration of diarrhea than patients who were treated with ciprofloxacin, as well as lower frequency of vomiting, fewer stools, and a lower stool output.32
A study was conducted in different parts of Odisha state of India during 2004 and 2013. Nine hundred nine V. cholerae isolates were obtained from 4,886 rectal swabs during the study period. Vibrio cholerae O1 showed uniformly high resistance to cotrimoxazole and furazolidone throughout the study period, which was similar to the report of this study.40 Another study was conducted in the rural coastal area of Mathbaria, Bangladesh, during 2010 and 2014. All V. cholerae O1 isolates were highly susceptible to azithromycin, and as high as 95% of the V. cholerae O1 isolates reported susceptibility to ciprofloxacin.41 Similar susceptibility has also been reported by our study during the 19-year study period. We do not have any ready explanation for such a susceptibility pattern that remains consistently high and close to 100% over a similar study period.
During the multidrug-resistant cholera outbreaks in Dhaka, antibiotic treatment guidelines for cases of severe cholera-like illnesses were changed, and single-dose ciprofloxacin was considered as the antibiotic of choice instead of tetracycline or doxycycline42; however, sooner, treating physicians anecdotally observed that the duration of illness was prolonged in most of the cases treated with ciprofloxacin. The questionable effectiveness of the ciprofloxacin prompted the review of the MIC levels of the drug, and the findings of the consistently increasing trend of MIC levels prompted clinicians to switch over to azithromycin in treating cases of cholera, both in young children and adults.7
CONCLUSION
As many antibiotics have shown their changing susceptibility patterns over time, close monitoring of antibiotic susceptibility and simultaneous reconsideration of switching to old conventional tetracycline to treat cases of cholera have become imperative. Such a notion is based on the increased susceptibility of the vast majority of V. cholerae O1 isolates to tetracycline in recent years.
Acknowledgments:
We thank the core donors for providing unrestricted support to the icddr,b for its operations and research. Current donors providing unrestricted support include the Government of the People’s Republic of Bangladesh; Global Affairs Canada (GAC); Swedish International Development Cooperation Agency (Sida) and the Department for International Development (UK Aid). We are grateful to these donors for their support and commitment to icddr,b’s research efforts. We would like to thank Dr. Mohammad Yunus, emeritus scientist of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), for his expert opinion and critical review of the manuscript.
REFERENCES
- 1.Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J, 2017. Cholera. Lancet 390: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 2.Sack DA, Sack RB, Nair GB, Siddique AK, 2004. Cholera. Lancet 363: 223–233. [DOI] [PubMed] [Google Scholar]
- 3.Legros D, 2018. Global cholera epidemiology: opportunities to reduce the burden of cholera by 2030. J Infect Dis 218 (Suppl_3): S137–S140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass RI, Becker S, Huq MI, Stoll BJ, Khan MU, Merson MH, Lee JV, Black RE, 1982. Endemic cholera in rural Bangladesh, 1966–1980. Am J Epidemiol 116: 959–970. [DOI] [PubMed] [Google Scholar]
- 5.Dhar U, Bennish ML, Khan WA, Seas C, Huq Khan E, Albert MJ, Abdus Salam M, 1996. Clinical features, antimicrobial susceptibility and toxin production in Vibrio cholerae O139 infection: comparison with V. cholerae O1 infection. Trans R Soc Trop Med Hyg 90: 402–405. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization , 2013. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 7.Faruque AS, et al. 2007. Emergence of multidrug-resistant strain of Vibrio cholerae O1 in Bangladesh and reversal of their susceptibility to tetracycline after two years. J Health Popul Nutr 25: 241–243. [PMC free article] [PubMed] [Google Scholar]
- 8.Khan WA, Saha D, Rahman A, Salam MA, Bogaerts J, Bennish ML, 2002. Comparison of single-dose azithromycin and 12-dose, 3-day erythromycin for childhood cholera: a randomised, double-blind trial. Lancet 360: 1722–1727. [DOI] [PubMed] [Google Scholar]
- 9.Saha D, Karim MM, Khan WA, Ahmed S, Salam MA, Bennish ML, 2006. Single-dose azithromycin for the treatment of cholera in adults. N Engl J Med 354: 2452–2462. [DOI] [PubMed] [Google Scholar]
- 10.Kitaoka M, Miyata ST, Unterweger D, Pukatzki S, 2011. Antibiotic resistance mechanisms of Vibrio cholerae. J Med Microbiol 60: 397–407. [DOI] [PubMed] [Google Scholar]
- 11.Stoll BJ, Glass RI, Huq MI, Khan MU, Holt JE, Banu H, 1982. Surveillance of patients attending a diarrhoeal disease hospital in Bangladesh. Br Med J (Clin Res Ed) 285: 1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qadri F, Das SK, Faruque A, Fuchs GJ, Albert MJ, Sack RB, Svennerholm A-M, 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol 38: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization , 1987. Manual for Laboratory Investigations of Acute Enteric infections. Geneva, Switzerland: WHO. [Google Scholar]
- 14.Rahman M, Sultana R, Ahmed G, Nahar S, Hassan ZM, Saiada F, Podder G, Faruque AS, Siddique A, Sack DA, 2007. Prevalence of G2P [4] and G12P [6] rotavirus, Bangladesh. Emerg Infect Dis 13: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isenberg HD, 1998. Essential Procedures for Clinical Microbiology. Washington, D.C.: ASM Press. [Google Scholar]
- 16.Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST, 1994. Bergey’s Manual of Determinative Bacteriology, 9th edition Baltimore, MD: A Waverly Company Williams and Wilkins. [Google Scholar]
- 17.Clinical Laboratory Standards Institute , 2006. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard. CLSI Document M2–A9 Wayne, PA: Clinical Laboratory Standards Institute. [Google Scholar]
- 18.Rossolini GM, Arena F, Pecile P, Pollini S, 2014. Update on the antibiotic resistance crisis. Curr Opin Pharmacol 18: 56–60. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary AS, 2016. A review of global initiatives to fight antibiotic resistance and recent antibiotics’ discovery. Acta Pharm Sin B 6: 552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization , 2014. Antimicrobial Resistance Global Report on Surveillance: 2014 Summary. Geneva, Switzerland: WHO. [Google Scholar]
- 21.Chokshi A, Sifri Z, Cennimo D, Horng H, 2019. Global contributors to antibiotic resistance. J Glob Infect Dis 11: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ab Rahman N, Teng CL, Sivasampu S, 2016. Antibiotic prescribing in public and private practice: a cross-sectional study in primary care clinics in Malaysia. BMC Infect Dis 16: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landers TF, Cohen B, Wittum TE, Larson EL, 2012. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep 127: 4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohanan M, Vera-Hernandez M, Das V, Giardili S, Goldhaber-Fiebert JD, Rabin TL, Raj SS, Schwartz JI, Seth A, 2015. The know-do gap in quality of health care for childhood diarrhea and pneumonia in rural India. JAMA Pediatr 169: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dancer SJ, 2003. The dangers of broad spectrum antibiotics. BMJ 326: 1111.12763980 [Google Scholar]
- 26.Martinez JL, 2008. Antibiotics and antibiotic resistance genes in natural environments. Science 321: 365–367. [DOI] [PubMed] [Google Scholar]
- 27.Barati H, Moradi G, Rasouli MA, Mohammadi P, 2015. Epidemiologic and drug resistance pattern of Vibrio cholerae O1 biotype El Tor, serotype Ogawa, in the 2011 cholera outbreak, in Alborz province, Iran. Jundishapur J Microbiol 8: e23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dengo-Baloi LC, Sema-Baltazar CA, Manhique LV, Chitio JE, Inguane DL, Langa JP, 2017. Antibiotics resistance in El Tor Vibrio cholerae 01 isolated during cholera outbreaks in Mozambique from 2012 to 2015. PLoS One 12: e0181496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandomando I, Espasa M, Valles X, Sacarlal J, Sigauque B, Ruiz J, Alonso P, 2007. Antimicrobial resistance of Vibrio cholerae O1 serotype Ogawa isolated in Manhica district hospital, southern Mozambique. J Antimicrob Chemother 60: 662–664. [DOI] [PubMed] [Google Scholar]
- 30.Ranjit K, Nurahan M, 2000. Tetracycline resistant cholera in Kelantan. Med J Malaysia 55: 143–145. [PubMed] [Google Scholar]
- 31.Glass RI, Huq I, Alim AR, Yunus M, 1980. Emergence of multiply antibiotic-resistant Vibrio cholerae in Bangladesh. J Infect Dis 142: 939–942. [DOI] [PubMed] [Google Scholar]
- 32.Saha D, Khan WA, Karim MM, Chowdhury HR, Salam MA, Bennish ML, 2005. Single-dose ciprofloxacin versus 12-dose erythromycin for childhood cholera: a randomised controlled trial. Lancet 366: 1085–1093. [DOI] [PubMed] [Google Scholar]
- 33.Ramamurthy T, Pal A, Bhattacharya MK, Bhattacharya SK, Chowdhury AS, Takeda Y, Takeda T, Pal SC, Nair GB, 1992. Serovar, biotype, phage type, toxigenicity and antibiotic susceptibility patterns of Vibrio cholerae isolated during two consecutive cholera seasons (1989–90) in Calcutta. Indian J Med Res 95: 125–129. [PubMed] [Google Scholar]
- 34.Garg P, Nandy RK, Chaudhury P, Chowdhury NR, De K, Ramamurthy T, Yamasaki S, Bhattacharya SK, Takeda Y, Nair GB, 2000. Emergence of Vibrio cholerae O1 biotype El Tor serotype Inaba from the prevailing O1 Ogawa serotype strains in India. J Clin Microbiol 38: 4249–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter CC, 1971. Cholera: diagnosis and treatment. Bull N Y Acad Med 47: 1192–1203. [PMC free article] [PubMed] [Google Scholar]
- 36.Greenough W, III, Gordon R, Jr., Rosenberg I, Davies B, Benenson A, 1964. Tetracycline in the treatment of cholera. Lancet 283: 355–357. [DOI] [PubMed] [Google Scholar]
- 37.Wallace CK, Anderson PN, Brown TC, Khanra SR, Lewis GW, Pierce NF, Sanyal SN, Segre GV, Waldman RH, 1968. Optimal antibiotic therapy in cholera. Bull World Health Organ 39: 239–245. [PMC free article] [PubMed] [Google Scholar]
- 38.Islam MR, 1987. Single dose tetracycline in cholera. Gut 28: 1029–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alam AN, Alam NH, Ahmed T, Sack DA, 1990. Randomised double blind trial of single dose doxycycline for treating cholera in adults. BMJ 300: 1619–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pal BB, Nayak SR, Khuntia HK, 2018. Epidemiology and antibiogram profile of Vibrio cholerae isolates between 2004–2013 from Odisha, India. Jpn J Infect Dis 71: 99–103. [DOI] [PubMed] [Google Scholar]
- 41.Rashed SM, Hasan NA, Alam M, Sadique A, Sultana M, Hoq MM, Sack RB, Colwell RR, Huq A, 2017. Vibrio cholerae O1 with reduced susceptibility to ciprofloxacin and azithromycin isolated from a rural coastal area of Bangladesh. Front Microbiol 8: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan W, Bennish ML, Seas C, Khan EH, Ronan A, Dhar U, Busch W, Salam MA, 1996. Randomised controlled comparison of single-dose ciprofloxacin and doxycycline for cholera caused by Vibrio cholerae O1 or O139. Lancet 348: 296–300. [DOI] [PubMed] [Google Scholar]