Abstract.
The ultrasensitive Alere Plasmodium falciparum Malaria Ag histidine-rich protein 2 rapid diagnostic test (Alere uRDT, Suwon City, South Korea) is a new diagnostic tool which is more expensive than other malaria rapid diagnostic tests (RDTs) routinely used in Ugandan clinics. The manufacturer recommends testing samples within 2 days and scoring results after 20 minutes, which may be impractical in high-volume resource-poor clinics. We compared testing by the Alere Ag rapid diagnostic test (uRDT), CareStart RDT, microscopy, and an ultrasensitive I8S rRNA quantitative reverse transcription polymerase chain reaction (qRT-PCR) using survey and clinical samples. For the Alere uRDT, we used survey blood samples stored at 4°C for 44 days and for some clinical samples deliberately scored results beyond 20 minutes. The Alere uRDT and qRT-PCR identified asymptomatic parasitemia cases in 56% and 72%, respectively, of survey samples originally scored as negative by the CareStart RDT. Using qRT-PCR as a gold standard, the Alere uRDT was superior to the CareStart RDT in estimating asymptomatic parasite prevalence in a cross-sectional survey (P = 0.007) and in detection of clinically significant malaria; both RDTs were comparable in detecting asymptomatic parasitemia in the clinic (P = 0.599). Scoring Alere uRDT results at 20 minutes produced valid results confirmed by the CareStart RDT, but there was a consistent background; scoring the Alere uRDT beyond 20 minutes produced false-positive results. The Alere uRDT outperformed the CareStart RDT (ACCESSBIO, Somerset, NJ) in a field survey in estimating malaria prevalence and in the clinic for symptomatic malarial illness. It produced reliable results using samples stored at 4°C for 44 days, but test results read beyond 20 minutes were invalid.
INTRODUCTION
Sustained malaria vector and parasite control interventions over the last decade have resulted in a significant reduction in malaria morbidity and mortality, across populations at risk.1,2 Widespread adoption of the WHO recommendation for malaria case management based on parasite diagnosis using microscopy and rapid diagnostic tests (RDTs) led to improved case detection and prompt treatment.3 However, the success of this strategy is undermined by the existence of submicroscopic asymptomatic parasite reservoirs and their potential to maintain human-to-mosquito transmission.4,5
The poor performance of microscopy and commercially available RDTs for the detection of low-density parasite infections limits their utility not only in low-transmission settings but also in high-transmission settings with asymptomatic parasitemias.6,7 Nucleic acid assays are reliable tools for detecting low-density infections in targeted populations, but remain impractical for many malaria programs in resource-limited settings because of the requirement for expensive equipment, reagents, and trained personnel.8–10 The renewed call for malaria elimination and eradication has sparked an urgent need for a simple, low-cost, rapid, and sensitive malaria diagnostics that require minimal laboratory infrastructure for use in low-transmission settings to detect low-density parasitemias. Recent reports indicate that the ultrasensitive Alere Plasmodium. falciparum Malaria Ag rapid diagnostic test (uRDT) based on histidine-rich protein 2 (HRP-2) detects much lower P. falciparum parasitemias than the standard RDT or microscopy.11–13 This uRDT is, therefore, promising, given the comparative sensitivity, ease of use, and short turnaround time. However, the higher cost of the uRDT may limit its usefulness to research settings rather than resource-poor clinics where such tools may be most needed. To date, most published studies of the uRDT were conducted in controlled reference laboratory settings by highly trained personnel.11–13 There is a paucity of studies carried out by public health facility workers under real-world resource-limited settings in malaria-endemic Africa.
Field and laboratory studies of the uRDT in Southeast Asia reported false-positive results.13 Such false-positive results in resource-poor clinics with no confirmatory diagnostic capability can lead to unnecessary treatments or overprescription of antimalarial drugs, thereby escalating the risk of antimalarial drug resistance. The reasons for false-positive results are not clear, but could be attributed to human errors, inherent test kit characteristics, and/or environmental or climatic factors. Furthermore, the manufacturer’s recommendations are that samples be analyzed within 2 days after storage at 4°C and that test results be scored after 20 minutes of initiating the test—the latter recommendation may be difficult for high-volume clinics to comply with given the limited personnel and resources at the height of a malaria transmission season with heavy demand from waiting patients.
In this study, we compared the ability of the Alere P. falciparum Malaria Ag HRP-2 rapid diagnostic test (Alere uRDT) and a conventional RDT in detecting asymptomatic parasitemia in a limited number of selected subject samples collected during a malaria survey in northeastern Uganda and also defined the fraction of infections detected by the uRDT compared with the conventional RDT during symptomatic/clinical infection. Blood samples from the cross-sectional studies for the uRDT were first deliberately stored in the refrigerator for over 1 month. We also compared both RDTs and microscopy under clinic conditions using more than 700 samples from the same region, and some clinic uRDT results were deliberately scored beyond the recommended 20 minutes. Our data indicate that the Alere uRDT was reliable and detected more asymptomatic parasitemias than the conventional RDT in field surveys, despite prolonged cold storage of samples and in detection of clinical malaria with freshly collected blood in a clinic setting. In addition, the uRDT produced false-positive results for tests read beyond 20 minutes.
MATERIALS AND METHODS
Study site and population.
The study population consisted of children aged > 5–17 years and adults who participated in the first cross-sectional survey, breastfeeding mothers and their infants aged < 2.5 years who participated in a clinic-based cross-sectional survey, and infants aged < 5 years who were prospectively followed up for 1 year in the clinic. All subjects were living in Usuk subcounty, Abwokodia Parish, Katakwi district in northeastern Uganda. Abwokodia Parish is made up of seven villages inhabited by a predominantly Nilo-Hamitic indigenous population whose main occupation is peasant agriculture and small-scale animal farming. The area is covered by savannah grassland interspersed with seasonal and established swamps.
Study design.
The samples used in this research were pulled from diverse studies, including cross-sectional surveys of asymptomatic subjects and a prospective follow-up of symptomatic infants in Katakwi district, northeastern Uganda. The prospective follow-up of children took place from November 2017 to August 2018 at St. Anne Health Center III where infants were followed up for malaria episodes by passive surveillance. Mothers were requested to bring their children to the clinic whenever the children were ill or had fever.
Ethical consideration.
Ethical clearance was obtained from the Research Ethical Committee of the Vector Control Division (Ministry of Health) and the Uganda National Council for Science and Technology. Blood sample collection was performed after the participants or their parents/guardians agreed to participate in the study and signed or thumb-printed the consent form. Individuals who refused to sign the consent form were not included in the study. Study participants with confirmed malaria diagnosis (by the RDT) were offered immediate treatment according to the national guidelines.
Study participants.
The study subjects who participated in these cross-sectional and longitudinal prospective studies were part of a cohort in Abwokodia Parish participating in a study investigating the effectiveness of home decoration for malaria control. Participants were mobilized by a field coordinator/social scientist aided by village health team members and Local Council chairpersons of the respective villages. In the field or clinic, details of the study subjects were verified by the field coordinator through checking the register, and demographic details and vital signs were taken by study nurses, followed by consultation, medical history taking, and examination by the study physician, and blood collection, malaria diagnosis, and treatment in that order.
Sample collection and preparation.
Approximately 2 mL of venous blood samples were collected in ethylenediaminetetraacetic acid (EDTA) vacutainer tubes from all participants in the first cross-sectional study, whereas finger-prick samples were collected from participants in the second cross-sectional study. Samples for rapid malaria diagnosis using the HRP-2–based CareStart™ RDT (ACCESSBIO) were immediately transferred onto the RDT cassettes, whereas blood samples for hemoglobin determination, dried blood spots (DBS) for quantitative reverse transcription polymerase chain reaction [qRT-PCR]), and microscopy were transferred using micropipettes onto HemoCue cuvettes 301, 3 MM Whatman paper Chr 3, and microscope slides, respectively. The remaining EDTA samples were then transported to Med Biotech Laboratories field office and laboratory in Katakwi town for storage within 6 hours of collection. During the time of the scheduled field survey, Alere uRDT test kits had not yet arrived, so whole blood samples from the first cross-sectional study were transported to Kampala and stored at 4°C. By the time the samples were tested by the Alere uRDT, they had been stored for exactly 44 days.
Malaria detection using CareStart RDT, Alere uRDT, and microscopy.
Malaria diagnosis by the CareStart RDT and thin and thick smear microscopy was carried out during the field survey. Because we were not sure about the performance of the Alere uRDT with samples stored beyond the recommended 2 days, we deliberately selected 25 CareStart RDT–negative samples (24 subjects aged 4–17 years and one subject aged 3 months) and 25 CareStart RDT–positive samples (25 subjects aged 1.5–16 years) from which the blood samples were stored as described earlier. Blood samples stored at 4°C were retrieved, brought to room temperature, and mixed by inverting the tube five times. Using the kit sample cup provided, approximately 5 µL of whole blood was transferred onto the Alere uRDT cassette. All tests were performed and interpreted according to the manufacturer’s instructions found in the package insert. Dried blood spots from these 50 samples were prepared in the field and used to estimate parasite density by Plasmodium 18S rRNA qRT-PCR. During the cross-sectional study in the clinic involving breastfeeding mothers and their children, the Alere uRDT, CareStart RDT, and microscopy (see below) were carried out on all blood samples. All children aged < 5 years in the passive surveillance follow-up were brought to the clinic whenever they had fever. Finger-prick blood samples were subjected to the three diagnostic tests to confirm malaria diagnosis.
Preparation of thick and thin blood smears and malaria microscopy.
Thin and thick blood smears were stained with 3% Giemsa for 30 minutes after fixing the thin smear with absolute methanol. A known positive smear was included with each new batch of working Giemsa stain for quality control. Smears were then examined using a ×100 oil immersion objective. Thin films were examined to confirm species identification on the thick film. All blood films were reread and checked for the second time by an experienced malaria microscopist blinded to the initial microscopy and RDT results. Discrepant readings were resolved by a third reader whose score was considered the final result. At least 100 high-power fields were examined before a thick smear was declared negative. A blood slide was declared positive when a concordant result was produced by two competent independent microscopists. Parasite densities were recorded as a ratio of parasites to white blood cells (WBCs) in thick films as described.14 Plasmodium falciparum parasites were counted against 200 WBCs. Five hundred WBCs were counted where less than nine parasites were counted after counting against 200 WBCs. Assuming a nominal density of 8,000 WBC/µL of blood, parasite densities (parasite/μL of whole blood) were calculated according to the formula:
Parasite detection and quantification using qRT-PCR.
Dried blood spots (50 μL spots) on Whatman 3 MM chromatography paper (Whatman, GE Healthcare GB) were air-dried and stored in gas-impermeable bags with silica desiccant and shipped at ambient temperature by a local courier to the University of Washington. Dried blood spots were laser-cut for contact-free processing as previously reported.15 Ribonucleic acid was extracted on an automated Abbott m2000sp molecular platform, and qRT-PCR was performed on the Abbott m2000rt platform for the P. falciparum 18S RNA using a method developed at the U. W. as reported.16 Results were based on positivity in the pan-Plasmodium and the P. falciparum–specific channels of the multiplex qRT-PCR assay; quantification is based on the pan-Plasmodium channel quantification results, provided that there was no evidence of mixed-species infection.
Statistical analysis.
Analyses were performed using Stata 12 (StataCorp LLC, College Station, TX). Prevalence estimates were reported as proportions and differences between age-groups, or diagnostic tests were compared using the chi-squared test. P-values < 0.05 were considered significant. GraphPad Prism 8.0.0 (GraphPad Inc, San Diego, CA) was used to make the correlation plan and to calculate the r2 value.
RESULTS
Population asymptomatic parasitemia prevalence by CareStart HRP-2 RDT and microscopy.
A cross-sectional community-based malaria survey involving 1,768 individuals living in Usuk subcounty, Katakwi district, northeastern Uganda was conducted between August and September 2017. One thousand seven hundred five subjects with complete datasets were included in the microscopy and conventional CareStart HRP-2 RDT prevalence analysis (Supplemental Figure 1). There were 42 subjects with fever (axillary temperature ≥ 37.5°C), giving an overall fever rate of 2.5% in the study population. Asymptomatic parasitemia was defined as having a positive malaria microscopy or RDT test result with normal axillary temperature (37°C) and no history of fever in the last 48 hours. The prevalence of asymptomatic parasitemia in subjects aged < 5, 5–14, and ≥ 15 years and overall for all subjects by the CareStart RDT and microscopy is presented in Table 1. For both techniques, the prevalence of asymptomatic parasitemia in the 5- to 14-year age-group was significantly higher than that in those aged < 5 years (P < 0.0001) or > 15 years (P < 0.0001). For the overall and age-specific prevalence, the CareStart RDT prevalence estimates were significantly higher than by microscopy (Table 1).
Table 1.
Population asymptomatic malaria prevalence by the CareStart histidine-rich protein 2 RDT and microscopy
| Age category (years) | Number sampled | Parasite prevalence, % (n/N) | P-value | |
|---|---|---|---|---|
| CareStart RDT | Microscopy | |||
| < 5 | 370 | 34.3 (127/370) | 27.0 (100/370) | 0.0314 |
| 5–14 | 633 | 54.6 (346/633) | 44.7 (283/633) | 0.0004 |
| ≥ 15 | 702 | 22.2 (156/702) | 17.4 (122/702) | 0.0241 |
| Total | 1,705 | 36.9 (629/1705) | 29.6 (505/1705) | < 0.0001 |
RDT = rapid diagnostic test.
We proceeded to assess the reliability of these estimates by the more sensitive Alere uRDT and qRT-PCR as a gold standard using 50 selected field survey samples stored at 4°C for 44 days. Among the 25 asymptomatic CareStart-positive samples, the Alere uRDT detected 24/25 (96%) and qRT-PCR detected 23/25 (92%) of the asymptomatic parasitemia cases identified by the CareStart RDT (Table 2). Overall, both Alere uRDT and qRT-PCR identified 22/25 (88%) of the asymptomatic parasitemia cases. Among the 25 CareStart-negative samples, the Alere uRDT was positive in 14/25 (56%) and qRT-PCR was positive in 18/25 (72%) (Table 2), indicating a high rate of low-density infections. There was excellent quantitative agreement between patient microscopic-positive samples and qRT-PCR (r2 = 0.84, data not shown). The Alere uRDT failed to identify 7/25 (28%) of the samples detected by the more analytically sensitive qRT-PCR. Among CareStart RDT–negative samples, microscopy was negative in 24/25 (96%)—the sole positive sample was Alere uRDT positive and had a qRT-PCR–estimated density of 148 parasites/µL blood, indicating that this sample may have been below the limit of detection for the CareStart RDT. However, among the samples positive by the CareStart RDT, microscopy was negative in 9/25 (36%). On closer inspection of these nine microscopically negative results, seven (28%) were likely false negatives because qRT-PCR returned high parasite counts of > 250 parasites/µL blood in two samples and lower counts (0.08–1 parasites/µL blood) in five samples—only two CareStart-negative/microscopy-negative samples were likely true negatives because qRT-PCR was also negative. Thus, overall there were a total of 33/50 (66%) asymptomatic parasitemia cases missed by microscopy compared with qRT-PCR. Among microscopically negative samples, qRT-PCR identified 20/25 (80%) as positive and 12 (48%) were CareStart RDT negative but Alere uRDT positive.
Table 2.
Comparison of test modalities for the detection of asymptomatic malaria
| Subject ID | Age (years) | Gender | Temp (°C) | Hemoglobin (g/dL) | Microscopy (para/µL) | CareStart RDT | Alere Pf Malaria Ag histidine-rich protein 2 rapid diagnostic test | Quantitative reverse transcription polymerase chain reaction (est. para/uL) |
|---|---|---|---|---|---|---|---|---|
| Known CareStart-negative samples | ||||||||
| A-AK-029 | 4 | F | 36.7 | 12.8 | − | − | + | − |
| A-AC-243 | 11 | F | 36.3 | 17.2 | − | − | + | − |
| A-AC-207 | 13 | F | 35.8 | 13 | − | − | − | − |
| A-AK-067 | 14 | M | 37.1 | ND | − | − | ND | − |
| A-AK-031 | 15 | F | 36.2 | 12.6 | − | − | + | − |
| A-AC-210 | 0.25 | F | 36.8 | 11.1 | − | − | − | − |
| A-AK-023 | 7 | M | 36.9 | 13.7 | − | − | − | < 0.02 |
| A-AC-208 | 11 | F | 36.7 | ND | − | − | − | 0.03 |
| A-AC-163 | 9 | M | 36.1 | 12.1 | − | − | + | 0.4 |
| A-AC-194 | 12 | M | 36.4 | ND | − | − | − | 0.5 |
| A-AK-015 | 9 | F | 36.1 | 9.7 | − | − | + | 4 |
| A-AC-239 | 17 | F | 37.0 | 21.4 | − | − | − | 4 |
| A-AC-232 | 15 | F | 36.8 | 15.3 | − | − | − | 8 |
| A-AK-034 | 11 | F | 36.2 | 12.7 | − | − | − | 10 |
| A-AK-026 | 12 | M | 36.5 | 13.6 | − | − | + | 16 |
| A-AK-019 | 16 | F | 35.1 | 13.4 | − | − | + | 23 |
| A-AC-251 | 14 | M | 36.4 | 12.3 | − | − | + | 25 |
| A-AC-245 | 16 | F | 36.1 | 12.2 | − | − | + | 38 |
| A-AK-039 | 12 | M | 36.7 | ND | − | − | + | 39 |
| A-AC-209 | 14 | M | 36.8 | 13.2 | − | − | + | 39 |
| A-AC-229 | 11 | M | 36.7 | 10.5 | − | − | + | 47 |
| A-AC-203 | 14 | F | 36.7 | 12.3 | −− | − | + | 47 |
| A-AK-004 | 8 | F | 37.1 | 12.2 | − | − | − | 66 |
| A-AC-193 | 13 | F | 36.1 | 12.8 | − | − | − | 142 |
| A-AC-228 | 15 | F | 36.4 | 19.6 | + (40) | − | + | 148 |
| Known CareStart-positive samples | ||||||||
| A-AK-035 | 2 | M | 36.7 | 10.4 | − | + | + | − |
| A-AK-070 | 8 | M | 36.5 | 11.8 | − | + | + | − |
| A-AK-036 | 2 | F | 36.9 | 13.6 | − | + | + | 0.08 |
| A-AK-033 | 6 | M | 36.4 | 12.7 | − | + | + | 0.18 |
| A-AK-054 | 13 | M | 36.4 | 11.7 | − | + | − | 0.8 |
| A-AK-001 | 1.5 | F | 36.1 | 10.6 | − | + | + | 1 |
| A-AK-063 | 11 | F | 37.0 | 10.9 | − | + | + | 1 |
| A-AC-221 | 13 | F | 36.4 | 16.6 | + (89) | + | + | 99 |
| A-AK-065 | 2.5 | M | 36.1 | 10.0 | − | + | + | 258 |
| A-AK-058 | 15 | M | 35.7 | 13.7 | − | + | + | 285 |
| A-AK-049 | 11 | M | 36.6 | 12.4 | + (364) | + | + | 301 |
| A-AK-002 | 5 | M | 35.7 | 8.2 | + (584) | + | + | 303 |
| A-AC-192 | 9 | M | 37.7 | 13.2 | + (196) | + | + | 405 |
| A-AK-062 | 8 | M | 36.7 | 14.4 | + (161) | + | + | 455 |
| A-AC-166 | 8 | M | 36.7 | 12.4 | + (380) | + | + | 516 |
| A-AK-011 | 15 | F | 37.4 | 9.9 | + (1,104) | + | + | 527 |
| A-AK-022 | 9 | M | 37.3 | 13.3 | + (1,076) | + | + | 1,023 |
| A-AK-028 | 5 | F | 36.3 | 12.2 | + (724) | + | + | 1,044 |
| A-AK-055 | 4.5 | F | 36.7 | 10.7 | + (6,060) | + | + | 2,204 |
| A-AK-043 | 12 | M | 36.4 | 12.7 | + (1,240) | + | + | 3,136 |
| A-AK-027 | 7 | F | 36.4 | 12.2 | + (2,168) | + | + | 3,527 |
| A-AK-005 | 6 | M | 36.6 | 12.2 | + (2,272) | + | + | 4,556 |
| A-AC-220 | 16 | M | 36.9 | 12.7 | + (2,724) | + | + | 13,215 |
| A-AK-021 | 11 | F | 36.4 | 13.5 | + (11,900) | + | + | 23,623 |
| A-AK-046 | 5 | M | 37.0 | 11.5 | + (18,940) | + | + | 98,177 |
| Overall prevalence | ||||||||
| Prevalence (all 50 samples) | 34% (17/50) | 50% (25/50) | 76% (38/49) | 84% (42/50) | ||||
| Prevalence (CareStart RDT–negative only) | 4% (1/25) | 0% (0/25) | 56% (14/24) | 76% (19/25) | ||||
| Prevalence (CareStart RDT–positive only) | 64% (16/25) | 100% (25/25) | 96% (24/25) | 92% (23/25) | ||||
g/dL = gram per deciliter; µL = microliter; °C = degree Celsius; est. = estimated; ND = not done rapid diagnostic test.
In summary, of the 50 field survey samples selected on the basis of CareStart RDT results, microscopy, CareStart RDT, and uRDT missed detecting 59.5, 45.2, and 19.0%, respectively, of asymptomatic parasitemia cases detected by qRT-PCR. There was 71.4% agreement between Alere uRDT and qRT-PCR, but the strength of agreement was poor (kappa = 0.058) probably because of small sample sizes.
Comparison of Alere uRDT, CareStart RDT, and microscopy for diagnosis of symptomatic and asymptomatic parasitemia in a clinic.
Symptomatic malaria was detected by the Alere uRDT, CareStart RDT, and microscopy in 55.2%, 53.5%, and 40.6%, respectively, during 475 clinic visits for fever by infants aged < 5 years from March to August 2018 (Table 3). Both RDTs performed comparably (P = 0.599), and both RDTs detected more cases than microscopy (Alere uRDT versus microscopy, P < 0.0001; CareStart RDT versus microscopy, P = 0.0001). Significantly, the CareStart RDT and Alere uRDT were positive in 61 and 69 febrile infants, respectively, when microscopy was negative. Thus, the Alere uRDT detected eight symptomatic malaria cases that were missed by the CareStart RDT. Among 127 asymptomatic breastfeeding mothers, the Alere uRDT, CareStart RDT, and microscopy were positive in 11.0%, 7.1%, and 4.7% of cases, respectively (no significant differences between tests: Alere uRDT versus CareStart RDT, P = 0.28; CareStart RDT versus microscopy, P = 0.418; Alere uRDT versus microscopy, P = 0.063). Among 123 breastfeeding nonfebrile infants aged < 5 years who accompanied their mothers to the clinic, there was also no difference in the prevalence of positivity by either RDTs (P = 1.000); microscopy was not carried out on these infants.
Table 3.
Comparison of the Alere Pf Malaria Ag histidine-rich protein 2 rapid diagnostic test, CareStart rapid diagnostic test, and microscopy for diagnosis of clinical and asymptomatic malaria in a clinic
| Population categories | Number sampled | Diagnostic test methods* | ||
|---|---|---|---|---|
| Microscopy positive, % (n/N) | CareStart positive, % (n/N) | Alere positive, % (n/N) | ||
| Clinic visits (symptomatic) | 475 | 40.6 (193/475) | 53.5 (254/475) | 55.2 (262/475) |
| Breastfeeding mothers (asymptomatic) | 127 | 4.7 (6/127) | 7.1 (9/127) | 11.0 (14/127) |
| Breastfeeding children (asymptomatic) | 123 | ND | 7.3 (9/123) | 7.3 (9/123) |
% = percent; n = number positive; N = number sampled; ND = not done.
Differences in malaria prevalence estimates for the different diagnostic methods are as follows: Clinic visits: CareStart versus microscopy, P = 0.0001; CareStart versus Alere, P = 0.599; Alere versus microscopy, P < 0.0001. Breastfeeding mothers: Microscopy versus CareStart, P = 0.418; CareStart versus Alere, P = 0.279; microscopy versus Alere, P = 0.063. Breastfeeding children: CareStart versus Alere, P = 1.000.
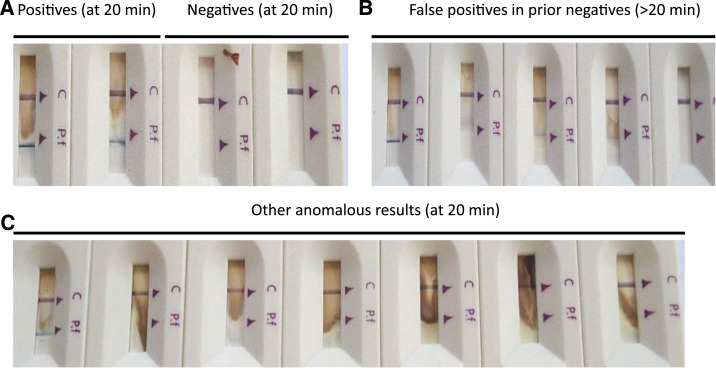
Abnormal Alere uRDT results observed in the clinic after extended incubation.
In the hands of three clinic health workers, reading the Alere uRDT test results at 20 minutes as recommended yielded the expected results, namely a single line corresponding to the control (C) and no line corresponding to P. falciparum for malaria-negative samples and two lines corresponding to the C and P. falciparum, respectively, for malaria-positive samples (Figure 1A). However, for uRDT-positive samples, there was a consistent background or dye backflow which varied from sample to sample and which was not apparent in Plasmodium-negative samples. Beyond 20 minutes, the background was prominent and with a faint or fuzzy line on the P. falciparum test window (Figure 1B). Other conspicuous anomalies observed even when results were scored at the recommended 20 minutes included a high background presenting as a brown smudge (Figure 1C) and failed and partial migration of the buffer (data not shown). By contrast, the CareStart RDT run in parallel did not show any of these anomalies or issues.
Figure 1.
Visual characteristics of uRDT results at different time points. (A) Positive and negative uRDT readings at 20 minutes of development. (B) False-positive Ag rapid diagnostic test (uRDT) readings exhibited by formerly negative uRDT kits read beyond 20 minutes of development (extreme left and right are true positive and negative results for comparison). (C) Other anomalies displayed by the Alere Pf Malaria Ag histidine-rich protein 2 rapid diagnostic test observed at 20 minutes of development (extreme left is true positive for comparison). Brightness and contrast were both increased 20% to improve printed visualization. All rapid diagnostic tests were from different samples. This figure appears in color at www.ajtmh.org.
DISCUSSION
In a field survey carried out in northeastern Uganda, the CareStart RDT and microscopy estimated that about one-third of infants aged < 5 years and one-half of children aged 5–14 years harbored asymptomatic P. falciparum infections and that the older children carried a significantly higher parasite burden. Our microscopy data confirmed an earlier estimate of the malaria parasite prevalence in infants aged < 5 years in northeastern Uganda.17 These prevalence estimates contrast with earlier reports in which 5- to 14-year-olds in a malaria-endemic region of Uganda had lower malaria parasite prevalence than younger infants.18 This reversal is a trend reported in neighboring Kenya as well.19–21 Here, it was important to establish the reliability of these prevalence estimates using the more sensitive Alere uRDT as a field deployable RDT and the qRT-PCR as the gold standard to establish infection status.
The Alere uRDT is a promising ultrasensitive RDT with a 10-fold lower limit of detection than conventional RDTs that were specifically developed to address the problem of low-density infections.11 We investigated the ability of the Alere uRDT against that of the CareStart RDT in detecting malaria parasitemia under field and clinic conditions in the same region. The Alere uRDT identified significantly more symptomatic and asymptomatic cases in infants attending a clinic and in selected samples from a field survey than the CareStart RDT and microscopy. The superior performance was observed even when field samples were stored for 44 days beyond the label recommendation of 2 days. Both Alere and CareStart RDTs gave false-negative results for asymptomatic parasitemia cases detected by qRT-PCR, which were expected given the limits of detection for each assay. The Alere uRDT is approximately five times more expensive than the CareStart RDT and showed higher background signal even at the recommended 20 minutes development period. Reading the Alere uRDT after 20 minutes of development led to false-positive results compared with when the same cassettes were scored within the recommended time frame. Although the manufacturer specifies that the uRDT should be read at 20 minutes, our anecdotal experience and discussion with others in the field are that in reality this is often not strictly followed in busy malaria clinics. We do not know whether the extended incubation produced positive results because of submicroscopic infections or if this was simply a false positive due to lingering HRP-2 antigens after parasite clearance. The Alere uRDT should be strictly read at 20 minutes as per the manufacturer’s instructions.
The higher analytical sensitivity of the Alere uRDT makes it potentially more useful in field surveys mapping malaria prevalence to guide national and international malaria intervention efforts. Our study confirmed the results of a large field survey in Myanmar in which the Alere uRDT detected more asymptomatic parasitemias than the conventional RDT and microscopy.13 The fact that samples can be stored for considerably longer periods without sacrificing the performance is convenient for large field trials in which it may be necessary to batch and store samples for some time for later careful analyses in the laboratory under more controlled conditions. This could remove the pressure and need to analyze samples in the field on the same day. In the Myanmar study, the sensitivity for both uRDT and SD Bioline Malaria Ag P. falciparum RDT under field conditions was approximately 40% lower than that in the laboratory.13 The Alere uRDT detected 60 and eight additional clinical malaria cases missed by microscopy and CareStart RDT, respectively, during clinic visits by febrile infants. The observed difference between the Alere uRDT and CareStart RDT in the number of detected clinical malaria cases strongly suggests that a significant proportion of clinically symptomatic malaria cases are characterized by low-density infections. Such low-density infections have been reported in febrile Ugandan infants aged 2–5 years.22 A single missed clinical malaria case in infants aged < 5 years may have disastrous and fatal consequences because treatment is usually dependent on confirmatory RDT testing. Therefore, wider adoption of the Alere uRDT in clinics in malaria-endemic countries could prevent unnecessary and preventable deaths caused by clinical malaria below the limit of detection of conventional RDTs.
Adoption and acceptability of the Alere uRDT in resource-poor settings for surveillance studies where accuracy for prevalence estimates is desirable might be constrained by several disadvantages. First, the Alere uRDT retail price is almost 5-fold higher than the CareStart RDT per test in Uganda (UGX 7,560 versus UGX 1,600, respectively). Second, the uRDT yields higher background even when the results are read at 20 minutes, which is not seen in the CareStart RDT. Third, the uRDT must be read at 20 minutes to avoid false-positive results. In high-volume clinics with limited human resources, it might be inevitable that sample results are read after a longer time window than the label recommendation. High false-positive results without additional confirmatory diagnostic tests may lead to overprescription and unnecessary consumption of antimalarial drugs, which increases the risk of drug resistance.23 False-positive Alere uRDT results have also been reported by others.12,13 However, these shortcomings must be balanced against its ability to detect malaria cases missed by conventional malaria RDTs and microscopy.
Microscopy missed most asymptomatic parasitemias detected by the Alere uRDT, CareStart RDT, and qRT-PCR. False negatives by microscopy were due to low parasitemias detectable by qRT-PCR and sometimes also occurred at qRT-PCR–defined densities that are within the expected range of detection by microscopy. Significantly, the Alere uRDT and the CareStart RDT missed asymptomatic parasitemias detected by qRT-PCR. One of the reasons for these false negatives may be deletion of hrp2 and hrp3 genes in some isolates because both RDTs detect HRP-2 or HRP-3 released into the blood by malaria parasites. Parasite isolates with deleted hrp2 and hrp3 genes confounding malaria diagnosis by HRP-2–based tests have been reported in Uganda and other African countries.24–26 There is a need for a large-scale survey in Uganda to determine the prevalence of parasite isolates with deleted hrp2 and hrp3 genes to inform the deployment of HRP-2–based RDTs in field surveys and in clinics. In resource-poor settings without a confirmatory qRT-PCR gold standard, it is prudent to retest negative HRP-2–based RDTs results with another malaria RDT with a different target such as lactate dehydrogenase (LDH) or alternatively use RDTs that detect both HRP-2 and LDH.27
The major limitation of our study is the small number of field samples analyzed by the Alere uRDT in the laboratory and the fact that these samples were preselected based on the outcome of CareStart RDT results in the field. The Alere uRDT was not initially available when the survey was carried out. This shortcoming notwithstanding, using a parallel ultrasensitive qRT-PCR enabled us to assess the relative performance of both RDTs in survey samples with greater precision. There is, therefore, a need to undertake more head-to-head comparisons between the Alere uRDT and other HRP-2–based RDTs in a larger number of field samples during surveys to estimate the proportion of asymptomatic parasitemia cases missed by conventional RDTs. One major strength of the study is that it investigated the Alere uRDT performance in a malaria clinic by health workers and tested the impact of noncompliance with the recommended readout time. Another strength is the parallel testing of the same samples by an ultrasensitive qRT-PCR which engendered more confidence in the results obtained with the field samples. The qRT-PCR results were quantitatively in strong agreement with microscopically defined parasite densities (r2 = 0.83) and additionally detected parasite 18S rRNA in most remaining samples. As the analytical sensitivity of field-deployable tests improves, comparison of such tests to a molecular gold standard such as qRT-PCR will help to rapidly assess the analytical sensitivity of novel RDTs such as Alere uRDT.
In conclusion, our study demonstrated that the Alere uRDT is a promising diagnostic tool in field surveys where reliable parasite prevalence estimates are needed. In the clinic, it detects clinical malaria cases missed by the conventional RDT and microscopy. However, in clinic settings where treatment based on more sensitive tests will not necessarily improve care, the more affordable microscopy and conventional RDTs will still remain the standard of care.
The current high cost and shortcomings of the Alere uRDT may constrain widespread adoption in the field in resource-poor settings where it is most needed.
Supplemental table
Acknowledgments:
We would like to thank the clinical and nursing staff of MBL clinic at St. Anne. We are also grateful the Usuk county-Katakwi district inhabitants for their participation in this study.
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation , 2016. World Malaria Report. Geneva, Switzerland: WHO. [Google Scholar]
- 3.World Health Organisation , 2015. Guidelines for the Treatment of Malaria, 3rd edition Geneva, Switzerland: WHO; Available at: http://www.who.int/malaria/publications/atoz/9789241549127/en. Accessed May 4, 2019. [Google Scholar]
- 4.Slater HC, et al. 2015. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature 528: S94–S101. [DOI] [PubMed] [Google Scholar]
- 5.Hemingway J, Shretta R, Wells TNC, Bell D, Djimdé AA, Achee N, Qi G, 2016. Tools and strategies for malaria control and elimination: what do we need to achieve a grand convergence in malaria? PLoS Biol 14: e1002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laban NM, Kobayashi T, Hamapumbu H, Sullivan D, Mharakurwa S, Thuma PE, 2015. Comparison of a PfHRP2-based rapid diagnostic test and PCR for malaria in a low prevalence setting in rural southern Zambia: implications for elimination. Malar J 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker DM, Landier J, von Seidlein L, Dondorp A, White L, Hanboonkunupakarn B, 2016. Limitations of malaria reactive case detection in an area of low and unstable transmission on the Myanmar–Thailand border. Malar J 15: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousema T, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 9.Zhao, et al. 2017. Comparison of methods for detecting asymptomatic malaria infections in the China–Myanmar border area. Malar J 16: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britton S, Cheng Q, McCarthy JS, 2016. Novel molecular diagnostic tools for malaria elimination: a review of options from the point of view of high-throughput and applicability in resource limited settings. Malar J 15: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das S, et al. 2017. Performance of a high-sensitivity rapid diagnostic test for Plasmodium falciparum malaria in asymptomatic individuals from Uganda and Myanmar and naïve human challenge infections. Am J Trop Med Hyg 97: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das S, Peck RB, Barney R, Jang IK, Kahn M, Zhu M, Domingo GJ, 2018. Performance of an ultra-sensitive Plasmodium falciparum HRP2-based rapid diagnostic test with recombinant HRP2, culture parasites, and archived whole blood samples. Malar J 17: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landier J, et al. 2018. Operational performance of a Plasmodium falciparum ultrasensitive rapid diagnostic test for detection of asymptomatic infections in eastern Myanmar. J Clin Microbiol 56: e00565–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization , 2010. Basic Malaria Microscopy. Available at: http://www.searo.who.int/LinkFiles/Malaria_malaria_microscopy_Learners_guide2010.pdf. Accessed June 14, 2019. [Google Scholar]
- 15.Murphy SC, Daza G, Chang M, Coombs R, 2012. Laser cutting eliminates nucleic acid cross-contamination in dried-blood-spot processing. J Clin Microbiol 50: 4128–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seilie AM, et al. 2019. Beyond blood smears: qualification of Plasmodium 18S rRNA as a biomarker for controlled human malaria infections. Am J Trop Med Hyg 100 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uganda Bureau of Statistics (UBOS) and ICF International , 2015. Uganda Malaria Indicator Survey 2014–15. Kampala, Uganda, and Rockville, Rockville, MD: UBOS and ICF International. [Google Scholar]
- 18.Egwang TG, Apio B, Riley E, Okello D, 2000. Plasmodium falciparum malariometric indices in Apac district, northern Uganda. East Afr Med J 77: 413–416. [PubMed] [Google Scholar]
- 19.Okiro EA, Al-Taiar A, Reyburn H, Idro R, Berkley JA, Snow RW, 2009. Age pattern of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar J 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walldorf JA, Cohee L, Coalson J, Bauleni A, 2015. School-age children are a reservoir of malaria infection in Malawi. PLoS One 10: e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai M, et al. 2014. Age-specific malaria mortality rates in the KEMRI/CDC health and demographic surveillance system in western Kenya, 2003–2010. PLoS One 9: e106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katrak S, Nayebare P, Rek J, Arinaitwe E, Nankabirwa JI, Kamya M, Dorsey G, Rosenthal PJ, Greenhouse B, 2018. Clinical consequences of submicroscopic malaria parasitaemia in Uganda. Malar J 17: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White NJ, 2004. Antimalarial drug resistance. J Clin Invest 113: 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nderu D, Kimani F, Thiong'o K, Karanja E, Akinyi M, Too E, Chege W, Nambati E, Meyer CG, Velavan TP, 2019. Plasmodium falciparum histidine-rich protein (PfHRP2 and 3) diversity in western and Coastal Kenya. Sci Rep 9: 1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson R, Beshir KB, Cunningham J, Baiden F, Bharmal J, Bruxvoort KJ, Maiteki-Sebuguzi C, Owusu-Agyei S, Staedke SG, Hopkins H, 2019. Pfhrp2 and Pfhrp3 gene deletions that affect malaria rapid diagnostic tests for Plasmodium falciparum: analysis of archived blood samples from 3 African countries. J Infect Dis 220: 1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wurtz N, et al. 2013. Pfhrp2 and pfhrp3 polymorphisms in Plasmodium falciparum isolates from Dakar, Senegal: impact on rapid malaria diagnostic tests. Malar J 12: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iqbal J, Munneer A, Khalid N, Ahmed MA, 2003. Performance of the OptiMAL test for malaria diagnosis among suspected malaria patients at the rural health centres. Am J Trop Med Hyg 68: 624–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.