Abstract.
We developed and evaluated the Global Health Wizard Hepatitis B Best Practice Alert (BPA) to increase primary care provider adherence to evidence-based guidelines for hepatitis B virus (HBV) infection screening in non–U.S.-born patients. We conducted a pilot study using nine clinics to test BPA effectiveness. Eligible patients were aged ≥ 12 years, from a country of origin with ≥ 2% HBV prevalence, had no electronic health record documentation of HBV screening, and were seen for primary care during July 2012–March 2013. The BPA triggered for > 4,500 patients and identified six previously unrecognized HBV-infected patients. The pilot project demonstrated BPA effectiveness and continued to be used at pilot clinics until 2018 and was expanded to additional clinics in 2019; 29 additional HBV-infected patients were identified. Although successful, BPA usage steadily decreased over time. Poor BPA usage limits the power to achieve the goal of improved population-based HBV screening.
Worldwide, about 400 million individuals are chronically infected with hepatitis B virus (HBV).1 In the United States, surveillance data estimate approximately 862,000 individuals older than 6 years are infected with HBV.2 However, HBV disproportionately affects non–U.S.-born persons,3 and national surveillance may underestimate the burden of HBV infection in this population. One evaluation estimated that there may be 1.32 million non–U.S.-born persons living in the United States with chronic HBV infection.4 Individuals with chronic HBV are often asymptomatic and unaware of their infection or health risks associated with untreated HBV infection such as cirrhosis, liver failure, and hepatocellular carcinoma.5 Identifying these individuals is important for patient and public health efforts because identification improves opportunities for provision of both appropriate disease management and HBV transmission prevention strategies.6 Both the U.S. Preventive Services Task Force and the CDC recommend screening populations at increased risk for HBV which includes person born in countries with ≥ 2% HBV prevalence.1,5 Unfortunately, aside from refugee screening, few primary care practices have translated these guidelines into routine care delivery.
HealthPartners Medical Group is a large U.S.-based integrated care system in the Midwest. As part of a system-wide initiative to identify and address disparities beginning in 2002, rooming staff were expected to ask for and record patient’s race, ethnicity, and preferred language during all medical encounters. In 2005, the country of origin was added to this standardized demographic data collection. To collect the country of origin, the rooming nurse asks the patient the country in which they were born and gives them the option of choosing not to answer. The objective of this project was to develop and evaluate a clinical decision support tool, the Global Health Wizard Hepatitis B Best Practice Alert (BPA), to increase HBV infection screening in non–U.S.-born populations originally from countries with ≥ 2% HBV prevalence. BPAs are automated alerts in the electronic health record (EHR) that appear, “trigger” or “fire” when a patient meets prespecified criteria.
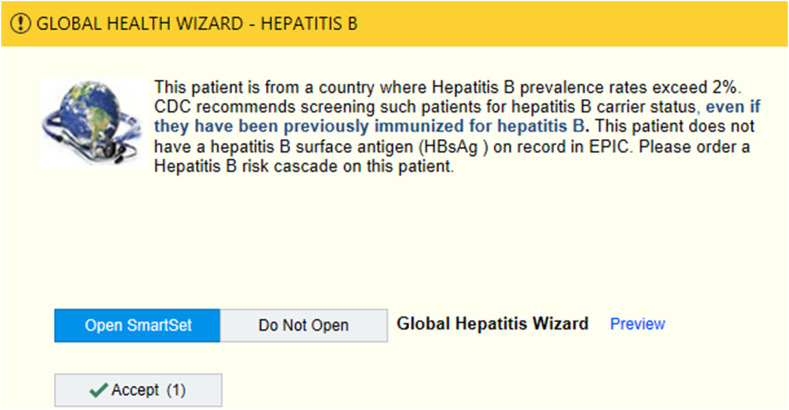
We conducted a two-arm, group-randomized pilot study to test BPA effectiveness. Nine primary care clinics (i.e., combination of family practice, internal medicine, and pediatrics) with > 450 non–English-speaking patients were matched by the proportion of non–English-speaking patients in each clinic and then randomized to either a passive or active intervention. In the passive intervention clinics, when the BPA was triggered, a pop-up educational alert recommending HBV testing only was displayed and could then be closed by the clinician. The active intervention included the same educational alert but also allowed providers to open an HBV screening “SmartSet,” an order set containing appropriate HBV screening orders for providers to select and sign. It also included pre-written documentation about the patient’s need for HBV screening and follow-up plans for subsequent testing if the screening test returned positive (Figure 1). The BPA triggered for eligible patients aged ≥ 12 years, from a country of origin with ≥ 2% HBV prevalence, with no EHR documentation of HBV screening, and who were seen for primary care at HealthPartners during July 2012–March 2013. We used documented country of origin or, if unavailable, preferred language as a proxy. Appropriate screening was defined as ordered and resulted hepatitis B surface antigen blood test.
Figure 1.
Screenshot of the Global Health Wizard Hepatitis B Best Practice Alert which allows providers to choose “Open SmartSet” or “Do Not Open.” This figure appears in color at www.ajtmh.org.
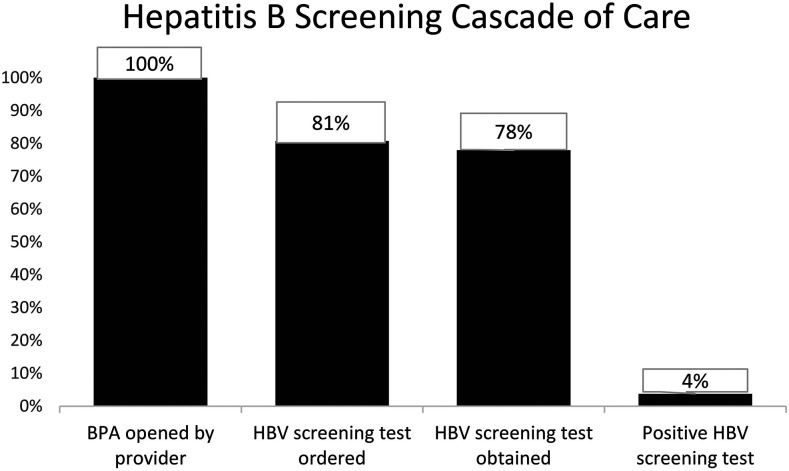
During the 9-month pilot study in the passive and active intervention clinics, the BPA triggered for 2,574 and 2,117 unique patients, and HBV screening tests were ordered for 325 (12.6%) and 333 (15.7%) patients, respectively. We identified six previously unrecognized chronic HBV patients using the intervention. The pilot project demonstrated BPA effectiveness. After pilot study completion, the active BPA continued to be used by the nine pilot clinics. BPA opening (uptake) ranged from 1.9% to 6.3% during 2012–2018, but clinician opening and appropriate screening decreased steadily over time (Table 1). Thirty-five patients (4% of tested patients, Figure 2) were identified with chronic HBV. In January 2019, the BPA was expanded to all primary care and select specialty departments in HealthPartners. Specialty departments were chosen based on the likelihood of prescribing immunosuppressive medications which may place patients with unidentified chronic, inactive HBV infection at risk for hepatitis B reactivation.
Table 1.
Hepatitis B BPA opening rate and results
| Year | Eligible patients, N | Opened, n (%) | Ordered, n (%) | Resulted, n (%) | Positive, n (%) |
|---|---|---|---|---|---|
| 2012* | 2,906 | 184 (6.3) | 136 (4.7) | 134 (4.6) | 3 (0.1) |
| 2013 | 4,313 | 230 (5.3) | 178 (4.1) | 174 (4.0) | 9 (0.2) |
| 2014 | 3,440 | 179 (5.2) | 136 (4.0) | 136 (4.0) | 5 (0.2) |
| 2015 | 2,142 | 113 (5.3) | 99 (4.6) | 98 (4.6) | 7 (0.3) |
| 2016 | 2,104 | 68 (3.2) | 58 (2.8) | 55 (2.6) | 4 (0.2) |
| 2017 | 2,148 | 40 (1.9) | 30 (1.4) | 29 (1.4) | 1 (0.1) |
| 2018 | 5,301 | 123 (2.3) | 105 (2.0) | 90 (1.7) | 6 (0.1) |
| All years | 13,707 | 919 (6.7) | 742 (5.4) | 716 (5.2) | 35 (0.3) |
BPA = Best Practice Alert. Numbers and percentages of patients who were eligible for the Global Health Wizard Hepatitis B BPA during primary care encounters at HealthPartners during 2012–2018 and for whom the BPA was opened by a provider (Opened), orders placed through BPA (Ordered), hepatitis B screening laboratory test resulted (Resulted), and the screening test returned positive (Positive). The discrepancy between screening tests ordered and resulted is due to patients not having their blood drawn after leaving the office visit.
Represents time period from July 1, 2012 to December 31, 2012.
Figure 2.
Hepatitis B screening cascade. Percentage of patients for whom each step in the hepatitis B screening cascade was complete from the point at which a healthcare provider opened the Global Health Wizard Hepatitis B Best Practice Alert to having a final positive screening test result, HealthPartners 2012–2018 (N = 919).
Based on provider feedback, we identified inaccurate BPA targeting of certain language groups (Spanish and French). We updated the BPA to improve accuracy by discontinuing the use of a preferred language as a trigger. We also added new countries to the triggering logic (Eswatini and North Macedonia). We expanded the BPA to include patients from age 0 to 75 years, instead of age 12–75 years. We developed and improved three comprehensive order sets to simplify provider ordering and documentation through an iterative, collaborative process with both primary care and gastroenterology. These include orders for HBV screening, management of newly diagnosed chronic HBV patients, and routine follow-up for known chronic HBV patients. Based on health system practices, the BPA was changed from a pop-up alert to a silent BPA which was available for view but did not interfere with workflow.
The Global Health Wizard Hepatitis B BPA increased primary care provider adherence to evidence-based guidelines for HBV screening and successfully identified 35 previously unrecognized HBV-infected patients, a life-altering diagnosis. Our results are similar to those of Chak et al. who created an EHR alert to improve HBV screening in Asian and Pacific Islanders within the UC Davis health system.7 Together, these studies illustrate an EHR alert’s ability to assist busy clinicians in providing comprehensive care to diverse patient populations. In both studies, BPA development required collection of granular demographic data (e.g., race, ethnicity, preferred language, and country of origin). As noted by Mitchell et al.,8 “Imported chronic hepatitis B cases account for approximately 95% of new U.S. cases. Earlier case identification and management of infected immigrants would strengthen the U.S. strategy to eliminate HBV transmission and could delay disease progression and prevent some deaths among new Americans.”
Despite its utility, BPA use in our system was low and decreased over time (4.7% in 2012–2.0% in 2018). Initial uptake of the BPA may have been higher because of direct education of providers by the research staff in the nine pilot clinics at the time the study began, as well as the pop-up nature of the initial BPA which garnered the attention of the clinician. Although no formal evaluation of the BPA was performed, we posit that suboptimal BPA uptake may be related to provider knowledge gaps and/or provider alert fatigue.7 The change to a silent BPA, as well as the lack of continued provider education about HBV after the initial pilot study education, likely contributed to the drop-off in BPA opening. In addition, providers may choose to ignore BPAs because of office visit time constraints or the need to address other medical issues. Future efforts should evaluate and address possible barriers to acceptance of BPAs designed to improve clinicians’ care for foreign-born patients, with the goal of increasing HBV screening and decreasing HBV-associated health disparities.
Acknowledgments:
We thank HealthPartners Medical Group leadership for their forethought in incorporating country of origin and preferred language in routinely collected demographic information as well as for their willingness to implement the Global Health Wizard during clinical encounters. We are indebted to the work of the HealthPartners Institute programming team for creation and maintenance of the Global Health Wizard. Finally, we thank Ann Settgast for her work throughout the development and implementation of the Global Health Wizard.
REFERENCES
- 1.Rajbhandari R, Chung RT, 2014. Screening for hepatitis B virus infection: a public health imperative. Ann Intern Med 161: 76–77. [DOI] [PubMed] [Google Scholar]
- 2.Patel EU, Thio CL, Boon D, Thomas DL, Tobian AAR, 2019. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011–2016. Clin Infect Dis 69: 709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le MH, Yeo YH, Cheung R, Henry L, Lok AS, Nguyen MH, 2019. Chronic hepatitis B prevalence among foreign-born and U.S.-born adults in the United States, 1999–2016. Hepatology 71: 431–443. [DOI] [PubMed] [Google Scholar]
- 4.Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL, 2012. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of Origin. Hepatology 56: 422–433. [DOI] [PubMed] [Google Scholar]
- 5.Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM; High Value Care Task Force of the American College of Physicians and the Centers for Disease Control and Prevention , 2017. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for disease Control and Prevention. Ann Intern Med 167: 794–804. [DOI] [PubMed] [Google Scholar]
- 6.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW; Centers for Disease Control and Prevention (CDC) , 2008. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 57: 1–20. [PubMed] [Google Scholar]
- 7.Chak E, Taefi A, Li CS, Chen Jr., MS, Harris AM, MacDonald S, Bowlus C, 2018. Electronic medical alerts increase screening for chronic hepatitis B: a randomized, double-blind, controlled trial. Cancer Epidemiol Biomarkers Prev 27: 1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA, 2011. The increasing burden of imported chronic hepatitis B—United States, 1974–2008. PLoS One 6: e27717. [DOI] [PMC free article] [PubMed] [Google Scholar]