Abstract.
The parasitic helminth infection neurocysticercosis (NCC) is the most common cause of adult-acquired epilepsy in the world. Despite the serious consequences of epilepsy due to this infection, an in-depth review of the distinct characteristics of epilepsy due to neurocysticercosis has never been conducted. In this review, we evaluate the relationship between NCC and epilepsy and the unique characteristics of epilepsy caused by NCC. We also discuss recent advances in our understanding of NCC-related epilepsy, including the importance of anti-inflammatory therapies, the association between NCC and temporal lobe epilepsy, and the recent discovery of biomarkers of severe epilepsy development in individuals with NCC and seizures.
INTRODUCTION
The parasitic infection neurocysticercosis (NCC) is the most common etiology of adult-acquired epilepsy (an enduring predisposition for seizures) worldwide, associated with 30–50% of such cases of epilepsy in endemic areas.1–12 The most common clinical presentation of NCC is seizures, occurring in up to 80% of infected individuals with symptoms.
Seizures can be classified, according to the International League Against Epilepsy, as either focal- or generalized-onset seizures.13 Focal seizures can be further divided into aware or impaired awareness. As generalized-onset seizures by definition involve networks on both sides of the brain at onset, they are always associated with impaired awareness. Both focal- and generalized-onset seizures are then classified by the type of symptoms experienced at the onset of the seizure, either motor symptoms (e.g., tonic, clonic, and myoclonic) or non-motor symptoms (e.g., changes in sensation or cognition).
Regardless of the seizure type, epilepsy of any etiology is believed to develop in the setting of an epileptic abnormality that impairs normal neuronal activity, an altered seizure threshold, and/or additional factors.14 In the case of NCC, the epileptic abnormality is typically caused by focal lesions,15 although other chronic changes resulting from NCC infection (such as perilesional gliosis or areas of infarcts from infection-associated vasculitis) may disturb normal neuronal activity.16,17 Additional factors that may also play a role in epilepsy development for some patients with NCC include a genetic predisposition toward a lowered seizure threshold and/or a strong host immune inflammatory response.18–20 Although NCC is a leading cause of epilepsy, little attention has been paid to the distinct characteristics of NCC-related epilepsy. In this review, we examine the unique characteristics of epilepsy development in NCC.
When discussing seizures associated with NCC, it is important to distinguish early-onset seizures provoked by an active NCC infection and resulting inflammation (non-epileptic early-onset seizures) from epileptic seizures, which are unprovoked. Early-onset–provoked seizures are thought to be primarily incited by an immune inflammatory response to antigens released either from a naturally degenerating lesion or following treatment.18,21 These seizures may have multiple relapses associated with episodic lesion-associated inflammation. What distinguishes these acute seizures from epileptic seizures is that acute seizures do not continue in the absence of an active immune/inflammatory response to the parasite. It is only when this inflammatory response is also accompanied by the formation of synchronous firing patterns and epileptic networks where epileptogenesis (epilepsy development) occurs. Once these functionally connected epileptic networks form, it is unclear if they ever go away.
Factors associated with prior NCC infection can acutely lower the seizure threshold even in the absence of active infection. This can occur because of 1) neurotoxicity of calcium released from calcified lesions22,23 or 2) transient release of NCC antigen during remodeling of calcified lesions causing an immune inflammatory response. An inflammatory response to NCC calcifications was demonstrated in a histologic examination of two calcified lesions.24,25 In subjects who have formed epileptic neural networks, an acute lowering of the seizure threshold can lead to additional epileptic seizures. Various studies have shown that up to 30% of NCC patients with epilepsy who were successfully treated for NCC can have persistent seizures despite being treated with both antiparasitic and anticonvulsant medications.18,21 These recurrent seizures are associated with worsened quality of life and increased mortality.21,26–35
Epilepsy due to NCC has many distinct characteristics that may lead to clinical differences and altered optimal treatment strategies compared with epilepsy of other etiologies. Because this syndrome is common, it is worth evaluating these distinct characteristics. In this review, we discuss the features of epilepsy in subjects with NCC and review new advances in our understanding of NCC-related epilepsy.
Clinical characteristics of epilepsy due to NCC.
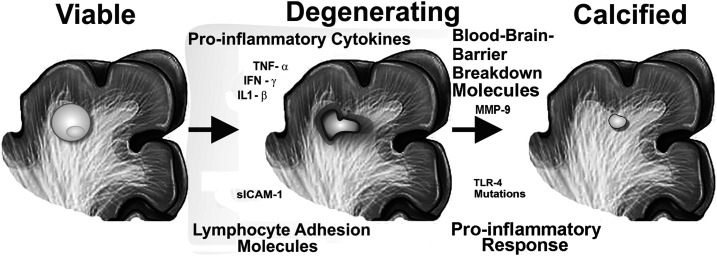
Neurocysticercosis occurs when an individual is infected by the ova of Taenia solium, most commonly via the fecal–oral route. The infection passes through several stages that can be distinguished on imaging studies, from a living (viable) cyst, to an inflamed (degenerating) lesion, and finally culminates in the formation of a granuloma that either resolves (is no longer visible on imaging) or forms a calcified lesion (Figure 1).23,36–38 The clinical presentation of seizures in NCC patients is likely impacted by whether the patients have multiple or single lesions, new-onset seizures, or established epilepsy, and whether or not they have been treated with antiparasitic medications.39,40 Despite this clinical heterogeneity, multiple studies have confirmed that the most common seizure types associated with NCC are focal (occurring in 22–61% of NCC patients with new-onset seizures) and secondarily generalized-focal seizures (15–61% of subjects).39,41
Figure 1.
Inflammation-associated factors associated with epilepsy development in neurocysticercosis (NCC) patients. The figure summarizes the stages of NCC lesions: living viable cysts (left), degenerating dying lesions with inflammation (center), and dead calcified lesions (right). Markers that are increased in those with active epilepsy compared with NCC-infected subjects without epilepsy are shown: 1) pro-inflammatory cytokines (tumor necrosis factor-α, interferon-γ, and interleukin 1-β) and blood–brain barrier breakdown molecules (MMP-9); 2) lymphocyte adhesion molecules; and 3) mutations in regulators of lymphocyte adhesion (Toll-like receptor 4) associated with pro-inflammatory conditions.
Various studies have evaluated for an association between characteristics of NCC lesions and seizure semiology (clinical signs and symptoms), with mixed results. In some studies, the frontal lobe lesions were more likely to present with focal seizures, whereas the temporal lesions and those in the degenerating stage were more likely to secondarily generalize.39,42–44 In a retrospective cohort study, a strong relationship was found between the semiologic classification of the seizure and the location of the NCC lesion,45 but other studies have not found this association. One explanation for this discrepancy could be that different studies may have included distinct subpopulations of patients with NCC. Many of the studies which did not find an association between semiology and lesion characteristics included primarily calcified lesions, which represent subjects at a late stage of disease who thus may present differently.46,47 These conflicting results also further highlight the exceedingly heterogenous nature of NCC.
Several studies have demonstrated a benefit of antiparasitic treatment in reducing the frequency of seizures in subjects with NCC. In a double-blind, placebo-controlled trial of subjects with viable NCC lesions, treatment with albendazole and dexamethasone was shown to decrease the number of generalized seizures during 30 months of follow-up. The number of total seizures, however, was not decreased by treatment. Unsurprisingly, those who were treated with albendazole in this study were more likely to have resolved their cystic lesions on follow-up imaging than those randomized to placebo.40 This finding is especially important as a separate randomized controlled study found a significant reduction in seizures in subjects treated for NCC; this reduction was primarily driven by the subgroup of subjects for whom the cyst resolved.48 However, the authors noted that the cyst resolution only partially reduced the likelihood of continued seizures, and therefore, additional methods capable of preventing recurrent epileptic seizures in NCC patients need to be identified.
Markers of increased risk for epilepsy development in patients with NCC.
Early intervention is thought to provide the best possibility for preventing epilepsy in subjects with an initial seizure, as each seizure may increase the brain’s ability to produce seizures.49 Unfortunately, regardless of the underlying cause of seizures, currently available anticonvulsant drugs do not prevent epileptogenesis or cure epilepsy.50 Therefore, for early intervention to prevent epilepsy in NCC patients to be possible, researchers must first 1) develop an increased understanding of the processes involved in epileptogenesis and 2) identify biomarkers capable of identifying subjects early in their clinical course who will go on to develop epilepsy. Such biomarkers would also help avoid unnecessary interventions in patients with a low likelihood of seizure recurrence and could identify novel therapeutic targets to prevent epilepsy.
To date, no biomarkers have been identified that can successfully predict which NCC patients with acute seizures will develop epilepsy. However, several imaging findings have shown promise in identifying those at increased risk for treatment-resistant epilepsy, as it occurs in up to 30% of subjects with NCC.18,21,26,33,51,52 These imaging findings include 1) an increased number of total, degenerating, and calcified lesions at baseline18 (Figure 2), 2) persistent perilesional edema on follow-up imaging studies53 (Figure 3), and 3) higher perilesional T2 values (likely indicative of gliosis)54 on posttreatment magnetic resonance imaging (MRI) scans. One potential drawback of perilesional edema as a predictive factor for persistent seizures is that this finding could either be caused by vasogenic edema from a seizure or could be due to immune-associated inflammation. One strategy to address this could be to reimage with MRI after a period of at least 2 months following a known seizure to evaluate edema persistence. This would not completely rule out that perilesional edema is due to seizures, however, as the patient could have subclinical ictal activity. A recent study examining calcified NCC lesions with perilesional edema using PET scans found that 12 of 13 new NCC lesions (including both degenerating cysts and areas of perilesional edema, 92.3%) expressed immune-mediated inflammatory proteins, which remained present from 2 to 9 months following treatment.55 Future studies using this technology could, therefore, help better understand the pathophysiology of perilesional edema.
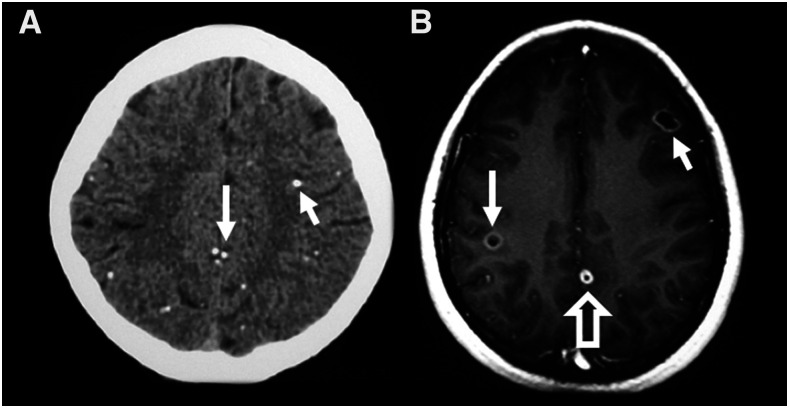
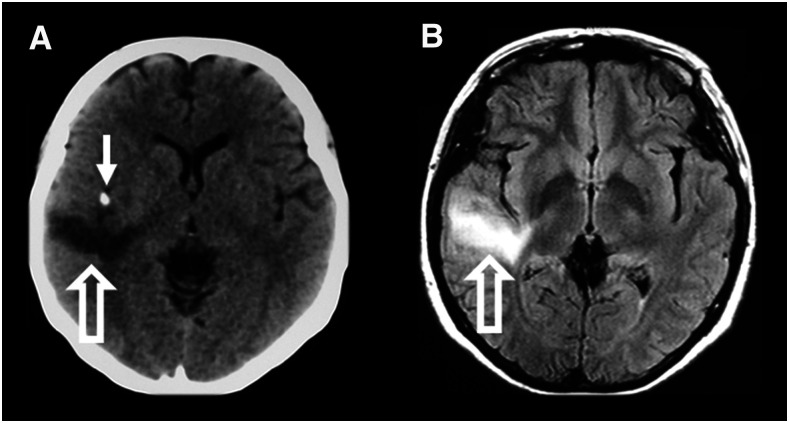
Figure 2.
Burden of disease. An increased number of lesions may be associated with an increased likelihood for treatment-resistant seizures in subjects with NCC. (A) Axial computed tomography (CT) scan of a patient with NCC who had multiple calcified lesions. Representative calcifications on this image are indicated by solid arrows. (B) Axial T1 MRI scan demonstrating multiple degenerating lesions. The image shows two lesions in the colloidal stage of degeneration that are indicated with solid arrows; a lesion in the nodular stage is indicated with a hollow arrow.
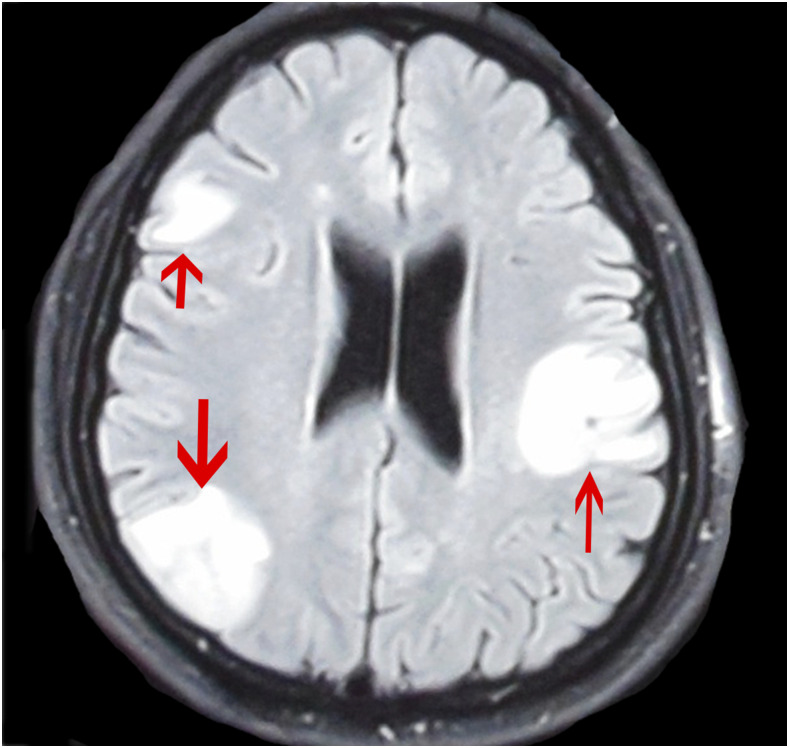
Figure 3.
Persistent edema on imaging studies. A 6-month posttreatment axial T2-FLAIR MRI scan from a patient with neurocysticercosis. The scan shows persistent edema in multiple areas, indicated by arrows. This figure appears in color at www.ajtmh.org.
Importance of the immune inflammatory response in epilepsy development.
In both porcine models of NCC and PET studies in humans, lesions of NCC showed evidence of perilesional inflammation.55,56 This inflammatory response in NCC appears to play an important role in epileptogenesis, natural involution of lesions, and response to treatment. Inflammation is thought to lead to epileptogenesis either by causing gliosis and/or by causing persistent areas of blood–brain barrier dysfunction.39
As evidence to support an association between the inflammatory response and epileptogenesis, subjects with active epilepsy due to NCC, compared with infected subjects without epilepsy, have 1) higher serum levels of blood–brain barrier breakdown molecules (MMP-9) and increase in pro-inflammatory cytokines (tumor necrosis factor [TNF]-α, interferon-γ, and interleukin [IL] 1-β), 2) increased expression of lymphocyte adhesion molecules, and 3) increased likelihood of mutations in the Toll-like receptor 4 that lead to an increased Th1 (pro-inflammatory) response (Figure 1).19,20,57,58 Finally, a strong serologic response (≥ 4 bands)8,59 to parasite antigen on NCC EITB was associated with both epilepsy development and with a more severe course once epilepsy was established.18,60
Given the importance of inflammation in epilepsy due to NCC, several studies have evaluated for a clinical utility of glucocorticoids such as prednisone and dexamethasone to reduce seizures or inhibit epileptogenesis. Steroids have been shown to reduce the incidence of acute seizures in NCC61,62 and may be able to impact disease progression.63 Steroids have proven beneficial in reducing the frequency of adverse events among subjects with 1) viable intraparenchymal lesions who are treated with antiparasitic medications,64 2) single enhancing or multicystic lesions,65 3) subarachnoid or spinal disease, and 4) those who require ventriculoperitoneal shunt placement.66 However, the lengthy treatment courses and high doses of steroids often required to control inflammation associated with NCC can lead to significant side effects, including hyperglycemia, avascular necrosis of joints, and glaucoma.67 Newer anti-inflammatory medications such as methotrexate and TNF-α inhibitors likely merit further study to assess their ability to suppress inflammation with potentially fewer side effects than steroids. Although data are limited, one case series included four patients with episodes of perilesional edema and seizures, all of whom reported clinical improvement after treatment with 25 or 50 mg of etanercept weekly for a median of 400.5 days.68 Further studies should be carried out with a larger number of patients to confirm these findings.
The role of calcified lesions and hippocampal sclerosis in epilepsy due to NCC.
Epilepsy rates have been shown to be higher in areas where NCC calcifications (the most frequent finding of NCC23,69) are common.26,70 Calcium is known to be neurotoxic, and other diseases with neurologic calcifications are also associated with epilepsy. In a select number of patients with cerebral calcifications (with causes including a calcified cavernous hemangioma or idiopathic calcification), the bisphosphonate drug disodium etidronate was found to decrease seizure recurrences, presumably by chelating solid-phase calcium phosphates.22 However, this has not yet been studied in calcified NCC lesions.
As the neuroanatomic locations of calcified NCC lesions do not always correlate with areas of abnormality on electroencephalogram (EEG), some researchers have suggested that calcifications may be only an incidental finding in epilepsy patients.23,47,71 It has recently been suggested that calcifications may be associated with hippocampal sclerosis71–74 (HS; a known cause of temporal lobe epilepsy,75,76 Figure 4), which could possibly explain this lack of concordance between NCC lesions and EEG abnormalities. Hippocampal sclerosis (HS) is defined as the presence of both hippocampal atrophy and visible sclerosis on MRI.77,78 NCC could lead to HS through two possible mechanisms: 1) the NCC lesion could cause chronic interictal or ictal discharges that injure and kindle the hippocampus or 2) NCC-associated inflammation could directly damage the hippocampus.79
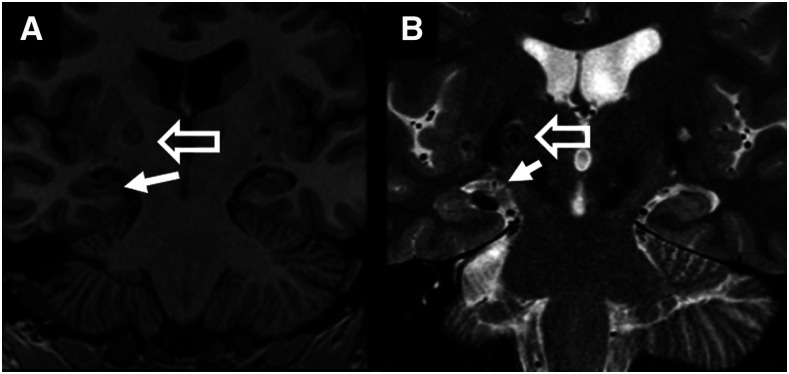
Figure 4.
Hippocampal sclerosis. Hippocampal sclerosis (defined as the presence of hippocampal atrophy and a hyperintense signal on long-repetition-time sequences of the hippocampus) is seen on the R-side (solid arrow) of the T1 (A) and T2-FLAIR (B) coronal MRI scans. An adjacent calcified lesion (hollow arrow) is also shown.
As evidence for a causative association between calcifications and HS, among patients presenting for an evaluation for epilepsy surgery, patients with HS were less likely to have another precipitating injury of epilepsy identified if they had NCC calcifications (4 of 18 subjects, 22.2% had another injury) compared with those with HS alone (19/36, 52.8%, P-value < 0.05).80 In addition, in a case–control study, patients with NCC had decreased hippocampal volumes compared with control subjects. On a subgroup analysis (of control subjects, subjects untreated for NCC, and treated NCC subjects), this decrease in hippocampal volumes among NCC patients was found to be primarily due to changes among untreated patients. Mean hippocampal volumes in this study were 3.44 cm3 on the right and 3.38 on the left for untreated NCC patients compared with 3.92 cm3 and 3.84, respectively, for control subjects (P < 0.001).81 Furthermore, in a cohort of patients surgically treated for mesial temporal lobe epilepsy, among patients with a single calcified NCC lesion, the lesion matched the side of the HS in 43 of 58 cases (74.1%), significantly more than would be expected because of chance (P-value = 0.008).82
Recent evidence suggests that calcifications may also lead to epilepsy through an immune inflammatory mechanism. NCC calcifications had long been thought of as immunologically inert, until Nash et al.83 demonstrated that calcified lesions of NCC could be associated with perilesional edema (inflamed calcifications, Figure 5). The presence of an inflamed calcification is associated with up to a 12-fold increased likelihood of ongoing seizures despite treatment with anticonvulsants.18,83–85 As evidence for an immune etiology of perilesional edema (as opposed to vasogenic edema), pathologic examination of a resected calcified NCC granuloma with recurrent episodes of perilesional edema showed a marked mononuclear infiltrate.25 Despite the immunologic causes that appear to underlie inflamed calcifications, the utility of corticosteroids or other immunosuppressive medications in this setting has not been established. Corticosteroid withdrawal has been shown to initiate (or exacerbate) pericalcific edema,86 and therefore, currently there is no sufficient evidence that the benefits of steroids outweigh the risks.
Figure 5.
Inflamed calcifications. CT scan (A) and concurrent T2-FLAIR MRI scan (B) demonstrating axial views of a calcified lesion with perilesional edema seen in the right temporal lobe of both the CT scan and the MRI scan. The calcified lesion is indicated by a solid arrow. Areas of perilesional edema are indicated by hollow arrows.
Because of the focal nature of NCC lesions, a promising option for a subset of patients with medically refractory epilepsy and NCC has been surgical resection. Although a minority of NCC patients would likely qualify for such treatment, patients may benefit from surgery if they have 1) intractable epilepsy with an NCC lesion within the boundaries of the epileptogenic zone or 2) developed HS in conjunction with NCC. In two small reviews,87,88 16 subjects who had calcified NCC lesions plus either evidence that a specific calcified lesion was the seizure-provoking focus (based on seizure semiology and/or EEG findings) or association of the calcification with unilateral HS were treated surgically. Those without evidence of HS underwent resection of the causative lesion, whereas those with HS were treated with resection of both the calcified lesion and the anterior temporal lobe (ATL). Following surgery, 93.8% (15/16) of subjects experienced complete resolution of their seizures. However, resection of solely the NCC lesion or the ATL alone was not found to be successful in subjects with both HS and a causative lesion due to NCC (4/5 subjects, 80%, continued to have seizures). Although surgical resection has been promising in this small group of patients, which surgical technique is best and what subset of patients are most likely to benefit from such an intervention are not yet known.
CONCLUSION
Recent studies have identified two primary processes, inflammation and calcification (in association with HS or not), which appear to be important for epilepsy development in patients with NCC. Factors associated with these processes (such as quantitative edema, an increased number of calcifications, and the presence of calcified lesions with perilesional edema) may be able to identify those at increased risk for severe seizure disorders early in their disease course. Both of these processes could potentially be treated with currently available medications that could be repurposed for use in NCC (either steroid-sparing anti-inflammatory medications such as methotrexate or etanercept or bisphosphonates). Studies of these treatments in NCC patients are needed as a large portion of those with NCC and epilepsy continue to seize despite treatment.
REFERENCES
- 1.Bern C, Garcia HH, Evans C, Gonzalez AE, Verastegui M, Tsang VC, Gilman RH, 1999. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis 29: 1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Brutto OH, Arroyo G, Del Brutto VJ, Zambrano M, Garcia HH, 2017. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: a large-scale, computed tomography-based population study in rural Ecuador. Epilepsia 58: 1955–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Brutto OH, et al. 2005. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia 46: 583–587. [DOI] [PubMed] [Google Scholar]
- 4.Garcia HH, Talley A, Gilman RH, Zorrilla L, Pretell J, 1999. Epilepsy and neurocysticercosis in a village in Huaraz, Peru. Clin Neurol Neurosurg 101: 225–228. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Noval J, Moreno E, de Mata F, Soto de Alfaro H, Fletes C, Craig PS, Allan JC, 2001. An epidemiological study of epilepsy and epileptic seizures in two rural Guatemalan communities. Ann Trop Med Parasitol 95: 167–175. [DOI] [PubMed] [Google Scholar]
- 6.Medina MT, Rosas E, Rubio-Donnadieu F, Sotelo J, 1990. Neurocysticercosis as the main cause of late-onset epilepsy in Mexico. Arch Intern Med 150: 325–327. [PubMed] [Google Scholar]
- 7.Millogo A, et al. 2012. Prevalence of neurocysticercosis among people with epilepsy in rural areas of Burkina Faso. Epilepsia 53: 2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moyano LM, et al. 2014. Neurocysticercosis as a cause of epilepsy and seizures in two community-based studies in a cysticercosis-endemic region in Peru. PLoS Negl Trop Dis 8: e2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pondja A, Neves L, Mlangwa J, Afonso S, Fafetine J, Willingham AL, 3rd, Thamsborg SM, Johansen MV, 2010. Prevalence and risk factors of porcine cysticercosis in Angonia district, Mozambique. PLoS Negl Trop Dis 4: e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad KN, Prasad A, Gupta RK, Nath K, Pradhan S, Tripathi M, Pandey CM, 2009. Neurocysticercosis in patients with active epilepsy from the pig farming community of Lucknow district, north India. Trans R Soc Trop Med Hyg 103: 144–150. [DOI] [PubMed] [Google Scholar]
- 11.Rottbeck R, et al. 2013. High prevalence of cysticercosis in people with epilepsy in southern Rwanda. PLoS Negl Trop Dis 7: e2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villaran MV, et al. 2009. Epilepsy and neurocysticercosis: an incidence study in a Peruvian rural population. Neuroepidemiology 33: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher RS, et al. 2017. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia 58: 522–530. [DOI] [PubMed] [Google Scholar]
- 14.Engel J, Jr., et al. 2013. Epilepsy biomarkers. Epilepsia 54 (Suppl 4): 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpio A, Romo ML, 2015. Multifactorial basis of epilepsy in patients with neurocysticercosis. Epilepsia 56: 973–974. [DOI] [PubMed] [Google Scholar]
- 16.Cantu C, Barinagarrementeria F, 1996. Cerebrovascular complications of neurocysticercosis. Clinical and neuroimaging spectrum. Arch Neurol 53: 233–239. [DOI] [PubMed] [Google Scholar]
- 17.Del Brutto OH, 1992. Cysticercosis and cerebrovascular disease: a review. J Neurol Neurosurg Psychiatry 55: 252–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrick JA, et al. Cysticercosis Working Group in Peru , 2018. Inflammation is a key risk factor for persistent seizures in neurocysticercosis. Ann Clin Transl Neurol 5: 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachuriya G, Garg RK, Jain A, Malhotra HS, Singh AK, Jain B, Kumar N, Verma R, Sharma PK, 2016. Toll-like receptor-4 polymorphisms and serum matrix metalloproteinase-9 in newly diagnosed patients with calcified neurocysticercosis and seizures. Medicine (Baltimore) 95: e3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma A, Prasad KN, Nyati KK, Singh SK, Singh AK, Paliwal VK, Gupta RK, 2011. Association of MMP-2 and MMP-9 with clinical outcome of neurocysticercosis. Parasitology 138: 1423–1428. [DOI] [PubMed] [Google Scholar]
- 21.Singh G, Burneo JG, Sander JW, 2013. From seizures to epilepsy and its substrates: neurocysticercosis. Epilepsia 54: 783–792. [DOI] [PubMed] [Google Scholar]
- 22.Loeb JA, Sohrab SA, Huq M, Fuerst DR, 2006. Brain calcifications induce neurological dysfunction that can be reversed by a bone drug. J Neurol Sci 243: 77–81. [DOI] [PubMed] [Google Scholar]
- 23.Nash TE, et al. 2004. Calcific neurocysticercosis and epileptogenesis. Neurology 62: 1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash TE, Bartelt LA, Korpe PS, Lopes B, Houpt ER, 2014. Calcified neurocysticercus, perilesional edema, and histologic inflammation. Am J Trop Med Hyg 90: 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ooi WW, Wijemanne S, Thomas CB, Quezado M, Brown CR, Nash TE, 2011. Short report: a calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: histopathological features. Am J Trop Med Hyg 85: 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno E, Bartoloni A, Zammarchi L, Strohmeyer M, Bartalesi F, Bustos JA, Santivanez S, Garcia HH, Nicoletti A; COHEMI Project Study Group , 2013. Epilepsy and neurocysticercosis in Latin America: a systematic review and meta-analysis. PLoS Negl Trop Dis 7: e2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeGiorgio CM, Medina MT, Duron R, Zee C, Escueta SP, 2004. Neurocysticercosis. Epilepsy Curr 4: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K, 2011. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long-term seizure outcome: a prospective study using serial magnetization transfer imaging. Epilepsia 52: 1918–1927. [DOI] [PubMed] [Google Scholar]
- 29.Begley CE, et al. 2000. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 41: 342–351. [DOI] [PubMed] [Google Scholar]
- 30.EUCARE , 2003. European white paper on epilepsy. Epilepsia 44 (Suppl 6): 1–88. [PubMed] [Google Scholar]
- 31.Forsgren L, Bucht G, Eriksson S, Bergmark L, 1996. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia 37: 224–229. [DOI] [PubMed] [Google Scholar]
- 32.Forsgren L, Hauser WA, Olafsson E, Sander JW, Sillanpaa M, Tomson T, 2005. Mortality of epilepsy in developed countries: a review. Epilepsia 46 (Suppl 11): 18–27. [DOI] [PubMed] [Google Scholar]
- 33.O’Neal SE, Flecker RH, 2015. Hospitalization frequency and charges for neurocysticercosis, United States, 2003–2012. Emerg Infect Dis 21: 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shihabuddin BS, Herlopian AS, Greenfield LJ, Jr., 2014. Ictal asystole in epilepsy patients undergoing inpatient video-EEG monitoring. Neurosciences (Riyadh) 19: 317–321. [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Chronic Disease Prevention and Health Promotion DoPH , 2017. Centers for Disease Control, At A Glance 2016: Epilepsy, One of the Nation’s Most Common Neurological Conditions. Available at: https://www.cdc.gov/chronicdisease/resources/publications/aag/epilepsy.htm. Accessed April 28, 2017. [Google Scholar]
- 36.Del Brutto OH, 2012. Neurocysticercosis: a review. ScientificWorldJournal 2012: 159821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia HH, Del Brutto OH, 2003. Imaging findings in neurocysticercosis. Acta Trop 87: 71–78. [DOI] [PubMed] [Google Scholar]
- 38.Lerner A, Shiroishi MS, Zee CS, Law M, Go JL, 2012. Imaging of neurocysticercosis. Neuroimaging Clin N Am 22: 659–676. [DOI] [PubMed] [Google Scholar]
- 39.Duque KR, Burneo JG, 2017. Clinical presentation of neurocysticercosis-related epilepsy. Epilepsy Behav 76: 151–157. [DOI] [PubMed] [Google Scholar]
- 40.Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, Herrera G, Evans CA, Gonzalez AE; Cysticercosis Working Group in Peru , 2004. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med 350: 249–258. [DOI] [PubMed] [Google Scholar]
- 41.Das K, Mondal GP, Banerjee M, Mukherjee BB, Singh OP, 2007. Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8 year randomised study. J Clin Neurosci 14: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 42.Del Brutto OH, Santibanez R, Noboa CA, Aguirre R, Diaz E, Alarcon TA, 1992. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology 42: 389–392. [DOI] [PubMed] [Google Scholar]
- 43.Prasad A, Gupta RK, Pradhan S, Tripathi M, Pandey CM, Prasad KN, 2008. What triggers seizures in neurocysticercosis? A MRI-based study in pig farming community from a district of north India. Parasitol Int 57: 166–171. [DOI] [PubMed] [Google Scholar]
- 44.Kelvin EA, Carpio A, Bagiella E, Leslie D, Leon P, Andrews H, Hauser WA, Ecuadorian Neurocysticercosis G, 2011. Seizure in people with newly diagnosed active or transitional neurocysticercosis. Seizure 20: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duque KR, Escalaya AL, Zapata W, Burneo JG, Bustos JA, Gonzales I, Saavedra H, Pretell EJ, Garcia HH; Cysticercosis Working Group in Peru , 2018. Clinical topography relationship in patients with parenchymal neurocysticercosis and seizures. Epilepsy Res 145: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murthy JM, Reddy VS, 1998. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure 7: 153–157. [DOI] [PubMed] [Google Scholar]
- 47.Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G, 2000. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia 41: 718–726. [DOI] [PubMed] [Google Scholar]
- 48.Garcia HH, et al. Cysticercosis Working Group in Peru , 2014. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis 14: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertram E, 2007. The relevance of kindling for human epilepsy. Epilepsia 48 (Suppl 2): 65–74. [DOI] [PubMed] [Google Scholar]
- 50.Temkin NR, 2001. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia 42: 515–524. [DOI] [PubMed] [Google Scholar]
- 51.Goyal M, Chand P, Modi M, Khandelwal N, Kharbanda PS, Lal V, Singla V, Sehgal R, 2015. Neurocysticercosis: an uncommon cause of drug-refractory epilepsy in North Indian population. Epilepsia 56: 1747–1752. [DOI] [PubMed] [Google Scholar]
- 52.Burneo JG, Cavazos JE, 2014. Neurocysticercosis and epilepsy. Epilepsy Curr 14: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia HH, Del Brutto OH; Cysticercosis Working Group in Peru , 2017. Antiparasitic treatment of neurocysticercosis - the effect of cyst destruction in seizure evolution. Epilepsy Behav 76: 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Souza A, Nalini A, Saini J, Thennarasu K, 2017. T2 relaxometry helps prognosticate seizure outcome in patients with solitary cerebral cysticercosis. J Neurol Sci 376: 1–6. [DOI] [PubMed] [Google Scholar]
- 55.Fujita M, Mahanty S, Zoghbi SS, Ferraris Araneta MD, Hong J, Pike VW, Innis RB, Nash TE, 2013. PET reveals inflammation around calcified Taenia solium granulomas with perilesional edema. PLoS One 8: e74052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nash TE, Mahanty S, Garcia HH; Cysticercosis Group in Peru , 2011. Corticosteroid use in neurocysticercosis. Expert Rev Neurother 11: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prasad A, Prasad KN, Gupta RK, Pradhan S, 2009. Increased expression of ICAM-1 among symptomatic neurocysticercosis. J Neuroimmunol 206: 118–120. [DOI] [PubMed] [Google Scholar]
- 58.Verma A, Prasad KN, Gupta RK, Singh AK, Nyati KK, Rizwan A, Pandey CM, Paliwal VK, 2010. Toll-like receptor 4 polymorphism and its association with symptomatic neurocysticercosis. J Infect Dis 202: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 59.Arroyo G, et al. 2017. Antibody banding patterns of the enzyme-linked immunoelectrotransfer blot (EITB) and brain imaging findings in patients with neurocysticercosis. Clin Infect Dis 66: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzales I, et al. 2015. Seizures, cysticercosis and rural-to-urban migration: the Peru MIGRANT study. Trop Med Int Health 20: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia HH, Gonzales I, Lescano AG, Bustos JA, Pretell EJ, Saavedra H, Nash TE; Cysticercosis Working Group in Peru , 2014. Enhanced steroid dosing reduces seizures during antiparasitic treatment for cysticercosis and early after. Epilepsia 55: 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mall RK, Agarwal A, Garg RK, Kar AM, Shukla R, 2003. Short course of prednisolone in Indian patients with solitary cysticercus granuloma and new-onset seizures. Epilepsia 44: 1397–1401. [DOI] [PubMed] [Google Scholar]
- 63.Cuello-Garcia CA, Roldan-Benitez YM, Perez-Gaxiola G, Villarreal-Careaga J, 2013. Corticosteroids for neurocysticercosis: a systematic review and meta-analysis of randomized controlled trials. Int J Infect Dis 17: e583–e592. [DOI] [PubMed] [Google Scholar]
- 64.White AC, Jr., Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A, Garcia HH, Nash TE, 2018. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 66: 1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao BC, et al. 2016. Albendazole and corticosteroids for the treatment of solitary cysticercus granuloma: a network meta-analysis. PLoS Negl Trop Dis 10: e0004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suastegui Roman RA, Soto-Hernandez JL, Sotelo J, 1996. Effects of prednisone on ventriculoperitoneal shunt function in hydrocephalus secondary to cysticercosis: a preliminary study. J Neurosurg 84: 629–633. [DOI] [PubMed] [Google Scholar]
- 67.Buchman AL, 2001. Side effects of corticosteroid therapy. J Clin Gastroenterol 33: 289–294. [DOI] [PubMed] [Google Scholar]
- 68.Nash TE, Ware JM, Coyle CM, Mahanty S, 2019. Etanercept to control inflammation in the treatment of complicated neurocysticercosis. Am J Trop Med Hyg 100: 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fleury A, et al. 2003. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology 22: 139–145. [DOI] [PubMed] [Google Scholar]
- 70.Tellez-Zenteno JF, Hernandez-Ronquillo L, 2017. Epidemiology of neurocysticercosis and epilepsy, is everything described? Epilepsy Behav 76: 146–150. [DOI] [PubMed] [Google Scholar]
- 71.Velasco TR, et al. 2006. Calcified cysticercotic lesions and intractable epilepsy: a cross sectional study of 512 patients. J Neurol Neurosurg Psychiatry 77: 485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Brutto OH, Salgado P, Lama J, Del Brutto VJ, Campos X, Zambrano M, Garcia HH, 2015. Calcified neurocysticercosis associates with hippocampal atrophy: a population-based study. Am J Trop Med Hyg 92: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oliveira MC, Martin MG, Tsunemi MH, Vieira G, Castro LH, 2014. Small calcified lesions suggestive of neurocysticercosis are associated with mesial temporal sclerosis. Arq Neuropsiquiatr 72: 510–516. [DOI] [PubMed] [Google Scholar]
- 74.de Oliveira Taveira M, Morita ME, Yasuda CL, Coan AC, Secolin R, Luiz Cunha da Costa A, Cendes F, 2015. Neurocysticercotic calcifications and hippocampal sclerosis: a case-control study. PLoS One 10: e0131180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thom M, 2014. Review: hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol 40: 520–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker MC, 2015. Hippocampal sclerosis: causes and prevention. Semin Neurol 35: 193–200. [DOI] [PubMed] [Google Scholar]
- 77.Bronen R, 1998. MR of mesial temporal sclerosis: how much is enough? AJNR Am J Neuroradiol 19: 15–18. [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson GD, Berkovic SF, Duncan JS, Connelly A, 1993. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am J Neuroradiol 14: 753–762. [PMC free article] [PubMed] [Google Scholar]
- 79.Bianchin MM, Velasco TR, Santos AC, Sakamoto AC, 2012. On the relationship between neurocysticercosis and mesial temporal lobe epilepsy associated with hippocampal sclerosis: coincidence or a pathogenic relationship? Pathog Glob Health 106: 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rathore C, Thomas B, Kesavadas C, Radhakrishnan K, 2012. Calcified neurocysticercosis lesions and hippocampal sclerosis: potential dual pathology? Epilepsia 53: e60–e62. [DOI] [PubMed] [Google Scholar]
- 81.Jama-Antonio JMC, Yasuda CL, Cendes F, 2019. Neurocysticercosis and hippocampal atrophy: MRI findings and the evolution of viable or calcified cysts in patients with neurocysticercosis. Front Neurol 10: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bianchin MM, et al. 2015. Neuroimaging observations linking neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res 116: 34–39. [DOI] [PubMed] [Google Scholar]
- 83.Nash TE, Pretell J, Garcia HH, 2001. Calcified cysticerci provoke perilesional edema and seizures. Clin Infect Dis 33: 1649–1653. [DOI] [PubMed] [Google Scholar]
- 84.Singh AK, Garg RK, Rizvi I, Malhotra HS, Kumar N, Gupta RK, 2017. Clinical and neuroimaging predictors of seizure recurrence in solitary calcified neurocysticercosis: a prospective observational study. Epilepsy Res 137: 78–83. [DOI] [PubMed] [Google Scholar]
- 85.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, Garcia HH; Cysticercosis Working Group in Peru , 2008. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol 7: 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mejia R, Nash TE, 2013. Corticosteroid withdrawal precipitates perilesional edema around calcified Taenia solium cysts. Am J Trop Med Hyg 89: 919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chandra PS, Bal C, Garg A, Gaikwad S, Prasad K, Sharma BS, Sarkar C, Singh MB, Padma VM, Tripathi M, 2010. Surgery for medically intractable epilepsy due to postinfectious etiologies. Epilepsia 51: 1097–1100. [DOI] [PubMed] [Google Scholar]
- 88.Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K, 2013. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia 54: 1815–1822. [DOI] [PubMed] [Google Scholar]