SUMMARY:
Thanatophoric dysplasia, achondroplasia, and hypochondroplasia belong to the fibroblast growth factor receptor 3 (FGFR3) group of genetic skeletal disorders. Temporal lobe abnormalities have been documented in thanatophoric dysplasia and hypochondroplasia, and in 1 case of achondroplasia. We retrospectively identified 13 children with achondroplasia who underwent MR imaging of the brain between 2002 and 2015. All children demonstrated a deep transverse temporal sulcus on MR imaging. Further common neuroimaging findings were incomplete hippocampal rotation (12 children), oversulcation of the mesial temporal lobe (11 children), loss of gray-white matter differentiation of the mesial temporal lobe (5 children), and a triangular shape of the temporal horn (6 children). These appearances are very similar to those described in hypochondroplasia, strengthening the association of temporal lobe malformations in FGFR3-associated skeletal dysplasias.
Achondroplasia is the most frequent form of short-limb dwarfism, affecting more than 250,000 people worldwide,1 with an incidence of 0.36–0.60 per 10,000 live births.2 Achondroplasia is inherited as an autosomal dominant condition, in which most cases result from a glycine-to-arginine substitution at codon 380 in the transmembrane domain of the fibroblast growth factor receptor 3 (FGFR3) gene.3 Thanatophoric dysplasia (a perinatal lethal skeletal dysplasia), achondroplasia, and hypochondroplasia belong to the FGFR3 group of genetic skeletal disorders; different allelic mutations result in the variable severity of expression.4,5
Temporal lobe abnormalities have been documented in thanatophoric dysplasia and hypochondroplasia.6–8 These changes were first reported in 1 case of an achondroplastic fetus in 20149 but are not commonly described findings in the postnatal brain imaging of affected individuals, despite relatively frequent neuroimaging of the brain and craniocervical junction in infants and children with achondroplasia.10,11 Well-documented features of MR imaging of the brain in children with achondroplasia are narrowing of the subarachnoid space at the level of the foramen magnum, ventriculomegaly, and bifrontal widening of the subarachnoid space.12
Abnormal gyration of the temporal lobes has been described in thanatophoric dysplasia on prenatal sonography13–15 and prenatal MR imaging, which showed abnormal gyration of the temporal and occipital lobes and deep sulci on the medial aspects of both temporal and occipital lobes.14 One case of prenatal MR imaging in an achondroplastic fetus was published in 2014 by Pugash et al,9 describing temporal and occipital lobe abnormalities characteristic of hypochondroplasia in addition to the finding of short bones. Achondroplasia was subsequently confirmed on postnatal clinical and genetic testing.
In a review of postmortem neuropathologic changes in cases of confirmed thanatophoric dysplasia, megalencephaly and hippocampal dysplasia were found in all patients. Other very common findings were the following: polymicrogyria, enlarged temporal lobes, deep transverse temporal sulci, and neuroglial heterotopia. The combination of megalencephaly, an enlarged temporal lobe, and aberrant deep sulci was suggested to be specific for thanatophoric dysplasia.6
We hypothesized that the association of temporo-occipital developmental brain malformations with FGFR3 osteochondrodysplasias extends to include individuals with achondroplasia, the most common nonlethal skeletal dysplasia.
Case Series
Approval for the study was obtained from the Royal Children's Hospital Melbourne Human Research Ethics Committee. A retrospective search was conducted of the Radiology Information System to identify children (from birth to 18 years of age) with a diagnosis of achondroplasia (determined by clinical, radiologic, or genetic evaluation) who had MR imaging of the brain performed between January 1, 2002, and December 31, 2015, for any clinical indication. Seventeen children with achondroplasia were retrospectively identified from the Radiology Information System; ranging in age from 3 months to 12 years, with a median age of 2.5 years at the time of MR imaging. Four patients were excluded due to inadequate sequences for evaluation of the specific imaging criteria. Thirteen subjects were, therefore, included in the study (On-line Table). In the 9 children who underwent genetic testing, a common G-to-A transition at nucleotide 1138 (c.1138G>A G380R mutation) in the FGFR3 gene was confirmed. In the remaining 4 children who did not undergo genetic testing, the diagnosis of achondroplasia was confirmed clinically and radiographically by a clinical geneticist.
The MR imaging studies were performed with 1 of three 1.5T imaging systems. The slice thickness of the T2WI sequences ranged from 1.5 to 4 mm. The T1WI was either reconstructed from 3D-T1WI or acquired with a slice thickness of 1.5–3 mm. Two pediatric neuroradiologists (S.A. Mandelstam, A.M.F.) and a pediatric neurologist (K.B.H.) reviewed the imaging for the presence of malformations of cortical development.
The MR images were assessed for the presence of the following: temporal lobe enlargement (defined as bulging of the surface of the temporal lobe beyond the contour of the adjacent frontal and parietal lobes), loss of gray-white matter differentiation of the mesial temporal lobe, incomplete hippocampal inversion, oversulcation of the mesial temporal lobe (assessed on coronal T2WI and 3D-T1WI), extension of oversulcation to the calcar avis, a deep transverse temporal sulcus (assessed separately on axial T1WI and sagittal T2WI), triangular shape of the temporal horn, ventriculomegaly (defined as frontal horns of the lateral ventricles measuring >10 mm), megalencephaly, polymicrogyria, and subependymal heterotopia. The results were consensus-based.
MR imaging studies in all children showed the presence of a deep transverse temporal sulcus, visible on axial T1WI (Fig 1) and sagittal T2WI (Fig 2). Incomplete hippocampal inversion (Fig 3) and ventriculomegaly were found in 12 children (92%) (Table). The next most prevalent finding was oversulcation of the mesial temporal lobe in 11 (85%) (Fig 4), with extension of oversulcation to involve the calcar avis in 9 (69%) (Fig 5). Further common findings were loss of gray-white matter differentiation of the mesial temporal lobe in 5 children (71% of the children older than 36 months of age in whom this could be accurately assessed), a triangular shape of the temporal horn in 6 (46%) (Fig 1), and megalencephaly in 5 (38%). In our cohort, all children with megalencephaly also exhibited ventriculomegaly. Subependymal heterotopia and polymicrogyria were not demonstrated in any children. In one of the children, the temporal lobe changes were also recognized antenatally at 30 weeks' gestation, after the detection of short limbs and mild ventriculomegaly in the fetus on sonography. Comparison of the postnatal with the fetal imaging (Figs 2C and 4C) illustrates the evolution of the findings with brain maturation.
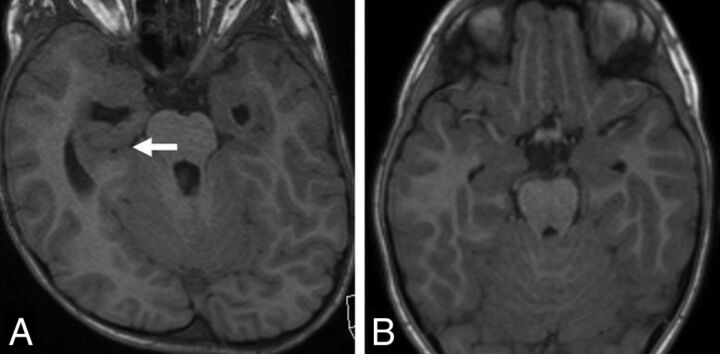
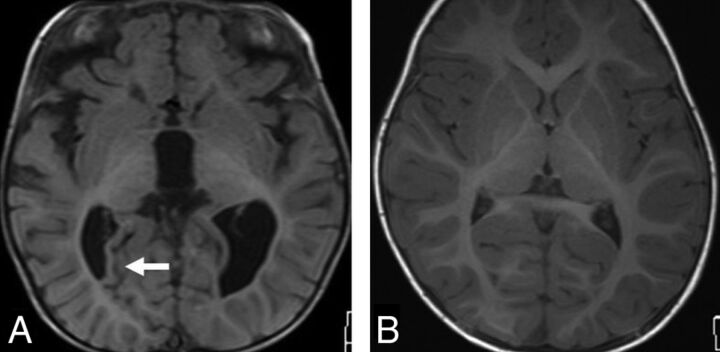
Fig 1.
A, Axial T1-weighted MR image in a 5-year-old boy with achondroplasia shows a deep transverse sulcus within the temporal lobe (arrow) and abnormal configuration of the temporal horns of the lateral ventricles. B, Axial T1-weighted MR image in a 5-year-old male control shows the normal configuration of the mesial temporal lobes and temporal horns.
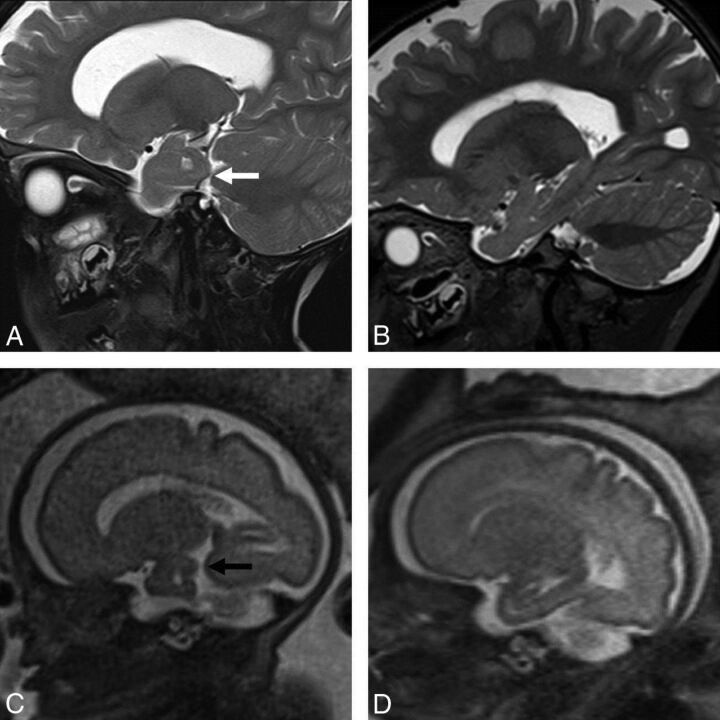
Fig 2.
A, Sagittal T2-weighted MR image in a 2-year-old boy with achondroplasia shows a sagittal cleft within the temporal lobe (white arrow). B, Sagittal T2-weighted MR image in a 2-year-old male control shows the normal appearance of the temporal lobe. C, Antenatal sagittal T2-weighted MR image of the child with achondroplasia in A demonstrates the prenatal appearance of the sagittal clefting within the temporal lobe at 30 weeks (black arrow). D, Antenatal sagittal T2-weighted MR image of a control fetus shows the normal appearance of the temporal lobe at the same gestation.
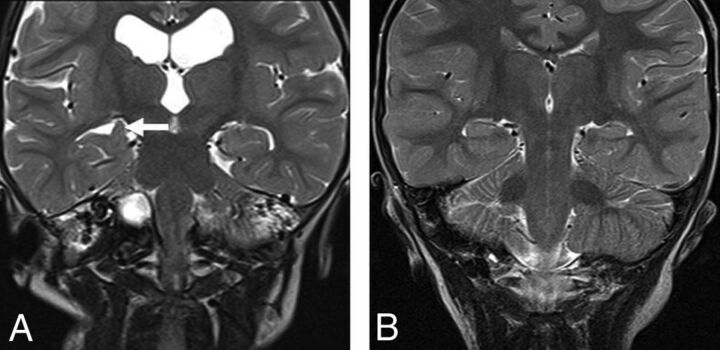
Fig 3.
A, Coronal T2-weighted MR image in a 30-month-old boy with achondroplasia shows incomplete hippocampal inversion (arrow) and ventriculomegaly. B, Coronal T2-weighted MR image in a 30-month-old male control shows the normal appearance of the hippocampi.
Prevalence of MRI findings in children with achondroplasia
| MRI Finding | Prevalence |
|---|---|
| Deep transverse temporal sulcus (axial T1WI) | 13/13 (100%) |
| Sagittal clefting in the medial temporal lobe (sagittal T2WI) | 13/13 (100%) |
| Incomplete hippocampal inversion | 12/13 (92%) |
| Ventriculomegaly | 12/13 (92%) |
| Oversulcation of the mesial temporal lobe | 11/13 (85%) |
| Extension of oversulcation to the calcar avis | 9/13 (69%) |
| Loss of gray-white matter differentiation of the mesial temporal lobe | 5/7a (71%) |
| Abnormal triangular shape of the temporal horn | 6/13 (46%) |
| Megalencephaly | 5/13 (38%) |
| Temporal lobe enlargement | 1/13 (8%) |
In the 6 children younger than 36 months of age, loss of gray-white matter differentiation of the mesial temporal lobe could not be accurately assessed on available sequences due to the incomplete myelination.
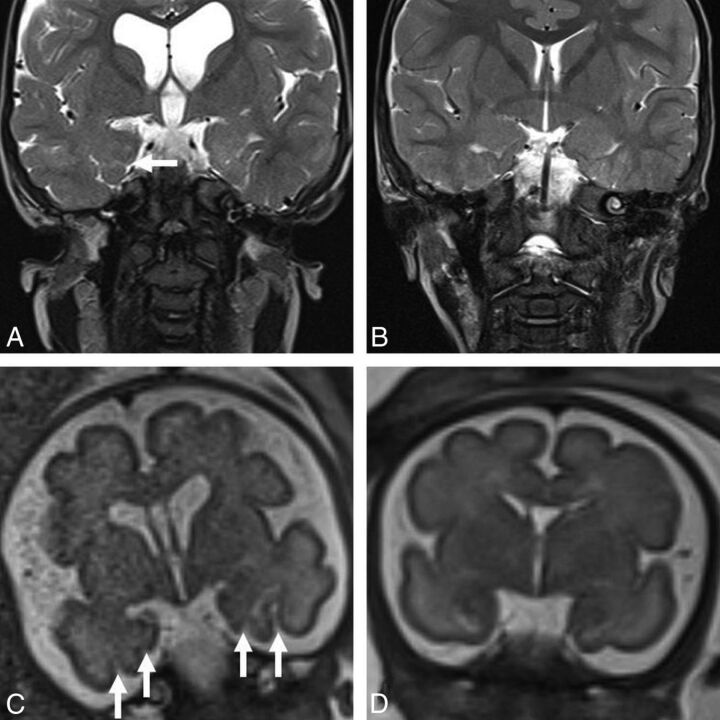
Fig 4.
A, Coronal T2-weighted MR image in a 30-month-old boy with achondroplasia shows oversulcation and loss of gray-white matter differentiation of the mesial temporal lobes (arrow) and ventriculomegaly. B, Coronal T2-weighted MR image in a 30-month-old male control shows the normal sulcation and gray-white matter differentiation of the temporal lobes. C, Antenatal coronal T2-weighted fetal MR image of the child with achondroplasia in A obtained at 30 weeks shows oversulcation of the mesial temporal lobes (white arrows) and mild ventriculomegaly. D, Antenatal coronal T2-weighted MR image of a control fetus shows the normal appearance of the temporal lobes at the same gestation.
Fig 5.
A, Axial T1-weighted MR image in a 1-year-old girl with achondroplasia shows oversulcation of the calcar avis (arrow) and moderate ventriculomegaly. B, Axial T1-weighted MR image in a 1-year-old female control shows the normal sulcation pattern of the calcar avis.
Seizures were reported in only 1 child who had focal seizures between 3 and 12 months of age. Seizures occurred in clusters and were characterized by behavioral arrest, apnea, facial suffusion, and tachycardia. Electroencephalography recorded seizures arising independently in the left and right temporal regions.
Discussion
Temporal lobe malformations have been previously described in other conditions composing the FGFR3 family of skeletal dysplasias and were first reported in 2014 by Pugash et al9 in 1 case of an achondroplastic fetus with prenatal and subsequent postnatal imaging. In 2012, Linnankivi et al7 described 8 patients with hypochondroplasia and an FGFR3 N540K mutation in whom brain MR imaging demonstrated bilateral temporal lobe dysgenesis, with abnormally shaped temporal horns and an aberrant hippocampal configuration. In a further cohort of children with hypochondroplasia, Philpott et al,8 in 2013, additionally described temporal lobe enlargement, deep transverse temporal sulci, overly sulcated mesial temporal lobes, megalencephaly, and mild ventriculomegaly. Correlating our findings with those of previous studies evaluating brain appearances in thanatophoric dysplasia and hypochondroplasia revealed common findings of temporal lobe sulcation abnormalities, incomplete hippocampal inversion, and ventriculomegaly.6,8 Polymicrogyria and subependymal heterotopia have been described in postmortem cases of thanatophoric dysplasia on neuropathology6 and in imaging of a case with hypochondroplasia,8 but these were not seen in our study. It may be that these are less common features that our sample size was not large enough to detect, or it may reflect differential effects of the different FGFR3 mutations on the brain. It is also possible that subtle cases of polymicrogyria or subependymal heterotopia may be present pathologically, however, not visible on the MR images assessed.
There is a growing body of literature elucidating the role of FGFR3 mutations in neural and skeletal development. FGFR is a membrane tyrosine kinase encoded by 4 genes (FGFR 1–4). In embryonic tissues in mice, FGFR3 expression is limited principally to neural tube derivatives (developing brain and spinal cord), cartilage rudiments of developing bone, the cochlea, and the lens.16 All 4 fibroblast growth factor receptors are expressed in the developing brain and contribute to its development.6 The FGFR3 gene in mice is expressed in a gradient in the developing cortex, with the highest levels adjacent to the hippocampal primordia and cortical hem.6 Mice with constitutive activation of FGFR3 in the forebrain demonstrated selective promotion of growth of the caudolateral (occipitotemporal) cortex, which is highly correlated with the gradient of FGFR3 expression in the ventricular zone at early stages of neurogenesis.17
Seizures are reported in FGFR3 disorders, but rarely in achondroplasia. Our patient had temporal lobe seizures in infancy, similar to that reported in hypochondroplasia7 and Muenke syndrome,18 raising the possibility that FGFR3-associated temporal lobe dysgenesis predisposes to seizures. It is of clinical importance, however, to note that in our series, most children did not have seizures despite having temporal lobe malformations. It is not clear whether this is the case in the other FGFR3 disorders.
Similar temporal lobe malformations are described in Apert syndrome, which is the result of localized gain-of-function mutations of fibroblast growth factor receptor 2 (FGFR2) and characterized by craniosynostosis and syndactyly of the hands and feet.19 The findings described in prenatal and postnatal imaging include expansion and overconvolution of the temporal lobes and temporal lobe clefts.20–22
The major limitations of our study are that it is retrospective, with a small sample size. It is not possible to determine what proportion of patients with achondroplasia have temporal lobe changes on the basis of such small numbers or to clarify the clinical and radiologic correlations. A further limitation is the qualitative rather than quantitative assessment of these cortical malformations on conventional MR imaging sequences.
In conclusion, this case series demonstrates the frequent presence of temporal lobe malformations in children with achondroplasia, the most common nonlethal skeletal dysplasia. The findings herein strengthen the association of temporal lobe malformations and skeletal dysplasias due to different mutations in the FGFR3 gene.
ABBREVIATION:
- FGFR3
fibroblast growth factor receptor 3
References
- 1. Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet 2007;370:162–72 10.1016/S0140-6736(07)61090-3 [DOI] [PubMed] [Google Scholar]
- 2. Waller DK, Correa A, Vo TM, et al. The population-based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. Am J Med Genet A 2008;146A:2385–89 10.1002/ajmg.a.32485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellus GA, Hefferon TW, Ortiz de Luna RI, et al. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet 1995;56:368–73 [PMC free article] [PubMed] [Google Scholar]
- 4. Naski MC, Wang Q, Xu J, et al. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat Genet 1996;13:233–37 10.1038/ng0696-233 [DOI] [PubMed] [Google Scholar]
- 5. Vajo Z, Francomano CA, Wilkin DJ. The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: the achondroplasia family of skeletal dysplasias, Muenke craniosynostosis, and Crouzon syndrome with acanthosis nigricans. Endocr Rev 2000;21:23–39 [DOI] [PubMed] [Google Scholar]
- 6. Hevner RF. The cerebral cortex malformation in thanatophoric dysplasia: neuropathology and pathogenesis. Acta Neuropathol 2005;110:208–21 10.1007/s00401-005-1059-8 [DOI] [PubMed] [Google Scholar]
- 7. Linnankivi T, Mäkitie O, Valanne L, et al. Neuroimaging and neurological findings in patients with hypochondroplasia and FGFR3 N540K mutation. Am J Med Genet A 2012;158A:3119–25 10.1002/ajmg.a.35642 [DOI] [PubMed] [Google Scholar]
- 8. Philpott C, Widjaja E, Raybaud C, et al. Temporal and occipital lobe features in children with hypochondroplasia/FGFR3 gene mutation. Pediatr Radiol 2013;43:1190–95 10.1007/s00247-013-2684-3 [DOI] [PubMed] [Google Scholar]
- 9. Pugash D, Lehman AM, Langlois S. Prenatal ultrasound and MRI findings of temporal and occipital lobe dysplasia in a twin with achondroplasia. Ultrasound Obstet Gynecol 2014;44:365–68 10.1002/uog.13359 [DOI] [PubMed] [Google Scholar]
- 10. Richette P, Bardin T, Stheneur C. Achondroplasia: from genotype to phenotype. Joint Bone Spine 2008;75:125–30 10.1016/j.jbspin.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 11. Trotter TL, Hall JG. Health supervision for children with achondroplasia. Pediatrics 2005;116:771–83 10.1542/peds.2005-1440 [DOI] [PubMed] [Google Scholar]
- 12. Kao SC, Waziri MH, Smith WL, et al. MR imaging of the craniovertebral junction, cranium, and brain in children with achondroplasia. AJR Am J Roentgenol 1989;153:565–69 10.2214/ajr.153.3.565 [DOI] [PubMed] [Google Scholar]
- 13. Miller E, Blaser S, Shannon P, et al. Brain and bone abnormalities of thanatophoric dwarfism. AJR Am J Roentgenol 2009;192:48–51 10.2214/AJR.08.1524 [DOI] [PubMed] [Google Scholar]
- 14. Fink AM, Hingston T, Sampson A, et al. Malformation of the fetal brain in thanatophoric dysplasia: US and MRI findings. Pediatr Radiol 2010;40(suppl 1):S134–37 10.1007/s00247-010-1697-4 [DOI] [PubMed] [Google Scholar]
- 15. Blaas HG, Vogt C, Eik-Nes SH. Abnormal gyration of the temporal lobe and megalencephaly are typical features of thanatophoric dysplasia and can be visualized prenatally by ultrasound. Ultrasound Obstet Gynecol 2012;40:230–34 10.1002/uog.11127 [DOI] [PubMed] [Google Scholar]
- 16. Peters K, Ornitz D, Werner S, et al. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol 1993;155:423–30 10.1006/dbio.1993.1040 [DOI] [PubMed] [Google Scholar]
- 17. Thomson RE, Kind PC, Graham NA, et al. FGF receptor 3 activation promotes selective growth and expansion of occipitotemporal cortex. Neural Dev 2009;4:4 10.1186/1749-8104-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grosso S, Farnetani M, Berardi R, et al. Medial temporal lobe dysgenesis in Muenke syndrome and hypochondroplasia. Am J Med Genet A 2003;120A:88–91 10.1002/ajmg.a.10171 [DOI] [PubMed] [Google Scholar]
- 19. Anderson J, Burns HD, Enriquez-Harris P, et al. Apert syndrome mutations in fibroblast growth factor receptor 2 exhibit increased affinity for FGF ligand. Hum Mol Genet 1998;7:1475–83 10.1093/hmg/7.9.1475 [DOI] [PubMed] [Google Scholar]
- 20. Tokumaru AM, Barkovich AJ, Ciricillo SF, et al. Skull base and calvarial deformities: association with intracranial changes in craniofacial syndromes. AJNR Am J Neuroradiol 1996;17:619–30 [PMC free article] [PubMed] [Google Scholar]
- 21. Raybaud C, Di Rocco C. Brain malformation in syndromic craniosynostoses, a primary disorder of white matter: a review. Childs Nerv Syst 2007;23:1379–88 10.1007/s00381-007-0474-7 [DOI] [PubMed] [Google Scholar]
- 22. Stark Z, McGillivray G, Sampson A, et al. Apert syndrome: temporal lobe abnormalities on fetal brain imaging. Prenat Diagn 2015;35:179–82 10.1002/pd.4515 [DOI] [PubMed] [Google Scholar]