Prospectively managed stroke data bases from 2 separate centers were retrospectively studied between 2009 and 2014 for records of tandem occlusions related to internal carotid dissection. The first step in the revascularization procedure was intracranial thrombectomy. Then, cervical carotid stent placement was performed depending on the functionality of the circle of Willis and the persistence of residual cervical ICA occlusion, severe stenosis, or thrombus apposition. Efficiency, complications, and radiologic and clinical outcomes were recorded. Thirty-four patients presenting with tandem occlusion stroke secondary to internal carotid dissection were treated during the study period. The mean age was 52.5 years, the mean initial NIHSS score was 17, and the mean delay between onset and groin puncture was 3.58 hours. Recanalization of TICI 2b/3 was obtained in 21 cases (62%). Fifteen patients underwent cervical carotid stent placement. There was no recurrence of ipsilateral stroke in the nonstented subgroup. The authors conclude that endovascular treatment of internal carotid dissection-related tandem occlusion stroke using the distal-to-proximal recanalization strategy appears to be feasible, with low complication rates and considerable rates of successful recanalization.
Abstract
BACKGROUND AND PURPOSE:
Internal carotid dissection is a frequent cause of ischemic stroke in young adults. It may cause tandem occlusions in which cervical carotid obstruction is associated with intracranial proximal vessel occlusion. To date, no consensus has emerged concerning endovascular treatment strategy. Our aim was to evaluate our endovascular “distal-to-proximal” strategy in the treatment of this stroke subtype in the first large multicentric cohort.
MATERIALS AND METHODS:
Prospectively managed stroke data bases from 2 separate centers were retrospectively studied between 2009 and 2014 for records of tandem occlusions related to internal carotid dissection. Atheromatous tandem occlusions were excluded. The first step in the revascularization procedure was intracranial thrombectomy. Then, cervical carotid stent placement was performed depending on the functionality of the circle of Willis and the persistence of residual cervical ICA occlusion, severe stenosis, or thrombus apposition. Efficiency, complications, and radiologic and clinical outcomes were recorded.
RESULTS:
Thirty-four patients presenting with tandem occlusion stroke secondary to internal carotid dissection were treated during the study period. The mean age was 52.5 years, the mean initial NIHSS score was 17.29 ± 6.23, and the mean delay between onset and groin puncture was 3.58 ± 1.1 hours. Recanalization TICI 2b/3 was obtained in 21 cases (62%). Fifteen patients underwent cervical carotid stent placement. There was no recurrence of ipsilateral stroke in the nonstented subgroup. Twenty-one patients (67.65%) had a favorable clinical outcome after 3 months.
CONCLUSIONS:
Endovascular treatment of internal carotid dissection–related tandem occlusion stroke using the distal-to-proximal recanalization strategy appears to be feasible, with low complication rates and considerable rates of successful recanalization.
Acute obstruction or occlusion of the extracranial ICA and additional intracranial ICA or MCA thrombus, so-called tandem occlusion, causes a severe form of ischemic stroke, which accounts for 10%–20% of major strokes and is associated with high rates of disability and death.1,2 Most stenotic or occlusive lesions of the ICA are caused by atherosclerotic disease or acute dissection. These 2 pathologies constitute distinct stroke etiologies that affect 2 different patient populations, each with their own prognosis. Internal carotid dissection (ICD) is a frequent cause of ischemic stroke, especially in young adults.3 In cervical carotid occlusive or near-occlusive disease, stroke is due to wall hematoma, accompanied by downstream intracranial embolus or hemodynamic impairment.4
Tandem occlusion stroke was known to have a very poor prognosis at the time when treatment consisted of intravenous thrombolysis alone.2,5,6 Endovascular treatment is now increasingly performed following the results from recent randomized controlled trials (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands [MR CLEAN], Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke [ESCAPE], Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial [EXTEND IA], Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours [REVASCAT], Solitaire With the Intention For Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke [SWIFT PRIME], and DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention with Trevo [DAWN]),7–12 which demonstrated the superiority of combined endovascular strategies over stand-alone intravenous treatment in large-vessel occlusion stroke. Endovascular strategies, however, remain understudied in the specific setting of tandem occlusions and were even excluded from some thrombectomy clinical trials, such as ESCAPE and SWIFT PRIME.7,8 Of several endovascular strategies that have been proposed during the past few years, the “proximal-to-distal” approach (the so-called antegrade approach) has been predominant.13–21 It consists of stepwise treatment of the arterial occlusion, starting from the cervical internal carotid lesion and finishing with the intracranial occlusion. We propose a different approach, the “distal-to-proximal” approach, in which the intracranial occlusion is treated first to quickly restore cerebral blood flow. Then, treatment of the internal carotid cervical dissection is performed, depending on functionality of the circle of Willis and persistence of an occlusive dissection. This method had previously demonstrated promising results.22
The present study describes the technical and clinical results of the first multicenter study evaluating the distal-to-proximal endovascular approach in cases of tandem occlusion related to ICD.
Materials and Methods
Sample
Records of all patients presenting with anterior circulation ischemic stroke treated with an endovascular approach in 2 separate institutions between August 2009 and April 2013 (Montpellier) and January 2010 and September 2014 (Bern) were retrospectively retrieved from prospectively maintained stroke data bases.
Patient Selection
Endovascular therapy was performed immediately after CT or MR imaging under the following conditions: 1) Diagnosis of ischemic stroke was established by CT/CTA/CT perfusion or MR/MRA/MR perfusion imaging; 2) the baseline NIHSS score was ≥4, or isolated aphasia or hemianopia was present; 3) hemorrhage had been excluded by cranial CT or MR imaging; 4) symptom duration was not longer than 24 hours; 5) no individual clinical or premorbid conditions or laboratory findings contraindicated treatment; and 6) tandem occlusion of the carotid-T or M1/M2 segment associated with cervical ICA obstruction or occlusion related to cervical ICA dissection was demonstrated by initial imaging and/or peri-interventional angiograms.
Decision-Making and Imaging
Initial NIHSS and Glasgow Coma Scale scores were assessed by a neurologist. CT and MR imaging were used, depending on the protocol of the center. The routine CT imaging protocol consisted of unenhanced CT and CT angiography and CT perfusion. The routine MR imaging protocol consisted of DWI, FLAIR, T2*/SWI, contrast-enhanced MR angiography including the supra-aortic trunks, and MR perfusion imaging. If tandem occlusion was suspected on initial imaging, it was confirmed with DSA based on the morphologic aspect of the cervical segment of the internal carotid artery (Fig 1) associated with a proximal intracranial vessel occlusion (carotid termination, M1, M1–M2 junction, and/or M2 segment of the middle cerebral artery). The ICD diagnosis was based on clinical, imaging, and angiographic data. We aimed to distinguish dissection from atheromatous cervical carotid artery lesions.
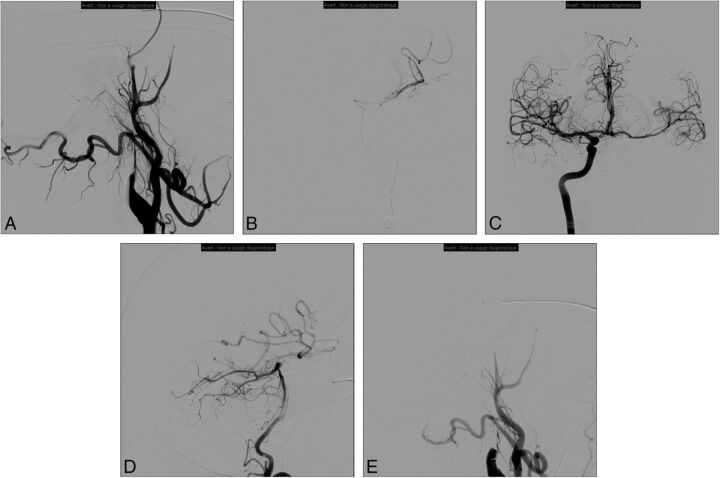
Fig 1.
Patient presenting with right hemiplegia and aphasia (NIHSS score = 20). Initial MR imaging revealed a DWI-ASPECTS of 6 after 4.5 hours since symptom onset associated with left tandem ICA and middle cerebral artery occlusions. Initial angiogram (A) demonstrates left internal carotid occlusion related to cervical dissection. We then carefully navigate the microcatheter through the dissected ICA to the intracranial occlusion (B); then thrombectomy is performed. After contralateral femoral puncture, the right ICA run shows a functional circle of Willis and no residual left M1 occlusion (C). The posterior communicating artery is also permeable as seen on the left vertebral artery run (D). Consequently, we decided not to treat the cervical ICA dissection, and the artery is left in its initial condition (E).
Intravenous Thrombolysis
Intravenous thrombolysis (0.9 mg/kg; 10% of the dose as a bolus and the remainder during 60 minutes) was administered to patients within a maximum of 4.5 hours after stroke onset. Conventional clinical and laboratory inclusion and exclusion criteria for intravenous thrombolysis were applied.
Endovascular Procedure
Endovascular treatment was performed with the patient under general anesthesia or conscious sedation. The decision on the type of sedation was made on an individual basis by the neurologist, neuroradiologist, and anesthesiologist on call. DSA was performed via a transfemoral approach using a biplane, high-resolution angiography system. In one center (Bern, Switzerland), a 4-vessel cerebral angiography was always performed before the intervention. In the other center (Montpellier, France), it was performed after intracranial recanalization through a second femoral approach to assess collateral circulation through the circle of Willis and leptomeningeal collaterals. An 8F guiding catheter or an 8F or 9F balloon-guiding catheter (Guider Softip XF, Boston Scientific, Fremont, California; Merci retriever, Concentric Medical, Mountain View, California) was introduced into the common carotid artery, and an angiographic run was performed to evaluate the occlusion. During this step, cervical internal carotid artery occlusion was demonstrated and eventually differentiated from carotid contrast agent stagnation related to isolated carotid termination thrombus. After we took into account both clinical data and angiographic morphology, ICD was distinguished from atheromatous occlusion.
In cases of obstruction related to cervical ICD, a 0.021-inch microcatheter (Headway microcatheter, MicroVention, Tustin, California; Prowler Select Plus microcatheter, Codman & Shurtleff, Raynham, Massachusetts) was navigated through the true lumen of the dissection over a 0.014-inch microwire (Transend 014, Stryker, Kalamazoo, Michigan; Traxcess, MicroVention; SilverSpeed-14, Covidien, Irvine, California). This maneuver was performed under flow arrest either through wedging of the guide catheter in the stenosis or inflation of the balloon-guide catheter to avoid thromboembolic events during crossing of the lesion. Once the microcatheter position in the true lumen had been confirmed distally by a careful contrast injection, an intermediate 5F guiding catheter (5MAX and 5MAX ACE Reperfusion Catheter, Penumbra, Alameda, California; Vasco +35ASPI, Balt Extrusion, Montmorency, France) was then advanced over the microcatheter into the distal internal carotid artery. After we crossed the thrombus over the microwire with the microcatheter, mechanical thrombectomy was performed with a Solitaire FR (Covidien) or Trevo stent (Stryker). The thrombectomy maneuver was also performed under flow arrest and manual aspiration through the intermediate catheter to prevent clot fragmentation and distal embolism. As an alternative access technique, the stent retriever was deployed first through the microcatheter; then, the intermediate catheter was advanced over the pusher wire of the stent retriever using the stent retriever as an intracranial anchor (so-called anchoring technique) to gain intracranial access with the intermediate catheter, especially in cases with very tortuous anatomy.
The result of intracranial recanalization was evaluated using the TICI score. Successful recanalization was defined as TICI 2b or 3. If intracranial recanalization was achieved, treatment of the cervical carotid dissection was left to the discretion of the operator, taking into account the functionality of the circle of Willis, residual cervical occlusion/severe stenosis or thrombus apposition, and difficulty of the endovascular access.
The 0.014-inch microwire was introduced into the petrous segment of the carotid artery to preserve distal access to the true arterial lumen for cervical stent placement. In 1 center (Bern, Switzerland), a filter protection device (FilterWire EZ; Boston Scientific) was placed distal to the dissection when anatomically possible. Then, the guiding catheter and the intermediate catheter were retrieved into the common carotid artery. A 250- to 500-mg bolus of aspirin was administered intravenously before stent placement. Cervical carotid artery stent placement was performed using the carotid Wallstent (Boston Scientific), Precise (Cordis, Fremont, California), Cristallo Ideale (Medtronic, Roncadelle, Italy), or, in case of distal cervical carotid dissection, the LEO (Balt Extrusion), Enterprise (Codman & Shurtleff), or Wingspan (Stryker) stent. Appropriate positioning and opening of the stent were immediately assessed angiographically, and additional angioplasty was performed if necessary. Several stents were used if the dissection was extensive and occlusive in the upper cervical and pre-petrous segments.
Follow-Up
Follow-up CT or MR imaging was performed 24 hours after the acute therapy to assess infarction volume and hemorrhagic status. Symptomatic intracranial hemorrhage (sICH) was defined as a documented hemorrhage associated with a decline of ≥4 points in the NIHSS score. If no hemorrhage occurred, double antiplatelet therapy was initiated and continued for 3–6 months. Single antiplatelet treatment was then maintained for 1 year or life-long, depending on the protocol of the responsible center.
NIHSS was measured following recovery from the anesthetic and throughout hospitalization until discharge. Routine clinical follow-up was performed at 3 months by an independent neurologist to evaluate the patient's recovery using the mRS. Clinical outcome was quantified by 3-month mRS and mortality. Favorable outcome was defined as a mRS ≤ 2.
Results
Patient Population
Between August 2009 and April 2013 (Montpellier) and January 2010 to September 2014 (Bern), 531 patients with anterior circulation ischemic stroke were treated in the 2 institutions. Tandem occlusion stroke related to ICD was identified in 34 of these patients (12 women and 22 men). The mean age was 52.47 ± 10.25 years (range, 30–73 years), and the mean NIHSS score was 17.29 ± 6.23 (range, 4–36). A history of high blood pressure was noted in 14 patients. Except for patients with unknown symptom onset, such as wake-up strokes, the average time from symptom onset to arterial puncture was 215.8 ± 66.07 minutes (range, 142–360 minutes). Intravenous thrombolysis was performed in 21 cases (61.8%). Baseline characteristics of the population are given in Table 1. Patients with presumed atheromatous tandem occlusions were excluded from the study.
Table 1:
Baseline characteristics of the population
| Baseline Characteristics | Values |
|---|---|
| Age (mean) (range) (yr) | 52.47 (30–73) |
| Sex (No.) (%) | |
| Female | 12 (35.3%) |
| Male | 22 (64.7%) |
| Initial NIHSS score (mean) (range) | 17.29 (4–36) |
| High blood pressure (No.) (%) | 14 (41.2%) |
| Associated IVT (No.) (%) | 21 (61.8%) |
Note:—IVT indicates intravenous thrombolysis.
Procedural Results and Early Evolution
Thirty-three (97%) patients were treated under general anesthesia, and 1 (3%) patient, under conscious sedation. Successful intracranial recanalization (TICI 2b or 3) was achieved in 23 patients (67.65%). The mean time from onset to revascularization was 329.71 ± 128.82 minutes (range, 180–855 minutes). The mean number of stent retriever passes was 2.03 (range, 1–4).
In our population of patients with ICD, 15 (44.12%) finally required cervical internal carotid stent deployment. Stent placement was technically feasible in all cases, and no distal thromboembolic events were associated with stent placement. Among the stented patient subgroup (n = 15), intracranial recanalization TICI 2b–3 was achieved in 8 patients (53.3%). In the nonstented subgroup, 15 patients (78.9%) benefited from revascularization, achieving TICI 2b–3. No periprocedural complications were observed in our multicenter cohort.
Representative endovascular strategies are illustrated in Figs 1 and 2.
Fig 2.
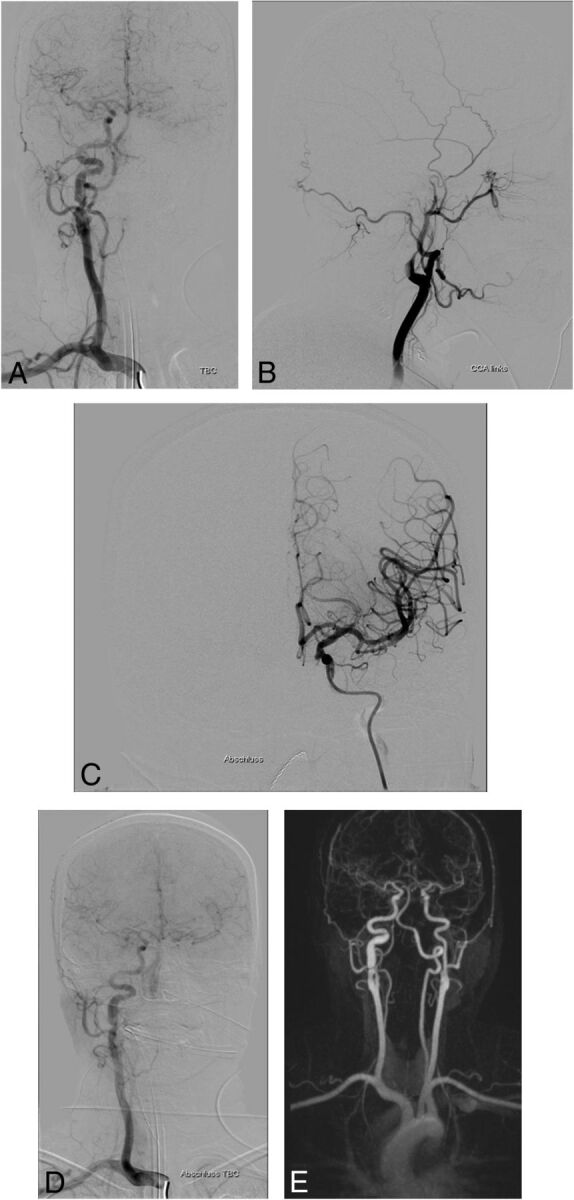
A 34-year-old woman presenting with right hemiplegia and aphasia (NIHSS score = 17). Initial contralateral angiogram depicting a left carotid-T occlusion with good leptomeningeal collaterals from the anterior cerebral artery (A). The left common carotid artery angiogram shows ICA occlusion related to cervical ICA dissection (B). After passing the dissection under proximal balloon occlusion with a microcatheter and an intermediate catheter, complete intracranial recanalization is achieved by mechanical thrombectomy using a Solitaire device (C). Control angiogram reveals extensive cervical ICA dissection with hemodynamic impairment (D). Contralateral injection shows functionality of the circle of Willis with good collateralization and patency of the left MCA (E). Therefore, the decision was made not to intervene on the left ICA. Control MRA after 3 months shows complete reconstitution of the left ICA under conservative management and without new neurologic events (mRS 1).
Two patients (5.9%) were affected by sICH. In 1 patient who received a cervical internal carotid artery stent, sICH in the basal ganglia occurred early despite a TICI 3 recanalization. Nevertheless, the patient had a favorable outcome with an mRS of 2 after 3 months. In the second patient, severe intracranial hemorrhage occurred early on while the patient was in the intensive care unit after unsuccessful mechanical revascularization (TICI 1) without cervical carotid artery stent placement, resulting in a mRS of 4 at 3-month follow-up.
Clinical Assessment after 3 Months
After 3 months, 23 (67.65%) of the 34 patients treated for ICD tandem occlusion demonstrated a favorable outcome (7 patients with mRS = 0; seven with mRS = 1; nine with mRS = 2). The mortality rate was 8.8% (3 patients). No patients were lost to clinical follow-up.
In the subgroup of patients who underwent ICA stent placement, 10 (66.7%) had mRS ≤ 2 at 3-month follow-up. In the unstented subgroup, 13 patients (68.4%) had a satisfactory clinical outcome. No stroke recurrence was observed in either of the subgroups.
The clinical results are summarized in Table 2 and the On-line Table.
Table 2:
Clinical results
| Procedural and Follow-Up Data | Values |
|---|---|
| Timing: onset-to-puncture (mean) (range) (min) | 215.8 (142–360) |
| Intracranial occlusion topography (No.) (%) | |
| Internal carotid terminus | 8 (23.5%) |
| M1 | 24 (70.6%) |
| M2 | 2 (5.9%) |
| No. of stent retriever passes (mean) (range) | 2.03 (1–4) |
| Cervical ICA stenting (No.) (%) | 15 (44.1%) |
| TICI 2b/3 (No.) (%) | 23 (67.65%) |
| Timing: onset-to-revascularization (mean) (range) (min) | 329.71 (180–855) |
| Favorable outcome (No.) (%) | 23 (67.65%) |
| Mortality (No.) (%) | 3 (8.8%) |
Note:—Stentriever is trademarked technology of Stryker (Trevo).
Discussion
Ischemic strokes due to a tandem occlusion caused by either atheromatous disease or a carotid dissection, if untreated, commonly have a poor prognosis and clinical outcome. Before the emergence of endovascular treatment, a very modest efficacy of intravenous thrombolysis was reported in the treatment of tandem occlusion.2,23 Linfante et al23 reported a very low rate of 31% recanalization on late 3-day imaging follow-up. Engelter et al5 reported a 36% rate of good outcome after intravenous thrombolysis in patients with tandem occlusions due to ICD.
Endovascular treatment approaches for tandem occlusions remain insufficiently studied. In some of the recent multicenter randomized trials, tandem occlusion strokes have even been considered an exclusion criterion.7,8 Most studies have analyzed tandem occlusion stroke by pooling atheromatous and ICD etiologies.13,15,18–21,24–29 In our opinion, these 2 entities involve 2 different diseases, each with a specific pathophysiologic origin and patient population. ICD usually affects young patients with patent supra-aortic trunks and circle of Willis. Cervical atheromatous disease affects an older population with chronic arterial lesions of the cervical and intracranial arteries and a high stroke recurrence rate.30 Thus, we consider these 2 etiologies completely different pathologies with different treatment strategies and prognoses.
The literature specifically addressing ICD tandem occlusion is scant.14,17,31–35 Reported satisfactory intracranial recanalization rates vary between 50% and 100%, and associated rates of favorable outcome, between 50% and 80%. Our article reports the results of the first multicenter study of endovascular treatment of this specific lesion subtype.
Different approaches and strategies have been proposed for the treatment of tandem occlusions. Most of the published studies have reported the results of antegrade recanalization.13,20 This consists of stepwise revascularization beginning from the cervical carotid artery lesion and ending by treating the intracranial occlusion. Despite overall satisfactory results, we believe that this approach can be improved. First, treating the cervical lesion with stent placement leads to significant prolongation of cerebral hypoperfusion. Cervical carotid stent placement may be difficult in a dissected vessel, and dissection can be extensive. Intracranial recanalization is performed as the second step, extending the duration of brain ischemia and potentially leading to larger infarct core volumes. The second disadvantage is that systematic cervical carotid stent placement necessitates antiplatelet medication, which may increase the risk of sICH.
Our approach is to focus first on intracranial revascularization.22 This shortens the duration of cerebral ischemia. The first step consists of navigating through the cervical carotid lesion up to the distal segments of the ICA. Avoiding the false lumen of the dissected vessel represents the main challenge. In our experience, it has never been impossible to reach the intracranial ICA via the true lumen. We routinely use a triaxial approach. If one positions the intermediate catheter beyond the dissection, the cervical occlusion is only crossed once to perform intracranial thrombectomy. This limits the maneuvers at the level of the dissected portion of the ICA, reducing the duration of the procedure and complication risk.
Our endovascular strategy also avoids cervical ICA stent placement in a significant number of cases. In our study population, the decision about stent placement was made taking into account cervical ICA residual steno-occlusion, circle of Willis functionality, intracranial recanalization TICI grade, and pretherapeutic infarct volume based on imaging. In many cases, after we navigated inside the dissected ICA through the true lumen, this simple maneuver was sufficient to reopen the ICA lumen enough to obtain efficient blood flow. In these cases, no further stent placement procedure of the ICA was required. Then, depending on circle of Willis functionality, we did not systematically stented the cervical ICA. When there was a likelihood that stent placement would be technically difficult and/or a high risk of hemorrhage due to antiplatelet therapy because of large cerebral infarct volume, we specifically studied circle of Willis functionality. Using previously published angiographic features demonstrating the functionality of the circle of Willis,36 we could decide not to stent the ICA if there was an efficient vascular supply from the contralateral ICA through the anterior or posterior communicating artery or leptomeningeal collaterals. Those 2 considerations leading to the decision not to stent the dissected ICA are supported by the low rate of delayed stroke recurrence after ICD.4 In the present study, no patient in the nonstented subgroup had a recurrence. Therefore, treatment of ICD by stent placement in tandem occlusion in patients with acute stroke should be carefully weighed in the light of the potentially benign course of the disease under conservative management and taking into account clinical and angiographic information in decision-making.
The sICH rate of only 2 cases (5.9%) was low. In the first case, the patient benefited from complete intracranial recanalization and cervical ICA stent placement. He presented with a favorable neurologic outcome (mRS, 2 after 3 months). In the second patient, sICH was likely related to extended cerebral infarction after failure of endovascular treatment (TICI score = 1), leading to a poor clinical outcome (mRS, 4 after 3 months).
Despite several limits of the present study, such as its retrospective nature, it is the largest study to date dealing with the endovascular treatment of acute stroke related to tandem occlusions due to ICD using the distal-to-proximal recanalization strategy. We also present the first reported multicenter experience with the recanalization treatment of this specific stroke subtype.
Conclusions
Endovascular treatment of ICD-related tandem occlusion stroke using the distal-to-proximal recanalization strategy appears feasible, with low complication rates and considerable rates of successful recanalization. This endovascular approach prioritizes the restoration of intracranial blood flow and focuses on making an informed decision about the need for stent placement of the cervical ICA. The decision should take into account clinical and angiographic information such as the degree of intracranial recanalization, dissection extent, infarct volume on initial imaging, and the functionality of the circle of Willis.
ABBREVIATIONS:
- ICD
internal carotid dissection
- sICH
symptomatic intracranial hemorrhage
Footnotes
Disclosures: Omer Faruk Eker—UNRELATED: Payment for Development of Educational Presentations: Stryker, Medtronic*. Jan Gralla—UNRELATED: Consulting Fee or Honorarium: Medtronic, Comments: Global Principal Investigator of the STAR study and SWIFT DIRECT trial, consultant for Medtronic*; Grants/Grants Pending: SNF, Comments: research grant for MRI stroke imaging*. Urs Fischer—UNRELATED: Consultancy: Medtronic, Stryker, Comments: Advisory Board and consultancy*; Grants/Grants Pending: Medtronic, Swiss National Science Foundation, Comments: research grants for SWIFT DIRECT, ELAN, and SWITCH trials.* Marcel Arnold—UNRELATED: Board Membership: Amgen, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Covidien, Daiichi Sankyo, Comments: honoraria for scientific advisory boards; Grants/Grants Pending: Swiss National Science Foundation, Swiss Heart Foundation*; Payment for Lectures Including Service on Speakers Bureaus: Bayer, Covidien, Comments: honoraria for lectures. Alain Bonafé—UNRELATED: Consultancy: Stryker, Medtronic, MicroVention. Vincent Costalat—UNRELATED: Consultancy: Stryker, Medtronic, Balt, Cerenovus; Grants/Grants Pending: Stryker, Medtronic*; Payment for Development of Educational Presentations: Stryker, Medtronic, Balt Extrusion. *Money paid to the institution.
REFERENCES
- 1. Grau AJ, Weimar C, Buggle F, et al. . Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001;32:2559–66 [DOI] [PubMed] [Google Scholar]
- 2. Rubiera M, Ribo M, Delgado-Mederos R, et al. . Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke 2006;37:2301–05 10.1161/01.STR.0000237070.80133.1d [DOI] [PubMed] [Google Scholar]
- 3. Arauz A, Hoyos L, Espinoza C, et al. . Dissection of cervical arteries: long-term follow-up study of 130 consecutive cases. Cerebrovasc Dis 2006;22:150–54 10.1159/000093244 [DOI] [PubMed] [Google Scholar]
- 4. Benninger DH, Georgiadis D, Kremer C, et al. . Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke 2004;35:482–85 10.1161/01.STR.0000109766.27393.52 [DOI] [PubMed] [Google Scholar]
- 5. Engelter ST, Rutgers MP, Hatz F, et al. . Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke 2009;40:3772–76 10.1161/STROKEAHA.109.555953 [DOI] [PubMed] [Google Scholar]
- 6. Engelter ST, Dallongeville J, Kloss M, et al. ; Cervical Artery Dissection and Ischaemic Stroke Patients-Study Group. Thrombolysis in cervical artery dissection: data from the Cervical Artery Dissection and Ischaemic Stroke Patients (CADISP) database. Eur J Neurol 2012;19:1199–206 10.1111/j.1468-1331.2012.03704.x [DOI] [PubMed] [Google Scholar]
- 7. Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 8. Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 9. Jovin T, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 10. Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 11. Berkhemer O, Fransen P, Beumer D, et al. . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 12. Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 13. Mpotsaris A, Bussmeyer M, Buchner H, et al. . Clinical outcome of neurointerventional emergency treatment of extra- or intracranial tandem occlusions in acute major stroke: antegrade approach with Wallstent and Solitaire stent retriever. Clin Neuroradiol 2013;23:207–15 10.1007/s00062-013-0197-y [DOI] [PubMed] [Google Scholar]
- 14. Lavallée PC, Mazighi M, Saint-Maurice JP, et al. . Stent-assisted endovascular thrombolysis versus intravenous thrombolysis in internal carotid artery dissection with tandem internal carotid and middle cerebral artery occlusion. Stroke 2007;38:2270–74 10.1161/STROKEAHA.106.481093 [DOI] [PubMed] [Google Scholar]
- 15. Cohen JE, Gomori JM, Rajz G, et al. . Extracranial carotid artery stenting followed by intracranial stent-based thrombectomy for acute tandem occlusive disease. J Neurointerv Surg 2015;7:412–17 10.1136/neurintsurg-2014-011175 [DOI] [PubMed] [Google Scholar]
- 16. Dababneh H, Bashir A, Hussain M, et al. . Endovascular treatment of tandem internal carotid and middle cerebral artery occlusions. J Vasc Interv Neurol 2014;14:26–31 [PMC free article] [PubMed] [Google Scholar]
- 17. Haussen DC, Jadhav A, Jovin T, et al. . Endovascular management vs intravenous thrombolysis for acute stroke secondary to carotid artery dissection: local experience and systematic review. Neurosurgery 2016;78:709–16 10.1227/NEU.0000000000001072 [DOI] [PubMed] [Google Scholar]
- 18. Malik AM, Vora NA, Lin R, et al. . Endovascular treatment of tandem extracranial/intracranial anterior circulation occlusions: preliminary single-center experience. Stroke 2011;42:1653–57 10.1161/STROKEAHA.110.595520 [DOI] [PubMed] [Google Scholar]
- 19. Papanagiotou P, Roth C, Walter S, et al. . Carotid artery stenting in acute stroke. J Am Coll Cardiol 2011;58:2363–69 10.1016/j.jacc.2011.08.044 [DOI] [PubMed] [Google Scholar]
- 20. Spiotta AM, Lena J, Vargas J, et al. . Proximal to distal approach in the treatment of tandem occlusions causing an acute stroke. J Neurointerv Surg 2015;7:164–69 10.1136/neurintsurg-2013-011040 [DOI] [PubMed] [Google Scholar]
- 21. Stampfl C, Ringleb P, Möhlenbruch M, et al. . Emergency cervical internal carotid artery stenting in combination with intracranial thrombectomy in acute stroke. AJNR Am J Neuroradiol 2014;35:741–46 10.3174/ajnr.A3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marnat G, Mourand I, Eker O, et al. . Endovascular management of tandem occlusion stroke related to internal carotid artery dissection using a distal to proximal approach: insight from the RECOST study. AJNR Am J Neuroradiol 2016;37:1281–88 10.3174/ajnr.A4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linfante I, Llinas RH, Selim M, et al. . Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke 2002;33:2066–71 10.1161/01.STR.0000021001.18101.A5 [DOI] [PubMed] [Google Scholar]
- 24. Kappelhof M, Marquering HA, Berkhemer OA, et al. . Intra-arterial treatment of patients with acute ischemic stroke and internal carotid artery occlusion: a literature review. J Neurointerv Surg 2015;7:8–15 10.1136/neurintsurg-2013-011004 [DOI] [PubMed] [Google Scholar]
- 25. Fischer U, Mono M-L, Schroth G, et al. . Endovascular therapy in 201 patients with acute symptomatic occlusion of the internal carotid artery. Eur J Neurol 2013;20:1017–24, e87 10.1111/ene.12094 [DOI] [PubMed] [Google Scholar]
- 26. Matsubara N, Miyachi S, Tsukamoto N, et al. . Endovascular intervention for acute cervical carotid artery occlusion. Acta Neurochir (Wien) 2013;155:1115–23 10.1007/s00701-013-1697-x [DOI] [PubMed] [Google Scholar]
- 27. Maurer C, Joachimski F, Berlis A. Two in one: endovascular treatment of acute tandem occlusions in the anterior circulation. Clin Neuroradiol 2015;25:397–402 10.1007/s00062-014-0318-2 [DOI] [PubMed] [Google Scholar]
- 28. Suh DC, Kim JK, Choi CG, et al. . Prognostic factors for neurologic outcome after endovascular revascularization of acute symptomatic occlusion of the internal carotid artery. AJNR Am J Neuroradiol 2007;28:1167–71 10.3174/ajnr.A0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lescher S, Czeppan K, Porto L, et al. . Acute stroke and obstruction of the extracranial carotid artery combined with intracranial tandem occlusion: results of interventional revascularization. Cardiovasc Intervent Radiol 2015;38:304–13 10.1007/s00270-014-1047-2 [DOI] [PubMed] [Google Scholar]
- 30. Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology 2004;62:569–73 10.1212/01.WNL.0000110311.09970.83 [DOI] [PubMed] [Google Scholar]
- 31. Baumgartner RW, Georgiadis D, Nedeltchev K, et al. . Stent-assisted endovascular thrombolysis versus intravenous thrombolysis in internal carotid artery dissection with tandem internal carotid and middle cerebral artery occlusion. Stroke 2008;39:e27–28 10.1161/STROKEAHA.107.500959 [DOI] [PubMed] [Google Scholar]
- 32. Fields JD, Lutsep HL, Rymer MR, et al. ; Merci Registry Investigators. Endovascular mechanical thrombectomy for the treatment of acute ischemic stroke due to arterial dissection. Interv Neuroradiol 2012;18:74–79 10.1177/159101991201800110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mourand I, Brunel H, Vendrell JF, et al. . Endovascular stent-assisted thrombolysis in acute occlusive carotid artery dissection. Neuroradiology 2010;52:135–40 10.1007/s00234-009-0597-5 [DOI] [PubMed] [Google Scholar]
- 34. Juszkat R, Liebert W, Stanisławska K, et al. . Extracranial internal carotid artery dissection treated with self-expandable stents: a single-centre experience. Cardiovasc Intervent Radiol 2015;38:1451–57 10.1007/s00270-015-1101-8 [DOI] [PubMed] [Google Scholar]
- 35. Pham M, Rahme RJ, Arnaout O, et al. . Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery 2011;68:856–66; discussion 866 10.1227/NEU.0b013e318209ce03 [DOI] [PubMed] [Google Scholar]
- 36. Abud DG, Spelle L, Piotin M, et al. . Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol 2005;26:2602–09 26/10/2602 [pii] [PMC free article] [PubMed] [Google Scholar]